Abstract
Curli fibrils, the best-characterized functional bacterial amyloids, are an important component of enterobacterial biofilms. We have previously shown that curli fibrils are recognized by the Toll-like receptor 2 (TLR2)/TLR1 heterodimer complex. Utilizing polarized T-84 cells, an intestinal epithelial cell line derived from colon carcinoma grown on semipermeable tissue culture inserts, we determined that infection with a Salmonella enterica serovar Typhimurium csgBA mutant, which does not express curli, resulted in an increase in intestinal barrier permeability and an increase in bacterial translocation compared to infection with curliated wild-type S. Typhimurium. When the TLR2 downstream signaling molecule phosphatidylinositol 3-kinase (PI3K) was blocked using wortmannin or LY294002, the difference in disruption of the intestinal epithelium and bacterial translocation was no longer observed. Additionally, disruption of polarized T-84 cells treated basolaterally with the TLR5 ligand flagellin was prevented when the polarized cells were simultaneously treated with the synthetic TLR2/TLR1 ligand Pam3CSK4 or with purified curli fibrils in the apical compartment. Similar to in vitro observations, C57BL/6 mice infected with the csgBA mutant suffered increased disruption of the intestinal epithelium and therefore greater dissemination of the bacteria to the mesenteric lymph nodes than mice infected with wild-type S. Typhimurium. The differences in disruption of the intestinal epithelium and bacterial dissemination in the mice infected with csgBA mutant or wild-type S. Typhimurium were not apparent in TLR2-deficient mice. Overall, these studies report for the first time that activation of the TLR2/PI3K pathway by microbial amyloids plays a critical role in regulating the intestinal epithelial barrier as well as monitoring bacterial translocation during infection.
INTRODUCTION
The intestinal epithelium represents a physical as well as an immunological barrier which is in constant contact with approximately 1013 to 1014 microorganisms (1–3). Bacteria comprise the vast majority of the intestinal organisms with at least 1,000 different species present within the community (4–6). Therefore, there is a critical need for mechanisms for protecting the host from hyperresponsive inflammatory processes due to the presence of an unprecedented amount of antigens while still supporting the growth of commensal bacteria which are beneficial to host health and function. As a first line of innate immune response, the intestinal epithelium has been found to play an important role in the maintenance and regulation of gastrointestinal homeostasis. For instance, it is currently known that the production of antimicrobial peptides and lectins by enterocytes and Paneth cells (7–11), the production of mucins by goblet cells (12, 13), and modulation of epithelial barrier integrity (14) all act in concert to regulate and maintain intestinal immune homeostasis.
Toll-like receptors (TLRs) comprise a family of innate pattern recognition receptors (PRRs) that sense conserved microbial structures known as pathogen-associated molecular patterns (PAMPs) and endogenous danger molecules (15–17). In the gut mucosa, various TLRs are involved in the recognition of microbial signature molecules. TLR5, expressed on the basolateral side of the epithelial cells, recognizes flagella of invading microbes and consequently activates nuclear factor kappa B (NF-κB), leading to the production of proinflammatory cytokines, including interleukin 8 (IL-8) (18). TLR2, a member of this family, recognizes a number of conserved molecular patterns, including lipopeptides, lipoteichoic acid, and zymosan, through the formation of heterodimers with TLR1 or with TLR6 (19–25). MyD88 (myeloid differentiation primary response gene 88) and Mal/TIRAP are both required for TLR2-dependent signaling where NF-κB is activated. While Mal/TIRAP is involved in bridging MyD88 to the TLR2 receptor complex and directing the recruitment of TRAF6, which is necessary for NF-κB activation, Mal binds to the p85α subunit of phosphatidylinositol 3-kinase (PI3K) upon activation of the TLR2/TLR6 heterodimer, resulting in Akt phosphorylation, which consequently leads to macrophage polarization and cell survival by inhibiting apoptosis. In contrast, TLR2/TLR1-mediated activation of PI3K occurs in the absence of Mal and MyD88, suggesting the presence of another adaptor molecule (26–29). Activation of the PI3K pathway as a downstream effect of TLR2 activation has also been shown to augment the tight-junction-associated epithelial barrier integrity, possibly by acting as a surveillance receptor which monitors luminal bacteria and translocation of pathogens (30–32).
Amyloids, which possess a fibrillar cross-β-sheet quaternary structure, are produced by both humans and bacteria. While amyloids in humans are associated mostly with complex diseases, functional amyloids that serve a role in physiological processes such as melanin production and blood clotting have been reported (33–38). In bacteria, amyloids function as a component of the extracellular matrix in biofilms of commensal organisms, such as spore-forming Bacillus subtilis and Pseudomonas fluorescens, or human pathogens, such as Mycobacterium tuberculosis, Salmonella enterica serovar Typhimurium, Citrobacter freundii, Enterobacter sakazakii, and Escherichia coli (39–46). Curli fibrils produced by enteric bacteria, including Salmonella spp. and E. coli, are the best-characterized bacterial amyloid to date. Earlier studies have shown that curli fibrils activate the immune system, inducing the production of inflammatory cytokines in a mouse model of sepsis as well as urinary tract infection induced by E. coli (47–52). Curli fibrils are indeed a pathogen-associated molecular pattern (PAMP) that is recognized by the TLR2/TLR1 heterodimer (48–50). Interestingly, TLR2 not only responds to curli fibrils but also recognizes host amyloids such as β-amyloid 1-40 and β-amyloid 1-42 of Alzheimer's plaques as well as serum amyloid A, an acute-phase protein (48, 53–57). In fact, TLR2 recognizes the conserved quaternary β-sheet structure that is common to amyloids of all distinct origins (48).
Amyloids have also been reported to be present in the biofilms of members of Bacteriodetes and Firmicutes, the predominant phyla found in the gastrointestinal tract (44, 58). In this study, we investigated whether recognition of amyloid fibrils could induce a TLR2-dependent response in intestinal epithelia contributing to the regulation of intestinal barrier integrity by using S. Typhimurium as a model.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. Typhimurium strain IR715 (wild type) is a fully virulent, nalidixic acid-resistant strain derived from strain ATCC 14028 (59). CT16 is a mutant strain derived from IR715 and contains an unmarked csgBA deletion (60). To induce the expression of curli fibrils, the bacterial strains were grown on tryptone agar (T-medium) plates at 28°C for 48 h (61). For in vivo experiments, bacterial strains were grown overnight with shaking at 37°C in Luria-Bertani (LB) broth (Fisher Bioreagents) supplemented with nalidixic acid (Fisher Bioreagents) at a final concentration of 0.05 mg/ml.
Cell culture.
The human intestinal epithelial cell (IEC) lines from colon carcinoma (T-84) and cervical carcinoma (HeLa) were obtained from the American Type Culture Collection. T-84 cells were grown in Dulbecco modified Eagle medium (DMEM)/F-12 (GIBCO) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (GIBCO). T-84 cells were grown to confluence on 0.4-μm semipermeable tissue culture inserts (Transwell; Corning) in a humidified incubator at 37°C and 5% CO2. T-84 cells achieved a polarized and differentiated state within 5 to 10 days and were used when the transepithelial resistance (TER) had reached >1,500 Ω cm2 (62).
Invasion assay.
