Abstract
Staphylococcus aureus is a human commensal and pathogen that is capable of forming biofilms on a variety of host tissues and implanted medical devices. Biofilm-associated infections resist antimicrobial chemotherapy and attack from the host immune system, making these infections particularly difficult to treat. In order to gain insight into environmental conditions that influence S. aureus biofilm development, we screened a library of small molecules for the ability to inhibit S. aureus biofilm formation. This led to the finding that the polyphenolic compound tannic acid inhibits S. aureus biofilm formation in multiple biofilm models without inhibiting bacterial growth. We present evidence that tannic acid inhibits S. aureus biofilm formation via a mechanism dependent upon the putative transglycosylase IsaA. Tannic acid did not inhibit biofilm formation of an isaA mutant. Overexpression of wild-type IsaA inhibited biofilm formation, whereas overexpression of a catalytically dead IsaA had no effect. Tannin-containing drinks like tea have been found to reduce methicillin-resistant S. aureus nasal colonization. We found that black tea inhibited S. aureus biofilm development and that an isaA mutant resisted this inhibition. Antibiofilm activity was eliminated from tea when milk was added to precipitate the tannic acid. Finally, we developed a rodent model for S. aureus throat colonization and found that tea consumption reduced S. aureus throat colonization via an isaA-dependent mechanism. These findings provide insight into a molecular mechanism by which commonly consumed polyphenolic compounds, such as tannins, influence S. aureus surface colonization.
INTRODUCTION
Staphylococcus aureus is a Gram-positive bacterium that exists both as a commensal, commonly colonizing humans, and as a deadly pathogen, possessing the ability to cause a multitude of infections (1–3). The ability of S. aureus to colonize surfaces contributes to its lifestyle as both a commensal and a pathogen (4). When colonizing a surface, S. aureus forms a structured community called a biofilm, in which cells are encased in a polymeric matrix. Although the exact composition of this matrix varies greatly from strain to strain and between different growth conditions, its components include extracellular DNA, polysaccharides, proteins, and amyloid fibers (5, 6). The variability among biofilms formed by S. aureus contributes to its ability to colonize humans and cause many different kinds of biofilm-associated infections, including osteomyelitis (7), endocarditis (8), and implanted device infections (9).
Management of biofilm infections is extremely difficult due to their inherent resistance both to antimicrobial chemotherapies and to the host immune response (4, 10).
New approaches are needed to overcome the challenge of antimicrobial resistance. Enzymatic disruption of the biofilm matrix and alteration of gene expression to induce biofilm disassembly are currently among the alternatives being investigated (6). In addition, much research has focused on understanding the environmental conditions and bacterial molecular mechanisms that influence S. aureus biofilm development. Several environmental factors, such as glucose, osmolarity, ethanol, hemoglobin, temperature, and anaerobiosis, have been reported to affect biofilm formation and disassembly (6, 11–13).
Beyond these examples, little is known about the contribution of other environmental conditions and the molecular mechanisms that respond to them to control biofilm development. It is hoped that a deeper understanding of these environmental cues will lead to innovative treatments for S. aureus biofilm infections. Therefore, we set out to look for novel environmental factors that could influence S. aureus biofilm development by screening a small chemical library.
This approach led to the finding that tannic acid inhibits S. aureus biofilm formation without inhibiting cell growth. Further analysis revealed increased levels of the protein IsaA (immunodominant staphylococcal antigen A) in culture supernatants when strains were grown in the presence of tannic acid. IsaA is expressed during infections and is antigenic, as high titers of antibody against IsaA are readily found in individuals that have had an S. aureus infectious disease (14). IsaA is a putative lytic transglycosylase and has been shown by zymography to cleave peptidoglycan (15). Lytic transglycosylases are a unique class of lysozyme-like enzymes that catalyze cleavage of the β-1,4-glycosidic bond between N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc) (16).
MATERIALS AND METHODS
Strains and plasmids.
The bacterial strains and plasmids used in this study are described in Table 1. Strains of Escherichia coli were grown in Luria-Bertani broth or Luria agar plates. For selection of chromosomal markers or maintenance of plasmids, E. coli antibiotic concentrations were 100 μg/ml for ampicillin and 10 μg/ml for chloramphenicol. Except where noted, S. aureus strains were grown in tryptic soy broth (TSB) or tryptic soy agar (TSA). For selection of chromosomal markers or maintenance of plasmids, S. aureus antibiotic concentrations were 10 μg/ml for erythromycin and 10 μg/ml for chloramphenicol. All reagents were purchased from Fisher Scientific (Pittsburg, PA) or Sigma (St. Louis, MO), unless otherwise indicated. pKP1 was created by PCR amplifying the isaA open reading frame from strain SH1000 using primers o82 (5′-ATGCGGTACCCTTGCACTACGACATTCAAATTC-3′) and o83 (5′-ATGCGAATTCCTCTCCCCAATTTCTATGGG-3′) and ligating this fragment into the multiple-cloning site of pALC2073. pKP1-IsaA.EQ was created by PCR amplifying the pKP1 vector with overlapping primers o172 (5′-TCATCGCTCGTCAATCA-3′) and o173 (5′-TGACCATTTGATTGACGAG-3′), both of which contain the desired point mutation. The PCR product was treated with DpnI to remove the template plasmid and transformed. Mutated plasmids were verified by DNA sequencing.
Table 1.
Strains and plasmidsa
| Strain or plasmid | Relevant genotype | Resistance | Reference or source |
|---|---|---|---|
| S. aureus strains | |||
| BB1209 | SH1000/pALC2073 | Cm | 17 |
| BB2146 | SH1000 spectinomycin resistant | Spec | 13 |
| BB2183 | isaA::Tetr | Tet | 15 |
| BB2184 | isaA::Tetr/pSK5630 | Cm | 15 |
| BB2185 | isaA::Tetr/pMEL4 | Cm | 15 |
| BB2242 | SH1000/pKP1 | Cm | This work |
| BB2333 | SH1000/pKP1.IsaA.EQ | Cm | This work |
| BB2515 | Dripper isolate | None | This work |
| BB2518 | Dripper isolate | None | This work |
| BB2519 | Dripper isolate | None | This work |
| BB2520 | Dripper isolate | None | This work |
| BB2521 | Dripper isolate | None | This work |
| BB2522 | Dripper isolate | None | This work |
| BB2523 | Dripper isolate | None | This work |
| BB2524 | Dripper isolate | None | This work |
| BB2527 | Dripper isolate | None | This work |
| BB2528 | Dripper isolate | None | This work |
| BB2529 | Dripper isolate | None | This work |
| BB2585 | Rat isolate | Spec | This work |
| BB2586 | Rat isolate | Spec | This work |
| BB2587 | Rat isolate | Spec | This work |
| BB2588 | Rat isolate | Spec | This work |
| BB2589 | Rat isolate | Spec | This work |
| BB2590 | Rat isolate | Spec | This work |
| BB204 | Newman | 18 | |
| BB206 | RN6390 | 19 | |
| BB207 | RN6911 | 20 | |
| BB248 | FRI1169 | 21 | |
| BB607 | Blood isolate | 5 | |
| BB608 | Blood isolate | 5 | |
| BB609 | Blood isolate | This work | |
| BB610 | Bone isolate | This work | |
| BB611 | Bone isolate | This work | |
| BB612 | Bone isolate | This work | |
| BB687 | MW2 | 22 | |
| BB1263 | LAC | 23 | |
| BB707 | Nasal isolate | This work | |
| BB759 | UAMS | 24 | |
| Plasmids | |||
| pSK5630 | Cm | 15 | |
| pMEL4 | isaA under the control of native promoter | Cm | 15 |
| pALC2073 | Cm | 17 | |
| pKP1 | isaA under the control of Tet promoter | Cm | This work |
| pKP1.IsaA.EQ | E183Q-isaA under the control of Tet promoter | Cm | This work |
Cm, chloramphenicol; Spec, spectinomycin; Tet, tetracycline.
