Abstract
Background
We studied nasopharyngeal (NP) colonization in a cohort of children to determine the impact of viral upper respiratory infections (URI) on non-pneumococcal alpha hemolytic streptococci (AHS) and otopathogen colonization in association with acute otitis media (AOM).
Methods
NP samples were collected routinely when children were aged 6, 9, 12, 15, 18, 24, and 30 months and during episodes of AOM. NP samples were prospectively obtained from 248 children during a 5-year time span; 1,018 during routine visits, 161 at the time of AOM and 59 at follow-up visits 3 weeks after AOM.
Results
The overall NP colonization rate of AHS was 50.8% during a non-AOM visit but declined to 38.3% during a viral URI with concurrent AOM (p=0.0006). Of 56 AOM visits with paired follow-ups, 6 (10.7%) had AHS in the NP at the time of viral URI and concurrent AOM whereas 29 (51.8%) had AHS at the follow-up (p<0.001). Lower NP colonization rates with AHS were associated with significant increases in Streptococcus pneumoniae carriage during non-AOM visits (p<0.001) and during viral URI and concurrent AOM visits (p=0.003). AHS NP colonization rates were not different when children had a viral URI without AOM versus when they were URI negative, but NP colonization with non-typeable Haemophilus influenzae rates increased (p<0.001) and Moraxella catarrhalis decreased (p<0.001) during viral URI.
Conclusion
Respiratory viral infections alter NP carriage rates of commensal AHS and otopathogens, including prior to AOM.
Keywords: Alpha Hemolytic Streptococci, Streptococcus pneumoniae, Haemophilus influenzae, M. catarrhalis acute otitis media
INTRODUCTION
The upper respiratory tract microbiome may include potential bacterial pathogens that cause acute otitis media (AOM). Viral upper respiratory infections facilitate an increase in otopathogen density and alter host defenses thereby increasing the risk of AOM.(1;2) Streptococcus pneumoniae (Spn), non-typeable Haemophilus influenzae (NTHi), and Moraxella catarrhalis (M cat) are the predominant otopathogens.(3–5) The frequency that Spn and NTHi are isolated from the same nasopharyngeal (NP) specimen is significantly less than would be predicted based on their relative prevalence.(6) This suggests a competition in the NP and indeed several studies have provided explanations of how Spn could inhibit NTHi colonization.(7–9) Jacoby, et al. observed positive associations between pairwise combinations of Spn and NTHi and between Spn and M cat in healthy Aboriginal children.(10;11) This is in contrast to the observations of Pettigrew, et al, of a negative interaction between Spn and NTHi, which can shift with the presence of M cat allowing for all three potential otopathogens to colonize together in children with a viral URI.(11) Our group recently showed that when co-colonizing the NP, NTHi predominates over Spn to cause AOM except serotype 19A strains.(12)
Non-pneumococcal α-hemolytic Streptococci (AHS) also are part of the microbiome of the NP beginning at an early age.(13) AHS are normally avirulent commensals that have been shown to inhibit growth of Spn in the NP.(14) Previous studies have shown an increase in the rate of NP colonization with Spn, NTHi and M cat among children experiencing AOM and a decrease in the commensal flora at the time of infection.(15) Also reduced quantities of AHS have been found in otitis prone children.(16) Furthermore, loss of AHS due to antibiotic treatment raises the risk of colonization by otopathogens as well as AOM.(17–19)
Children younger than 2 years old suffer an average of six to eight respiratory infections per year, which coincides with the highest AOM occurrence.(20) Ninety-Four percent of children who develop AOM have a concurrent viral URI at diagnosis.(20;21) Respiratory syncytial virus (RSV) load in the presence of Spn, has been associated with increased risk of AOM in children.(22) No prior study has examined the influence of respiratory viral infections on AHS NP carriage or the associated effects on colonization with otopathogens in a prospective cohort of children at onset of AOM compared to without development of AOM. Therefore, we sought to determine alterations of the bacterial NP in the presence or absence of a respiratory viral infection with a specific focus on AHS and potential otopathogens in association with AOM.