The invasion assay was carried out as described previously (49). Briefly, T-84 monolayers were infected with 3.5 ×105 of wild-type IR715 and csgBA mutant CT16 bacterial strains (multiplicity of infection [MOI] of 7) grown under conditions optimal for curli expression or type III secretion system 1 (T3SS-1) expression. Bacteria were allowed to invade cells for an hour. This was then followed by replacement of the medium containing 1 mg/ml gentamicin (Invitrogen) to eliminate extracellular bacteria and incubation for 1.5 h. Epithelial cells were then lysed with 1% Triton-X (Sigma). Cell lysates were then plated on LB agar plates supplemented with nalidixic acid at a final concentration of 0.05 mg/ml. Invasion assays were repeated three times.
IL-8 production.
Polarized T-84 cells were infected with wild-type IR715 and the csgBA mutant CT16 as described above. At 24 h postinfection, 100 μl of the supernatant was removed from the basolateral compartment of the Transwell. The IL-8 concentration was determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Biolegend).
Translocation studies.
For translocation studies, polarized T-84 epithelial layers grown on 3.0-μm semipermeable tissue culture inserts (Transwell; Corning) were infected apically with S. Typhimurium (wild type) or its csgBA mutant (CT16) for 1 h as described above. One-hundred-microliter samples from the basolateral medium were taken at 1 h postinfection, and appropriate dilutions were plated on LB agar plates containing nalidixic acid. For studies involving the PI3K inhibitor LY294002, the polarized epithelial layer was incubated with 50 μM LY294002 for 1 h prior to bacterial infection.
Epithelial integrity.
Polarized T-84 cells were infected with wild-type IR715 and the csgBA mutant CT16 as described above. At 5 h or 24 h postinfection, 5 μl of 10-mg/ml fluorescein isothiocyanate-labeled dextran (FITC-dextran) (average molecular weight, 3,000 to 5,000; Sigma) was added to the apical side of the Transwell chamber. Two hours after the addition of FITC-dextran, medium from the basolateral side of the Transwell chamber was collected and fluorescence intensity was measured using an Omega plate reader (BMG Labtech) at 485-nm excitation and 520-nm emission wavelengths (63). To study the role played by flagellin and curli fibrils in intestinal epithelial integrity, flagellin (FLA-ST; Invivogen) was added to the Transwells basolaterally at a final concentration of 0.01 μg/ml. Purification of curli fibrils from the S. Typhimurium msbB mutant (RPW3) was performed according to an established protocol (61). Briefly, bacterial cells were removed from T-medium plates and lysed by sonication. This was followed by enzymatic digestion and preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Insoluble material (curli fibrils) retained in the well of the SDS-polyacrylamide gel was collected after the electrophoresis was complete. The protein concentration of curli fibrils was determined with the bicinchoninic acid (BCA) protein assay (Calbiochem). Curli fibrils (10 μg/ml) or the triacylated synthetic TLR2/TLR1 ligand Pam3CSK4 (0.1 μg/ml; Invivogen) was added either alone or simultaneously with basolateral flagellin treatment to the apical chamber of the Transwell. To block PI3K, polarized epithelial cells were incubated either with 20 μM wortmannin (Calbiochem) for 30 min or with 50 μM LY294002 (Cell Signal) for 1 h prior to bacterial infection. Experiments were repeated three times.
Mouse experiments.
Six- to 8-week-old female C57BL/6 mice were obtained from Jackson Laboratory. TLR2-deficient mice (B6.129-TLR2tm1Kir/J) were purchased from Jackson Laboratory and were maintained and bred in Temple University's animal facility. The Temple University Animal Care and Use Committee approved all animal studies.
The use of FITC-dextran to assess intestinal permeability in vivo has been previously described (12). Briefly, groups of 3 or 4 mice were either orally inoculated with 1 × 109 bacteria in LB or mock infected with sterile LB. At 72 h postinfection, 150 μl of 80-mg/ml FITC-dextran was administered orally. Mice were sacrificed 4 h later, and blood was collected via cardiac puncture. Blood was collected into microcentrifuge tubes coated with a mixture of the anticoagulant heparin (15 mg/ml) and acid citrate-dextrose (20 mM citric acid, 100 mM sodium citrate, 5 mM dextrose). Blood was then spun at 1,000 rpm for 20 min to separate serum from whole blood cells. Fluorescence intensity in the serum was then determined using the Omega plate reader (BMG Labtech) at 485-nm excitation and 520-nm emission wavelengths.
To assess bacterial numbers, oral inoculation of bacteria as described above was performed. Mice were sacrificed 72 h later, and tissue samples from the cecum, liver, spleen, mesenteric lymph nodes, and Peyer's patches were collected. Colonic content was collected in 1 ml of sterile phosphate-buffered saline (PBS). Organ samples were homogenized in sterile PBS, and appropriate serial dilutions were plated on LB-nalidixic acid agar plates. All the animal experiments were repeated 3 times.
PCR.
To examine the expression of TLR1 and TLR2 by epithelial cells, T-84 cells were grown to confluence on permeable tissue culture inserts as described above. RNA was extracted in 0.5 ml of TriReagent. Following RNA isolation, 2 μg of total RNA was reverse transcribed using murine leukemia virus (MuLV) reverse transcriptase. Two microliters of cDNA was subjected to PCR amplification using a high-fidelity PCR Supermix (Invitrogen) and the primers listed in Table 1. The following program was used for PCR amplification: 95°C for 120 s, followed by 35 cycles of 95°C for 60s, 55 to 58°C for 45 s (annealing temperatures were optimized for each TLR primer pair used), and 72°C for 60 s. As a positive control, HeLa cells were stably transfected with a plasmid expressing either TLR1 or TLR2 as described previously (48). HeLa cells transfected with an empty vector were employed as a negative control. The resultant PCR products were then analyzed on a 1.5% agarose gel.
Table 1.
Primers
| Gene | Primer sequence |
|
|---|---|---|
| Forward | Reverse | |
| hTLR1 | 5′-CTATACACCAAGTTGTCAGC-3′ | 5′-GTCTCCAACTCAGTAAGGTG-3′ |
| hTLR2 | 5′-GCCAAAGTCTTGATTGATTGG-3′ | 5′-TTGAAGTTCTCCAGCTCCTG-3′ |
Statistical analysis.
The Student t test was used to calculate statistically significant differences (P < 0.05). For analysis of bacterial numbers, values were logarithmically converted prior to statistical analysis.
RESULTS
TLR2 and TLR1 are expressed by polarized T-84 epithelial cells.
