Abstract
The NadA adhesin is a major component of 4CMenB, a novel vaccine to prevent meningococcus serogroup B (MenB) infection. Under in vitro growth conditions, nadA is repressed by the regulator NadR and poorly expressed, resulting in inefficient killing of MenB strains by anti-NadA antibodies. Interestingly, sera from children infected with strains that express low levels of NadA in laboratory growth nevertheless recognize the NadA antigen, suggesting that NadA expression during infection may be different from that observed in vitro. In a strain panel covering a range of NadA levels, repression was relieved through deleting nadR. All nadR knockout strains expressed high levels of NadA and were efficiently killed by sera from subjects immunized with 4CMenB. A selected MenB strain, NGP165, mismatched for other vaccine antigens, is not killed by sera from immunized infants when the strain is grown in vitro. However, in an in vivo passive protection model, the same sera effectively protected infant rats from bacteremia with NGP165. Furthermore, we identify a novel hydroxyphenylacetic acid (HPA) derivative, reported by others to be produced during inflammation, which induces expression of NadA in vitro, leading to efficient antibody-mediated killing. Finally, using bioluminescent reporters, nadA expression in the infant rat model was induced in vivo at 3 h postinfection. Our results suggest that during infectious disease, NadR repression is alleviated due to niche-specific signals, resulting in high levels of NadA expression from any nadA-positive (nadA+) strain and therefore efficient killing by anti-NadA antibodies elicited by the 4CMenB vaccine.
INTRODUCTION
The human pathogen Neisseria meningitidis is an encapsulated Gram-negative diplococcus which asymptomatically colonizes the naso- and oropharynx of 10% to 15% of healthy adults. For reasons not yet fully understood, it occasionally crosses the mucosal epithelial barrier to cause severe septicemia and meningitis (1, 2). Each year, there are an estimated 1.2 million cases of invasive meningococcal disease and 135,000 deaths (http://www.who.int/mediacentre/en/), and infants represent the population at highest risk of infection. Individuals surviving the disease often suffer from permanent disabilities, including brain damage responsible for hearing loss or learning difficulties, as well as amputation of limbs (1). Of the 12 known serogroups classified by the immunochemistry of their capsular polysaccharides, six, A, B, C, X, Y, and W, regularly cause disease (3–5). Meningococcal disease progresses rapidly, and in its early stages, it is easily misdiagnosed (1), making vaccination the best public health option and the most effective way to prevent it. Polysaccharide and glycoconjugate vaccines are available against serogroups A, C, Y, and W, but there is no broadly protective vaccine against meningococcus serogroup B (MenB).
A novel vaccine against MenB named 4CMenB has been developed (6) and has progressed through clinical trials that have demonstrated its safety (7) and its efficacy in inducing a protective immune response in infants, children, adolescents, and adults to potentially the majority of MenB strains (8, 9). The 4CMenB vaccine is composed of the recombinant protein Neisserial adhesin A (NadA) (10), the factor H binding protein (fHbp) (11) and Neisserial Heparin-Binding Antigen (NHBA) (12) fused with the meningococcal gene product GNA2091 or GNA1030, and Outer Membrane Vesicles (OMVs) from the meningococcus B NZ98/254 strain in which PorA serosubtype 1.4 represents the major antigen. In order to evaluate 4CMenB vaccine coverage, an assay, the Meningococcal Antigen Typing System (MATS), which assesses simultaneously the cross-reactivity and the expression of the antigens present on the surface of an unknown test strain with respect to a reference MenB strain, has been developed (13). The MATS relative potency (MATS RP), obtained by applying MATS to unknown strains, correlates with data from the human Serum Bactericidal Antibody (hSBA) assay, the surrogate of protection accepted for meningococcal infection (14–17), and may predict whether a strain would be killed due to antibodies elicited by the 4CMenB vaccine (13). A MATS RP threshold value for complement-mediated killing of MenB by antibodies against NadA, fHbp, and NHBA antigens was established and termed the Positive Bactericidal Threshold (PBT). Using MATS, it has been estimated that 78% of circulating MenB strains in Europe would have at least one antigen rated above the PBT and therefore would be covered by the 4CMenB vaccine. However, the estimated contribution of the NadA antigen to the vaccine coverage appears to be very low (18).
The nadA gene is carried by about 30% of pathogenic isolates collected from patients in 5 European countries and the United States and is always present in members of three of four major meningococcal hypervirulent lineages (ST8, ST11, and ST32 complexes) (10, 19). Despite the presence of the gene, the quantities of NadA protein that are expressed by bacteria cultured in vitro differ greatly in different strains due to complex mechanisms of nadA regulation. The nadA gene shows growth-phase-dependent expression, reaching a maximal level in the stationary phase (20). It is also subject to phase variation, through the presence of a variable-length tetranucleotide repeat upstream of its promoter. It has been shown that different strains comprising different phase variants of nadA express the protein at different levels in vitro (20). However, the major mediator of the phase-variable expression of nadA is NadR, which binds to two high-affinity sites on the promoter of nadA, repressing it. When nadR is knocked out (KO), the level of expression of NadA is induced to almost comparable levels in all tested strains, suggesting that the differential ability of NadR to repress different phase variants of nadA is the cause of the variability of NadA within and between strains (20).
NadR belongs to the MarR family of regulators, which are known to respond to small-molecule inducers, often low-molecular-weight phenolic compounds (21). It has been demonstrated that NadR responds to 4-hydroxyphenylacetic acid (4-HPA), which is able to alleviate the binding of the repressor on nadA, thereby inducing its expression (20). 4-HPA is a catabolite of aromatic amino acids and is commonly found in human saliva (22). It has also been recently reported that human saliva itself can induce NadA expression as well as 4-HPA, suggesting that signals capable of inducing NadA expression, which can be mimicked by 4-HPA in vitro, are present in the oropharyngeal niche of meningococcal colonization (23). Moreover, it has also been demonstrated that sera from children convalescent after meningococcal disease are able to recognize NadA, suggesting that NadA is expressed during invasive human infection to a level which is sufficient to elicit anti-NadA antibodies (24). Importantly, NadA elicited strong reactivity in convalescent patients previously infected with nadA-positive (nadA+) strains relative to uninfected subjects. Taken together, these observations suggest that the level of expression of NadA during invasive disease may be very different from the levels that are measured under in vitro growth conditions.
In this report, we address the possibility that the contribution of the NadA antigen to 4CMenB vaccine coverage is underestimated due to the conditions used for performing the hSBA and the MATS assay. We provide data showing that strains able to cause disease in humans, even with low MATS RP, are in fact able to induce anti-NadA antibodies during infection. We demonstrate that once NadR repression is alleviated, strains carrying nadA express high levels of NadA and therefore have the potential to be killed due to antibodies elicited by the 4CMenB vaccine. Finally, we have assessed our hypothesis in a case study, showing that MenB strain NGP165, which exhibits a NadA MATS RP under the PBT and therefore is not killed by anti-NadA antibodies in vitro, is killed in an in vivo infant rat model by passive protection conferred by sera of infants immunized with 4CMenB.
MATERIALS AND METHODS
Ethics statement.
The trials described in this paper were conducted following good clinical practice and the principles outlined in the Declaration of Helsinki. The studies were approved in different countries by local ethical committees. All animal experiments were performed in accordance with European and Italian guidelines regarding the protection of animals used for experimental and other scientific purposes.
Bacterial strains and culture conditions.