Growth assays.
SH1000 cultures were grown in TSBg (66% TSB with 0.2% glucose). The optical density at 600 nm (OD600) was measured every 30 min, and ODs from early and late log phase were used to calculate doubling times. Doubling times presented are averages from three biological replicates. To calculate population density in stationary phase, cultures were grown for 24 h, washed in phosphate-buffered saline (PBS), bath sonicated for 4 min, serially diluted, and plated on TSA. The numbers of CFU were counted on the following day. CFU counts presented are averages from four biological replicates.
Biofilm assays.
Microtiter plate biofilms were grown as previously described (11). Briefly, late-log-phase S. aureus cultures were diluted 1:200 in a final volume of 200 μl 66% TSB in wells of a 96-well microtiter plate (164688; Nunc). Glucose was added to a final concentration of 0.2% to induce biofilm formation. Plates were incubated overnight at 37°C with shaking at 200 rpm. After incubation, medium was removed by pipetting and wells were gently washed with 150 μl sterile water. One hundred microliters of 0.1% crystal violet was added, and the mixture was allowed to sit for 10 min. Crystal violet was removed by pipetting, and wells were again washed with 150 μl sterile water. Plates were air dried and photographed. To quantitate crystal violet stain, 150 μl of 40 mM HCl in ethanol was added to each well, the contents were pipetted to mix, and the plates were allowed to sit for 5 min. The contents of the wells were again mixed, 100 μl of stain was moved to a new plate, and the absorbance at 595 nm was measured. All microtiter plate quantitations with multigroup comparisons were analyzed by analysis of variance and found to have P values of <0.05. Data sets were analyzed post hoc by a Dunnett's test, and the P values of these tests are listed in the applicable figure legends.
Drip-flow biofilms were set up and grown as described previously (5), with the growth medium being 2% tryptic soy broth (0.6 g/liter) with 0.2% glucose (2 g/liter). After 5 days of growth, coupons were removed with sterile tweezers and biofilm cells were harvested into 10 ml sterile PBS. Samples were bath sonicated for 10 min, serially diluted, and plated in plate count agar. Colonies were counted on the following morning.
Flow-cell biofilms were grown in 2% tryptic soy broth (0.6 g/liter) with 0.2% glucose (2 g/liter) supplemented with tannic acid, as indicated in the figure legends. Confocal scanning laser microscopy and image analysis were performed as described previously (11). Biofilms were treated with 330 nM Syto9 (LIVE/DEAD BacLight bacterial viability kit; Invitrogen, Carlsbad, CA) 15 min prior to visualization.
Cotton rat oropharynx colonization model.
To assess the ability of S. aureus to colonize the throat, a cotton rat throat colonization model was developed on the basis of previous studies done in mice studying Streptococcus pyogenes throat colonization (25). Animal work was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council (26). The protocol was approved by the Committee on Use and Care of Animals (UCUCA) of the University of Michigan (permit number 10394). All efforts were made to minimize pain and discomfort during the procedure. Female cotton rats were obtained from Harlan Laboratories and housed at 3 per cage in a room kept at 23°C ± 2°C with 50 to 60% relative humidity and a 12-h light and 12-h dark cycle. Rats were given tap water and rodent chow ad libitum and were acclimated to the laboratory environment for a minimum of 6 days before inoculation. S. aureus (strain BB2146 or BB2497) was grown overnight in TSB, harvested by centrifugation, washed, and resuspended in PBS. Female cotton rats were anesthetized, and a 100-μl aliquot containing 1 × 105 CFU was instilled into the throat of anesthetized animals via gavage. Throat swab samples for culture were taken using an alginate swab inserted into the oropharynx of anesthetized rats at the indicated time points. The swab was streaked onto mannitol salt agar containing spectinomycin at 100 μg/ml, and these plates were incubated for 24 h at 37°C. The growth of S. aureus colonies on these plates was interpreted as the animal being colonized. Tea was given to the indicated animals at 2, 5, and 8 days after the initial S. aureus colonization by slowly delivering 200 μl of room temperature tea (prepared as described below) into the anesthetized animal's throat via gavage. Control animals were given the same volume of water. Animals were held upright during gavage and monitored closely to avoid pulmonary aspiration.
Protein gel and Western blot analyses.
Cultures were grown overnight at 37°C with shaking in 66% TSB with 0.2% glucose. Cultures were normalized by the OD600, and cells were removed by centrifugation. Culture supernatants were concentrated by trichloroacetic acid (TCA) precipitation, boiled for 10 min in SDS running buffer, run on a 14% polyacrylamide gel, and stained with Coomassie. Western blotting was performed by boiling normalized culture supernatants for 10 min in SDS running buffer and separating on a 14% polyacrylamide gel. Proteins were transferred to a polyvinylidene difluoride membrane and probed with a polyclonal anti-IsaA antibody. The IsaA antibody was generated in rabbits using the peptide DQLNAAPIKDGAYD, which corresponds to amino acids 48 to 61 of the IsaA protein.
Tea.
Black tea was brewed by adding 100 ml boiling water to one bag of Twinings English breakfast tea and steeped for 7 min. Tea was cooled to room temperature and filter sterilized. Milk was made from powder to a concentration of 100 mg/ml (approximately the same concentration used to make milk from powder for consumption). To precipitate tannins from tea, 5 μl milk was mixed with 25 μl tea and 20 μl water. The mixture was allowed to sit at room temperature for 1 h before use.
RESULTS
Tannic acid inhibits S. aureus biofilm formation in multiple biofilm models.