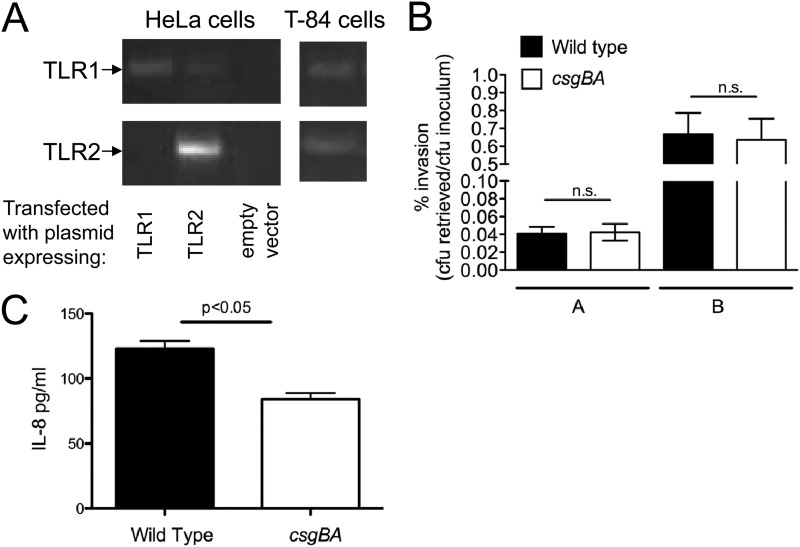
In humans, TLR2 is expressed at the apical pole of the intestinal epithelium (64–66). Likewise, mouse intestinal epithelial cells express TLR2 (67). Earlier studies, using germfree mice, demonstrated that expression of TLR2 is increased by stimuli derived from commensal bacteria (14). Since many bacteria belonging to Firmicutes and Bacteriodetes, two predominant phyla in the gut, produce amyloids as a component of their extracellular matrix (44), we hypothesized that detection of amyloids via TLR2 may affect immune responses in the intestinal epithelium in the gut. To unravel the immune responses generated against amyloid fibrils, we used a human colon carcinoma cell line, T-84. When grown on semipermeable tissue culture inserts, T-84 epithelial cells are able to differentiate and polarize to take on functional and morphological characteristics that are specific to the intestinal epithelium, with apical microvilli and a basolateral surface that can be likened to the cellular surface in contact with the subepithelial lamina propria (62, 68, 69). To ensure that the T-84 cell lines indeed expressed TLR2, RNA from these epithelial cell lines was extracted and subjected to reverse transcription and PCR amplification. As controls, HeLa cells were transfected with an empty human expression vector (negative control) or a vector containing the human TLR2 gene. T84 cells were found to express TLR2. Since curli amyloid fibrils have been reported to signal through TLR2 complexed with TLR1 (48), TLR1 expression was also confirmed via PCR (Fig. 1A).
Fig 1.
(A) Expression of TLR2 and TLR1 was determined by reverse transcription-PCR (RT-PCR) on RNA extracted from HeLa cells transfected with an empty vector or a TLR2 or TLR1 expression vector as well as from T84 cells. (B) Invasion of polarized T-84 epithelial cells by wild-type S. Typhimurium and its isogenic csgBA mutant. Bacteria were grown under conditions optimal for expression of curli fibrils (bars A) or optimal for expression of the T3SS-1 (bars B) prior to inoculation of cells. The number of bacteria recovered from the gentamicin protection assay is expressed as the percentage of the number present in the inoculum. Data are shown as geometric means from three independent experiments ± standard deviation. (C) IL-8 secretion in the supernatants of polarized T-84 cells infected with wild-type S. Typhimurium and its isogenic csgBA mutant was determined after 24 h by ELISA. Significant statistical differences are indicated above the bars (P < 0.05). n.s., not significant.
Deletion of csgBA decreases IL-8 secretion by S. Typhimurium-infected epithelial cells.
S. Typhimurium invades the intestinal epithelium by using its type III secretion system (T3SS), encoded by Salmonella pathogenicity island 1(SPI-1) (70). After invasion, conserved bacterial surface structures or effector proteins encoded by the T3SS are recognized by the innate immune system (18, 71, 72). This results in the activation of the downstream transcription factor NF-κB, which then triggers the expression of cytokines and chemokines, including IL-8. Previous studies have shown that wild-type S. Typhimurium and its csgBA mutant invade HT-29 cells, another epithelial cell line derived from colon carcinoma, and bovine intestinal epithelium equally (49, 50, 73).
To determine whether differences in host responses were due to differences in invasiveness between bacterial strains, we infected polarized T-84 epithelial cells with wild-type S. Typhimurium or its isogenic csgBA mutant, grown under optimal conditions for curli expression or T3SS-1 expression, and performed a gentamicin protection assay. We did not observe any difference in invasiveness between the wild-type S. Typhimurium and the csgBA mutant under both conditions (Fig. 1B). However, increased levels of IL-8 were observed in the basolateral compartment of cells infected by wild-type S. Typhimurium compared to those infected by the csgBA mutant grown under curli-inducing conditions (Fig. 1C).
Epithelial integrity is ensured through the activation of TLR2/PI3K pathway by curli amyloid fibrils on bacteria.
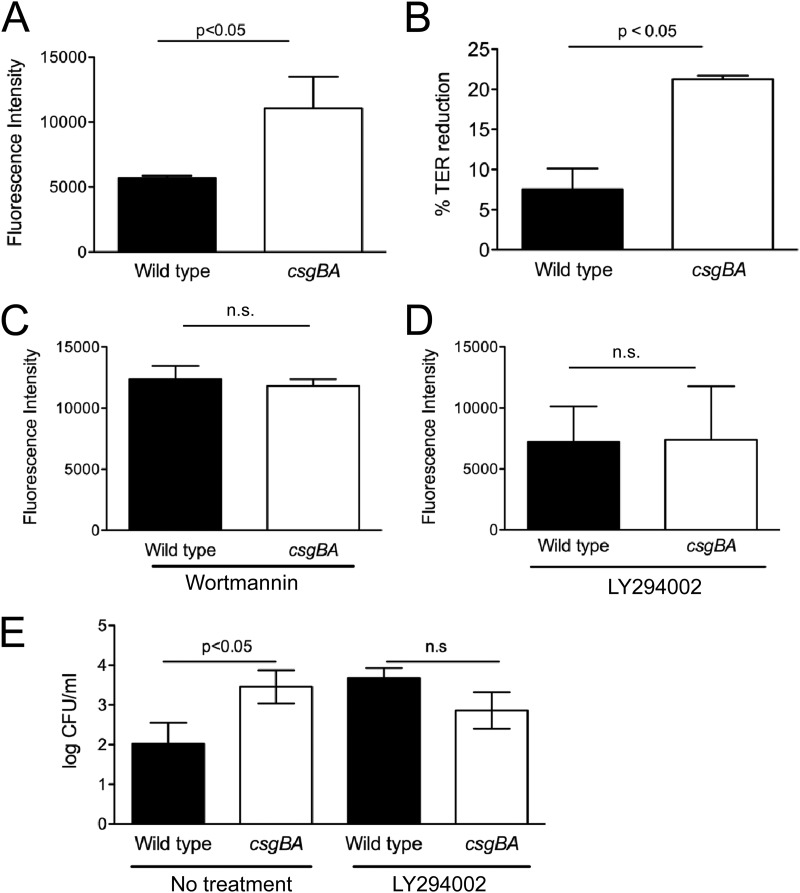
Previous studies have shown that TLR2 signaling selectively enhances the tight-junction-associated barrier integrity through the activation of the PI3K/Akt pathway via MyD88 (31). Thus, we investigated the effect of curli amyloid fibrils on epithelial integrity via TLR2/PI3K pathway activation. We measured epithelial permeability by applying FITC-dextran to the apical compartment of the polarized T-84 cells at 24 h after infection with either wild-type S. Typhimurium or the csgBA mutant. Two hours following FITC-dextran application, fluorescence in the basolateral compartment was determined. Interestingly, csgBA mutant-infected wells exhibited increased fluorescence compared to the wells infected with wild-type S. Typhimurium (Fig. 2A). To see if the increased fluorescence observed in the basolateral compartments of csgBA mutant-infected wells indeed corresponded with a disruption in the epithelial membrane, the transepithelial electrical resistance (TER) across the permeable tissue culture insert was determined prior to the infection as well as at 24 h postinfection. The percent change in TER was then determined. TER was significantly reduced when the polarized cells were infected with the csgBA mutant compared to the wild-type S. Typhimurium at 24 h postinfection (Fig. 2B). The PI3K inhibitors LY294002 and wortmannin are often used to block PI3K activity (74). To determine if the csgBA-dependent increase in epithelial permeability was due to activation of the TLR2/PI3K pathway, we pretreated the polarized epithelial cells with the irreversible PI3K inhibitor wortmannin or LY294002. Treatment with either inhibitor abolished the epithelial permeability difference observed after infection with the wild-type S. Typhimurium and the csgBA mutant (Fig. 2C and D). Furthermore, at 60 min after infection, we determined that the number of csgBA mutant cells in the basolateral compartment of the polarized T-84 cells was significantly higher (P < 0.05) than that of the wild-type S. Typhimurium. However, this difference was no longer observed when the cells were treated with the PI3K inhibitor LY294002 (Fig. 2E). Similar experiments using polarized Caco-2 cells revealed parallel results, showing that activation of TLR2/PI3K pathway with curli fibrils maintains epithelial integrity (data not shown).
