Abstract
The three human ficolins (H-, L-, and M-ficolins) and mannan-binding lectin are pattern recognition molecules of the innate immune system mediating activation of the lectin pathway of the complement system. These four human proteins bind to some microorganisms and may be involved in the resolution of infections. We investigated binding selectivity by examining the binding of M-ficolin to a panel of more than 100 different streptococcal strains (Streptococcus pneumoniae and Streptococcus mitis), each expressing distinct polysaccharide structures. M-ficolin binding was observed for three strains only: strains of the pneumococcal serotypes 19B and 19C and a single S. mitis strain expressing a similar polysaccharide structure. The bound M-ficolin, in association with MASP-2, mediated the cleavage of complement factor C4. Binding to the bacteria was inhibitable by N-acetylglucosamine, indicating that the interaction with the bacterial surface takes place via the fibrinogen-like domain. The common N-acetylmannosamine residue present in the structures of the four capsular polysaccharides of group 19 is linked via a phosphodiester bond. This residue is apparently not a ligand for M-ficolin, since the lectin binds to two of the group 19 polysaccharides only. M-ficolin bound strongly to serotype 19B and 19C polysaccharides. In contrast to those of serotypes 19A and 19F, serotype 19B and 19C polysaccharides contain an extra N-acetylmannosamine residue linked via glycoside linkage only. Thus, this extra residue seems to be the M-ficolin ligand. In conclusion, we were able to demonstrate specific binding of M-ficolin to some capsular polysaccharides of the opportunistic pathogen S. pneumoniae and of the commensal bacterium S. mitis.
INTRODUCTION
An efficient immune system is a necessity for the body to mount defenses against microbial infections. The immune system is also needed for homeostasis, since a large number of apoptotic and necrotic cells are marked for removal by the immune system. The immune system is composed of the adaptive and the innate immune system. An important part of the innate immune system is the complement system. It consists of several recognition molecules and a number of proenzymes that can be activated, leading to proinflammatory reactions that may eliminate pathogens. In the present report, we study the interaction between the human pathogen Streptococcus pneumoniae and recognition molecules of the lectin pathway of the complement system.
The lectin pathway is initiated when mannan-binding lectin (MBL) or one of the three ficolins (H-ficolin, L-ficolin, and M-ficolin) binds to a fitting pattern of carbohydrates. MBL and the ficolins thus function as pattern recognition molecules (PRMs) sensing foreign as well as altered self molecules, as seen on ischemic reperfused tissues, apoptotic cells, and cancer cells. Analogously to the C1 complex of the classical pathway, consisting of C1q in complex with the serine proteases C1r and C1s, MBL and ficolins form complexes with MBL-associated serine proteases (MASPs), which enables them to activate complement upon binding to suitable structures (1). A fifth PRM of the lectin pathway, CL-K1 (also known as collectin 11 [CL-11]), has been described recently (2). CL-K1 forms complexes with MASP-1 and MASP-2 and is able to recognize Escherichia coli, Candida albicans, and S. pneumoniae (3, 4).
M-ficolin (also known as ficolin-1) is the only ficolin that is produced in the bone marrow and by peripheral leukocytes (5–8). Like the other two ficolins, M-ficolin is built of a number of identical polypeptide chains. The polypeptide chain comprises a collagen-like domain and a C-terminal fibrinogen-like (FBG) recognition domain. The three chains together form a structural subunit that oligomerizes further (1). M-ficolin thus possesses many recognition domains and recognizes patterns of N-acetylated residues via this FBG domain (9–12). Uniquely among the ficolins, M-ficolin also recognizes N-acetylated residues in sialic acids (10, 12). In addition to its abundant presence within monocytes and granulocytes (13), M-ficolin is also located on the external surfaces of these cells, possibly via binding to sialic acids through the FBG domain (14), via interaction with the G-protein-coupled receptor GPCR43 (15), or through binding to leukosialin (CD43) (16). Upon stimulation of neutrophils, intracellular M-ficolin is mobilized, resulting in the upregulation of surface-bound M-ficolin (17) and its release to the surroundings (18). Presumably, if the secreted M-ficolin then binds to nearby microorganisms, it can activate the complement system and thus initiate an antimicrobial reaction.
Recently, we reported the binding of M-ficolin to strains of Streptococcus agalactiae (group B Streptococcus [GBS]) (13). In the present study, we investigated the binding of M-ficolin to the opportunistic pathogen Streptococcus pneumoniae (pneumococcus) and to the closely related commensal bacterium Streptococcus mitis. The pneumococcus is part of the normal microflora in the upper respiratory tract, although this species may give rise to otitis media, a common infection in childhood, and to invasive diseases such as pneumonia, sepsis, and meningitis (19). The chemical structures of many of the capsular polysaccharides of the 93 known pneumococcal serotypes have been revealed (20–23). Each serotype possesses a polysaccharide with a distinct chemical structure, and together they represent a unique panel of naturally occurring carbohydrate patterns. In order to recognize natural PRM ligands, it is relevant to examine such panels for the ability to bind PRMs. Thus, we examined more than 100 different streptococcal strains, each with a unique capsular structure, and found distinct binding of M-ficolin to pneumococcal strains of serotype 19B or 19C and to a single S. mitis strain, which possesses a polysaccharide with a structure similar to the structures of the group 19 polysaccharides. We show that such recognition leads to complement activation.
MATERIALS AND METHODS
Bacteria.