Methods
Study design and population
The samples evaluated in this report were collected as part of a prospective study that commenced in July 2006 and is ongoing. For this analysis, we included samples collected between January 2007 and October 2011. The children were enrolled at 6 months of age and followed prospectively. NP and OP samples were obtained at 6, 9, 12, 15, 18, 24, and 30 months of age for microbial culture. Inclusion criteria were: healthy, full term birth, no craniofacial anomalies, no known immune deficits, and no AOM events prior to enrollment at 6 months of age. AOM was diagnosed by validated otoscopists relying on criteria recommended by the American Academy of Pediatrics but with a requirement for a bulging tympanic membrane.(23;24) Middle ear fluid (MEF; obtained by tympanocentesis) confirmed the diagnosis and established the etiology of AOM when a child developed their first and any subsequent AOM episodes. NP and OP samples were obtained at the time of AOM and 3 weeks later (follow-up samples). Patients enrolled prior to June 2010 received the PCV-7 vaccine, and patients enrolled after June 1, 2010 received PCV-13 vaccine. The study was approved by the University of Rochester and subsequently by the Rochester General Hospital IRB and written informed consent was obtained from parents.
Sampling NP and MEF samples were collected and processed as previously described.(25)
Demographics, Risk Factors, and Antibiotic Use
Upon enrollment, demographics and risk factors were elicited from the parents via questionnaire. The information collected included sex, breast-feeding status, tobacco smoke exposure in the household, family history of AOM, number of siblings, and participation in daycare. At every visit parents were asked if their child had antibiotics within the prior 30 days, and if so, what type, at what dose, and the duration of use.
Symptoms of Viral Upper Respiratory Infection
In the 2009–2010 and 2010–2011 winter respiratory seasons clinical viral upper respiratory infection (URI) symptoms were assessed by a physician investigator prospectively and samples for viral detection were collected. As described by Kalu et al, we defined a viral URI clinically by the occurrence of acute onset of rhinorrhea, cough, decreased appetite, and malaise with or without fever.(26) The number of days the symptoms were present prior to the sampling was obtained from the parents.
Bacteriology
NP swabs, nasal washes (NW), and MEF were plated on Chocolate II agar (BD) and TSA with 5% sheep blood (BD) and then isolates were selected based on colony morphology for further identification.
For the detection of Spn, colony morphology, α-hemolysis on trypticase soy agar (TSA) with 5% sheep blood, and inhibition by optochin were used to confirm the species identity. If inhibition by optochin was inconclusive, dissolution of colony by desoxycholate was used to test if it was Spn. AHS was determined by colony morphology, α-hemoylsis on TSA with 5% sheep blood, and growth in the presence of optochin were used to identify AHS. Identification of NTHi was based on colony morphology, gram stain, growth in quadrants III and IV on QuadID plates, no hemolysis in quadrant IV on QuadID plate, and inability to synthesize porphyrin. M cat identification was based on colony morphology, gram stain, oxidase reaction, and reaction to the catarrhalis disk (Remel). For each isolate, sub-culturing was used to ensure the presence of a pure isolate.
Statistical analysis
The main outcomes of interest were the relationships between AHS, in the presence or absence of URI symptoms, and other otopathogens that reside in the NP of children. All statistical analyses were conducted by using STATA version 12.0 (College Station, TX). We examined colonization of the NP by non-pneumococcal alpha hemolytic streptococci, Spn, NTHi, and M cat by using repeated measures logistic regression with an unstructured (UN) correlation structure using the command xtmelogit (a command similar to xtmixed, but for use with binary outcomes). A repeated measure design was utilized due to the longitudinal nature of this study, with children observed every 3 months from 6 months of age to 3 years, with AOM visits interspersed. This design will account for variability both within the subject and between the subjects.(27) The model for AHS colonization included the following covariates: colonization by other otopathogens (Spn, NTHi, and M cat), type of visit (Non-AOM, AOM, Follow-Up), presence of URI symptoms at visit, and antibiotic exposure 30 days prior to visit. Host factors included sex, breast-feeding (formula, <6 months, ≥6 months, combination of formula and breastfeeding), daycare attendance (home setting, center, both), exposure to tobacco smoke, and family history of ear infections. Results for the model were expressed as odds ratios with a 95% confidence interval.
Results
NP samples were obtained from 248 children; 1,018 were collected during a routine scheduled visit and 161 samples were collected at the time of an AOM event. Of the 248 children sampled, 135 (54.4%) were male, 147 (59.3%) were Non-Hispanic White, and 93 had a family history of otitis media (Table 1). The average age at enrollment was 6.9 months. Of the 161 AOM events, 106 (65.8%) patients had bacteria isolates in the MEF and an additional 29.1% detected by multiplex PCR (data not shown). In a subset analysis of available samples collected during a clinical viral URI (N=89), 47% of the samples were PCR positive for respiratory syncytial virus A or B, parainfluenza 3, or Influenzae A or B; PCR methods were not applied. The positive results suggest that the clinical diagnosis of a viral upper respiratory infection was adequately supported by PCR results in the samples available for testing.