Fig 2.
(A) Epithelial permeability in polarized T-84 cells after infection with wild-type S. Typhimurium and its isogenic csgBA mutant was determined after 24 h. FITC-dextran was added to the apical chamber and left for 2 h, and then fluorescence in the basolateral supernatants was determined using a BMG Omega plate reader. (B) Changes in the transepithelial resistance (TER) were measured at 5 h and 24 h postinfection, and the percent TER reduction was calculated. (C and D) T-84 cells were treated with the specific PI3K inhibitor wortmannin (20 μM) for 30 min (C) or LY294002 (50 μM) for 1 h (D) prior to infection with wild-type S. Typhimurium and its isogenic csgBA mutant. After 24 h, epithelial permeability was determined after treatment with FITC-dextran in the apical chamber for 2 h. (E) Bacterial translocation in the basolateral compartment of T-84 cells which were not treated or were pretreated with LY294002 for 1 h (50 μM) prior to infection with wild-type S. Typhimurium and its isogenic csgBA mutant was determined. Significant statistical differences are indicated above the bars (P < 0.05). n.s., not significant.
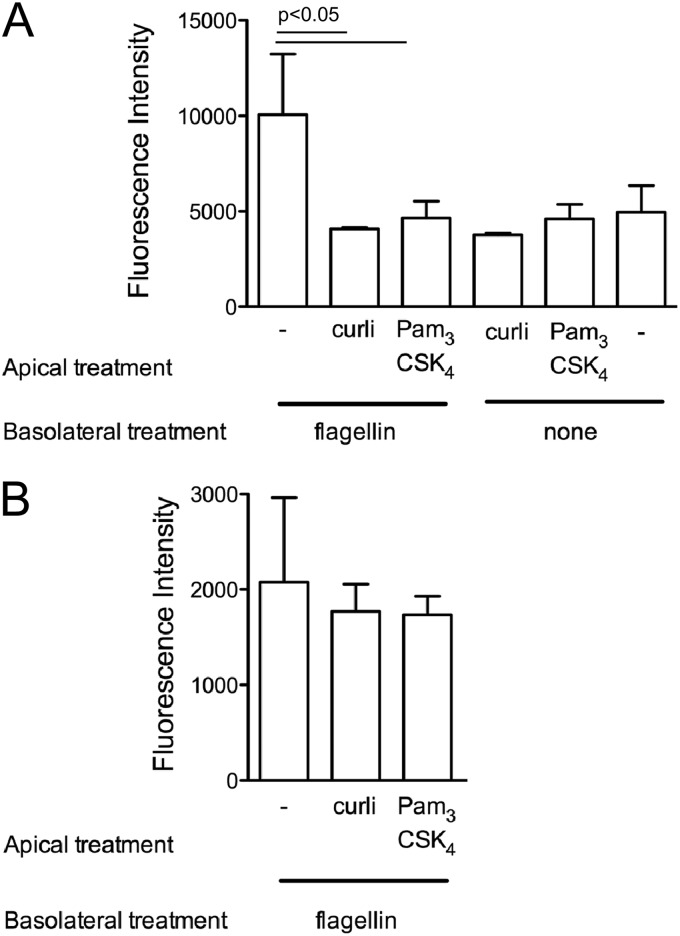
Flagellin, the major protein subunit of flagella, is a conserved surface structure of bacteria that activates TLR5 expressed basolaterally in the epithelium (18). To simulate the effects of activation of TLR2 and TLR5 by bacterial components during invasion on epithelial integrity, we stimulated polarized T-84 cells basolaterally with flagellin and/or apically with curli fibrils or Pam3CSK4 and measured the epithelial permeability by applying FITC-dextran in the apical chamber. While basolateral addition of flagellin to polarized epithelia resulted in an increase in the epithelial permeability as measured by increased fluorescence in the basolateral chamber, addition of purified curli amyloid fibrils or Pam3CSK4 did not affect the permeability. However, simultaneous addition of curli amyloid fibrils or the synthetic TLR2/TLR1 ligand Pam3CSK4 to the apical chamber and of flagellin to the basolateral chamber helped polarized epithelia to remain unaffected from flagellin treatment (Fig. 3A). When the polarized epithelium was pretreated with the specific PI3K inhibitor LY294002, the observed effects were abolished (Fig. 3B). Overall, these results suggested that the detection of curli amyloid fibrils helps polarized epithelia to maintain the epithelial barrier integrity via TLR2/PI3K activation.
Fig 3.
(A) Polarized T-84 cells were treated with either flagellin (0.01 μg/ml) or PBS in the basolateral chamber. Simultaneously, curli fibrils (10 μg/ml) or the synthetic TLR2 ligand Pam3CSK4 (0.1 μg/ml) was added to the apical chamber. (B) Polarized T-84 cells were pretreated with the specific PI3K inhibitor LY294002 (10 μM) for 1 h prior to the experiment. Cells were then treated with flagellin (0.01 μg/ml) in the basolateral chamber alone, or simultaneously curli fibrils (10 μg/ml) or the synthetic TLR2 ligand Pam3CSK4 (0.1 μg/ml) was added to the apical chamber. Epithelial permeability was determined by treatment for 2 h with FITC-dextran added after 24 h to the apical chamber. Fluorescence in the basolateral supernatants using a BMG Omega plate reader was determined.
Activation of TLR2 by curli fibrils in vivo decreases epithelial permeability and reduces bacterial translocation.
To determine whether curli fibrils are an important modulator of TLR2-mediated epithelial barrier integrity generated by bacteria in vivo, C57BL/6 mice were intragastrically infected with wild-type S. Typhimurium or the csgBA mutant. At 72 h postinfection, 150 μl of 80-mg/ml FITC-dextran was administered intragastrically. At 4 h after FITC-dextran administration, fluorescence in serum was quantified. Consistent with the in vitro data, sera from mice infected with the csgBA mutant exhibited significantly higher levels of fluorescence (P < 0.05) than sera of mice infected with wild-type S. Typhimurium (Fig. 4A). When the same experiment was repeated using TLR2-deficient mice, no significant changes in the fluorescence levels in the sera of mice infected with wild-type S. Typhimurium or with the csgBA mutant were observed (Fig. 4B).