Ninety-one different S. pneumoniae strains, representing all known pneumococcal serotypes except for 11E and 6D (serotypes 1, 2, 3, 4, 5, 6A, 6B, 6C, 7F, 7A, 7B, 7C, 8, 9A, 9L, 9N, 9V, 10F, 10A, 10B, 10C, 11F, 11A, 11B, 11C, 11D, 12F, 12A, 12B, 13, 14, 15F, 15A, 15B, 15C, 16F, 16A, 17F, 17A, 18F, 18A, 18B, 18C, 19F, 19A, 19B, 19C, 20, 21, 22F, 22A, 23F, 23A, 23B, 24F, 24A, 24B, 25F, 25A, 27, 28F, 28A, 29, 31, 32F, 32A, 33F, 33A, 33B, 33C, 33D, 34, 35F, 35A, 35B, 35C, 36, 37, 38, 39, 40, 41F, 41A, 42, 43, 44, 45, 46, 47F, 47A, and 48) (strain collection, Institute of Biomedicine, Aarhus University), were cultured in Todd-Hewitt broth over night (O/N) at 37°C under 5% CO2. The bacteria were fixed in formaldehyde at a final concentration of 1% (vol/vol). Residual aldehyde groups were blocked with Tris-buffered saline (TBS) (10 mM Tris-HCl, 140 mM NaCl [pH 7.4]) with 15 mM NaN3 (TBS-azide) or with 0.1 M ethanolamine (pH 9). In addition, fixed cells were prepared from 10 S. mitis strains (SK597, SK1124, SK608, SK569, SK575, SK564, SK611, SK637, SK137, and SK271; Institute of Biomedicine, Aarhus University) and from the two acapsular (“rough”) pneumococcal strains ATCC 11733 and ATCC 27336 (from the American Type Culture Collection). The bacteria were stored at 4°C and were washed three times by centrifugation in TBS-azide immediately before use. The concentration of the bacteria was estimated by reading the optical density at 600 nm (OD600); an OD600 of 1 corresponded to ≈1.8 × 108 bacteria/ml (24).
Binding assay.
The binding of ficolins and MBL to S. pneumoniae in serum was first tested with bacteria in suspension. A fixed number (see below) of formalin-fixed bacterial cells was sedimented (2,000 × g, 5 min, room temperature [RT]) and was washed once in binding buffer (TBS-azide, 5 mM CaCl2, 1% human serum albumin [HSA]; Statens Serum Institut, Denmark). The bacterial cells were sedimented, resuspended in 360 μl binding buffer, and added to 40 μl normal human serum (NHS) (a serum pool prepared from the sera of nine healthy individuals). As a positive control, 40 μl sedimented acetylated HSA-derivatized Sepharose beads (AcHSA beads) (25) was resuspended in 320 μl binding buffer and was added to 40 μl NHS. In order to construct standard curves, dilution series of pneumococcal serotype 19B and 19C cells were sedimented by centrifugation and were tested as described above. All samples were incubated with rotation for 2 h at RT. After removal of the bacteria by centrifugation (10,000 × g, 10 min, 4°C), the concentrations of MBL, M-ficolin, and H-ficolin in the supernatants were measured using in-house assays, which have been described in detail previously (26–28). The in-house H- and M-ficolin assays are based on the use of antibody-coated microtiter wells in which samples were incubated and bound proteins detected with biotin-labeled antibodies. This was followed by incubation with europium-labeled streptavidin, and the amount of bound europium was measured by time-resolved fluorometry. We found that the purified recombinant M-ficolin (rM-ficolin) prepared in Chinese hamster ovary (CHO) cells as described below is more similar to natural M-ficolin in terms of oligomerization than the rM-ficolin used previously, which was produced by transient expression in HEK293F cells (27). The corrected median concentration of M-ficolin in serum for 350 healthy blood donors, obtained by using the rM-ficolin produced in CHO cells, is 556 ng/ml, not 1,072 ng/ml as reported previously (27). Our MBL assay is based on the use of mannan-coated microtiter wells, with a europium-labeled antibody for detection. The concentration of L-ficolin was measured by use of a commercial L-ficolin enzyme-linked immunosorbent assay (ELISA) kit (catalog no. HK336; Hycult Biotech, Uden, The Netherlands) or our own in-house assay (28).
Inhibition assays.
The specificity of M-ficolin binding to S. pneumoniae strains was examined in a competitive inhibition assay with various monosaccharides and capsular polysaccharides.
S. pneumoniae strains of serotypes 19B and 19C (≈109 bacterial cells) were suspended in 360 μl binding buffer containing either 100 mM glucose (β-d-glucose; G5767; Sigma-Aldrich, St. Louis, MO) or 100 mM N-acetylglucosamine (GlcNAc) (A8625; Sigma-Aldrich) and were incubated with 40 μl NHS added. After centrifugation, the contents of MBL and ficolins in the supernatants were tested as described above. To examine the nature of the ligand on the bacteria, the two strains representing serotypes 19B and 19C (109 formalin-fixed cells) were treated with 60 μg proteinase K (catalog no. 03 115 852 001; Roche) in 300 μl 20 mM Tris (pH 8) for 30 min at 37°C so as to digest any proteins on the bacteria. After the incubation, 700 μl binding buffer with 5 mM phenylmethylsulfonyl fluoride (PMSF) was added to inactivate the proteinase K activity. The protease-treated bacteria were sedimented (2,000 × g, 10 min, RT), washed once in 1 ml binding buffer, and tested for the binding of MBL and ficolins from NHS as described above.
We also tested the binding of M-ficolin to bacteria that had been applied to microtiter wells. To test the binding of rM-ficolin, FluoroNunc microtiter plate wells were coated with 107 formalin-fixed whole cells of S. pneumoniae serotype 19B or 19C in 100 μl coating buffer (15 mM Na2CO3, 35 mM NaHCO3, 15 mM NaN3 [pH 9.6]) by O/N incubation at 4°C. Plates were then blocked with 200 μg HSA in 200 μl TBS-azide for 1 h at RT. The wells were washed three times in TBS-azide with 0.05% Tween 20 and 5 mM CaCl2 (TBS-azide–Tw–Ca2+) before the addition of 50 ng of rM-ficolin in 100 μl binding buffer containing mannose (M2069; Sigma-Aldrich), glucose, N-acetylmannosamine (ManNAc) (A8176; Sigma-Aldrich), GlcNAc, d-ribose (R7500; Sigma-Aldrich), l-rhamnose monohydrate (R3875; Sigma-Aldrich), or purified pneumococcal serotype 19F or 19C capsular polysaccharide (76958 and 76961, respectively; Statens Serum Institut). The plates were incubated O/N at 4°C, washed three times in TBS-azide–Tw–Ca2+, and analyzed for M-ficolin as described previously (27).