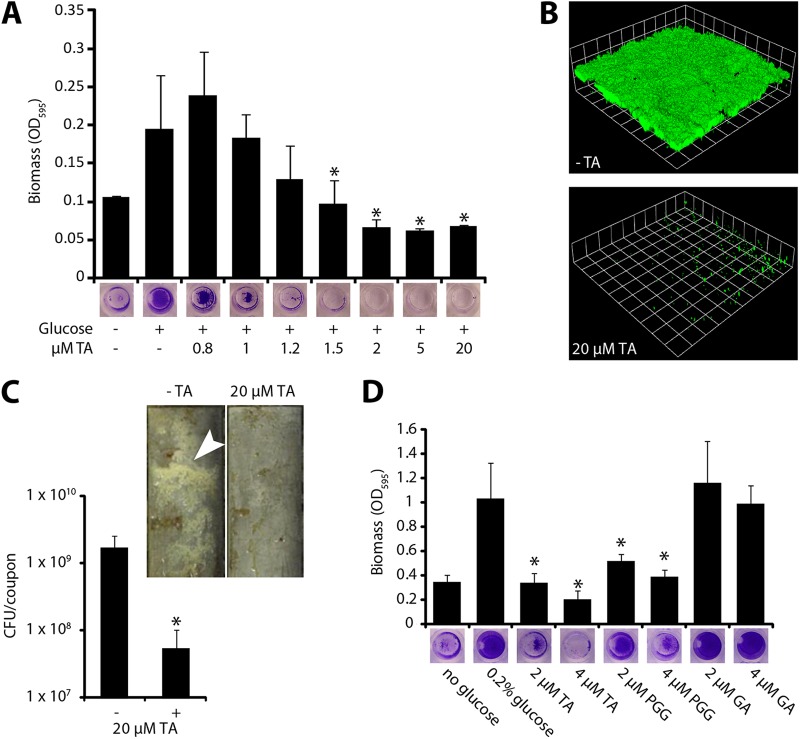
To identify chemicals and environmental conditions that influence S. aureus biofilm formation, we screened a collection of compounds contained in Biolog plates PM1 to PM20 for the ability to inhibit surface colonization by strain SH1000. Briefly, the contents of each well of the Biolog plates were resuspended in 102 μl distilled water, and 50 μl of this resuspension was added to a microtiter plate assay and screened for the ability to inhibit biofilm formation. Of the 1,920 conditions tested, 41 inhibited S. aureus biofilm formation (for a complete list, see Table S1 in the supplemental material). Among these compounds was tannic acid, a common component in teas and other plant-derived foods. Because S. aureus colonizes the oropharynx and oral cavity (2, 27, 28) and is likely to encounter this compound during colonization, we focused our efforts on tannic acid. We first confirmed and expanded the result from the screen, showing that tannic acid inhibited S. aureus biofilm formation at low micromolar concentrations in a concentration-dependent manner (Fig. 1). Tannic acid inhibited surface colonization in several biofilm model systems (Fig. 1B and C).
Fig 1.
Tannic acid inhibits biofilm formation in S. aureus. (A) S. aureus does not form a biofilm in a microtiter plate assay when grown with micromolar concentrations of tannic acid (TA). Biofilm formation was induced by supplementing the growth medium with 0.2% glucose. Error bars represent standard deviations. *, P < 0.005 compared to the control with glucose and without tannic acid. (B) S. aureus biofilm formation in a flow cell is dramatically reduced by treatment with 20 μM tannic acid. Shown are three-dimensional image reconstructions of a z series created with Velocity software. Confocal laser scanning microscopy images are representative of three separate experiments, and each side of a grid square represents 15 μm. (C) S. aureus forms significantly less biofilm in drip reactors when grown with 20 μM tannic acid. Drip-reactor biofilms were grown for 3 days and photographed before harvesting. Arrowhead indicates visible biofilm. Cells were harvested from four replicate drip reactors, and the numbers of CFU were counted. Error bars represent standard deviations. *, P < 0.01 compared to untreated control. (D) Two major components of commercial tannic acid are compared for antibiofilm activity. Pentagalloyl glucose (PGG) significantly inhibits biofilm formation. Gallic acid (GA) causes no significant inhibition. Error bars represent standard deviations. *, P < 0.01 compared to the control with glucose and without tannic acid.
To ensure that tannic acid's biofilm-inhibitory activity was not specific to SH1000, we tested its effect on biofilms of 15 other strains in microtiter plate assays (see Fig. S1 in the supplemental material). These strains included both clinical isolates and established lab strains. Because many of these strains did not grow robust biofilms in the tryptic soy broth used for SH1000, we also tested these 15 strains in a peptone-based medium that promotes amyloid fiber formation in the biofilms (5). The overwhelming majority of these strains formed less robust biofilms in the presence of tannic acid, suggesting that this effect is broadly applicable.
Tannic acid is a mix of plant-derived polyphenolic compounds, and two of the most abundant and consistently isolated components of commercially available tannic acid are gallic acid and pentagalloyl glucose (29). Therefore, we also tested the ability of these compounds to inhibit S. aureus biofilm formation. Gallic acid failed to inhibit S. aureus biofilm formation, whereas pentagalloyl glucose inhibited S. aureus biofilm formation at concentrations similar to those observed with tannic acid (Fig. 1D).
Tannic acid at 20 μM does not inhibit growth of S. aureus.
Because tannic acid is known to have antimicrobial activity, we investigated whether it affected S. aureus growth at the concentrations where we see antibiofilm activity. We grew cultures in 66% TSBg supplemented with up to 20 μM tannic acid and observed growth (Table 2). In log phase, the doubling times of cultures grown with and without tannic acid were not statistically significantly different. We also allowed the cultures to grow to stationary phase and counted the numbers of CFU in each culture. There was no statistically significant difference between final CFU counts.
Table 2.
Growth rate and final culture density when grown in the presence of tannic acid
| TA concn (μM) | Doubling time (min) | No. of CFU (109)/ml after 24 h |
|---|---|---|
| 0 | 35.6 ± 0.4 | 1.35 ± 0.77 |
| 5 | 34.6 ± 2.6 | 1.4 ± 0.66 |
| 20 | 36.2 ± 0.8 | 1.65 ± 1.03 |
S. aureus supernatants display increased levels of IsaA in the presence of tannic acid.
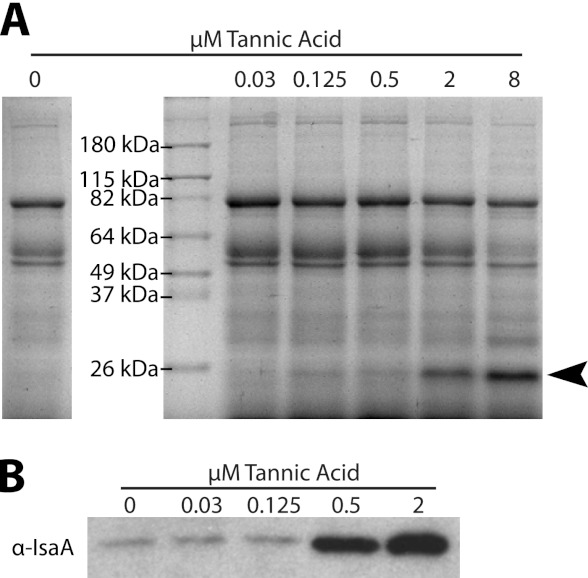
To begin elucidating how tannic acid exerts its antibiofilm effect, we grew planktonic cultures of S. aureus in the presence of various concentrations of tannic acid. We examined changes in the extracellular protein profile of culture supernatants by SDS-PAGE. One band, migrating at approximately 26 kDa, became more pronounced with increasing concentrations of tannic acid (Fig. 2A). This protein band was excised and identified by mass spectrometric analysis to be immunodominant staphylococcal antigen A (IsaA). IsaA is a putative lytic transglycosylase that has previously been implicated in cleaving peptidoglycan (15). A polyclonal antibody was generated against IsaA, and subsequent Western blot analysis confirmed that increased levels of IsaA were present in culture supernatants supplemented with tannic acid (Fig. 2B).
Fig 2.
Tannic acid increases levels of IsaA in S. aureus culture supernatants. (A) Western blot of TCA-precipitated supernatants from overnight cultures of S. aureus grown in 66% TSB supplemented with 0.2% glucose and with the indicated concentrations of tannic acid. When cultures are grown with higher concentrations of tannic acid, a band (indicated with an arrowhead) appears with an apparent molecular mass slightly below 26 kDa. The band was excised, and the protein was identified by mass spectrometry as IsaA. (B) Western blot of unprecipitated supernatants from overnight cultures probed with polyclonal anti-IsaA antibody. IsaA levels in culture supernatants increase when the culture is grown with tannic acid.