Fig 4.
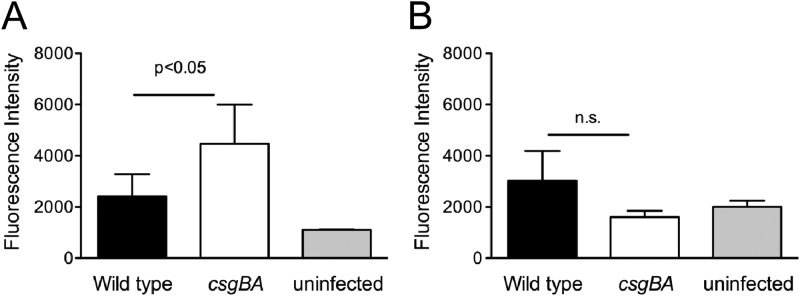
C57BL/6 (A) or TLR2-deficient (B) mice were infected intragastrically with 1 × 109 CFU wild-type S. Typhimurium or isogenic csgBA mutant or were mock treated (LB) for 72 h, and 150 μl of 80-mg/ml FITC-dextran was administered intragastrically 4 h prior to sacrifice of animals. Blood was collected, and fluorescence in the serum was measured using a BMG Omega plate reader. Significant statistical differences are indicated (P < 0.05).
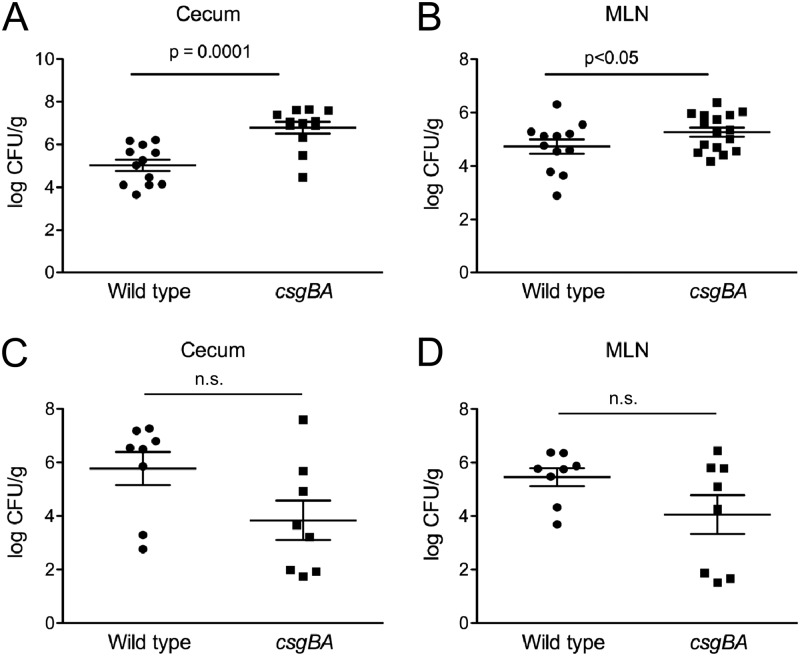
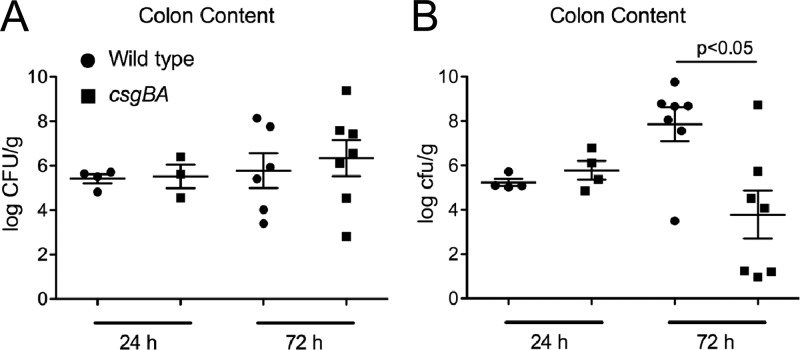
The bacterial numbers in cecal tissue and mesenteric lymph nodes of infected mice were determined. Significantly higher bacterial numbers were recovered from the ceca and mesenteric lymph nodes of C57BL/6 mice infected with the csgBA mutant strain than from those of C57BL/6 mice infected with wild-type S. Typhimurium (Fig. 5A and B). In TLR2-deficient mice, however, lower numbers of the csgBA mutant strain than of wild-type S. Typhimurium were recovered from the cecal tissue and the mesenteric lymph nodes (Fig. 5C and D). To ensure that the mice were equally infected, bacteria in the colon contents of mice were enumerated at 24 and 72 h postinfection. At 24 h postinfection, there were no significant differences between the numbers of wild-type S. Typhimurium and the csgBA mutant in infected C57BL/6 mice or TLR2-deficient mice. However, at 72 h, the wild-type S. Typhimurium bacterial numbers in colon contents of TLR2-deficient mice increased 2 logs over the bacterial numbers at 24 h. This result suggests that TLR2 may play a role in controlling bacterial infection with wild-type S. Typhimurium. Interestingly, TLR2-deficient mice infected with the csgBA mutant had significantly lower bacterial numbers in the colon contents than mice infected with wild-type S. Typhimurium at 72 h (Fig. 6A and B). Overall, these results suggest that the activation of TLR2 by curli amyloid fibrils in wild-type S. Typhimurium-infected mice promoted the maintenance of the intestinal epithelial barrier, whereas mice infected with the csgBA mutant exhibited a more permeable epithelium as seen by the increased translocation of the csgBA mutant into the cecal tissue and the mesenteric lymph nodes.
Fig 5.
C57BL/6 mice were infected intragastrically with 1 × 109 CFU wild-type S. Typhimurium or isogenic csgBA mutant or were mock treated (LB) for 72 h. (A and B) Bacterial numbers in the cecal tissue (A) and mesenteric lymph nodes (MLN) (B) were enumerated by plating serial dilutions on medium. (C and D) A similar experiment was conducted with TLR2-deficient animals, and bacterial numbers in the cecal tissue (C) and mesenteric lymph nodes (D) were enumerated. Significant statistical differences are indicated (P < 0.05 or P = 0.0001).
Fig 6.
Bacterial numbers in the colon contents of C57BL/6 mice (A) or TLR2-deficient mice (B) infected with 1 × 109 CFU wild-type S. Typhimurium (●) or its isogenic csgBA mutant (■) for 24 and 72 h were enumerated. Significant statistical differences are indicated (P < 0.05).