The N. meningitidis strains used in this study are listed in Table 1 and include the respective NadR null mutant derivatives, which have been described elsewhere (23). The NGP165 NHBA null mutant was generated as described previously (12). In addition, the previously reported clinical isolates Nm036, Nm037, Nm066, Nm067, Nm069, Nm081, Nm088, Nm100, Nm119, Nm145, Nm154, Nm156, Nm188, and Nm191 (24) were used. All strains were routinely cultured in GC-based medium (Difco) and stocked as previously described (25). When required, indicated small molecules were added to culture media to achieve a final concentration of 2 or 5 mM in aqueous solution. All small molecules were obtained from Sigma-Aldrich, with the exception of the 3Br4-HPA, which was purchased from Chemsigma.
Table 1.
Selected strains used in this study
| Strain | Clonal complex(es) | STa | Yr of collection | Countryb | Typing resultc | fHbp IDd | No. of isolates |
|
|---|---|---|---|---|---|---|---|---|
| NHBA | NadA variant | |||||||
| 5/99 | ST-8 complex/cluster A4 | 1349 | 1999 | B:2b:P1.5,2 | N | 23 | 20 | 2 |
| 961-5945 | ST-8 complex/cluster A4 | 153 | 1996 | AUS | B:2b:P1.21,16 | 16 | 20 | 2 |
| LNP17094 | ST-8 complex/cluster A4 | 153 | 1999 | F | B:2b:P1.10 | 16 | 22 | 2 |
| B3937 | ST-18 complex | 6344 | 1995 | D | B:22:P1.16 | 17 | 23 | 3 |
| M10574 | ST-32 complex/ET5 complex | 803 | 2003 | USA | B:NT:P1.7–2,13-1 | 76 | 3 | 1 |
| M14933 | ST-32 complex/ET5 complex | 32 | 2006 | USA | B:ND:P1.22-1,14 | 76 | 3 | 1 |
| MC58 | ST-32 complex/ET5 complex | 74 | 1985 | UK | B:15:P1-7,16b | 1 | 3 | 1 |
| NGP165 | ST11 complex/ET-37 complex | 11 | 1974 | N | B:NT:P1.2 | 29 | 29 | 2 |
ST, multilocus sequence type.
AUS, Austria; D, Denmark; F, France; N, Norway; UK, United Kingdom; USA, United States of America.
No strains match the PorA P1.4 allele in the OMV_NZ vaccine component.
The fHbp allele identification numbers (ID) are reported here according to Oxford database nomenclature.
Generation of lux reporter strains.
To generate bacterial luciferase transcriptional fusions of the promoter under study at a chromosomal location between the two converging open reading frames (ORFs) NMB1074 and NMB1075, flanked on both sides with transcriptional terminators, plasmid pSL-LuxFla was constructed for allelic exchange in N. meningitidis strains. Briefly, the promoterless luxCDABE operon and cat cassette were subcloned from pSB1075 (21) into pBluescript II (26) as an EcoRI-BamHI fragment and then cloned as a 6.5-kb XhoI-BamHI fragment into pSL-furlacZ (27), replacing 4.7 kb containing an erythromycin cassette and fur-lacZ fusion, generating pSL-LuxFla. The nadA promoter was cloned as a 250-bp Xho-KpnI fragment upstream of the luxCDABE operon, generating pSLPnadA-lux. The pSL-LuxFla and pSLPnadA-lux plasmids were used for transformation of the MC58 and MC58-Δ1843 strains, generating the MC58-lux and MC58-PnadA-lux strains and the MC58-Δ1843-lux and MC58-Δ1843-PnadA-lux strains, respectively, for the in vitro reporter analyses, and the 2996 strain, generating 2996-lux and 2996-PnadA-lux, respectively, for the in vivo reporter analysis.
Western blot analysis.
Liquid cultures were grown to mid-exponential phase (optical density at 600 nm [OD600] = 0.4 to 0.5), harvested, and resuspended in GC, GC plus a 2 mM concentration of the indicated molecule, or GC plus a 5 mM concentration of the indicated molecule or 10%, 50%, or 90% (vol/vol) human saliva (prepared as described previously [23]) in GC containing EDTA-free protease inhibitor cocktail (Roche). After 1 h of incubation at 37°C, total protein extracts were prepared and Western blot experiments were performed as previously described (23). After quantification of the signal of the bands, statistical analyses were performed to evaluate the significance of the results.
In silico docking experiments.
The generation of a tridimensional structural model of NadR and the description of docking experiments were fully reported by Brier and colleagues (28). The coordinates of the 4-HPA molecule were obtained from the Protein Data Bank (PDB) web site (identification [ID] no. 4HP). AutoDockTools 1.5.4 (ADT) software was used to prepare the ligand for docking (29).
Immunization of mice.
To prepare antisera, 20 μg of NadA, NHBA-GNA1030, or GNA2091-fHbp antigen or a combination of 20 μg each of NHBA-GNA1030, GNA2091-fHbp, and NadA with or without 10 μg of deoxycholate-extracted OMVs derived from the NZ98/254 strain was used to immunize 6-week-old CD1 female mice (Charles River). Five to 10 mice per group were used. The antigens were administered intraperitoneally (i.p.), together with aluminum hydroxide (3 mg/ml), on days 0, 21, and 35.
MATS and Serum Bactericidal Assay (SBA).
The MATS assay was performed as previously described (13). When required, bacteria were grown overnight with 4-HPA and 3Cl4-HPA supplementation on Chocolate Agar plates (bioMérieux). Raw data reduction and analysis were performed by StatLIA (Brendan Technologies).
Serum bactericidal antibody activity against N. meningitidis strains with mice antisera was evaluated as previously described (30), with pooled baby rabbit serum used as the complement source (rSBA). Serum bactericidal antibody assays with human complement (hSBA) were performed as described by Borrow et al. (30). When required, 4-HPA and 3Cl4-HPA in aqueous solution were added to plates at a final concentration of 5 mM.
Human serum samples.
Serum samples before and after immunization were obtained from the following clinical trials. Study 1 was a clinical trial conducted in healthy adults and laboratory workers. Pooled sera were derived from 23 subjects before and after 3 doses of 4CMenB administered at 0, 2, and 6 months. Study 2 was a clinical study evaluating the safety, immunogenicity, and lot consistency of 4CMenB administered to infants at 2, 4, and 6 months of age. Extensions of this clinical study investigated a fourth (booster) dose at 12 months of age. Pooled sera were derived from 107 infants at 7 months of age who received the primary series of 3 doses of routine vaccine at 2, 4, and 6 months of age and from 141 infants who received the primary series of 3 doses of 4CMenB at 2, 4, and 6 months of age plus a booster in the second year of life. Study 3 was a clinical study evaluating the safety, tolerability, and immunogenicity of 4CMenB administered to infants at 2, 4, and 6 months of age. Extensions of this clinical study investigated a fourth (booster) dose at 12, 18, or 24 months of age. Pooled sera were derived from 109 infants at 5 months of age who received the primary series of 3 doses of routine vaccine at 2, 3, and 4 months of age and from 69 infants who received the primary series of 3 doses of 4CMenB at 2, 4, and 6 months of age plus a booster in the second year of life.
Passive protection and in vivo imaging in infant rats.
The ability of anti-NadA antibodies to confer passive protection against N. meningitidis bacteremia was tested in infant rats challenged intraperitoneally (i.p.) as previously described (31). In vivo imaging of the bioluminescence of the PnadA-lux reporter and control strains was monitored in infant rats infected i.p.