An isaA mutant resists tannic acid-mediated biofilm inhibition.
Since tannic acid affected IsaA abundance, we asked whether IsaA has a role in tannic acid biofilm inhibition. An isogenic isaA mutant was assessed for its ability to form biofilms in the presence of tannic acid (Fig. 3A and B). In contrast to what we observed with the wild type, increasing the tannic acid concentration up to 20 μM had no effect on the ability of an isaA mutant to form a biofilm. However, complementation of the isaA mutant by expressing isaA from its native promoter on a plasmid restored the susceptibility of this strain to the antibiofilm effects of tannic acid. Taken together these results suggest that the antibiofilm effects of tannic acid are dependent upon the presence of IsaA.
Fig 3.
isaA is necessary for tannic acid-induced biofilm inhibition. (A) Strain SH1000 (BB386), a mutant isaA::Tetr strain (ΔisaA; BB2183), an isaA::Tetr strain with an empty vector (ΔisaA + EV; BB2184), and a mutant isaA::Tetr strain with an isaA complement (ΔisaA + isaA; BB2185) were assayed in a microtiter plate for tannic acid-induced biofilm inhibition. Strains lacking functional isaA were resistant to inhibition. *, P ≪ 0.001 compared to isogenic untreated control. (B) SH1000 (BB386), a mutant isaA::Tetr strain with an empty vector (BB2184), and a mutant isaA::Tetr strain with an isaA complement (BB2185) were assayed in a flow cell for tannic acid-induced biofilm inhibition. The isaA mutant was resistant to inhibition.
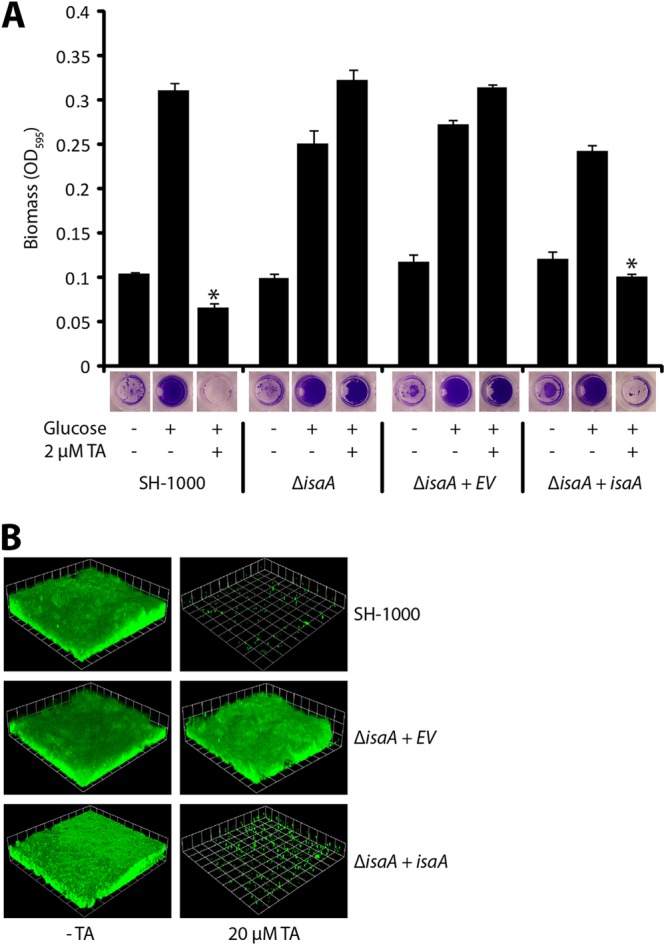
IsaA expression prevents S. aureus biofilm formation.
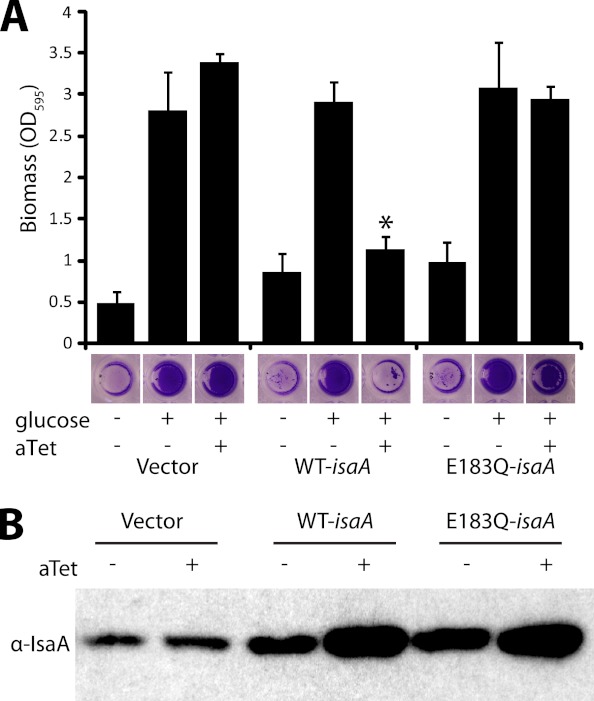
Since tannic acid increases the level of IsaA found in culture supernatants and results in reduced biofilm formation, we hypothesized that overexpression of IsaA would inhibit biofilm formation. To test this hypothesis, we cloned the isaA gene behind an inducible promoter and assessed biofilm formation. Induction of IsaA expression did not interfere with growth (data not shown) and resulted in no biofilm formation, whereas noninducing conditions or the empty vector allowed biofilm formation (Fig. 4A). The overexpression vector pALC2073 that we used is known to be leaky (30), and therefore, IsaA levels in the absence of inducer were higher than those in the empty vector controls (Fig. 4B). This increase in IsaA abundance under the noninducing conditions did not affect biofilm formation. Taken together, these results suggest that it is possible to increase IsaA levels somewhat without having an effect but that once IsaA levels hit a certain threshold, biofilm formation is inhibited.
Fig 4.
Induced expression of IsaA inhibits biofilm formation. (A) SH1000 harboring empty vector (BB1209), isaA (BB2242), or E183Q-isaA (BB2333) under a tetracycline-inducible promoter was grown with 0.2% glucose and 250 ng/ml anhydrotetracycline (aTet). No biofilm formed when IsaA was overexpressed. A biofilm formed when E183Q-IsaA was overexpressed. Error bars represent standard deviations. WT, wild type; *, P ≪ 0.001 compared to the isogenic control with glucose and without anhydrotetracycline. (B) Wild-type IsaA and E183Q-IsaA are overexpressed when induced with 250 ng/ml anhydrotetracycline. The Western blot is of SH1000 harboring strains with an empty vector (BB1209), isaA (BB2242), or E183Q-isaA (BB2333) under a tetracycline-inducible promoter, with and without anhydrotetracycline induction, probed with polyclonal anti-IsaA antibody.
The putative transglycosylase active site is necessary for IsaA's antibiofilm activity.