Table 1.
Demographics of study patients at enrollment (N=248)
| No. | % | |
|---|---|---|
| Sex | ||
| Male | 135 | 54.4 |
| Female | 113 | 45.6 |
|
| ||
| Ageα (months) | ||
| Mean | 6.9 | |
| Range | 5.8–15.2 | |
|
| ||
| Race | ||
| Non-Hispanic White | 147 | 59.3 |
| African American | 48 | 19.4 |
| Asian | 2 | 0.8 |
| Hispanic | 12 | 4.8 |
| Other | 39 | 15.7 |
|
| ||
| Family History of OM | ||
| Maternal | 34 | 13.8 |
| Paternal | 15 | 6.1 |
| Sibling | 24 | 9.7 |
| >1 Family Member | 20 | 8.1 |
| None/Missing | 155 | 62.3 |
|
| ||
| Daycareα | ||
| Home | 49 | 60.5 |
| Center | 29 | 35.8 |
| Combination (Home and Center) | 3 | 3.7 |
|
| ||
| Breast-feedingα | ||
| <6 months | 28 | 11.3 |
| ≥6 months | 33 | 13.3 |
| Formula | 86 | 34.7 |
| Combination (Formula and breast milk) | 81 | 31.6 |
|
| ||
| Antibiotic Exposure (Number of visits) | ||
| Use in 0–30 days prior to visit | 106 | 8.6 |
| No antibiotic Use or 30+ days prior to visit | 1133 | 91.4 |
Variables collected at enrollment; daycare had 167 missing values, breast-feeding had 20 missing values
The colonization rate of AHS and 3 potential otopathogens with and without viral URI symptoms during non-AOM visits
URI symptom data was available for 475 NP samplings during 2009–2011 when children were evaluated at pre-scheduled visits, of which 331 (69.7%) did not have viral URI symptoms while 144 (30.3%) did. Having a viral URI was not associated with any change in AHS colonization frequency (168 versus 74 out of 144 samples). Viral URI also did not significantly effect Spn colonization rates (83 (25.1%) vs. 48 (33.3%). Viral URI was associated with a significant increase in NTHi carriage, increasing from 14 (4.2%) to 30 (20.8%), p< 0.001. Viral URI was also associated with a significant increase in M cat carriage from 88 (26.6%) to 68 (47.2%), p< 0.001.
Colonization rate of AHS and 3 potential otopathogens in non-AOM versus viral URI infections with concurrent AOM visits
In contrast to the above findings, when viral URI progressed to AOM AHS carriage was significantly reduced (38.3% of 60 children) compared to, the 51.4% of children who had a viral URI not associated with AOM, p=0.006. The opposite occurred with otopathogens. At the time of a viral URI with concurrent AOM, 30 (50.0%), 19 (31.7%), and 29 (48.3%) carried Spn, NTHi, and M cat, respectively. Whereas at non-AOM, viral URI negative visits, 83 (25.1%), 14 (4.3%), and 88 (26.6%) carried Spn, NTHi, and M cat, respectively. The increases in Spn, NTHi, and M cat carriage during a viral URI and concurrent AOM event were significant (p<0.001).
Colonization rate of AHS at the time of respiratory viral infection and concurrent AOM versus recovery
Of the 56 AOM visits with paired NP samples, 6 (10.7%) of the samples had AHS in the NP at the time of viral URI and concurrent AOM whereas 29 (51.8%) had AHS in the follow-up samplings 3 weeks after recovery from infection, a significant increase (p<0.001).
Effect of AHS colonization on Spn colonization during non-AOM visits
Of the 1018 non-AOM samplings, Spn colonization without AHS was 44.6% (N=484), while Spn colonization with AHS was 13.9% (N=534 p<0.001). Colonization rates of NTHi and M cat with AHS were 8.1% and 37.1% respectively. Colonization rates of NTHi and M cat without AHS were 9.1% and 38.4% respectively. The difference in colonization rates by NTHi or M cat with and without AHS was not significantly different.