On the morning of the challenge/infection, the bacteria were grown, washed, and diluted in phosphate-buffered saline (PBS) to obtain 105 CFU/ml. For passive protection, groups of 3 to 19 animals were treated i.p. at time zero with 100-μl doses of different dilutions of test or control antisera. Three hours later, the animals were challenged i.p. with a 100-μl dose of 105 CFU of N. meningitidis strain NGP165 or NGP165 NHBA KO. Eighteen hours after the bacterial challenge, blood samples were obtained by cheek puncture with a syringe containing 25 U of heparin without preservative (American Pharmaceutical Partners), and CFU levels were measured. Rats were considered infected when >10 CFU were counted on plates carrying 100 μl of blood. Counts above the threshold were verified for positivity by examining plates carrying 10- and 100-fold dilutions of blood.
For in vivo imaging of bioluminescent reporters, groups of 5 animals were inoculated i.p. at time zero with 100-μl doses of 104 CFU of the 2996-lux or 2996-Pnad-lux strain. Rats were then anesthetized using a constant flow of 2.5% isoflurane mixed with oxygen. Bioluminescence measurements of ventral views of each group of rats were taken at time zero and at 3 and 24 h, using an IVIS 100 system (Xenogen Corp., Alameda, CA) according to instructions from the manufacturer. Analysis and acquisition were performed using Living Image 3.1 software (Xenogen Corp.). Quantification was performed using the photons emitted per second by each rat. Two rats infected with the 2996 wild-type strain under the same conditions of acquisition were used for subtracting the background. At 24 h after infection, blood samples were obtained and CFU counts measured. Statistical analyses were performed to assess the relevance of the results obtained.
RESULTS
Strains with MATS RP ≤ PBT express NadA in an immunogenic form during invasive disease.
Litt and colleagues (24) observed that many protein antigens, including the NadA protein, were recognized by antibodies present in sera of children convalescing after meningococcal disease. Importantly, NadA was significantly more strongly recognized by sera of convalescent patients infected with nadA-positive strains than by sera of uninfected control subjects (24). We have extended this study by subjecting the 14 nadA-positive isolates, matched to the sera of the Litt study, to Western blot and MATS analysis in order to visualize the in vitro levels of NadA expression of the infecting strain. In Fig. 1A, Western blots reveal that while the regulator NadR is expressed comparably by all strains tested, the levels of NadA are variable, and some strains (Nm067, Nm100, and Nm188) failed to express the protein at detectable levels under the in vitro growth conditions used. When analyzed by MATS, the level of NadA expression, calculated as relative potency compared to that of a reference strain (5/99), correlated well with Western blot results (Fig. 1B; see also Table S1 in the supplemental material). As shown, only 5 strains (Nm036, Nm037, Nm081, Nm088, and Nm154) have RP values above the PBT of NadA, which is 0.009, while the remaining strains show RP ≤ PBT. However, sera from children infected either by strains that failed to express NadA in vitro (MATS RP = 0) or by strains with a NadA RP < PBT are nonetheless able to recognize at least one form of the NadA recombinant proteins used in the dot blot experiments of Litt and colleagues (24) (Fig. 1C) more efficiently than sera from subjects infected by nadA knockout meningococcal strains.
Fig 1.
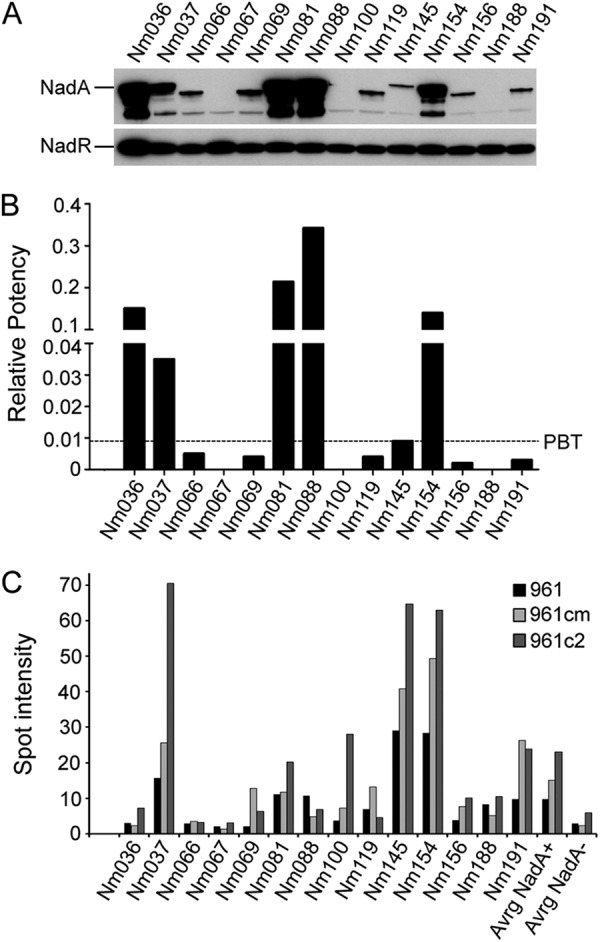
Strains with MATS RP ≤ PBT express NadA in an immunogenic form during infection. (A) Western blot analyses of the wild-type nadA+ strains from the Litt study (24), showing NadA and NadR expression. (B) MATS relative potency (RP) of NadA determined by the MATS ELISA. A black dashed line represents the positive bactericidal threshold (PBT) for NadA. The RPs of each strain are reported in Table S1 in the supplemental material. (C) Spot intensity of dot blot experiments adjusted from the data of Litt and colleagues (24). Reactivities of sera from children infected with the reported isolated strains are reported (961 = full-length NadA, 961 cm and 961c2 = truncated forms comprising the extracellular portion of NadA). The average values for 14 nadA+ strains and 17 nadA knockout strains are reported (Avrg NadA+ and Avrg NadA-, respectively).
These data show that strains with MATS RP values below the PBT are nevertheless able to express the NadA protein in an immunogenic form during invasive disease, driving a robust humoral response. This observation suggests that the levels of expression of NadA under in vitro growth conditions may differ from, and be lower than, those reached during infection in the human host.
All strains carrying the nadA gene can express high levels of the NadA protein and can therefore be killed by vaccine-induced bactericidal antibodies.
The main mediator of various expression levels of NadA within and between strains is the transcriptional regulator NadR, through its ability to differentially repress the phase-variant promoter of nadA (20). To understand the relevance of NadR repression to the variable expression levels of NadA observed in different MenB strains under in vitro conditions, a representative panel of strains covering a range of NadA expression levels was selected (Table 1) and the nadR gene was deleted in each of them. We evaluated the implications of the alleviation of NadR repression under in vitro conditions by Western blotting, MATS, and SBA analysis (Fig. 2 and Tables 2 and 3).
Fig 2.
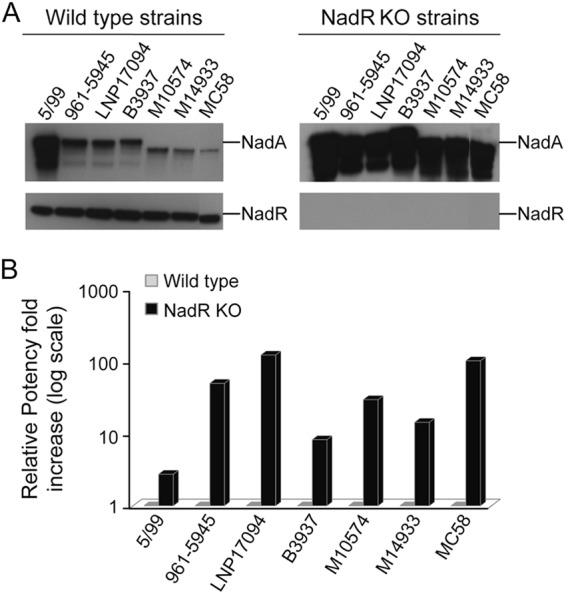
NadA espression levels in a panel of wild-type and nadR knockout strains. (A) Western blot analyses of wild-type and relative nadR knockout (KO) strains. The NadA and NadR levels of expression are shown. (B) Ratio of NadA MATS RPs of nadR knockout strains versus the wild-type strains. The RP values of wild-type strains and the fold increase of NadA RP of the nadR knockout strain, calculated by dividing the RP value of nadR knockout strains by the RP value of the relative wild-type strains, are reported in the graph.