To investigate if the putative transglycosylase activity was required for the antibiofilm activity of IsaA, we constructed a mutant with a point mutation in the conserved transglycosylase active site that would be expected to abolish activity. Family 1 lytic transglycosylases, including IsaA, share a conserved E-S motif, with the glutamyl residue being essential for catalysis (31, 32). In Salmonella enterica, the peptidoglycan-digesting activity of two lytic transglycosylases was dramatically reduced by replacing the conserved glutamyl residue with a glutamine (33). We made the analogous mutation in IsaA's conserved active site (E183Q) and expressed it from the plasmid pKP1.IsaA.EQ. Western blot analysis revealed that this construct produced a protein whose size was consistent with the size of the wild-type protein (Fig. 4B). However, overexpression of pKP1.IsaA.EQ did not result in biofilm inhibition (Fig. 4A), suggesting that the IsaA putative transglycosylase activity is responsible for the protein's antibiofilm effects.
Spontaneous mutants that resist tannic acid biofilm inhibition fail to produce IsaA.
Although tannic acid inhibits biofilm formation in drip reactors grown for 3 days (Fig. 1), we noticed that extending the drip biofilm growth period to 5 days allowed a significant biofilm to form in the presence of tannic acid. This tannic acid-resistant biofilm was broken up by sonication and plated onto nutrient agar to isolate single colonies. In testing 11 isolates, we found that 3 resisted tannic acid biofilm inhibition (Fig. 5A). Western blot analysis of culture supernatants revealed that the three tannic acid-resistant isolates also failed to produce IsaA (Fig. 5B), further strengthening the link between IsaA and tannic acid-mediated biofilm inhibition.
Fig 5.
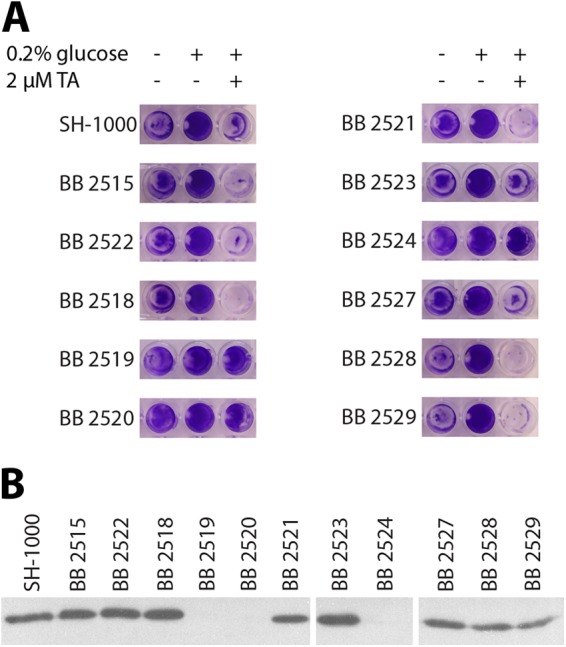
Biofilm resistance to tannic acid is coincident with a reduction in IsaA expression. Strains derived from 11 S. aureus colonies isolated from a tannic acid-treated biofilm were tested for resistance to tannic acid-induced biofilm inhibition, as well as for IsaA production. (A) Three of 11 strains (BB2519, BB2520, and BB2524) are robustly resistant to tannic acid-induced biofilm inhibition in a microtiter plate biofilm assay. (B) The same 3 strains also do not have detectable IsaA in their culture supernatants. The Western blot is of culture supernatants from the 11 strains isolated from a tannic acid-resistant biofilm (along with wild type) probed with polyclonal anti-IsaA antibody.
Tea inhibits S. aureus biofilm formation in an isaA-dependent manner.
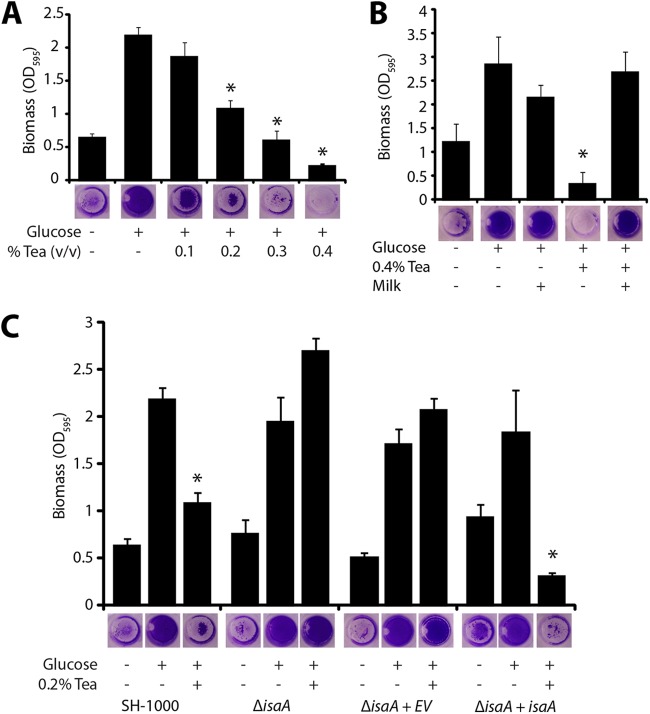
Tannic acid is a variable mixture of plant-derived polyphenols, consisting primarily of gallotannins (29), that has historically been used to precipitate proteins from solution (34). Tannins are an abundant component of vascular plant tissue and help protect plants against bacterial and fungal infection (35). In addition, tannins are thought to be partially responsible for the astringent taste of red wines and tea (36, 37). We therefore wondered if a tannin-containing drink, such as tea, would directly affect biofilms by the same isaA-dependent mechanism as tannic acid. We added various concentrations of brewed black tea to a biofilm formation assay. In the wild-type background, very low concentrations of brewed tea (1:500 dilution of brewed tea) significantly inhibited biofilm formation (Fig. 6A). However, an isaA mutant formed a biofilm in the presence of tea at any concentration tested, and this phenotype could be complemented by expression of isaA from its native promoter on a plasmid (Fig. 6C).
Fig 6.
Black tea inhibits biofilm formation in S. aureus. (A) Black tea inhibits biofilm formation in a dose-dependent manner. Biofilm formation was induced in a microtiter plate biofilm assay by supplementing the medium with 0.2% glucose. Error bars represent standard deviations. *, P ≪ 0.001 compared to the control with glucose and without tea. (B) When black tea is mixed with milk, the tea loses its biofilm-inhibitory effect. Error bars represent standard deviations. *, P ≪ 0.001 compared to the control with glucose, without tea, and without milk. (C) isaA is necessary for tea-induced biofilm inhibition. Strain SH1000 (BB386), a mutant isaA::Tetr strain (BB2183), a mutant isaA::Tetr mutant strain with an empty vector (BB2184), and a mutant isaA::Tetr mutant strain with an isaA complement (BB2185) were assayed in microtiter plates for tea-induced biofilm inhibition. Strains lacking functional isaA were resistant to inhibition. Error bars represent standard deviations. *, P ≪ 0.001 compared to an isogenic control with glucose and without tea.
Since tannins and proteins readily coprecipitate (38), we tested whether the addition of milk (protein) to tea would affect the antibiofilm properties of tea. Milk was added to freshly brewed black tea at a concentration of 5 mg/ml. The mixture was vortexed briefly and allowed to sit at room temperature for 1 h before it was directly added to microtiter plate biofilm assays. Unlike tea alone, the tea-milk mixture failed to inhibit biofilm formation (Fig. 6B). This lack of biofilm inhibition corresponded to the removal of polyphenols from the tea (39, 40).