Multilevel modeling of NP colonization with AHS and covariates
Repeated measures logistic regression models predicting colonization by AHS are shown in Table 2. A positive association between AHS in the NP is indicated by an odds ratio (OR) ≥ 1; a negative association is indicated by an OR<1. In our model, AHS was negatively associated with Spn and AOM visit type. When the dataset was split into non-AOM visits and AOM visits and our model was analyzed, the same negative association between AHS and Spn was observed in both non-AOM and AOM visits (data not shown). Of the seven host characteristics in our model, only breast-feeding for 6 months or less was associated with colonization of NP by AHS (p=0.053). Antibiotic exposure was not associated with differences in AHS colonization.
Table 2.
Predicted outcome of colonization with AHS in young children (N=1146 visits)
| Parameters | OR (95% CI) | P-value |
|---|---|---|
| Colonization by S. pneumoniae | ||
| No (reference) | 1.0 | |
| Yes | 0.172 (0.125–0.238) | <0.001 |
|
| ||
| Colonization by H. influenzae | ||
| No (reference) | 1.0 | |
| Yes | 1.254 (0.803–1.957) | 0.320 |
|
| ||
| Colonization by M. catarrhalis | ||
| No (reference) | 1.0 | |
| Yes | 1.271 (0.963–1.676) | 0.090 |
|
| ||
| Visit type | ||
| β AOM | 1.0 | |
| Non-AOM | 2.135(1.355–3.365) | 0.001 |
| Follow Up | 2.122(0.966–4.665) | 0.061 |
|
| ||
| Presence of URI symptoms | ||
| No (reference) | 1.0 | |
| Yes | 1.139 (0.782–1.660) | 0.498 |
|
| ||
| Sex | ||
| Male | 1.0 | |
| Female | 1.161 (0.868–1.554) | 0.313 |
|
| ||
| Exposure to tobacco smoke | ||
| No (reference) | 1.0 | |
| Yes | 0.820 (0.533–1.263) | 0.369 |
|
| ||
| Age (months) | 1.010 (0.990–1.032) | 0.316 |
|
| ||
| Breastfed | ||
| Formula(reference) | 1.0 | |
| Less than 6 months | 1.787 (0.992–3.219) | 0.053 |
| More than 6 months | 1.164 (0.761–1.780) | 0.483 |
| Combination (Formula + Breastfeeding) | 0.883 (0.631–1.237) | 0.471 |
|
| ||
| Daycare | ||
| Home | 1.0 | |
| Center | 0.817 (0.477–1.401) | 0.463 |
| Both | 0.362 (0.088–1.486) | 0.158 |
|
| ||
| Antibiotic Exposure in the last 30 days | ||
| No (reference) | 1.0 | |
| Yes | 0.969 (0.533–1.761) | 0.920 |
|
| ||
| Family History | ||
| No (reference) | 1.0 | |
| Yes | 1.150 (0.862–1.535) | 0.342 |
Associations with a significant p-value (p 0.05) are indicated in bold lettering
When the model included Non-AOM as the reference for visit type, the OR and p-value were; 0.488, p=0.002
Discussion
In this study among children in the peak age range of incidence of AOM we determined the impact of viral infection on AHS NP colonization and on NP colonization rates of Spn, NTHi, and M cat. The study occurred in a population of children that had been PCV7 vaccinated and herd immunity likely established since our community commenced PCV7 vaccination immediately after licensure in 2000. We found: 1) AHS and Spn carriage was the same, while NTHi, and M cat carriage were higher when children experienced a viral URI compared to without viral URI. 2) In contrast, AHS carriage was reduced and Spn, NTHi and M cat carriage was increased when children had a viral URI that was associated with AOM. 3) The frequency of isolation of AHS at onset of viral URI and concurrent AOM was decreased two-fold compared to after recovery from infection. 4) The frequency of isolation of Spn when AHS was present was two-fold lower than when AHS was absent.
A viral URI is known to initiate an inflammatory response in the NP, associated with damage to epithelial cells, dysfunction of cilia, increased viscosity of nasal mucus, up-regulation of epithelial cell receptors for respiratory bacteria, up-regulation of inflammatory cytokines, recruitment of polymorphonuclear neutrophils, and down-regulation of other innate and adaptive immune responses.(28–31) We have shown that viral URI perturbs the polymicrobial balance in the NP.