Table 2.
rSBA performed with immunized mice sera and rabbit complement on wild-type and nadR knockout strainsa
| Strain | rSBA titer |
|||
|---|---|---|---|---|
| NHBA | fHbp | NadA | 4CMenBb | |
| 5/99 | 512 | <16 | >65,536 | >32,768 |
| 5/99 nadR KO | 128 | <16 | >65,536 | >65,536 |
| 961-5945 | 1,024 | 1,024 | 1,024 | 4,096 |
| 961-5945 nadR KO | 1,024 | 2,048 | >65,536 | >65,536 |
| LNP17094 | 1,024 | <16 | 128 | 4,096 |
| LNP17094 nadR KO | 512 | <16 | 32,768 | >65,536 |
| B3937 | <16 | 1,024 | 512 | 2,048 |
| B3937 nadR KO | <16 | 512 | >65,536 | >65,536 |
| M10574 | 4,096 | 128 | >8,192 | >8,192 |
| M10574 nadR KO | 4,096 | 64 | >32,768 | >32,768 |
| M14933 | 4,096 | <16 | 512 | >8,192 |
| M14933 nadR KO | 4,096 | <16 | >32,768 | >32,768 |
| NGP165 | 128 | <16 | 128 | 512 |
| NGP165 nadR KO | 128 | <16 | >32,768 | >32,768 |
Strains were considered killed if pooled mouse sera achieved an rSBA titer ≥ 128. nadR KO, nadR knockout.
Vaccine formulation with both the three recombinant major antigens and the OMVs, as described in the text.
Table 3.
hSBA performed on wild-type and nadR knockout mutant selected strainsa
| Strain | Study 1 (adults) SBA titer |
Study 2 (infants) SBA titer |
||
|---|---|---|---|---|
| Preimmune | 4CMenB post 3b | Routine | 4CMenB post 4c | |
| 5/99 | <4 | 256 | <4 | >512 |
| 961-5945 | <4 | 16 | <2 | 16 |
| 961-5945 nadR KO | <4 | >512 | <4 | >512 |
| LNP17094 | <4 | 16 | <2 | 16 |
| LNP17094 nadR KO | <4 | >512 | <4 | >512 |
| B3937 | <4 | 8 | <2 | <2 |
| B3937 nadR KO | <4 | >512 | 8 | >512 |
| M10574 | <4 | 32 | <2 | 64 |
| M10574 nadR KO | <4 | >512 | <4 | >512 |
| M14933 | <4 | 16 | <2 | 32 |
| M14933 nadR KO | <4 | >512 | 8 | >512 |
| NGP165 | <4 | <4 | 2 | 4 |
| NGP165 nadR KO | <4 | >256 | 4 | >256 |
Strains were considered killed if pooled sera from different age groups who received the 4CMenB achieved an SBA titer ≥ 8.
4CMenB post 3, 3 doses of the vaccine given at 0, 2, and 6 months.
4CMenB post 4, 3 doses of the vaccine given at 0, 2, and 6 months plus 1 boost between 12 and 24 months.
As previously shown (20), all the nadR knockout strains expressed considerably more NadA than their wild-type forebears, confirming that deletion of nadR results in strong induction of NadA. Furthermore, all nadR knockout strains expressed comparable high levels of the NadA antigen measured by Western blotting (Fig. 2A). The MATS assay performed on wild-type and nadR knockout strain pairs demonstrated that the ratio of the RP values for NadA increased from 3-fold to up to 100-fold in the mutant strains (Fig. 2B), indicating that all these strains can express high levels of immunogenically relevant NadA antigen when NadR repression is abolished. The MATS assay correlates with the hSBA (13) at values of RP higher than the PBT. Therefore, we compared the ability of immune sera to kill the nadR knockout strains and their related wild types. Table 2 shows that sera from mice immunized with NadA alone or with the 4CMenB vaccine have an increased NadA-specific bactericidal activity on nadR knockout strains compared with wild-type strains. The only exception is for strain 5/99, in which, as expected, there is no significant difference in SBA titers between the wild-type and the nadR knockout strains. In this strain, the NadR-mediated repression of NadA is minimal: NadA is highly expressed in the wild-type strain. Sera from immunizations with NHBA and fHbp (Table 2) had invariant activity toward wild-type and nadR knockout strains, confirming that the knockout of nadR does not alter the susceptibility of these strains in the bactericidal assay and suggesting that neither NHBA expression nor fHbp expression is regulated in a NadR-dependent way. The results of a hSBA assay performed on the mutant strains demonstrated that sera from clinical trial subjects of different age groups immunized with the 4CMenB vaccine formulations were efficiently able to kill all nadR knockout strains and exhibited extremely high bactericidal titers (Table 3). Of note, antibodies present in sera from some age groups and apparently ineffective in the killing of certain strains (e.g., B3937 and NGP165) show the ability to efficiently kill the equivalent recombinant strains once NadR repression has been relieved.
Taken together these data demonstrate that all the strains carrying nadA can potentially express NadA to a level which is sufficient to be recognized and to mediate killing by the bactericidal antibody elicited by the 4CMenB vaccine.
NadA expression can be induced in vitro by different physiologically relevant signals in strain NGP165.
The 4CMenB vaccine has been formulated in order to confer protection by targeting multiple antigens on the surface of as many strains as possible. To evaluate the contribution of NadA to vaccine coverage and to test the hypothesis that analysis of the levels of NadA expression in vitro could underestimate the predicted efficacy of bactericidal antibody in mediating the killing of NadA-positive strains during infection, we selected strain NGP165 for a case study. NGP165 and 4CMenB are mismatched with respect to fHbp and PorA (carrying fHbp variant 3.29 and PorA serosubtype 1.2 and carrying fHbp variant 1.1 and PorA serosubtype 1.4, respectively), and, with respect to NHBA, it had MATS RP below the PBT and almost negative rSBA titers in the preclinical studies (Table 2). Thus, only the NadA antigen could plausibly contribute to 4CMenB-induced antibody-mediated killing of this strain.