Tea inhibits S. aureus throat colonization in an isaA-dependent manner.
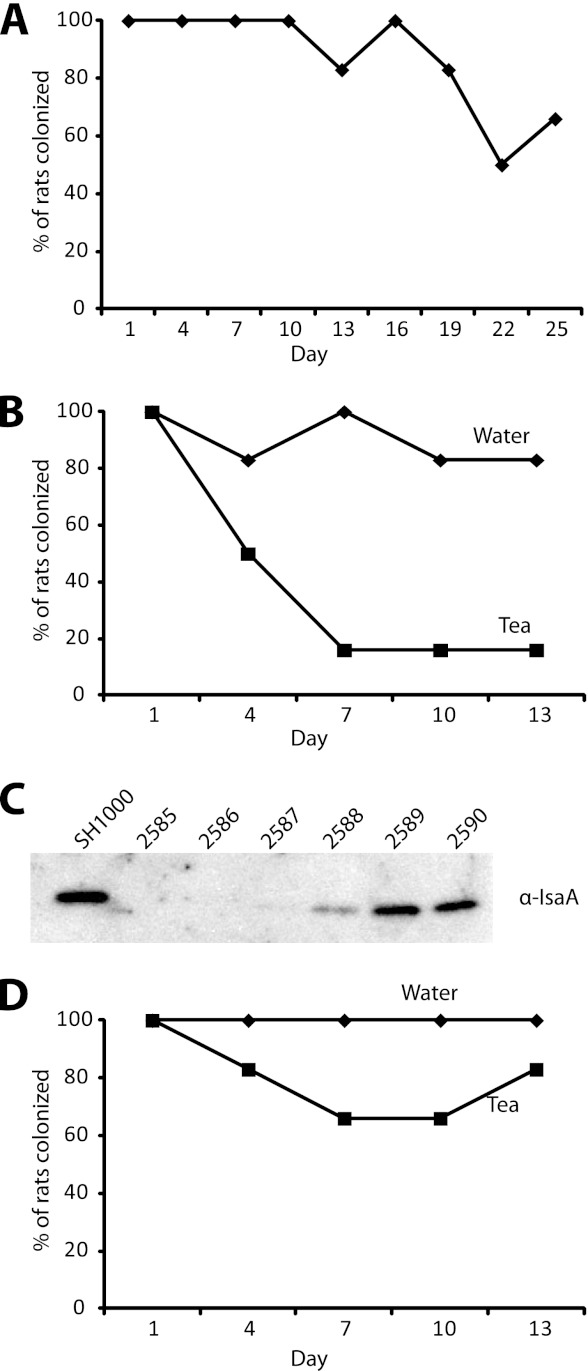
Emerging evidence suggests that, in addition to the nasopharynx, S. aureus commonly colonizes the oropharynx and oral cavity (2, 27, 28). Since tea is a commonly consumed beverage that inhibits surface colonization in our in vitro models, we tested if tea could impact S. aureus colonization in a rodent model. The cotton rat has previously been used to study S. aureus nasal colonization (41), so we first assessed if S. aureus would also colonize the cotton rat oropharynx. Oral inoculation with 1 × 105 S. aureus CFU resulted in reproducible oropharynx colonization, with 5 out of 6 animals displaying detectable oropharynx colonization over a period of 19 days (Fig. 7A).
Fig 7.
S. aureus throat colonization is reduced by tea in an isaA-dependent manner. (A) Cotton rats colonized with S. aureus via gavage to the oropharynx remain consistently throat colonized for 19 days (n = 6). (B) Tea ingestion via gavage at days 2, 5, and 8 reduces the number of animals colonized with wild-type S. aureus in the oropharynx to 1 out of 6 animals at days 7, 10, and 13 (n = 6). Control water ingestion resulted in oropharynx colonization of 6 out of 6 animals at day 7 and 5 out of 6 animals at days 10 and 13 (n = 6). (C) Western blot with anti-IsaA antibody on culture supernatants from six colonies isolated from the one rat that remained colonized after tea ingestion. Three of the six colonies tested did not produce detectable levels of IsaA in culture supernatants. (D) Colonization of cotton rats with an isaA mutant. All animals remained colonized after water ingestion via gavage at days 2, 5, and 8, and tea ingestion via gavage resulted in 4 out of 6 animals being colonized at days 7 and 10 and 5 out of 6 being colonized at day 13.
To determine if tea ingestion influenced S. aureus oropharynx colonization, 200 μl of black tea was administered via gavage to colonized rats at 2, 5, and 8 days after initial colonization. Tea ingestion reduced the number of animals colonized with wild-type S. aureus in the oropharynx, with 5 out of 6 animals not being colonized after tea ingestion versus 1 out of 6 animals in a water gavage control group not being colonized (Fig. 7B). In addition, we tested six colonies from the rat that remained colonized with S. aureus after tea ingestion for IsaA production. Three of the six isolates produced no detectable IsaA, and one produced dramatically lower levels of IsaA than the wild type (Fig. 7C). Finally, an isaA mutant appeared to maintain higher levels of colonization upon tea ingestion. Four out of six animals colonized with an isaA mutant versus one out of six colonized with the isogenic wild-type parent remained colonized after tea ingestion (Fig. 7D and B). These results suggest that consumption of polyphenolic compounds, like those in tea, may reduce S. aureus oropharynx colonization in an isaA-dependent manner.
DISCUSSION
The ability of S. aureus to colonize surfaces and form biofilms contributes to its success as a commensal and pathogen. S. aureus lives as a commensal attached to surfaces such as the skin, nasopharynx, and oropharynx (2). As a pathogen, S. aureus can attach to internal tissues such as bone, heart valves, or implanted medical devices (7–9). Colonization by S. aureus increases the incidence of infection, and biofilm infections represent a serious clinical situation, based on their recalcitrance to antibiotics, their persistence, and the propensity of organisms to detach and colonize new sites (6). Relatively little is known regarding how natural products that are common in the human diet can influence S. aureus colonization and biofilm development. Therefore, understanding how S. aureus responds to natural products and different environmental conditions is an important issue that warrants further investigation.
In this work we demonstrate that the polyphenolic compound tannic acid can inhibit S. aureus surface colonization in a multitude of biofilm models (Fig. 1). Analysis of liquid culture supernatants revealed increased levels of the protein IsaA when strains were grown in the presence of tannic acid (Fig. 2). An isaA mutant was not susceptible to the biofilm inhibition effects of tannic acid, and this phenotype was complemented by expressing isaA under the control of its native promoter in trans (Fig. 3). Expression of IsaA from an inducible promoter inhibited biofilm formation, and this was dependent upon a catalytic residue at the putative IsaA transglycosylase active site (Fig. 4). After prolonged incubation in drip-flow biofilms, isolates that were resistant to the antibiofilm effect of tannic acid appeared, and these isolates failed to produce IsaA (Fig. 5). Black tea, a common source of tannic acid in the human diet, inhibited biofilm formation in vitro in an isaA-dependent manner (Fig. 6). Finally, we developed an animal model for S. aureus throat colonization and found that tea reduced throat colonization in an isaA-dependent manner (Fig. 7).