The rM-ficolin was produced in CHO cells by using the Flp-In system (Invitrogen). The transfected cells were grown in F-12 medium (GIBCO, Invitrogen) supplemented with 10% fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. The culture supernatant (CS) was harvested 72 h after confluence, preserved by the addition of 15 mM NaN3, and stored at 4°C. For the tests described above, the CS was diluted 2.3-fold to reach a concentration of 500 ng rM-ficolin/ml.
C4 deposition assay.
Complement activation by M-ficolin/MASP-2 complexes bound to bacterial cells was examined by a modification of the assay for C4 deposition induced by MBL/MASP on mannan (29). FluoroNunc microtiter plate wells were coated with whole cells of S. pneumoniae serotype 19B, 19C, or 19F as described above. After washing, dilutions of rM-ficolin in TBS-azide-Tw–Ca2+ were added, and the plates were incubated for 3 h at RT. The plates were washed, and 2 ng rMASP-2 (diluted recombinant CS) (30) in 100 μl TBS-azide–Tw–Ca2+ was added per well, followed by O/N incubation at 4°C. The plates were washed three times, and to each well was added 0.5 μg purified C4 (31) in 100 μl barbital buffer (4 mM barbital, 145 mM NaCl, 2 mM CaCl2, 1 mM MgCl2 [pH 7.4]). The plates were incubated for 1.5 h at 37°C and were then washed three times, and 50 ng biotinylated anti-C4 antibody (Hyb 162-2; BioPorto A/S, Gentofte, Denmark) (biotinylated with 167 μg biotin N-hydroxysuccinimide ester [H1759; Sigma-Aldrich] per mg antibody) in 100 μl TBS-azide–Tw–Ca2+ was added per well. After incubation for 2 h at RT, the plates were washed three times, and 10 ng europium-labeled streptavidin (1244-360; PerkinElmer, Waltham, MA) in 100 μl TBS-azide–Tw with 25 μM EDTA was added per well. The plates were incubated for 1 h at RT once more, and after three final washes, 200 μl enhancement buffer (PerkinElmer) was added to each well. The amount of europium in the wells was measured by time-resolved fluorometry (Victor 3; PerkinElmer). As a negative control, rM-ficolin or rMASP-2 was omitted.
Flow cytometry.
The binding of M-ficolin to pneumococcal serotype 19B and 19C strains was studied further by flow cytometry. Bacteria (≈108) were washed once in binding buffer and were incubated with 500 ng rM-ficolin in 1 ml binding buffer for 2 h at RT with rotation. Afterwards, the bacteria were washed twice in binding buffer and were incubated for 30 min at 4°C with rotation together with 2 μg of a fluorescein-labeled anti-M-ficolin antibody (fluorescein-7G1) or 2 μg of fluorescein-labeled mouse IgG1(κ) as an isotype control (13). The bacteria were subsequently washed twice and were fixed in 0.9% (vol/vol) formaldehyde in phosphate-buffered saline (PBS) (140 mM NaCl, 8.1 mM Na2HPO4, 2.7 mM KCl, 1.5 mM KH2PO4 [pH 7.4]). GlcNAc or glucose added to the buffer (50 mM) during the binding of rM-ficolin to the bacteria served as a negative or positive control, respectively. Bacteria incubated without rM-ficolin served as an additional negative control.
Western blotting.
Bacteria of serotypes 19F, 19B, and 19C (≈5 × 108) were incubated with NHS as described above. After centrifugation and removal of the supernatant, the bacteria were washed once in 1 ml binding buffer. The bacteria were sedimented and the supernatant discarded. To elute the M-ficolin, 200 μl binding buffer containing 100 mM GlcNAc was added to the bacteria, followed by incubation for 30 min with rotation and centrifugation (10,000 × g, 10 min, 4°C). Samples of the supernatants were added to ¼ volume sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (30 mM Tris-HCl, 10% [vol/vol] glycerol, 8 M urea, 3% [wt/vol] SDS, 0.1% [wt/vol] bromophenol blue [pH 8.9]), and the proteins were separated by SDS-PAGE on 4-to-15% Criterion TGX gels (567-1083; Bio-Rad, Hercules, CA) under nonreducing conditions. The proteins in the gels were blotted onto nitrocellulose membranes (170-4159; Bio-Rad). The membranes were blocked by incubation for 30 min at RT in TBS–0.1% (vol/vol) Tween 20, washed, and developed with a monoclonal anti-M-ficolin antibody (1 μg/ml; ABS 036-05; BioPorto, Gentofte, Denmark) in primary buffer (TBS-azide–Tw, 1 mg HSA/ml, 100 μg normal human IgG [007815; CSL Behring GmbH, Hattersheim am Main, Germany] per ml, 1 mM EDTA [pH 7.4]). The membranes were subsequently washed and were then incubated with a horseradish peroxidase (HRP)-conjugated rabbit anti-mouse Ig antibody (P0260; Dako, Glostrup, Denmark) diluted 1/4,000 in a secondary buffer (TBS-Tw, 100 μg normal human IgG/ml, 1 mM EDTA [pH 7.4]). After washing, the blot was developed with a SuperSignal West Dura extended-duration substrate (Pierce, Rockford, IL), and the light emitted was recorded by a charge-coupled device (CCD) camera.
RESULTS
The aim of the present study was to study the selectivity of human soluble PRMs by screening their binding to a large number of streptococcal polysaccharides with distinct and different chemical structures. In the initial screening of 91 S. pneumoniae strains of different serotypes, we found specific binding of M-ficolin only to strains of the two serotypes 19B and 19C (Fig. 1). In contrast to M-ficolin, two other PRMs, MBL and H-ficolin, exhibited very low or no binding to strains of any of the 91 serotypes (data not shown). L-ficolin was found to bind to some degree to strains of different serotypes, which confirmed our previous observations (24).