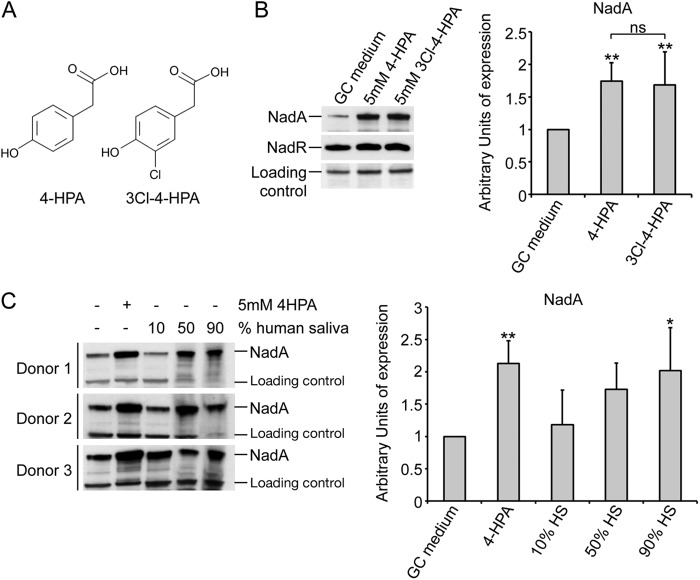
It has been previously shown that NadR-mediated repression of the nadA promoter can be alleviated by 4-HPA, a catabolite of aromatic amino acids which is commonly found in human saliva (20, 22). Human saliva has been shown to induce NadA expression to the same level as 4-HPA in strain MC58, suggesting that, in vivo, the expression of the nadA gene might be induced by signals present in saliva (23). Mass spectrometry and hydrogen/deuterium exchange analysis (MS-HDX) have recently identified a substrate binding pocket which is involved in the interaction of the 4-HPA ligand and the NadR repressor (28). We used the resultant inducer-NadR interaction model (see Fig. S1A in the supplemental material) for in silico docking experiments to screen a number of molecules structurally similar to 4-HPA in order to identify candidates for other potentially physiologically relevant inducers of NadA expression. Any molecule that was identified in silico as able to dock in the binding pocket of NadR was tested for its ability to induce NadA expression in MC58 in in vitro-grown cultures (see Fig. S1B in the supplemental material). Among the molecular species tested, some (2-HPA, 2,4-HPA, and 3,4-HPA) were unable to induce NadA expression, while others (3Cl4-HPA, 3Br4-HPA, NO2-4HPA, and, to a lesser extent, 3-HPA) increased expression of NadA to a level comparable to that of 4-HPA itself. We then verified which of the newly found inducers might have a significant role during meningococcal infection. Interestingly, 3Cl4-HPA, which is structurally similar to 4-HPA (Fig. 3A), has been shown to be produced during inflammatory processes as a catabolite of chlorinated aromatic amino acids (32) and therefore represents a possible natural ligand that the meningococcus might encounter during infection of the host. Due to their physiological relevance, we accordingly decided to test both the 4-HPA and the 3Cl4-HPA molecules in in vitro assays using the selected NGP165 strain in order to assess the putative level of NadA expression in the host.
Fig 3.
Induction of NadA by different physiologically relevant signals in the NGP165 selected strain. (A) Representation of the chemical structures of the 4-HPA and the 3Cl4-HPA compounds. (B) Western blot analyses of the level of expression of NadA in the NGP165 strain. The experiment was repeated with three biological replicates, and the band signals were quantified using a loading control as reference (fHbp). One representative Western blot is shown together with a histogram summarizing the results of three replicates. (** = P < 0.01; ns = not significant). (C) Induction of the NadA expression in the NGP165 strain with human saliva (HS). On the left, Western blot analyses of total protein from mid-log-phase cultures of NGP165 under the indicated conditions are shown. On the right, a histogram reporting the average values of NadA expression determined in 5 independent experiments is shown. Western blot bands were normalized for a nonspecific band (loading control) in order to avoid 4-HPA- or NadR-independent effects on NadA expression due to possible protein degradation in saliva. * = P < 0.05; ** = P < 0.01 (comparing all condition to the basal GC medium level). Levels of NadA induction comparable to those seen with 4-HPA and saliva were obtained with the 3Cl4-HPA molecule (data not shown). In addition, neither 4HPA nor 3Cl-4HPA had any effect on the expression level of NHBA or fHbp in NGP165 (data not shown).
Figure 3B shows that NadA expression is induced by 4-HPA and 3Cl4-HPA to similar levels, with no statistically significant difference seen between the inductions achieved by the two molecular species in three biological replicates. As previously reported for strain MC58 (23), human saliva from different donors is able to induce NadA expression in a dose-dependent manner to the same extent as 4-HPA (or 3Cl4-HPA; data not shown) in NGP165 (Fig. 3C).
These observations support the proposition that NadA expression in NGP165 can be induced in vivo in the human host by alleviating NadR repression through the action of signals either present in saliva or produced during inflammation. The use of HPA derivatives in in vitro assays achieves levels of NadA expression similar to those seen with ex vivo human saliva and may mimic the predicted levels in the host.
hSBA and MATS analysis performed with 3Cl4-HPA predict 4CMenB vaccine coverage of the NGP165 strain.
We performed the hSBA and the MATS assays on the NGP165 wild-type and the nadR knockout mutant strains grown in the absence or presence of 3Cl4-HPA (Table 4) in order to evaluate the 4CMenB vaccine coverage prediction for NGP165 by taking into account the putative level of NadA expression in the host. The NGP165 wild-type strain expressing low levels of NadA in vitro (Fig. 3B) has a NadA MATS RP = 0.005, below the PBT for NadA (0.009). Results of the hSBA demonstrated that NGP165 was indeed resistant to killing by pooled sera from infants who received 4 doses of 4CMenB. When grown in the presence of 3Cl4-HPA, the MATS RP of NGP165 increased to 0.028 (Table 4) and the strain was rendered susceptible in the hSBA using the same infants' sera. Bactericidal titers increased from 4 with preimmune sera to 128 with immunized sera (Table 4). As seen for other strains tested, a more pronounced increase in NadA expression was seen in the nadR knockout mutant, in which the nadA gene is fully derepressed (NadA MATS RP = 0.503). This situation correlates with positive bactericidal titers of >256 in hSBA.
Table 4.
hSBA and MATS of the NGP165 strain performed with the 3Cl4-HPA moleculea
| Strain | Inducer | NadA MATS RP | hSBA titer (study 3 [infants]) |
|
|---|---|---|---|---|
| Routine | 4CMenB post 4 | |||
| NGP165 | None | 0.005 | 2 | 4 |
| NGP165 | 3Cl4-HPA | 0.028 | 4 | 128 |
| NGP165 nadR KO | None | 0.503 | 2 | >256 |
Strains were considered killed if pooled sera from infants who received three immunizations plus one booster of 4CMenB achieved an SBA titer ≥ 8.
In conclusion, using a modified in vitro growth protocol (with HPA supplementation) that we consider more accurately reflects the level of NadA expression that occurs in vivo, the MATS and hSBA assays predicted that NGP165 would be efficiently killed during infection by anti-NadA antibodies in sera of subjects immunized with the 4CMenB vaccine.
Sera from 4CMenB-immunized infants protect infant rats from infection with strain NGP165.