Tannic acid has long been known to have antibacterial properties (42), bacteria are known to actively modulate gene expression in response to tannins (43, 44), and recently, it has been suggested to have antibiofilm properties (45). Pentagalloyl glucose (one of the major components of commercial tannic acid) and ellagic acid (another plant-derived polyphenolic compound) have also been shown to inhibit biofilm formation in S. aureus (46, 47). To the best of our knowledge, no genetic mechanism for the antibiofilm properties of tannic acid or related polyphenols in S. aureus has been proposed. In this report, we show that tannic acid causes an increase in extracellular IsaA levels and that increased levels of IsaA are able to inhibit biofilm formation in S. aureus. At this time, the molecular mechanism leading to increased extracellular IsaA levels is not known. Several possible mechanisms that would lead to this outcome are under investigation, including differential regulation of isaA and altered protein stability.
Lytic transglycosylases have been extensively studied in E. coli, where they have been shown to cleave peptidoglycan at the β-1,4-glycosidic bond between N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc) (16). By virtue of their ability to cleave the polysaccharide backbone of the peptidoglycan layer, lytic transglycosylases are thought to play a role in synthesis and degradation of the peptidoglycan. It has been proposed that lytic transglycosylases play important roles in cellular elongation, septation, recycling of muropeptides, and pore formation (48).
To the best of our knowledge, this is the first report describing a specific function of the lytic transglycosylase IsaA in S. aureus. The mechanism by which IsaA leads to biofilm inhibition remains unclear, but the evidence that we present in this paper demonstrates that this activity depends on IsaA's catalytic function as a lytic transglycosylase. There are several ways in which cleavage of peptidoglycan could lead to a reduction in biofilm formation. For example, peptidoglycan cleavage could change the composition of proteins and teichoic acids displayed on the cell wall, cleaving away factors necessary for surface colonization. Alternatively, peptidoglycan cleavage could release a signaling molecule (49), leading to modulation of biofilm-related gene expression. Work is under way to elucidate how IsaA activity leads to biofilm inhibition.
S. aureus nasal colonization is a significant risk factor for several infections, including bacteremia, postoperative infections, and diabetic foot ulcer infections, and contributes to the spread of this pathogen in hospital environments (50–53). Many hospitals employ rigorous S. aureus infection control policies, including active surveillance of nasal colonization for patients and personnel, contact precautions, and isolation of colonized patients (54). Current decolonization strategies involve applying topical agents such as mupirocin to the nose. Several recent studies have identified the oropharynx as another common site of S. aureus colonization (2, 27, 28). Because hospital efforts to track and eliminate S. aureus colonization focus primarily on the nasal cavity, these approaches are not likely to affect throat carriage, and therefore, this reservoir for future infection may persist.
Our finding that tea inhibited S. aureus biofilm development in vitro and reduced throat colonization in an animal model may have important consequences. In addition to helping us understand the function and role of IsaA, it gives us a safe, effective tool for decolonizing a second common site of S. aureus colonization, aiding in hospitals' efforts to reduce the risk of infection and spread of this deadly pathogen. Hot tea or coffee has previously been associated with reduced methicillin-resistant S. aureus nasal colonization, suggesting that our results may translate to human colonization (55). Understanding the effects of tannic acid and tea in decolonizing this reservoir, as well as the genetic mechanism underlying this effect, could lead us to more effective treatments for S. aureus colonization and infection.
Supplementary Material
ACKNOWLEDGMENTS
We extend our gratitude to Adnan Syed, Kelly Schwartz, Rachel Stephenson, Matt Sekedat, Raymond Barbehenn, and members of the M. R. Chapman laboratory for their helpful discussions and suggestions.
This material is based upon work supported by the NSF GRFP under grant no. DGE 0718128 (to D.E.P.) and the NIH NIAID AI081748 (to B.R.B.).
Footnotes
Published ahead of print 3 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00877-12.
REFERENCES
- 1. Acton DS, Plat-Sinnige MJ, van Wamel W, de Groot N, van Belkum A. 2009. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur. J. Clin. Microbiol. Infect. Dis. 28:115–127 [DOI] [PubMed] [Google Scholar]
- 2. Lee CJ, Sankaran S, Mukherjee DV, Apa ZL, Hafer CA, Wright L, Larson EL, Lowy FD. 2011. Staphylococcus aureus oropharyngeal carriage in a prison population. Clin. Infect. Dis. 52:775–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters PJ, Brooks JT, Limbago B, Lowery HK, McAllister SK, Mindley R, Fosheim G, Gorwitz RJ, Guest JL, Hageman J, Fridge J, Rimland D. 2010. Methicillin-resistant Staphylococcus aureus colonization in HIV-infected outpatients is common and detection is enhanced by groin culture. Epidemiol. Infect., p 1–11 [DOI] [PubMed] [Google Scholar]
- 4. Parsek MR, Singh PK. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677–701 [DOI] [PubMed] [Google Scholar]
- 5. Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. 2012. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 8:e1002744 doi:10.1371/journal.ppat.1002744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boles BR, Horswill AR. 2011. Staphylococcal biofilm disassembly. Trends Microbiol. 19:449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zuluaga AF, Galvis W, Saldarriaga JG, Agudelo M, Salazar BE, Vesga O. 2006. Etiologic diagnosis of chronic osteomyelitis: a prospective study. Arch. Intern. Med. 166:95–100 [DOI] [PubMed] [Google Scholar]
- 8. Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021 [DOI] [PubMed] [Google Scholar]
- 9. Gomez-Barrena E, Esteban J, Medel F, Molina-Manso D, Ortiz-Perez A, Cordero-Ampuero J, Puertolas JA. 2012. Bacterial adherence to separated modular components in joint prosthesis: a clinical study. J. Orthop. Res. 30:1634–1639 [DOI] [PubMed] [Google Scholar]
- 10. del Pozo JL, Patel R. 2007. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 82:204–209 [DOI] [PubMed] [Google Scholar]
- 11. Boles BR, Horswill AR. 2008. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 4:e1000052 doi:10.1371/journal.ppat.1000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gotz F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367–1378 [DOI] [PubMed] [Google Scholar]
- 13. Pynnonen M, Stephenson RE, Schwartz K, Hernandez M, Boles BR. 2011. Hemoglobin promotes Staphylococcus aureus nasal colonization. PLoS Pathog. 7:e1002104 doi:10.1371/journal.ppat.1002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lorenz U, Ohlsen K, Karch H, Hecker M, Thiede A, Hacker J. 2000. Human antibody response during sepsis against targets expressed by methicillin resistant Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 29:145–153 [DOI] [PubMed] [Google Scholar]