Fig 1.
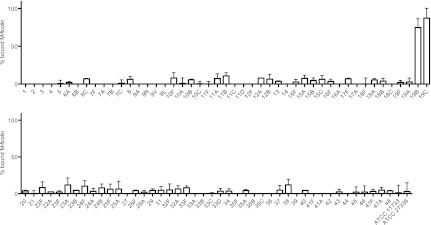
Binding of M-ficolin to S. pneumoniae. Formalin-fixed bacteria were first incubated with 10% NHS and then spun down, and the M-ficolin remaining in the supernatant was measured. The percentage of bound M-ficolin was calculated based on the concentration in the supernatants compared to the concentration in 10% NHS. The designations of the serotypes are given on the x axis. Data are representative of two individual experiments. Means and standard deviations are shown.
Based on the results mentioned above and on our previous investigations of the binding of L-ficolin to the capsular polysaccharides of various pneumococcal serotypes (24), we selected 13 serotypes for detailed investigations. We found that 109 bacterial cells of the two serotypes 19B and 19C were able to remove nearly all the M-ficolin present in 40 μl serum (Fig. 2A), and we showed dose-dependent depletion of M-ficolin with these two serotypes (Fig. 2E). Virtually no binding of MBL (Fig. 2B) or H-ficolin (Fig. 2C) to strains of any of the serotypes was seen (<15%). In agreement with previous observations (24), L-ficolin bound strongly to strains of serotypes 35A and 35C and to some degree (20 to 50%) to strains of serotypes 11F, 11A, and 11D (Fig. 2D). We used AcHSA beads as a positive control in the assay, because ficolins are known to bind efficiently to the acetyl groups on these beads (13). As expected, no H-, L-, or M-ficolin was detected in the supernatants after incubation with AcHSA beads.
Fig 2.
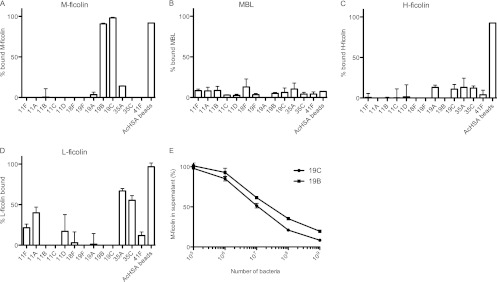
Binding of PRMs to S. pneumoniae. (A to D) Formalin-fixed pneumococci (109) of selected serotypes were incubated with 400 μl 10% NHS, and the lectin pathway PRMs remaining in the supernatant after the removal of the bacteria by centrifugation were measured. The percentage of bound PRMs was calculated based on the concentration in the supernatants compared to the concentration in 10% NHS. (A) M-ficolin binding; (B) MBL binding; (C) H-ficolin binding; (D) L-ficolin binding. The designations of the serotypes are given on the x axis. Data are representative of two individual experiments. Means and standard deviations are shown. (E) Concentration-dependent binding of M-ficolin to cells of pneumococcal serotypes 19B and 19C. Data are representative of two individual experiments.
To examine the selectivity of the binding of M-ficolin in serum to serotype 19B and 19C strains, we added GlcNAc or glucose to the incubation mixture. We found that GlcNAc, but not glucose, prevented binding (Fig. 3A). These observations confirm the requirement of the N-acetyl group for the binding of ficolins (25). Treatment of serotype 19B and 19C cells with proteinase K (a promiscuous serine protease) had no influence on the binding of serum M-ficolin (Fig. 3A), indicating that the bacterial proteins are not involved in the process. To further examine the ability of acetylated compounds to compete for binding to the bacteria, we tested the binding of rM-ficolin to serotype 19B and 19C whole cells applied directly to wells on microtiter plates. The binding observed was dose-dependently inhibited by GlcNAc and ManNAc. Approximately 50% inhibition was seen with either compound at 30 mM on a 19B strain (Fig. 3B) and at 60 mM on a 19C strain (Fig. 3C), but no inhibition was seen at any concentration of glucose or mannose. Purified 19C capsular polysaccharide, but not purified 19F capsular polysaccharide, was able to inhibit the binding. In this case, less than 0.03 μg/ml 19C polysaccharide reduced the binding on a 19B strain by 50%; on a 19C strain (homologous inhibition), 0.16 μg/ml was needed for the same degree of inhibition (Fig. 3B and C, respectively). Addition of d-ribose or l-rhamnose did not influence the binding (data not shown). In conclusion, these results show a specific interaction between the FBG domain of M-ficolin and residues on serotype 19B and 19C capsular polysaccharides, and M-ficolin apparently shows a higher affinity for the 19C than for the 19B polysaccharide.
Fig 3.
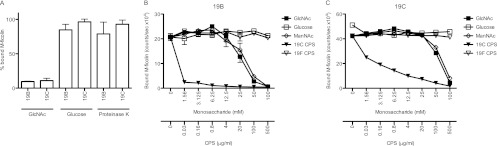
Inhibition of binding of M-ficolin to S. pneumoniae strains of serotypes 19B and 19C. (A) The binding of serum M-ficolin to 19B and 19C strains (in the presence of 100 mM GlcNac or 100 mM glucose) and to proteinase K-treated bacteria was tested as described for Fig. 2. The data are presented as percentages of the amount of serum M-ficolin that was bound if no carbohydrate was added and are representative of two individual experiments. Means and standard deviations are shown. (B and C) The binding of rM-ficolin to microtiter wells coated with pneumococcal cells of serotype 19B (B) or 19C (C) was tested in the presence of different monosaccharides or purified 19C or 19F capsular polysaccharide (CPS). Data are given as counts per second on the y axis and are representative of two individual experiments. The amounts of the various potential inhibitors used are given in millimolar concentrations for the monosaccharides and in micrograms per milliliter for the capsular polysaccharides.