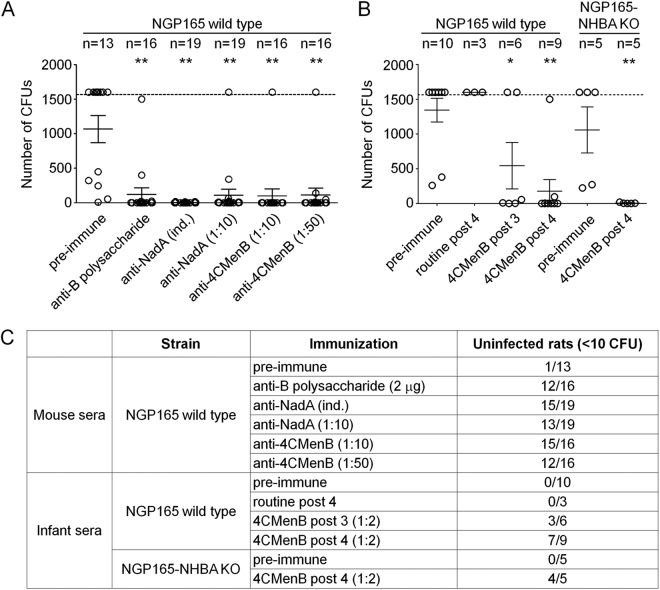
To determine whether NGP165 would be killed in vivo by anti-NadA antibodies, we performed a passive protection assay in the infant rat model (33). Figure 4 reports the results of these experiments. Groups of infant rats were inoculated i.p. with an infectious dose (105 CFU) of NGP165 after being treated with control serum or pre- or postimmune sera from mice and infants immunized with either NadA or the 4CMenB vaccine. Administration of preimmune sera from either mice or human infants had no effect on the ability of NGP165 to infect infant rats: 105 CFU of NGP165 led to sustained infection in all but 1 of the 19 animals tested. Sera from mice immunized with the NadA antigen alone resulted in the protection of 15 of 19 infant rats challenged with NGP165. Sera from mice or human infants immunized with the 4CMenB vaccine formulation conferred protection on the infant rats as well (15 of 16 or 4 of 4 rats, respectively, protected in two experiments). Treatment with the same human sera resulted in protection of infant rats from infection with a NGP165 NHBA KO strain (4 of 5 rats), demonstrating that killing of the strain is not due to anti-NHBA antibodies present in the sera. Taken together, these data suggest that in this in vivo model, NadA is expressed to a sufficient level to be recognized by specific anti-NadA antibodies elicited by NadA in the 4CMenB vaccine and to mediate killing of the bacterium.
Fig 4.
Passive protection in the in vivo rat model. (A) Plot of the number of CFU counted for each infant rat alternatively injected with either preimmune mouse sera or sera from immunized mice, as indicated below the chart. (B) Plot of the number of CFU counted for each infant rat, alternatively injected with either preimmune human sera or sera from human immunized with the 4CMenB vaccine, as indicated. Infant rats were infected with either the NGP165 wild-type or NGP165 NHBA KO strain. Circles indicate single infant rats, while solid horizontal black lines indicate the average of CFU counted for each condition; error bars are also reported. A horizontal dashed line indicates the limit of quantification of the CFU. * = P < 0.05; ** = P < 0.01 (comparing data determined under all described conditions to those determined for rats injected with preimmune sera). No statistical difference between data corresponding to the protection of infant rats from infection by either the NGP165 wild-type or the NHBA KO strain is present. (C) Table showing the results obtained in the in vivo passive protection model.
PnadA is activated in vivo during infection of the infant rat model.
In order to directly evaluate the expression and induction of nadA during infection, we generated reporter strains carrying the promoterless luciferase operon (negative control) or carrying the operon under the control of the nadA promoter (PnadA-lux). The bioluminescence of the resulting strains was evaluated in in vitro experiments. Interestingly, the PnadA-lux strain is significantly less bioluminescent than the negative control (data not shown), indicating that the nadA promoter is efficiently repressed under in vitro conditions, and, as expected, 4HPA-specific induction of PnadA-lux (10-fold) and the derepression of PnadA-lux in the NadR KO background (385-fold) were observed (see Fig. S2 in the supplemental material). The infective dose of 104 CFU of the negative control and the PnadA-lux reporter strains was used to infect groups of 5 infant rats, and images of ventral views of intraperitoneally infected rats were collected either immediately or 3 and 24 h after infection. Immediately after infection, both the negative control and the PnadA reporter strains were poorly bioluminescent (less than 1.5-fold change from the background) (dark gray and white circles, respectively, in Fig. 5A); however, at 3 h postinfection, expression of the PnadA-lux reporter strain was significantly induced (6-fold on average) whereas the negative control maintained low levels of bioluminescence (Fig. 5). It is worth noting that, in Pnad-luxA-infected rats, a widespread bioluminescence was observed over the entire rat that is indicative of bioluminescence from bacteria in a systemic infection. At 24 h after infection, the bioluminescent signals from both the negative control and the reporter strains were almost indistinguishable from the background (Fig. 5A). These data produced in the infant rat model of septicemia indicate that expression of the nadA promoter is induced during infection in vivo, suggesting that NadA is expressed during invasive disease.
Fig 5.
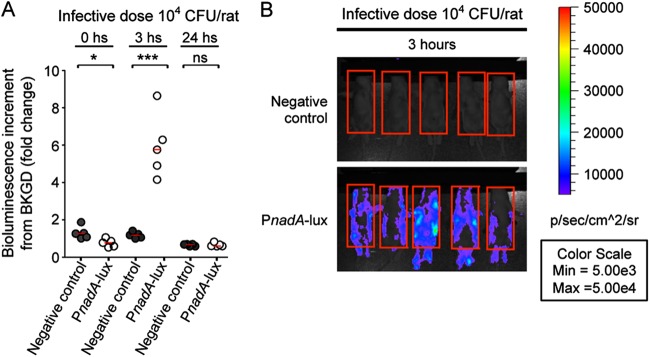
Direct visualization of PnadA expression in the in vivo infant rat model. (A) Histogram representing the bioluminescence increment from the background (infant rats infected with the 2996 wild-type strain) of groups of 5 infant rats infected with 104 CFU of the 2996 negative control (dark gray circles) or the 2996 nadA-lux reporter strain (white circles) as well as median values of bioluminescence increment of each group (solid horizontal red lines) at the time points indicated. * = P < 0.05; *** = P < 0.001; ns = not significant. (B) Panel of ventral views of groups of 5 infant rats infected with 104 CFU of the negative control or the PnadA-lux reporter, as indicated, taken 3 h after infection. Red boxes indicates the regions of interest (ROI) that were taken into consideration by the Live Imaging software to quantify bioluminescent values. Blood samples were recovered 24 h after infection, and CFU counts confirm that the bacterial loads within infant rats infected with different strains were similar.
DISCUSSION
In the absence of an efficacious broadly protective vaccine, MenB is the leading cause of bacterial meningitis and septicemia in many industrialized countries. A novel multicomponent vaccine, 4CMenB, is able to induce bactericidal antibodies against strains expressing vaccine antigens, but because MenB clinical isolates are diverse, it is necessary to evaluate the coverage of circulating strains and therefore the potential public health impact of this vaccine. The MATS assay, which assesses the relative contributions of the 4 major components present in the 4CMenB vaccine, predicts whether a given isolate can be killed or not (13). Using this assay, an evaluation of more than 1,000 MenB strains from 5 European Union countries predicted that 73% to 87% would be covered by 4CMenB vaccination. However, the relative contribution of NadA to this combined-coverage prediction is estimated to be significantly lower than that predicted by the number of strains carrying the nadA gene (18).
The role of NadA in eliciting bactericidal antibodies protecting against circulating strains has been unclear due to NadR-mediated repression of NadA expression under the in vitro growth conditions used for MATS and hSBA assays, which are performed in the early phase of growth, when NadR maximally represses nadA expression (20). For this reason, it is reasonable to anticipate that nadA expression in vitro could be different from the level reached in the host. Litt and colleagues (24) previously showed the presence of antibodies that recognized recombinant NadA in children convalescing after meningococcal disease. Interestingly, we report here that sera from children infected by isolates that failed to express NadA in culture (MATS RP = 0) or that expressed low NadA levels (MATS RP value < PBT) are able to recognize NadA at significantly higher levels than sera from subjects infected by nadA knockout strains (Fig. 1). Taken together, these observations suggest that, despite the low levels of NadA expression in vitro, all these strains express NadA in an immunogenic form in the setting of invasive disease. Furthermore, anti-NadA antibodies are also found in healthy individuals, with levels tending to increase with age (34), suggesting that NadA is expressed in vivo, at a level sufficient to drive the immune response. NadA expression is also induced in the ex vivo model of human saliva (reference 23 and this study), again suggesting high expression in the niche of meningococcal colonization, which can be mimicked in vitro by addition of 4-HPA or 3Cl4-HPA, representing natural inducers of NadA present in the host.