Viral URIs predispose children to AOM.(30) The very high rate of antecedent/concurrent viral URI we observed is consistent with many prior reports.(2;30–33) Our results suggest the NP microenvironment with viral URI and concurrent AOM must differ from that of viral URI not associated with AOM. AHS colonization rates were the same comparing children with and without viral URI but if viral URI was associated with AOM then AHS colonization rates were lower, consistent with a loss of the protective role of AHS during URIs that are followed by AOM. We interpret this finding to suggest that viral URI itself does not diminish AHS NP colonization but rather the pro-inflammatory conditions of viral URI that is associated with AOM provides a favorable environment for Spn to increase in density/inoculum. A second observation supporting a distinctive effect of viral URI on Spn was that viral URI was associated with an increase in NTHi and M cat carriage but when viral URI was followed by AOM an increase in Spn was also detected. A viral URI creates a more favorable micro-environment for NTHi and M cat resulting in a synergistic interaction.(34) Our results suggest that the presence of NTHi and M cat creates a more favorable micro-environment for Spn colonization, which then can lead to an AOM event.
As an extension of the possible protective role of AHS in the polymicrobial environment of the NP, we found that AHS colonization in the NP was significantly lower at the onset of viral URI and concurrent AOM compared to follow-up samplings in the same children after recovery from illness. The result suggests that after an episode of AOM part of the recovery process includes AHS re-colonization. However, in some cases AHS strains that re-colonize the NP after antibiotic therapy for AOM may be more resistant to antibiotics.(17)
The influence of normal upper respiratory commensals, specifically non-pneumococcal AHS, as interfering organisms for otopathogen colonization has received prior study.(7;8;13;16;17;35) Our result regarding competition between AHS and Spn in the NP is consistent with earlier reports and with a protective role of AHS colonization when given to children as a probiotic therapy to prevent AOM caused by Spn.(14;16;35;36) Unlike a previous report, we could not correlate any inhibition of NTHi colonization with the isolation of non-pneumococcal AHS.(35) AHS’s production of hydrogen peroxide has been suggested to be the main mechanism by which it inhibits other pathogens.(8) Similarly some Spn strains are also capable of producing hydrogen peroxide and have a similar inhibitory effect on other species.(9) It was unexpected that AHS was not associated with a reduction in NTHi or M cat carriage since Pericone et al had demonstrated that hydrogen peroxide produced by Spn was capable of inhibitory and bactericidal activity against NTHi and M cat.(9) However, there are many factors that have not been well studied with regard to interspecies interactions in the NP and further research is needed.(7;9;37)
Our results from the multilevel model confirm previous reports regarding the negative association between AHS and Spn.(17;38) This negative association between Spn and AHS colonization was observed in both AOM visits and non-AOM visits. The relationship between AHS and either NTHi or M cat was unclear. In our repeated measures model, a Non-AOM visit (OR=2.135; p=0.001) and follow-up visit (OR=2.122; p=0.061) were positively associated with AHS colonization. When the reference group was changed in the model (to Non-AOM), AHS colonization was negatively associated with an AOM visit (OR=0.488, p=0.002). During an AOM event, colonization by either (singly or in combination) Spn, NTHi and M cat increases, outcompeting AHS by potentially depleting the NP of necessary nutrients or creating an inhospitable microenvironment.(38) The effect of other covariates in the model, i.e. age, daycare attendance, etc., were not significantly associated with AHS colonization (p<0.05). This was most likely due to a lack of variability for these covariates as these samples were drawn from a middle-to-upper middle class population. Our results from the multilevel model for breastfeeding suggest that breastfeeding for less than 6 months increases AHS colonization (OR=1.787; p=0.053), which agrees with previous reports regarding the protective effect of breastfeeding.(39–41) The significance around this statistic are borderline (p>0.05); more samples would confirm this result (only 6.8% of visits reported breastfeeding for < 6 months). Our study has limitations regarding viral identification. As observed with many prior studies, specific identification of the viral pathogen causing viral URI illnesses was not achieved for all samples.