We have shown previously that M-ficolin bound to AcHSA can bind and activate MASP-2, thereby eliciting activation of the complement system (11). By the addition of increasing amounts of rM-ficolin to wells coated with serotype 19C or 19B whole cells, a dose-dependent amount of M-ficolin was bound in the wells, whereas no binding was seen to wells coated with the 19F strain (Fig. 4A). More binding was seen to the 19C-coated wells than to the 19B-coated wells; however, we do not know whether this is due to higher avidity for the 19C cells or whether cells of the serotype 19C strain bind more efficiently to the plastic surface in the wells than do serotype 19B cells. After incubation of coated wells with rM-ficolin, MASP-2 was added to allow the formation of complexes. Subsequently, complement factor C4 was added, and the C4 fragments deposited in the wells were detected with an anti-C4 antibody. We observed M-ficolin concentration-dependent complement activation in wells coated with serotype 19B or 19C cells, but not in wells coated with serotype 19F whole cells (Fig. 4B). Inhibition of rM-ficolin binding by the addition of GlcNAc prevented C4 deposition (Fig. 4B), and the omission of rMASP-2 or rM-ficolin resulted in no deposition of C4b (data not shown). Comparison of the detection of M-ficolin bound in the bacterium-coated wells (Fig. 4A) with the detection of deposited C4 fragments (Fig. 4B) indicates that the latter test is more sensitive.
Fig 4.
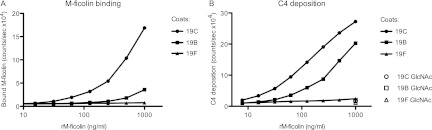
Complement deposition on S. pneumoniae by rM-ficolin/rMASP-2 complexes. (A) rM-ficolin was incubated in wells coated with cells of serotype 19B, 19C, and 19F strains. Bound rM-ficolin was detected with an anti-M-ficolin antibody. (B) In parallel wells in which rMASP-2 was associated with the bound rM-ficolin, complement activation was probed by adding C4, incubating at 37°C, and then detecting the C4b deposited. Data are representative of two individual experiments.
To evaluate the binding of M-ficolin by a different procedure, we used a flow cytometric assay to analyze bacterial cells incubated with rM-ficolin, and we detected bound M-ficolin with an anti-M-ficolin antibody. By this technique, we confirmed the binding of M-ficolin to pneumococcal cells of serotypes 19B and 19C (Fig. 5A). This binding was blocked by GlcNAc but not by glucose (Fig. 5B). As expected from the results presented above, rM-ficolin did not bind to 19F cells (Fig. 5A).
Fig 5.
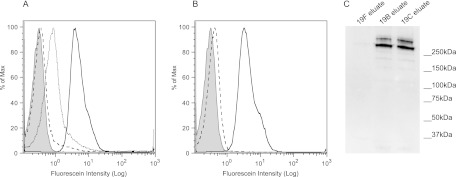
Flow cytometric and Western blot analyses of the binding of M-ficolin to S. pneumoniae. (A) Binding of rM-ficolin to serotype 19B (dotted line), 19C (solid line), and 19F (dashed line) strains. (B) Inhibition of the binding of rM-ficolin to a serotype 19C strain by GlcNAc (dashed line) or glucose (solid line). Shaded curves represent rM-ficolin-treated 19C cells with no anti-M-ficolin antibody added (negative control). Isotype controls [IgG1(κ)] gave results identical to those represented by the shaded curves. Data are representative of two individual experiments. (C) Western blot analysis. M-ficolin bound to pneumococcal 19F, 19B, or 19C cells after incubation with NHS and washing was eluted with GlcNAc, and the eluates were examined by SDS-PAGE under nonreducing conditions, followed by blotting onto a nitrocellulose membrane and detection with a monoclonal anti-M-ficolin antibody. Data are representative of two individual experiments. Molecular markers are shown on the right.
For further analysis by Western blotting, we incubated formalin-fixed pneumococcal serotype 19F, 19B, and 19C cells with NHS, and we eluted proteins bound to the cells with GlcNAc. Western blotting of the GlcNAc eluates showed that two oligomeric forms of serum M-ficolin were bound to and released from 19B and 19C cells, whereas no M-ficolin was eluted from 19F cells (Fig. 5C). Neither MBL, H-ficoln, nor L-ficolin could be detected in the GlcNAc eluates by sensitive time-resolved immunofluorometric assays (TRIFMAs) (data not shown), confirming that these three serum PRMs do not bind to strains of the serotypes examined.
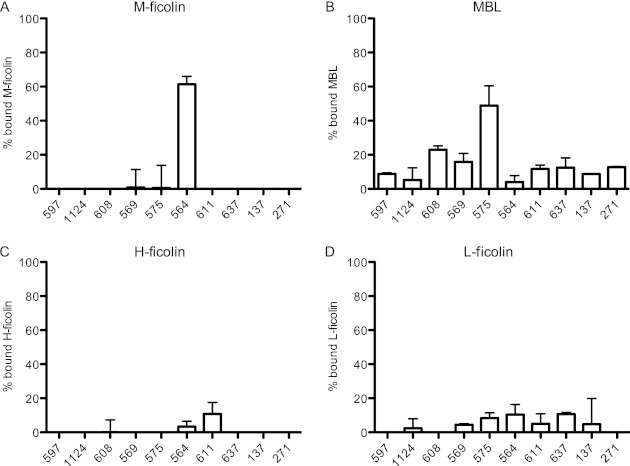
In a final series of experiments, we examined the binding of MBL and of H-, L-, and M-ficolins to 10 different strains of S. mitis, each with a unique polysaccharide (our own observations, to be published). S. mitis is a commensal bacterium closely related to S. pneumoniae (32). We incubated serum with the bacteria, centrifuged the samples, and tested for PRMs left in the supernatants. One of the strains (SK564) bound serum M-ficolin when incubated with NHS (Fig. 6A). MBL bound to another strain (SK575) (Fig. 6B), whereas H-ficolin (Fig. 6C) and L-ficolin (6D) only bound weakly to a few of the S. mitis strains examined.