Using the recombinant nadR knockout strains and the HPA inducers as a model for in vivo expression, we have validated NadA as a potent immunogen for patients of all ages and a valid target for protective responses. Once repression mediated by NadR is relieved and NadA is expressed at high levels, strains normally resistant to killing by 4CMenB immune sera are rendered highly susceptible to killing in SBA. The molecular mechanism of both NadR repression of nadA and induction mediated by HPA compounds is conserved in a wide panel of N. meningitidis strains belonging to different clonal complexes (Fig. 2 and reference 23). These observations suggest that any strain carrying nadA could potentially be targeted by bactericidal antibody elicited by the 4CMenB vaccine when NadR repression is relieved during infection.
The ability of passively administered vaccinees' sera to protect mice from infection of NGP165, and to return protective results in the modified MATS and hSBA assays when NGP165 is grown in vitro with added HPA, suggests that this treatment mimics the in vivo status of NGP165 with respect to NadA expression. It is noteworthy that passively administered sera from mice immunized with NadA recombinant protein alone protect infant rats from infection with NGP165, demonstrating that the level of expression reached by NadA alone in vivo is sufficient to promote bacterial killing. Finally, a bioluminescent nadA-lux fusion demonstrates the induction of the nadA promoter 3 h after infection in the infant rat model, demonstrating the expression of nadA during bacteremia in vivo.
The expression of NadA in vivo in the host is in line with its putative role during meningococcal infection. Its adhesive role is important in the course of colonization of the human upper respiratory tract (10, 35). Following the passage across the epithelium, meningococcus invades tissues and blood, where NadA can interact with human blood leukocytes and lead to enhanced immune stimulation (36–39). For these reasons, NadA is to be considered an important meningococcal virulence factor, involved in progressively invasive steps of infection. In accordance with this statement, we demonstrated here that different signals are able to modulate the NadR-repressive activity on NadA (see Fig. S1 in the supplemental material). Signals present in saliva, which we can mimic by using HPA molecules at a high concentration during in vitro growth, may mediate NadA induction during the colonization of the oropharynx. However, because NadA could be required during other steps of the pathogenesis, multiple signals in niches other than the pharynx could modulate NadA expression as soon as the bacterium passes the epithelial barrier. We demonstrate indeed in the infant rat model of bacteremia that NadA is expressed widely 3 hours postinfection and that this upregulation could be due to molecules such as 3CI4-HPA and NO2-4HPA (see Fig. S1 in the supplemental material), which are produced by leukocytes during inflammatory processes (32, 40). Furthermore, we cannot exclude the possibility that other molecules can act on NadR by increasing its repressive action on its targets.
From our data, current methods used to predict coverage of strains by the 4CMenB vaccine are underestimating the contribution of the NadA antigen, suggesting that all nadA+ meningococcal strains may be susceptible to bactericidal anti-NadA antibodies elicited by vaccination. A more accurate prediction may be obtained by addition of physiologically relevant inducers to in vitro-grown bacteria, resulting in NadA expression levels similar to those measured in ex vivo and in vivo models of infection. This report provides new insights into the expression of the NadA antigen during infection which are fundamental for the implementation of prophylactic strategies such as vaccines and evaluation of their impact on public health.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to the Active Bacterial Core surveillance team for providing the Nm isolates M10574 and M14933. We thank Phil Boucher, Davide Serruto, Marirosa Mora, Maurizio Comanducci, Kate Seib, and Ana Antunes for critical reading of the manuscript. We are grateful to Fabio Rigat and Giacomo Frosi for their support in evaluating the sample numerosity for passive protection experiments. We also thank Mauro Agnusdei, Isabella Simmini, Angela Spagnuolo, and the animal care facility at Novartis Vaccines for technical support. We thank Giorgio Corsi for artwork.
L.F. is the recipient of a Novartis fellowship from the Ph.D. program in Functional Biology of Molecular and Cellular Systems of the University of Bologna.
Footnotes
Published ahead of print 10 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01085-12.
REFERENCES
- 1. Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378–1388 [DOI] [PubMed] [Google Scholar]
- 2. Tinsley C, Nassif X. 2001. Meningococcal pathogenesis: at the boundary between the pre- and post-genomic eras. Curr. Opin. Microbiol. 4:47–52 [DOI] [PubMed] [Google Scholar]
- 3. Boisier P, Nicolas P, Djibo S, Taha MK, Jeanne I, Mainassara HB, Tenebray B, Kairo KK, Giorgini D, Chanteau S. 2007. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin. Infect. Dis. 44:657–663 [DOI] [PubMed] [Google Scholar]
- 4. Jarvis GA, Vedros NA. 1987. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect. Immun. 55:174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stephens DS, Greenwood B, Brandtzaeg P. 2007. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 369:2196–2210 [DOI] [PubMed] [Google Scholar]
- 6. Giuliani MM, Adu-Bobie J, Comanducci M, Arico B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, Cartocci E, Ciucchi L, Di Marcello F, Ferlicca F, Galli B, Luzzi E, Masignani V, Serruto D, Veggi D, Contorni M, Morandi M, Bartalesi A, Cinotti V, Mannucci D, Titta F, Ovidi E, Welsch JA, Granoff D, Rappuoli R, Pizza M. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. U. S. A. 103:10834–10839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esposito S, Vesikari T, Kimura A, Ypma E, Toneatto D, Dull P. 2010. Tolerability of a three-dose schedule of an investigational, multicomponent meningococcal serogroup B vaccine and routine infant vaccines in a lot consistency trial, p 168. Abstr. 17th International Pathogenic Neisseria Conference (IPNC), Banff, Alberta, Canada [Google Scholar]
- 8. Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, Oster P, Miller E, Pollard AJ. 2010. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin. Infect. Dis. 51:1127–1137 [DOI] [PubMed] [Google Scholar]
- 9. Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, Findlow J, Yu LM, Borrow R, Ypma E, Toneatto D, Pollard AJ. 2010. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr. Infect. Dis. J. 29:e71–e79 [DOI] [PubMed] [Google Scholar]