The dynamics of polymicrobial NP colonization with commensals, such as AHS, and potential otopathogens is not well understood. Complicating the study of polymicrobial NP colonization and the presence of different URI viruses is age of the child reflected in a maturing innate and adaptive immune response and other environmental and epidemiologic risk factors as important covariates. Despite the complexity, in this study we have been able to make distinct observations regarding the influence of clinical viral URIs on NP colonization patterns involving AHS, Spn, NTHi and M cat. Study of the underlying mechanisms that may account for viral infection influencing polymicrobial interactions in humans is now an area of active study by our group. The introduction of the new 13-valent pneumococcal conjugate vaccine creates a new condition in the NP. The role of AHS as a competitor in the NP niche for Spn epithelial cell adherence and as a donor of antimicrobial resistance genes will deserve careful study.(17;42;43)
Figure 1.
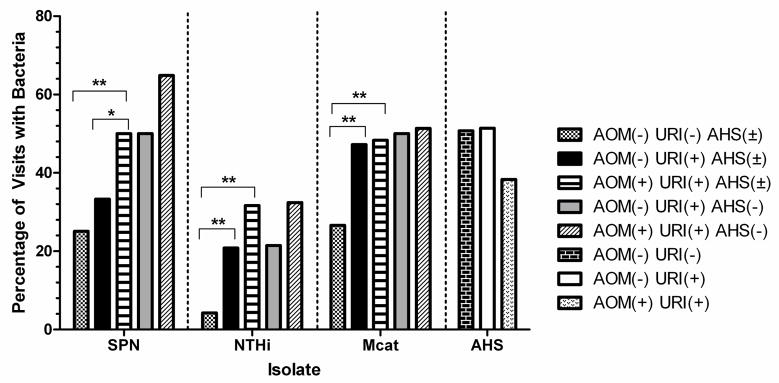
Frequency of isolation of otopathogens under various criteria. AOM(±) indicates the presence or absence of an AOM event. URI(±) indicates the presence of absence of URI symptoms at the visit. AHS(±) indicates the presence or absence of AHS colonization in the NP during the visit. * indicates a significance of P = 0.05. ** indicates a significance of P < 0.001.
Acknowledgments
This study was supported by NIH NIDCD RO1 08671. We thank Janet Casey, MD, the nurses and staff of Legacy Pediatrics and the collaborating pediatricians from Sunrise Pediatrics, Westfall Pediatrics, Lewis Pediatrics and Long Pond Pediatrics, the parents who consented and the children who participated in this study.
Reference List
- 1.Ruuskanen O, Arola M, Putto-Laurila A, et al. Acute otitis media and respiratory virus infections. Pediatr Infect Dis J. 1989 Feb;8(2):94–9. [PubMed] [Google Scholar]
- 2.Bakaletz LO. Immunopathogenesis of polymicrobial otitis media. J Leukoc Biol. 2010 Feb;87(2):213–22. doi: 10.1189/jlb.0709518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musher DM. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin Infect Dis. 1992 Apr;14(4):801–7. doi: 10.1093/clinids/14.4.801. [DOI] [PubMed] [Google Scholar]
- 4.Leibovitz E, Jacobs MR, Dagan R. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J. 2004 Dec;23(12):1142–52. [PubMed] [Google Scholar]
- 5.Karalus R, Campagnari A. Moraxella catarrhalis: a review of an important human mucosal pathogen. Microbes Infect. 2000 Apr;2(5):547–59. doi: 10.1016/s1286-4579(00)00314-2. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Rodriguez JA, Fresnadillo Martinez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother. 2002 Dec;50(Suppl S2):59–73. doi: 10.1093/jac/dkf506. [DOI] [PubMed] [Google Scholar]
- 7.Tano K, Hakansson EG, Holm SE, Hellstrom S. Bacterial interference between pathogens in otitis media and alpha-haemolytic Streptococci analysed in an in vitro model. Acta Otolaryngol. 2002 Jan;122(1):78–85. doi: 10.1080/00016480252775788. [DOI] [PubMed] [Google Scholar]
- 8.Tano K, Grahn HE, Wallbrandt P, Ronnqvist D, Holm SE, Hellstrom S. Is hydrogen peroxide responsible for the inhibitory activity of alpha-haemolytic streptococci sampled from the nasopharynx? Acta Otolaryngol. 2003 Aug;123(6):724–9. doi: 10.1080/00016480310000403. [DOI] [PubMed] [Google Scholar]