Fig 6.
Binding of ficolins and MBL to S. mitis. The binding of the lectin pathway PRMs was assessed as described in the legend to Fig. 1. (A) M-ficolin; (B) MBL; (C) H-ficolin; (D) L-ficolin. The designations of the strains are given on the x axis. Data are representative of two individual experiments. Means and standard deviations are shown.
DISCUSSION
The innate immune system is essential for defense during invasive infections. A number of host PRMs have thus evolved to enable the recognition of constituents of bacteria, viruses, and fungi. The membrane-bound Toll-like receptors (TLRs) TLR2, TLR4, and TLR9, the intracellular nucleotide oligomerization domain (NOD)-like receptors NLRP3 and NOD2, and the intracellular DNA sensor AIM2 are all involved in the defense against S. pneumoniae (33). In contrast, the present report is focused on the study of soluble PRMs.
Most bacteria that cause invasive diseases, such as bacteremia and meningitis, are encapsulated. The polysaccharide capsules are hydrophilic and usually anionic and hide the pathogen from the immune system unless specific antibodies or other recognition molecules are present. Microbial carbohydrates have been suggested to be targets for PRM binding, and it has been proposed that recognition by PRMs may prevent infections caused by some pathogens. Few studies have examined the binding of PRMs to well-defined bacterial strains in a systematic way, and only limited information is available on the bacterial binding repertoire of M-ficolin (9, 10, 13). In the present study, we examined the binding of MBL and of H-, L-, and M-ficolins to 91 different serotypes of the opportunistic pathogen S. pneumoniae. Each of these serotypes expresses a unique capsular polysaccharide, and many of the chemical structures have been described previously (20). We also examined the binding of the four serum proteins to 10 strains of S. mitis, a commensal bacterium closely related to S. pneumoniae.
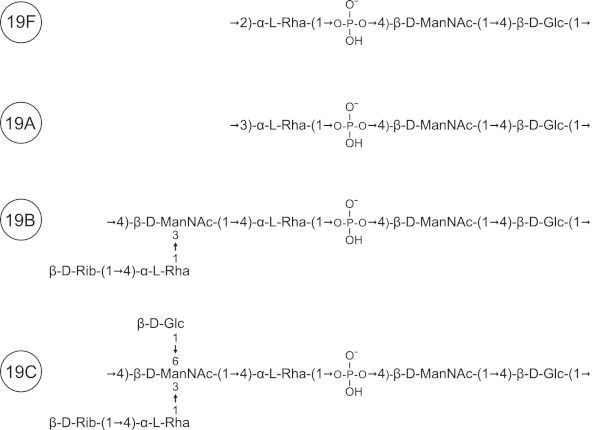
We demonstrate here that of the 91 strains of S. pneumoniae examined, M-ficolin binds selectively only to capsular polysaccharides of the two serotypes 19B and 19C and not to serotype 19F and 19A polysaccharides, which have chemical structures quite similar to those of 19B and 19C polysaccharides (group 19) (Fig. 7; also see below). Like glucose and rhamnose, N-acetylmannosamine residues are present in the structures of all four of the polysaccharides of group 19 (19F, -A, -B, and -C) (Fig. 7). The common N-acetylmannosamine residues are linked via a phosphodiester bond (Fig. 7), which may prevent the binding of M-ficolin to this subunit (i.e., no binding to serotype 19F and 19A polysaccharides). In contrast to those of serotypes 19F and 19A, the capsular polysaccharides of the two serotypes 19B and 19C each contain an extra N-acetylmannosamine residue linked only via glycoside linkages. This residue seems to be part of the molecular pattern responsible for the binding of M-ficolin, since none of the other residues present in the structures of the 19B and 19C polysaccharides (glucose, rhamnose, and ribose) were able to inhibit the binding of M-ficolin (Fig. 3; also see above).
Fig 7.
Capsular structures of S. pneumoniae serogroup 19. The repeating unit of the four capsular polysaccharides of serogroup 19 is shown. Serotype 19B and 19C polysaccharides differ from serotype 19A and 19F polysaccharides by having an extra ManNAc residue with a branched terminal d-ribose linked to l-rhamnose. In addition, the capsular polysaccharide of serotype 19C also has a d-glucose residue linked to the extra ManNAc residue.
N-Acetylmannosamine residues linked via glycoside linkages are also present in the structures of type 4 polysaccharide and in the four polysaccharides of serogroup 9 (9A, 9L, 9N, and 9V) (20). However, we observed no binding of M-ficolin to any of these five polysaccharides. This result, together with the conclusions given above, indicates that M-ficolin recognizes a molecular pattern consisting of more than a single monosaccharide residue. M-ficolin is a multivalent molecule with multiple FBG recognition domains, and the spacing between the binding sites influences the overall binding avidity, since the best-fitting pattern of structures must resemble this spacing.
Among the 10 different strains of S. mitis that we examined, a single strain specifically bound M-ficolin. This strain (SK564) possesses a surface polysaccharide with a chemical structure closely similar or identical to that of the 19C polysaccharide (our own observations, to be published).