- 15. Stapleton MR, Horsburgh MJ, Hayhurst EJ, Wright L, Jonsson IM, Tarkowski A, Kokai-Kun JF, Mond JJ, Foster SJ. 2007. Characterization of IsaA and SceD, two putative lytic transglycosylases of Staphylococcus aureus. J. Bacteriol. 189:7316–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holtje JV, Mirelman D, Sharon N, Schwarz U. 1975. Novel type of murein transglycosylase in Escherichia coli. J. Bacteriol. 124:1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bateman BT, Donegan NP, Jarry TM, Palma M, Cheung AL. 2001. Evaluation of a tetracycline-inducible promoter in Staphylococcus aureus in vitro and in vivo and its application in demonstrating the role of sigB in microcolony formation. Infect. Immun. 69:7851–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duthie ES, Lorenz LL. 1952. Staphylococcal coagulase; mode of action and antigenicity. J. Gen. Microbiol. 6:95–107 [DOI] [PubMed] [Google Scholar]
- 19. Peng HL, Novick RP, Kreiswirth B, Kornblum J, Schlievert P. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365–4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967–3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reiser RF, Hinzman SJ, Bergdoll MS. 1987. Production of toxic shock syndrome toxin 1 by Staphylococcus aureus restricted to endogenous air in tampons. J. Clin. Microbiol. 25:1450–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. CDC 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997-1999. MMWR Morb. Mortal. Wkly. Rep. 48:707–710 [PubMed] [Google Scholar]
- 23. Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175:3907–3919 [DOI] [PubMed] [Google Scholar]
- 24. Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 63:3373–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Husmann LK, Yung DL, Hollingshead SK, Scott JR. 1997. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infect. Immun. 65:1422–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Research Council 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC [Google Scholar]
- 27. Nurjadi D, Lependu J, Kremsner PG, Zanger P. 2012. Staphylococcus aureus throat carriage is associated with ABO-/secretor status. J. Infect. 65:310–317 [DOI] [PubMed] [Google Scholar]
- 28. Ohara-Nemoto Y, Haraga H, Kimura S, Nemoto TK. 2008. Occurrence of staphylococci in the oral cavities of healthy adults and nasal oral trafficking of the bacteria. J. Med. Microbiol. 57:95–99 [DOI] [PubMed] [Google Scholar]
- 29. Salminen J-P, Karonen M. 2011. Chemical ecology of tannins and other phenolics: we need a change in approach. Funct. Ecol. 25:325–338 [Google Scholar]
- 30. Corrigan RM, Foster TJ. 2009. An improved tetracycline-inducible expression vector for Staphylococcus aureus. Plasmid 61:126–129 [DOI] [PubMed] [Google Scholar]
- 31. Blackburn NT, Clarke AJ. 2001. Identification of four families of peptidoglycan lytic transglycosylases. J. Mol. Evol. 52:78–84 [DOI] [PubMed] [Google Scholar]
- 32. Thunnissen AM, Dijkstra AJ, Kalk KH, Rozeboom HJ, Engel H, Keck W, Dijkstra BW. 1994. Doughnut-shaped structure of a bacterial muramidase revealed by X-ray crystallography. Nature 367:750–753 [DOI] [PubMed] [Google Scholar]
- 33. Monteiro C, Fang X, Ahmad I, Gomelsky M, Romling U. 2011. Regulation of biofilm components in Salmonella enterica serovar Typhimurium by lytic transglycosylases involved in cell wall turnover. J. Bacteriol. 193:6443–6451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haslam E, Lilley TH, Cai Y, Martin R, Magnolato D. 1989. Traditional herbal medicines—the role of polyphenols. Planta Med. 55:1–8 [DOI] [PubMed] [Google Scholar]
- 35. Scalbert A. 1991. Antimicrobial properties of tannins. Phytochemistry 30:3875–3883 [Google Scholar]
- 36. Bandyopadhyay P, Ghosh AK, Ghosh C. 2012. Recent developments on polyphenol-protein interactions: effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 3:592–605 [DOI] [PubMed] [Google Scholar]
- 37. Vidal S, Francis L, Noble A, Kwiatkowski M, Cheynier V, Waters E. 2004. Taste and mouth-feel properties of different types of tannin-like polyphenolic compounds and anthocyanins in wine. Anal. Chim. Acta 513:57–65 [Google Scholar]
- 38. Thomas AW, Frieden A. 1923. The gelatin-tannin reaction. Ind. Eng. Chem. 15:839–841 [Google Scholar]
- 39. Anderson RA, Polansky MM. 2002. Tea enhances insulin activity. J. Agric. Food Chem. 50:7182–7186 [DOI] [PubMed] [Google Scholar]
- 40. Morton JF. 1979. Tea with milk. Science 204:909. [PubMed] [Google Scholar]
- 41. Kokai-Kun JF. 2008. The cotton rat as a model for Staphylococcus aureus nasal colonization in humans: cotton rat S. aureus nasal colonization model. Methods Mol. Biol. 431:241–254 [DOI] [PubMed] [Google Scholar]
- 42. Henis Y, Volcani R, Tagari H. 1964. Effect of water extracts of carob pods, tannic acid, and their derivatives on morphology and growth of microorganisms. Appl. Microbiol. 12:204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quan S, Koldewey P, Tapley T, Kirsch N, Ruane KM, Pfizenmaier J, Shi R, Hofmann S, Foit L, Ren G, Jakob U, Xu Z, Cygler M, Bardwell JC. 2011. Genetic selection designed to stabilize proteins uncovers a chaperone called Spy. Nat. Struct. Mol. Biol. 18:262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zoetendal EG, Smith AH, Sundset MA, Mackie RI. 2008. The BaeSR two-component regulatory system mediates resistance to condensed tannins in Escherichia coli. Appl. Environ. Microbiol. 74:535–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chusri S, Phatthalung PN, Voravuthikunchai SP. 2012. Anti-biofilm activity of Quercus infectoria G. Olivier against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 54:511–517 [DOI] [PubMed] [Google Scholar]
- 46. Lin MH, Chang FR, Hua MY, Wu YC, Liu ST. 2011. Inhibitory effects of 1,2,3,4,6-penta-O-galloyl-beta-d-glucopyranose on biofilm formation by Staphylococcus aureus. Antimicrob. Agents Chemother. 55:1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Quave CL, Estevez-Carmona M, Compadre CM, Hobby G, Hendrickson H, Beenken KE, Smeltzer MS. 2012. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS One 7:e28737 doi:10.1371/journal.pone.0028737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scheurwater E, Reid CW, Clarke AJ. 2008. Lytic transglycosylases: bacterial space-making autolysins. Int. J. Biochem. Cell Biol. 40:586–591 [DOI] [PubMed] [Google Scholar]
- 49. Shah IM, Laaberki MH, Popham DL, Dworkin J. 2008. A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell 135:486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Munoz P, Hortal J, Giannella M, Barrio JM, Rodriguez-Creixems M, Perez MJ, Rincon C, Bouza E. 2008. Nasal carriage of S. aureus increases the risk of surgical site infection after major heart surgery. J. Hosp. Infect. 68:25–31 [DOI] [PubMed] [Google Scholar]
- 51. Stanaway S, Johnson D, Moulik P, Gill G. 2007. Methicillin-resistant Staphylococcus aureus (MRSA) isolation from diabetic foot ulcers correlates with nasal MRSA carriage. Diabetes Res. Clin. Pract. 75:47–50 [DOI] [PubMed] [Google Scholar]
- 52. von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 344:11–16 [DOI] [PubMed] [Google Scholar]
- 53. Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705 [DOI] [PubMed] [Google Scholar]
- 54. Lee BY, Bailey RR, Smith KJ, Muder RR, Strotmeyer ES, Lewis GJ, Ufberg PJ, Song Y, Harrison LH. 2010. Universal methicillin-resistant Staphylococcus aureus (MRSA) surveillance for adults at hospital admission: an economic model and analysis. Infect. Control Hosp. Epidemiol. 31:598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matheson EM, Mainous AG, III, Everett CJ, King DE. 2011. Tea and coffee consumption and MRSA nasal carriage. Ann. Fam. Med. 9:299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.