- 9.Pericone CD, Overweg K, Hermans PW, Weiser JN. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun. 2000 Jul;68(7):3990–7. doi: 10.1128/iai.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacoby P, Watson K, Bowman J, et al. Modelling the co-occurrence of Streptococcus pneumoniae with other bacterial and viral pathogens in the upper respiratory tract. Vaccine. 2007 Mar 22;25(13):2458–64. doi: 10.1016/j.vaccine.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis. 2008 Oct;14(10):1584–91. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q, Casey JR, Chang A, Pichichero ME. When Co-colonizing the Nasopharynx Haemophilus influenzae Predominates Over Streptococcus pneumoniae Except Serotype 19A Strains to Cause Acute Otitis Media. Pediatr Infect Dis J. 2012 Jun;31(6):638–40. doi: 10.1097/INF.0b013e31824ba6f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long SS, Henretig FM, Teter MJ, McGowan KL. Nasopharyngeal flora and acute otitis media. Infect Immun. 1983 Sep;41(3):987–91. doi: 10.1128/iai.41.3.987-991.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowe CC, Sanders WE, Jr, Longley S. Bacterial interference. II. Role of the normal throat flora in prevention of colonization by group A Streptococcus. J Infect Dis. 1973 Oct;128(4):527–32. doi: 10.1093/infdis/128.4.527. [DOI] [PubMed] [Google Scholar]
- 15.Revai K, Mamidi D, Chonmaitree T. Association of nasopharyngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin Infect Dis. 2008 Feb 15;46(4):e34–e37. doi: 10.1086/525856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein JM, Sagahtaheri-Altaie S, Dryja DM, Wactawski-Wende J. Bacterial interference in nasopharyngeal bacterial flora of otitis-prone and non-otitis-prone children. Acta Otorhinolaryngol Belg. 1994;48(1):1–9. [PubMed] [Google Scholar]
- 17.Ghaffar F, Friedland IR, Katz K, et al. Increased carriage of resistant non-pneumococcal alpha-hemolytic streptococci after antibiotic therapy. J Pediatr. 1999 Nov;135(5):618–23. doi: 10.1016/s0022-3476(99)70061-2. [DOI] [PubMed] [Google Scholar]
- 18.Brook I, Gober AE. The effects of treatment of acute otitis media with a low dose vs a high dose of amoxicillin on the nasopharyngeal flora. Arch Otolaryngol Head Neck Surg. 2009 May;135(5):458–61. doi: 10.1001/archoto.2008.506. [DOI] [PubMed] [Google Scholar]
- 19.Brook I. Effects of exposure to smoking on the microbial flora of children and their parents. Int J Pediatr Otorhinolaryngol. 2010 May;74(5):447–50. doi: 10.1016/j.ijporl.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Heikkinen T, Chonmaitree T. Importance of respiratory viruses in acute otitis media. Clin Microbiol Rev. 2003 Apr;16(2):230–41. doi: 10.1128/CMR.16.2.230-241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arola M, Ziegler T, Ruuskanen O. Respiratory virus infection as a cause of prolonged symptoms in acute otitis media. J Pediatr. 1990 May;116(5):697–701. doi: 10.1016/s0022-3476(05)82650-2. [DOI] [PubMed] [Google Scholar]
- 22.Pettigrew MM, Gent JF, Pyles RB, Miller AL, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J Clin Microbiol. 2011 Nov;49(11):3750–5. doi: 10.1128/JCM.01186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diagnosis and management of acute otitis media. Pediatrics. 2004 May;113(5):1451–65. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 24.Kaleida PH, Stool SE. Assessment of otoscopists’ accuracy regarding middle-ear effusion. Otoscopic validation. Am J Dis Child. 1992 Apr;146(4):433–5. doi: 10.1001/archpedi.1992.02160160053013. [DOI] [PubMed] [Google Scholar]
- 25.Kaur R, Adlowitz DG, Casey JR, Zeng M, Pichichero ME. Simultaneous assay for four bacterial species including Alloiococcus otitidis using multiplex-PCR in children with culture negative acute otitis media. Pediatr Infect Dis J. 2010 Aug;29(8):741–5. doi: 10.1097/INF.0b013e3181d9e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalu SU, Ataya RS, McCormick DP, Patel JA, Revai K, Chonmaitree T. Clinical spectrum of acute otitis media complicating upper respiratory tract viral infection. Pediatr Infect Dis J. 2011 Feb;30(2):95–9. doi: 10.1097/INF.0b013e3181f253d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer JD. Using SAS PROC MIXED to Fit Multilevel Models, Hierarchical Models, and Individual Growth Models. Journal of Educational and Behavioral Statistics. 1998;24(4):323–355. [Google Scholar]