- 10. Comanducci M, Bambini S, Brunelli B, Adu-Bobie J, Arico B, Capecchi B, Giuliani MM, Masignani V, Santini L, Savino S, Granoff DM, Caugant DA, Pizza M, Rappuoli R, Mora M. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serruto D, Spadafina T, Ciucchi L, Lewis LA, Ram S, Tontini M, Santini L, Biolchi A, Seib KL, Giuliani MM, Donnelly JJ, Berti F, Savino S, Scarselli M, Costantino P, Kroll JS, O'Dwyer C, Qiu J, Plaut AG, Moxon R, Rappuoli R, Pizza M, Arico B. 2010. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc. Natl. Acad. Sci. U. S. A. 107:3770–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, Moxon ER, Stella M, Comanducci M, Bambini S, Muzzi A, Andrews W, Chen J, Santos G, Santini L, Boucher P, Serruto D, Pizza M, Rappuoli R, Giuliani MM. 2010. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc. Natl. Acad. Sci. U. S. A. 107:19490–19495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gotschlich EC, Goldschneider I, Artenstein MS. 1969. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J. Exp. Med. 129:1367–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gotschlich EC, Goldschneider I, Artenstein MS. 1969. Human immunity to the meningococcus. V. The effect of immunization with meningococcal group C polysaccharide on the carrier state. J. Exp. Med. 129:1385–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Donnelly J, Medini D, Giuliani MM, Boccadifuoco G, Stella M, Frosi G, Comanducci M, Bambini S, Muzzi A, Pizza M, Rappuoli R, Findlow J, Borrow R, Gilchrist S, Thompson D, Ledroit M, Hong E, Taha MK, Abad R, Vazquez J, Mastrantonio P, Stefanelli P, Fazio C, Carannante A, Oksnes J, Caugant DA, Claus H, Vogel U. 2011. Estimating the potential strain coverage in Europe of a multicomponent vaccine targeting serogroup B meningococci, p 17–18 Abstr. 11th European Monitoring Group on Meningococci Congress, Ljubljana, Slovenia. [Google Scholar]
- 19. Wang X, Cohn A, Comanducci M, Andrew L, Zhao X, MacNeil JR, Schmink S, Muzzi A, Bambini S, Rappuoli R, Pizza M, Murphy E, Hoiseth SK, Jansen KU, Anderson AS, Harrison LH, Clark TA, Messonnier NE, Mayer LW. 2011. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. Vaccine 29:4739–4744 [DOI] [PubMed] [Google Scholar]
- 20. Metruccio MM, Pigozzi E, Roncarati D, Berlanda Scorza F, Norais N, Hill SA, Scarlato V, Delany I. 2009. A novel phase variation mechanism in the meningococcus driven by a ligand-responsive repressor and differential spacing of distal promoter elements. PLoS Pathog. 5:e1000710 doi:10.1371/journal.ppat.1000710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perera IC, Lee YH, Wilkinson SP, Grove A. 2009. Mechanism for attenuation of DNA binding by MarR family transcriptional regulators by small molecule ligands. J. Mol. Biol. 390:1019–1029 [DOI] [PubMed] [Google Scholar]
- 22. Takahama U, Oniki T, Murata H. 2002. The presence of 4-hydroxyphenylacetic acid in human saliva and the possibility of its nitration by salivary nitrite in the stomach. FEBS Lett. 518:116–118 [DOI] [PubMed] [Google Scholar]
- 23. Fagnocchi L, Pigozzi E, Scarlato V, Delany I. 2012. In the NadR regulon, adhesins and diverse meningococcal functions are regulated in response to signals in human saliva. J. Bacteriol. 194:460–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Litt DJ, Savino S, Beddek A, Comanducci M, Sandiford C, Stevens J, Levin M, Ison C, Pizza M, Rappuoli R, Kroll JS. 2004. Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease. J. Infect. Dis. 190:1488–1497 [DOI] [PubMed] [Google Scholar]
- 25. Ieva R, Roncarati D, Metruccio MM, Seib KL, Scarlato V, Delany I. 2008. OxyR tightly regulates catalase expression in Neisseria meningitidis through both repression and activation mechanisms. Mol. Microbiol. 70:1152–1165 [DOI] [PubMed] [Google Scholar]
- 26. Niimi Y, Kambara H, Matsui T, Yoshioka D, Fukuyama H. 2006. Real-space imaging of alternate localization and extension of quasi-two-dimensional electronic states at graphite surfaces in magnetic fields. Phys. Rev. Lett. 97:236804 doi:10.1103/PhysRevLett.97.236804 [DOI] [PubMed] [Google Scholar]
- 27. Delany I, Ieva R, Alaimo C, Rappuoli R, Scarlato V. 2003. The iron-responsive regulator fur is transcriptionally autoregulated and not essential in Neisseria meningitidis. J. Bacteriol. 185:6032–6041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brier S, Fagnocchi L, Donnarumma D, Scarselli M, Rappuoli R, Nissum M, Delany I, Norais N. 2012. Structural insight into the mechanism of DNA-binding attenuation of the neisserial adhesin repressor NadR by the small natural ligand 4-hydroxyphenylacetic acid. Biochemistry 51:6738–6752 [DOI] [PubMed] [Google Scholar]
- 29. Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. 2009. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30:2785–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borrow R, Aaberge IS, Santos GF, Eudey TL, Oster P, Glennie A, Findlow J, Hoiby EA, Rosenqvist E, Balmer P, Martin D. 2005. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup b, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin. Diagn. Lab. Immunol. 12:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moe GR, Tan S, Granoff DM. 1999. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect. Immun. 67:5664–5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mani AR, Ippolito S, Moreno JC, Visser TJ, Moore KP. 2007. The metabolism and dechlorination of chlorotyrosine in vivo. J. Biol. Chem. 282:29114–29121 [DOI] [PubMed] [Google Scholar]
- 33. Granoff DM, Moe GR, Giuliani MM, Adu-Bobie J, Santini L, Brunelli B, Piccinetti F, Zuno-Mitchell P, Lee SS, Neri P, Bracci L, Lozzi L, Rappuoli R. 2001. A novel mimetic antigen eliciting protective antibody to Neisseria meningitidis. J. Immunol. 167:6487–6496 [DOI] [PubMed] [Google Scholar]
- 34. Jacobsson S, Molling P, Olcen P. 2009. Seroprevalence of antibodies against fHbp and NadA, two potential vaccine antigens for Neisseria meningitidis. Vaccine 27:5755–5759 [DOI] [PubMed] [Google Scholar]
- 35. Capecchi B, Adu-Bobie J, Di Marcello F, Ciucchi L, Masignani V, Taddei A, Rappuoli R, Pizza M, Arico B. 2005. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol. Microbiol. 55:687–698 [DOI] [PubMed] [Google Scholar]
- 36. Mazzon C, Baldani-Guerra B, Cecchini P, Kasic T, Viola A, de Bernard M, Arico B, Gerosa F, Papini E. 2007. IFN-gamma and R-848 dependent activation of human monocyte-derived dendritic cells by Neisseria meningitidis adhesin A. J. Immunol. 179:3904–3916 [DOI] [PubMed] [Google Scholar]
- 37. Franzoso S, Mazzon C, Sztukowska M, Cecchini P, Kasic T, Capecchi B, Tavano R, Papini E. 2008. Human monocytes/macrophages are a target of Neisseria meningitidis Adhesin A (NadA). J. Leukoc. Biol. 83:1100–1110 [DOI] [PubMed] [Google Scholar]
- 38. Tavano R, Franzoso S, Cecchini P, Cartocci E, Oriente F, Arico B, Papini E. 2009. The membrane expression of Neisseria meningitidis adhesin A (NadA) increases the proimmune effects of MenB OMVs on human macrophages, compared with NadA− OMVs, without further stimulating their proinflammatory activity on circulating monocytes. J. Leukoc. Biol. 86:143–153 [DOI] [PubMed] [Google Scholar]
- 39. Cecchini P, Tavano R, Polverino de Laureto P, Franzoso S, Mazzon C, Montanari P, Papini E. 2011. The soluble recombinant Neisseria meningitidis adhesin NadA(Delta351-405) stimulates human monocytes by binding to extracellular Hsp90. PLoS One 6:e25089 doi:10.1371/journal.pone.0025089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fukuyama N, Ichimori K, Su Z, Ishida H, Nakazawa H. 1996. Peroxynitrite formation from activated human leukocytes. Biochem. Biophys. Res. Commun. 224:414–419 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.