DISCUSSION
The intestinal epithelial barrier plays an extremely pivotal role in the maintenance and control of gastrointestinal homeostasis and immunity while interacting with large numbers of commensal microorganisms that provide essential nutrients for the host. While recognition of commensal microflora is required for intestinal homeostasis, mice deficient in TLR signaling exhibit higher antibody titers to intestinal commensal organisms, suggesting that optimal TLR signaling is key for the maintenance of the intestinal barrier (14, 75). One TLR implicated in the maintenance of intestinal homeostasis and pathogen surveillance in the intestinal mucosa is TLR2, which is expressed by both epithelial cells and antigen-presenting cells. TLR2 has been shown to regulate intestinal barrier function by augmenting tight-junction formation (30, 31, 76). In a chemically induced colitis model (dextran sodium sulfate [DSS]-induced colitis), TLR2-deficient mice exhibited an exacerbated inflammatory response and an increased susceptibility, allowing luminal commensal bacteria to infiltrate the underlying lamina propria, compared to those of wild-type mice (14). Conversely, the treatment of C57BL/6 mice with a synthetic TLR2 ligand, Pam3CSK4, was reported to ameliorate DSS-induced colitis and improve the intestinal epithelial barrier (31). Consistent with these observations, in a pathogen-induced colitis model, TLR2-deficient mice exhibited 45% to 75% mortality compared to wild-type mice (77). Overall, these data suggest that a bacterial component found in commensal as well as pathogenic bacteria may be detected by the surveillance receptor TLR2 modulating intestinal epithelial integrity.
Most studies looking at the actions of TLR2 in modulating intestinal epithelial integrity have focused on the recognition of lipopeptides by TLR2 complexes. These studies have successfully shown that TLR2 complexes are involved in modulating intestinal epithelial integrity mainly by using synthetic lipopeptides (30, 31). This is in part because of the difficulties in constructing bacterial strains deficient in lipoprotein production due to its high abundance and the deleterious effects of these mutations on bacteria.
Bacteria form biofilms to provide protection from environmental insults in various niches, including the gastrointestinal tract (78–81). Bacterial species express amyloid fibrils as a major component of their extracellular matrix in biofilms. In addition to members of the Proteobacteria, members of two of the major bacterial phyla to which most intestinal commensals belong, Bacteroidetes and Firmicutes, have been reported to express bacterial amyloids as a component of their biofilms (39, 41, 43, 44, 82, 83). Since amyloids are common across several bacterial phyla and have a highly conserved quaternary structure, amyloid fibrils may serve as targets for immune surveillance by giving the immune system an opportunity to detect the presence of many microorganisms by recognizing one conserved molecule expressed by all.
Two signaling cascades subsequent to TLR2 activation result in the activation of cytokine expression and augmentation of epithelial barrier through NF-κB activation and PI3K activation, respectively (26–29). The innate immune recognition of the best-characterized bacterial amyloid curli, expressed in the biofilms of members of the bacterial family Enterobacteriaceae, requires TLR1 dimerization with TLR2 (48). Our recent investigations have shown that the expression of curli amyloid fibrils by S. Typhimurium resulted in the expression of interleukin 17 (IL-17)/IL-22, produced by direct or indirect activation of T cells, via TLR2 activation in the gastrointestinal tract of mice (60). Here, we have focused on determining the effect of TLR2 activation by amyloid fibrils on epithelial cell function in the gastrointestinal tract. Although epithelial damage caused by both curliated wild-type S. Typhimurium and the csgBA mutant was evident in vitro and in vivo, infection with the csgBA mutant caused more pronounced damage to the epithelium, allowing more bacteria to translocate to the basolateral side of the epithelium, and this effect was abolished in the presence of PI3K inhibitors (Fig. 2) or in the absence of TLR2 (Fig. 4), suggesting that the curli amyloid fibrils on bacteria activate the TLR2/PI3K pathway in intestinal epithelial cells, resulting in the reinforcement of the epithelial barrier.
During infection, multiple TLRs, including TLR2 and TLR5, found in the apical and basolateral sides of the epithelium, respectively, are activated (18, 71, 84). In this study, we mimicked the conditions that the epithelium encounters during bacterial infection using purified ligands, curli amyloid fibrils, and flagellin. Our in vitro results using polarized epithelial cells suggested that apical activation of TLR2 by curli amyloid fibrils restored the epithelial damage introduced by basolateral flagellin treatment (Fig. 3). Therefore, the greater permeability seen in the in vitro experiments (Fig. 2) as well as in the gastrointestinal tracts of mice infected with the csgBA mutant in vivo (Fig. 4) is possibly due to the lack of TLR2 activation while typical TLR5 activation occurs, damaging the epithelium and allowing increased translocation of bacteria.
Previous studies have shown that MyD88 and TLR2 deficiency impaired intestinal barrier repair during infection with another enteric pathogen, Citrobacter rodentium (77, 85). Although there were no differences in the bacterial numbers in the colon contents of both wild-type C57BL/6 and TLR2-deficient mice at 24 h postinfection, consistent with these reports, we determined that wild-type S. Typhimurium numbers were increased (P > 0.05), whereas csgBA mutant bacterial counts were significantly decreased (P < 0.05), in the colon contents of the TLR2-deficient mice at 72 h postinfection compared to the bacterial numbers in C57BL/6 mice (Fig. 6A and B). Interestingly, curli fibrils are required for efficient colonization of the intestinal epithelia by E. coli strains, while this phenotype is not observed with S. Typhimurium (47, 86–88). Recently, we determined that TLR2-deficient animals acquire less inflammation during S. Typhimurium infection, which could be measured by a decreased expression of IL-17A and IL-22 (60). Thus, under conditions of low or no inflammation, the presence of curli fibrils may provide the wild-type S. Typhimurium a colonization and growth advantage similar to what is observed with E. coli. Nonetheless, we are currently investigating the mechanism underlying this phenotype.
In the studies of chronic Citrobacter infection, the activation of TLR2 was attributed to the recognition of bacterial lipoproteins (77, 89). To our interest, similar to the case for E. coli and S. Typhimurium, Citrobacter spp. also express curli fibrils (45). Even though, diacylated and triacylated bacterial lipoproteins have been shown to trigger TLR2 activation, when bacterial amyloids are present, they exist as the predominant TLR2 ligand on bacteria, due to lipopeptides being buried in the outer membrane or bacterial cell wall while bacterial amyloids are being secreted to the cell surface (48). In conclusion, our data point to bacterial amyloids as a TLR2 ligand that enables epithelial cells to monitor bacterial translocation from the gut.
ACKNOWLEDGMENTS
Work in C.T.'s laboratory was supported by Scientist Development grant 0835248N from the American Heart Association and by Mid-Atlantic Regional Center for Excellence for Biodefense and Emerging Infectious Diseases Research grant U54 AI57168 from the National Institutes of Health. G.J.R. was supported by a grant from the Pennsylvania Department of Health.
The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, and conclusions.