A recent study of mice deficient in ficolin-A, the murine equivalent of human L-ficolin, and on mice deficient in the lectin pathway effector enzyme MASP-2 has demonstrated that the lectin pathway of complement activation is a critical component of the immune defense against S. pneumoniae infections, since both MASP-2- and ficolin-A-deficient mice are severely compromised when challenged intranasally with the S. pneumoniae serotype 2 strain D39 (4). Another recent study has also investigated mice deficient in ficolin-B (the murine ortholog to human M-ficolin), in addition to ficolin-A-deficient mice, by using a similar infection model (34). The binding of ficolin-B, as well as that of ficolin-A, to S. pneumoniae D39 was observed, and survival rates were significantly lower for both single and double ficolin knockout mice than for the wild type (34). In contrast, mice deficient in both murine MBL genes (i.e., MBL-A and MBL-C double-deficient mice) showed no increase in mortality rates (4). The reported binding of ficolin-B to S. pneumoniae serotype 2 strain D39 contrasts with our observation of no M-ficolin binding to an S. pneumoniae serotype 2 strain (Fig. 1). In the study by Ali et al., binding of CL-K1 to nine strains of S. pneumoniae was observed (4). Future challenge studies including mice lacking CL-K1 may reveal the influence of this PRM on S. pneumoniae infections.
We have documented that M-ficolin specifically recognizes only two (19B and 19C) of the more than 90 known pneumococcal serotypes, and M-ficolin may contribute to the prevention of infections caused by these two serotypes, although this has not been shown yet. It is, however, unlikely that M-ficolin is involved directly in protection against infections caused by the large majority of pneumococcal serotypes, which are equipped with capsules of various chemical structures to avoid recognition by the immune system and, as shown here, avoid recognition by M-ficolin.
ACKNOWLEDGMENTS
This work was supported by The Danish Council for Independent Research, Medical Sciences.
We thank Lisbeth Jensen, Mette L. G. Nikolajsen, and Herdis B. Johansen (all at the Institute of Biomedicine, Aarhus University, Aarhus, Denmark) for technical support and Charlotte Christie Petersen (FACS Core Facility, The Faculty of Health Sciences, Aarhus University, Aarhus, Denmark) for help with flow cytometry. We thank Rune T. Kidmose (Department of Molecular Biology and Genetics, Aarhus University, Aarhus, Denmark) for providing purified C4. Streptococcal strains were kindly provided by Mogens Kilian (Institute of Biomedicine, Aarhus University, Aarhus, Denmark).
Footnotes
Published ahead of print 26 November 2012
REFERENCES
- 1. Thiel S. 2007. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol. Immunol. 44:3875–3888 [DOI] [PubMed] [Google Scholar]
- 2. Keshi H, Sakamoto T, Kawai T, Ohtani K, Katoh T, Jang SJ, Motomura W, Yoshizaki T, Fukuda M, Koyama S, Fukuzawa J, Fukuoh A, Yoshida I, Suzuki Y, Wakamiya N. 2006. Identification and characterization of a novel human collectin CL-K1. Microbiol. Immunol. 50:1001–1013 [DOI] [PubMed] [Google Scholar]
- 3. Hansen S, Selman L, Palaniyar N, Ziegler K, Brandt J, Kliem A, Jonasson M, Skjoedt MO, Nielsen O, Hartshorn K, Jorgensen TJ, Skjodt K, Holmskov U. 2010. Collectin 11 (CL-11, CL-K1) is a MASP-1/3-associated plasma collectin with microbial-binding activity. J. Immunol. 185:6096–6104 [DOI] [PubMed] [Google Scholar]
- 4. Ali YM, Lynch NJ, Haleem KS, Fujita T, Endo Y, Hansen S, Holmskov U, Takahashi K, Stahl GL, Dudler T, Girija UV, Wallis R, Kadioglu A, Stover CM, Andrew PW, Schwaeble WJ. 2012. The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathog. 8:e1002793 doi:10.1371/journal.ppat.1002793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Endo Y, Sato Y, Matsushita M, Fujita T. 1996. Cloning and characterization of the human lectin P35 gene and its related gene. Genomics 36:515–521 [DOI] [PubMed] [Google Scholar]
- 6. Harumiya S, Takeda K, Sugiura T, Fukumoto Y, Tachikawa H, Miyazono K, Fujimoto D, Ichijo H. 1996. Characterization of ficolins as novel elastin-binding proteins and molecular cloning of human ficolin-1. J. Biochem. 120:745–751 [DOI] [PubMed] [Google Scholar]
- 7. Lu J, Tay PN, Kon OL, Reid KB. 1996. Human ficolin: cDNA cloning, demonstration of peripheral blood leucocytes as the major site of synthesis and assignment of the gene to chromosome 9. Biochem. J. 313(Part 2): 473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hummelshoj T, Fog LM, Madsen HO, Sim RB, Garred P. 2008. Comparative study of the human ficolins reveals unique features of Ficolin-3 (Hakata antigen). Mol. Immunol. 45:1623–1632 [DOI] [PubMed] [Google Scholar]
- 9. Teh C, Le Y, Lee SH, Lu J. 2000. M-ficolin is expressed on monocytes and is a lectin binding to N-acetyl-d-glucosamine and mediates monocyte adhesion and phagocytosis of Escherichia coli. Immunology 101:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Y, Endo Y, Iwaki D, Nakata M, Matsushita M, Wada I, Inoue K, Munakata M, Fujita T. 2005. Human M-ficolin is a secretory protein that activates the lectin complement pathway. J. Immunol. 175:3150–3156 [DOI] [PubMed] [Google Scholar]
- 11. Frederiksen PD, Thiel S, Larsen CB, Jensenius JC. 2005. M-ficolin, an innate immune defence molecule, binds patterns of acetyl groups and activates complement. Scand. J. Immunol. 62:462–473 [DOI] [PubMed] [Google Scholar]