- 28.McGillivary G, Mason KM, Jurcisek JA, Peeples ME, Bakaletz LO. Respiratory syncytial virus-induced dysregulation of expression of a mucosal beta-defensin augments colonization of the upper airway by non-typeable Haemophilus influenzae. Cell Microbiol. 2009 Sep;11(9):1399–408. doi: 10.1111/j.1462-5822.2009.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakir M, Yagci A, Ulger N, Akbenlioglu C, Ilki A, Soyletir G. Asymtomatic carriage of Neisseria meningitidis and Neisseria lactamica in relation to Streptococcus pneumoniae and Haemophilus influenzae colonization in healthy children: apropos of 1400 children sampled. Eur J Epidemiol. 2001;17(11):1015–8. doi: 10.1023/a:1020021109462. [DOI] [PubMed] [Google Scholar]
- 30.Chonmaitree T, Revai K, Grady JJ, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008 Mar 15;46(6):815–23. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alper CM, Winther B, Mandel EM, Hendley JO, Doyle WJ. Rate of concurrent otitis media in upper respiratory tract infections with specific viruses. Arch Otolaryngol Head Neck Surg. 2009 Jan;135(1):17–21. doi: 10.1001/archotol.135.1.17. [DOI] [PubMed] [Google Scholar]
- 32.Heikkinen T, Chonmaitree T. Viral-Bacterial Synergy in Otitis Media: Implications for Management. Curr Infect Dis Rep. 2000 Apr;2(2):154–9. doi: 10.1007/s11908-000-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heikkinen T. Role of viruses in the pathogenesis of acute otitis media. Pediatr Infect Dis J. 2000 May;19(5 Suppl):S17–S22. doi: 10.1097/00006454-200005001-00004. [DOI] [PubMed] [Google Scholar]
- 34.Davis BM, Aiello AE, Dawid S, Rohani P, Shrestha S, Foxman B. Influenza and Community-acquired Pneumonia Interactions: The Impact of Order and Time of Infection on Population Patterns. Am J Epidemiol. 2012 Mar 1;175(5):363–7. doi: 10.1093/aje/kwr402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tano K, Grahn-Hakansson E, Holm SE, Hellstrom S. Inhibition of OM pathogens by alpha-hemolytic streptococci from healthy children, children with SOM and children with rAOM. Int J Pediatr Otorhinolaryngol. 2000 Dec 22;56(3):185–90. doi: 10.1016/s0165-5876(00)00428-6. [DOI] [PubMed] [Google Scholar]
- 36.Roos K, Hakansson EG, Holm S. Effect of recolonisation with “interfering” alpha streptococci on recurrences of acute and secretory otitis media in children: randomised placebo controlled trial. BMJ. 2001 Jan 27;322(7280):210–2. doi: 10.1136/bmj.322.7280.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lysenko ES, Ratner AJ, Nelson AL, Weiser JN. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog. 2005 Sep;1(1):e1. doi: 10.1371/journal.ppat.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders E. Bacterial interference. I. Its occurrence among the respiratory tract flora and characterization of inhibition of group A streptococci by viridans streptococci. J Infect Dis. 1969 Dec;120(6):698–707. doi: 10.1093/infdis/120.6.698. [DOI] [PubMed] [Google Scholar]
- 39.Duncan B, Ey J, Holberg CJ, Wright AL, Martinez FD, Taussig LM. Exclusive breast-feeding for at least 4 months protects against otitis media. Pediatrics. 1993 May;91(5):867–72. [PubMed] [Google Scholar]
- 40.Abrahams SW, Labbok MH. Breastfeeding and otitis media: a review of recent evidence. Curr Allergy Asthma Rep. 2011 Dec;11(6):508–12. doi: 10.1007/s11882-011-0218-3. [DOI] [PubMed] [Google Scholar]
- 41.Sassen ML, Brand R, Grote JJ. Breast-feeding and acute otitis media. Am J Otolaryngol. 1994 Sep;15(5):351–7. doi: 10.1016/0196-0709(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 42.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010 Apr;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichichero ME, Casey JR. Evolving microbiology and molecular epidemiology of acute otitis media in the pneumococcal conjugate vaccine era. Pediatr Infect Dis J. 2007 Oct;26(10 Suppl):S12–S16. doi: 10.1097/INF.0b013e318154b25d. [DOI] [PubMed] [Google Scholar]