Footnotes
Published ahead of print 3 December 2012
REFERENCES
- 1. Xu J, Gordon JI. 2003. Honor thy symbionts. Proc. Natl. Acad. Sci. U. S. A. 100:10452–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. 2007. The human microbiome project. Nature 449:804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. 2006. Metagenomic analysis of the human distal gut microbiome. Science 312:1355–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD. 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang T, Breitbart M, Lee WH, Run JQ, Wei CL, Soh SW, Hibberd ML, Liu ET, Rohwer F, Ruan Y. 2006. RNA viral community in human feces: prevalence of plant pathogenic viruses. PLoS Biol. 4:e3 doi:10.1371/journal.pbio.0040003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hooper LV. 2009. Do symbiotic bacteria subvert host immunity? Nat. Rev. Microbiol. 7:367–374 [DOI] [PubMed] [Google Scholar]
- 7. Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113–118 [DOI] [PubMed] [Google Scholar]
- 8. Satchell DP, Sheynis T, Shirafuji Y, Kolusheva S, Ouellette AJ, Jelinek R. 2003. Interactions of mouse Paneth cell alpha-defensins and alpha-defensin precursors with membranes. Prosegment inhibition of peptide association with biomimetic membranes. J. Biol. Chem. 278:13838–13846 [DOI] [PubMed] [Google Scholar]
- 9. Cunliffe RN, Rose FR, Keyte J, Abberley L, Chan WC, Mahida YR. 2001. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 48:176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cash HL, Whitham CV, Behrendt CL, Hooper LV. 2006. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313:1126–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christa L, Carnot F, Simon MT, Levavasseur F, Stinnakre MG, Lasserre C, Thepot D, Clement B, Devinoy E, Brechot C. 1996. HIP/PAP is an adhesive protein expressed in hepatocarcinoma, normal Paneth, and pancreatic cells. Am. J. Physiol. 271:G993–G1002 [DOI] [PubMed] [Google Scholar]
- 12. Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA. 2010. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 6:e1000902 doi:10.1371/journal.ppat.1000902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. 2006. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131:117–129 [DOI] [PubMed] [Google Scholar]
- 14. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229–241 [DOI] [PubMed] [Google Scholar]
- 15. Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511 [DOI] [PubMed] [Google Scholar]
- 16. Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449:819–826 [DOI] [PubMed] [Google Scholar]
- 17. Takeda K, Akira S. 2007. Toll-like receptors. Curr. Protoc. Immunol. Chapter 14:Unit 14.12. [DOI] [PubMed] [Google Scholar]
- 18. Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. 2001. Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882–1885 [DOI] [PubMed] [Google Scholar]
- 19. Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443–451 [DOI] [PubMed] [Google Scholar]
- 20. Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10–14 [DOI] [PubMed] [Google Scholar]
- 21. Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736–739 [DOI] [PubMed] [Google Scholar]
- 22. Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732–736 [DOI] [PubMed] [Google Scholar]
- 23. Takeda K, Akira S. 2004. TLR signaling pathways. Semin. Immunol. 16:3–9 [DOI] [PubMed] [Google Scholar]
- 24. Takeuchi O, Hoshino K, Akira S. 2000. TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392–5396 [DOI] [PubMed] [Google Scholar]
- 25. Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933–940 [DOI] [PubMed] [Google Scholar]
- 26. Franke TF, Kaplan DR, Cantley LC. 1997. PI3K: downstream AKTion blocks apoptosis. Cell 88:435–437 [DOI] [PubMed] [Google Scholar]
- 27. Santos-Sierra S, Deshmukh SD, Kalnitski J, Kuenzi P, Wymann MP, Golenbock DT, Henneke P. 2009. Mal connects TLR2 to PI3Kinase activation and phagocyte polarization. EMBO J. 28:2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mansell A, Brint E, Gould JA, O'Neill LA, Hertzog PJ. 2004. Mal interacts with tumor necrosis factor receptor-associated factor (TRAF)-6 to mediate NF-kappaB activation by Toll-like receptor (TLR)-2 and TLR4. J. Biol. Chem. 279:37227–37230 [DOI] [PubMed] [Google Scholar]
- 29. Verstak B, Nagpal K, Bottomley SP, Golenbock DT, Hertzog PJ, Mansell A. 2009. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. J. Biol. Chem. 284:24192–24203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cario E, Gerken G, Podolsky DK. 2004. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 127:224–238 [DOI] [PubMed] [Google Scholar]
- 31. Cario E, Gerken G, Podolsky DK. 2007. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132:1359–1374 [DOI] [PubMed] [Google Scholar]
- 32. Podolsky DK, Gerken G, Eyking A, Cario E. 2009. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 137:209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leonhardt RM, Vigneron N, Rahner C, Van den Eynde BJ, Cresswell P. 2010. Endoplasmic reticulum export, subcellular distribution, and fibril formation by Pmel17 require an intact N-terminal domain junction. J. Biol. Chem. 285:16166–16183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pfefferkorn CM, McGlinchey RP, Lee JC. 2010. Effects of pH on aggregation kinetics of the repeat domain of a functional amyloid, Pmel17. Proc. Natl. Acad. Sci. U. S. A. 107:21447–21452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Theos AC, Truschel ST, Raposo G, Marks MS. 2005. The Silver locus product Pmel17/gp100/Silv/ME20: controversial in name and in function. Pigment Cell Res. 18:322–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aigelsreiter A, Janig E, Stumptner C, Fuchsbichler A, Zatloukal K, Denk H. 2007. How a cell deals with abnormal proteins. Pathogenetic mechanisms in protein aggregation diseases. Pathobiology 74:145–158 [DOI] [PubMed] [Google Scholar]
- 37. Hull RL, Westermark GT, Westermark P, Kahn SE. 2004. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 89:3629–3643 [DOI] [PubMed] [Google Scholar]
- 38. Brandan E, Inestrosa NC. 1993. Extracellular matrix components and amyloid in neuritic plaques of Alzheimer's disease. Gen. Pharmacol. 24:1063–1068 [DOI] [PubMed] [Google Scholar]
- 39. Romero D, Aguilar C, Losick R, Kolter R. 2010. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. U. S. A. 107:2230–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alteri CJ, Xicohtencatl-Cortes J, Hess S, Caballero-Olin G, Giron JA, Friedman RL. 2007. Mycobacterium tuberculosis produces pili during human infection. Proc. Natl. Acad. Sci. U. S. A. 104:5145–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dueholm MS, Petersen SV, Sonderkaer M, Larsen P, Christiansen G, Hein KL, Enghild JJ, Nielsen JL, Nielsen KL, Nielsen PH, Otzen DE. 2010. Functional amyloid in Pseudomonas. Mol. Microbiol. 77:1009–1020 [DOI] [PubMed] [Google Scholar]
- 42. Blanco LP, Evans ML, Smith DR, Badtke MP, Chapman MR. 2012. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 20:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ. 2002. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295:851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Larsen P, Nielsen JL, Dueholm MS, Wetzel R, Otzen D, Nielsen PH. 2007. Amyloid adhesins are abundant in natural biofilms. Environ. Microbiol. 9:3077–3090 [DOI] [PubMed] [Google Scholar]
- 45. Zogaj X, Bokranz W, Nimtz M, Romling U. 2003. Production of cellulose and curli fimbriae by members of the family Enterobacteriaceae isolated from the human gastrointestinal tract. Infect. Immun. 71:4151–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Collinson SK, Clouthier SC, Doran JL, Banser PA, Kay WW. 1996. Salmonella enteritidis agfBAC operon encoding thin, aggregative fimbriae. J. Bacteriol. 178:662–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kai-Larsen Y, Luthje P, Chromek M, Peters V, Wang X, Holm A, Kadas L, Hedlund KO, Johansson J, Chapman MR, Jacobson SH, Romling U, Agerberth B, Brauner A. 2010. Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog. 6:e1001010 doi:10.1371/journal.ppat.1001010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tukel C, Nishimori JH, Wilson RP, Winter MG, Keestra AM, van Putten JP, Baumler AJ. 2010. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cell. Microbiol. 12:1495–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tukel C, Raffatellu M, Humphries AD, Wilson RP, Andrews-Polymenis HL, Gull T, Figueiredo JF, Wong MH, Michelsen KS, Akcelik M, Adams LG, Baumler AJ. 2005. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol. Microbiol. 58:289–304 [DOI] [PubMed] [Google Scholar]