- 12. Gout E, Garlatti V, Smith DF, Lacroix M, Dumestre-Perard C, Lunardi T, Martin L, Cesbron JY, Arlaud GJ, Gaboriaud C, Thielens NM. 2010. Carbohydrate recognition properties of human ficolins: glycan array screening reveals the sialic acid binding specificity of M-ficolin. J. Biol. Chem. 285:6612–6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kjaer TR, Hansen AG, Sorensen UB, Nielsen O, Thiel S, Jensenius JC. 2011. Investigations on the pattern recognition molecule M-ficolin: quantitative aspects of bacterial binding and leukocyte association. J. Leukoc. Biol. 90:425–437 [DOI] [PubMed] [Google Scholar]
- 14. Honoré C, Rorvig S, Hummelshoj T, Skjoedt MO, Borregaard N, Garred P. 2010. Tethering of Ficolin-1 to cell surfaces through recognition of sialic acid by the fibrinogen-like domain. J. Leukoc. Biol. 88:145–158 [DOI] [PubMed] [Google Scholar]
- 15. Zhang J, Yang L, Ang Z, Yoong SL, Tran TT, Anand GS, Tan NS, Ho B, Ding JL. 2010. Secreted M-ficolin anchors onto monocyte transmembrane G protein-coupled receptor 43 and cross talks with plasma C-reactive protein to mediate immune signaling and regulate host defense. J. Immunol. 185:6899–6910 [DOI] [PubMed] [Google Scholar]
- 16. Moreno-Amaral AN, Gout E, Danella-Polli C, Tabarin F, Lesavre P, Pereira-da-Silva G, Thielens NM, Halbwachs-Mecarelli L. 2012. M-ficolin and leukosialin (CD43): new partners in neutrophil adhesion. J. Leukoc. Biol. 91:469–474 [DOI] [PubMed] [Google Scholar]
- 17. Rørvig S, Honoré C, Larsson LI, Ohlsson S, Pedersen CC, Jacobsen LC, Cowland JB, Garred P, Borregaard N. 2009. Ficolin-1 is present in a highly mobilizable subset of human neutrophil granules and associates with the cell surface after stimulation with fMLP. J. Leukoc. Biol. 86:1439–1449 [DOI] [PubMed] [Google Scholar]
- 18. Schlapbach LJ, Kjaer TR, Thiel S, Mattmann M, Nelle M, Wagner BP, Ammann RA, Aebi C, Jensenius JC. 2012. M-ficolin concentrations in cord blood are related to circulating phagocytes and to early-onset sepsis. Pediatr. Res. 71:368–374 [DOI] [PubMed] [Google Scholar]
- 19. Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144–154 [DOI] [PubMed] [Google Scholar]
- 20. Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, Collins M, Donohoe K, Harris D, Murphy L, Quail MA, Samuel G, Skovsted IC, Kaltoft MS, Barrell B, Reeves PR, Parkhill J, Spratt BG. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:e31 doi:10.1371/journal.pgen.0020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calix JJ, Nahm MH. 2010. A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene. J. Infect. Dis. 202:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45:1225–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jin P, Kong F, Xiao M, Oftadeh S, Zhou F, Liu C, Russell F, Gilbert GL. 2009. First report of putative Streptococcus pneumoniae serotype 6D among nasopharyngeal isolates from Fijian children. J. Infect. Dis. 200:1375–1380 [DOI] [PubMed] [Google Scholar]
- 24. Krarup A, Sørensen UB, Matsushita M, Jensenius JC, Thiel S. 2005. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect. Immun. 73:1052–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zacho RM, Jensen L, Terp R, Jensenius JC, Thiel S. 2012. Studies of the pattern recognition molecule H-ficolin: specificity and purification. J. Biol. Chem. 287:8071–8081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frederiksen PD, Thiel S, Jensen L, Hansen AG, Matthiesen F, Jensenius JC. 2006. Quantification of mannan-binding lectin. J. Immunol. Methods 315:49–60 [DOI] [PubMed] [Google Scholar]
- 27. Wittenborn T, Thiel S, Jensen L, Nielsen HJ, Jensenius JC. 2010. Characteristics and biological variations of M-ficolin, a pattern recognition molecule, in plasma. J. Innate Immun. 2:167–180 [DOI] [PubMed] [Google Scholar]
- 28. Krarup A, Thiel S, Hansen A, Fujita T, Jensenius JC. 2004. L-ficolin is a pattern recognition molecule specific for acetyl groups. J. Biol. Chem. 279:47513–47519 [DOI] [PubMed] [Google Scholar]
- 29. Petersen SV, Thiel S, Jensen L, Steffensen R, Jensenius JC. 2001. An assay for the mannan-binding lectin pathway of complement activation. J. Immunol. Methods 257:107–116 [DOI] [PubMed] [Google Scholar]
- 30. Stengaard-Pedersen K, Thiel S, Gadjeva M, Moller-Kristensen M, Sorensen R, Jensen LT, Sjoholm AG, Fugger L, Jensenius JC. 2003. Inherited deficiency of mannan-binding lectin-associated serine protease 2. N. Engl. J. Med. 349:554–560 [DOI] [PubMed] [Google Scholar]
- 31. Kidmose RT, Laursen NS, Dobo J, Kjaer TR, Sirotkina S, Yatime L, Sottrup-Jensen L, Thiel S, Gal P, Andersen GR. 2012. Structural basis for activation of the complement system by component C4 cleavage. Proc. Natl. Acad. Sci. U. S. A. 109:15425–15430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kilian M, Poulsen K, Blomqvist T, Havarstein LS, Bek-Thomsen M, Tettelin H, Sorensen UB. 2008. Evolution of Streptococcus pneumoniae and its close commensal relatives. PLoS One 3:e2683 doi:10.1371/journal.pone.0002683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koppe U, Suttorp N, Opitz B. 2012. Recognition of Streptococcus pneumoniae by the innate immune system. Cell. Microbiol. 14:460–466 [DOI] [PubMed] [Google Scholar]
- 34. Endo Y, Takahashi M, Iwaki D, Ishida Y, Nakazawa N, Kodama T, Matsuzaka T, Kanno K, Liu Y, Tsuchiya K, Kawamura I, Ikawa M, Waguri S, Wada I, Matsushita M, Shwaeble WJ, Fujita T. 2012. Mice deficient in ficolin, a lectin complement pathway recognition molecule, are susceptible to Streptococcus pneumoniae infection. J. Immunol. 189:5860–5866 [DOI] [PubMed] [Google Scholar]