- 50. Tukel C, Wilson RP, Nishimori JH, Pezeshki M, Chromy BA, Baumler AJ. 2009. Responses to amyloids of microbial and host origin are mediated through Toll-like receptor 2. Cell Host Microbe 6:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bian Z, Brauner A, Li Y, Normark S. 2000. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J. Infect. Dis. 181:602–612 [DOI] [PubMed] [Google Scholar]
- 52. Bian Z, Yan ZQ, Hansson GK, Thoren P, Normark S. 2001. Activation of inducible nitric oxide synthase/nitric oxide by curli fibers leads to a fall in blood pressure during systemic Escherichia coli infection in mice. J. Infect. Dis. 183:612–619 [DOI] [PubMed] [Google Scholar]
- 53. Cheng N, He R, Tian J, Ye PP, Ye RD. 2008. TLR2 is a functional receptor for acute-phase serum amyloid A. J. Immunol. 181:22–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. He RL, Zhou J, Hanson CZ, Chen J, Cheng N, Ye RD. 2009. Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood 113:429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jana M, Palencia CA, Pahan K. 2008. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer's disease. J. Immunol. 181:7254–7262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. 2009. CD14 and Toll-like receptors 2 and 4 are required for fibrillar Aβ-stimulated microglial activation. J. Neurosci. 29:11982–11992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Udan ML, Ajit D, Crouse NR, Nichols MR. 2008. Toll-like receptors 2 and 4 mediate Abeta(1-42) activation of the innate immune response in a human monocytic cell line. J. Neurochem. 104:524–533 [DOI] [PubMed] [Google Scholar]
- 58. Lay C, Sutren M, Rochet V, Saunier K, Dore J, Rigottier-Gois L. 2005. Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environ. Microbiol. 7:933–946 [DOI] [PubMed] [Google Scholar]
- 59. Stojiljkovic I, Baumler AJ, Heffron F. 1995. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J. Bacteriol. 177:1357–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nishimori JH, Newman TN, Oppong GO, Rapsinski GJ, Yen JH, Biesecker SG, Wilson RP, Butler BP, Winter MG, Tsolis RM, Ganea D, Tukel C. 2012. Microbial amyloids induce interleukin 17A (IL-17A) and IL-22 responses via Toll-like receptor 2 activation in the intestinal mucosa. Infect. Immun. 80:4398–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Collinson SK, Emody L, Muller KH, Trust TJ, Kay WW. 1991. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J. Bacteriol. 173:4773–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Madara JL, Dharmsathaphorn K. 1985. Occluding junction structure-function relationships in a cultured epithelial monolayer. J. Cell Biol. 101:2124–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lambert D, O'Neill CA, Padfield PJ. 2005. Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem. J. 387:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cario E, Podolsky DK. 2000. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68:7010–7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609–1616 [DOI] [PubMed] [Google Scholar]
- 66. Abreu MT. 2010. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 10:131–144 [DOI] [PubMed] [Google Scholar]
- 67. Chabot S, Wagner JS, Farrant S, Neutra MR. 2006. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J. Immunol. 176:4275–4283 [DOI] [PubMed] [Google Scholar]
- 68. Chantret I, Barbat A, Dussaulx E, Brattain MG, Zweibaum A. 1988. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 48:1936–1942 [PubMed] [Google Scholar]
- 69. Hidalgo IJ, Raub TJ, Borchardt RT. 1989. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749 [PubMed] [Google Scholar]
- 70. Galan JE, Curtiss R., III 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. U. S. A. 86:6383–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gewirtz AT, Simon PO, Jr, Schmitt CK, Taylor LJ, Hagedorn CH, O'Brien AD, Neish AS, Madara JL. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Invest. 107:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bruno VM, Hannemann S, Lara-Tejero M, Flavell RA, Kleinstein SH, Galan JE. 2009. Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog. 5:e1000538 doi:10.1371/journal.ppat.1000538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. 2009. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. 2000. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 6:909–919 [DOI] [PubMed] [Google Scholar]
- 75. Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD, Bercik P, Verdu EF, McCoy KD, Macpherson AJ. 2009. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science 325:617–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ey B, Eyking A, Gerken G, Podolsky DK, Cario E. 2009. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J. Biol. Chem. 284:22332–22343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gibson DL, MA C, Rosenberger CM, Bergstrom KS, Valdez Y, Huang JT, Khan MA, Vallance BA. 2008. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10:388–403 [DOI] [PubMed] [Google Scholar]
- 78. Bollinger RR, Everett ML, Wahl SD, Lee YH, Orndorff PE, Parker W. 2006. Secretory IgA and mucin-mediated biofilm formation by environmental strains of Escherichia coli: role of type 1 pili. Mol. Immunol. 43:378–387 [DOI] [PubMed] [Google Scholar]
- 79. Swidsinski A, Loening-Baucke V, Lochs H, Hale LP. 2005. Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J. Gastroenterol. 11:1131–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Swidsinski A, Schlien P, Pernthaler A, Gottschalk U, Barlehner E, Decker G, Swidsinski S, Strassburg J, Loening-Baucke V, Hoffmann U, Seehofer D, Hale LP, Lochs H. 2005. Bacterial biofilm within diseased pancreatic and biliary tracts. Gut 54:388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. 2005. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J. Clin. Microbiol. 43:3380–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jordal PB, Dueholm MS, Larsen P, Petersen SV, Enghild JJ, Christiansen G, Hojrup P, Nielsen PH, Otzen DE. 2009. Widespread abundance of functional bacterial amyloid in mycolata and other gram-positive bacteria. Appl. Environ. Microbiol. 75:4101–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Larsen P, Nielsen JL, Otzen D, Nielsen PH. 2008. Amyloid-like adhesins produced by floc-forming and filamentous bacteria in activated sludge. Appl. Environ. Microbiol. 74:1517–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cario E, Brown D, McKee M, Lynch-Devaney K, Gerken G, Podolsky DK. 2002. Commensal-associated molecular patterns induce selective Toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am. J. Pathol. 160:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gibson DL, Ma C, Bergstrom KS, Huang JT, Man C, Vallance BA. 2008. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10:618–631 [DOI] [PubMed] [Google Scholar]
- 86. Torres AG, Cieza RJ, Rojas-Lopez M, Blumentritt CA, Souza CS, Johnston RK, Strockbine N, Kaper JB, Sbrana E, Popov VL. 2012. In vivo bioluminescence imaging of Escherichia coli O104:H4 and role of aerobactin during colonization of a mouse model of infection. BMC Microbiol. 12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang X, Rochon M, Lamprokostopoulou A, Lunsdorf H, Nimtz M, Romling U. 2006. Impact of biofilm matrix components on interaction of commensal Escherichia coli with the gastrointestinal cell line HT-29. Cell. Mol. Life Sci. 63:2352–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Weening EH, Barker JD, Laarakker MC, Humphries AD, Tsolis RM, Baumler AJ. 2005. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in mice. Infect. Immun. 73:3358–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gibson DL, Montero M, Ropeleski MJ, Bergstrom KS, Ma C, Ghosh S, Merkens H, Huang J, Mansson LE, Sham HP, McNagny KM, Vallance BA. 2010. Interleukin-11 reduces TLR4-induced colitis in TLR2-deficient mice and restores intestinal STAT3 signaling. Gastroenterology 139:1277–1288 [DOI] [PubMed] [Google Scholar]