Abstract
Shigella flexneri is a Gram-negative intracellular pathogen that infects the intestinal epithelium and utilizes actin-based motility to spread from cell to cell. S. flexneri actin-based motility has been characterized in various cell lines, but studies in intestinal cells are limited. Here we characterized S. flexneri actin-based motility in HT-29 intestinal cells. In agreement with studies conducted in various cell lines, we showed that S. flexneri relies on neural Wiskott-Aldrich Syndrome protein (N-WASP) in HT-29 cells. We tested the potential role of various tyrosine kinases involved in N-WASP activation and uncovered a previously unappreciated role for Bruton's tyrosine kinase (Btk) in actin tail formation in intestinal cells. We showed that Btk depletion led to a decrease in N-WASP phosphorylation which affected N-WASP recruitment to the bacterial surface, decreased the number of bacteria displaying actin-based motility, and ultimately affected the efficiency of spread from cell to cell. Finally, we showed that the levels of N-WASP phosphorylation and Btk expression were increased in response to infection, which suggests that S. flexneri infection not only triggers the production of proinflammatory factors as previously described but also manipulates cellular processes required for dissemination in intestinal cells.
INTRODUCTION
Shigella flexneri is a Gram-negative pathogen that invades the colonic and rectal mucosa of humans, causing bacillary dysentery (1, 2). Immediately after internalization into intestinal cells, S. flexneri lyses the primary vacuole and escapes into the cytosol, where it induces actin polymerization at one bacterial pole. The force generated by actin polymerization propels the bacterium throughout the cytosol of infected cells (3). S. flexneri actin-based motility relies on an IcsA bacterial factor and a host factor, neural Wiskott-Aldrich Syndrome protein (N-WASP) (4–7). IcsA is secreted at bacterial poles (8, 9), where it recruits the nucleation-promoting factor N-WASP. The recruitment of N-WASP leads to the recruitment and activation of the actin nucleator, the Arp2/3 complex (6, 10).
WASP and N-WASP are multidomain proteins harboring an N-terminal small GTPase binding domain (GBD) which interacts with Cdc42 and a C-terminal verprolin central acidic (VCA) domain which interacts with the ARP2/3 complex (11). Under nonstimulating conditions, WASP/N-WASP are folded into an autoinhibitory conformation due to interactions between the N-terminal GBD and the C-terminal VCA domain (12). In vitro binding of the small GTPase Cdc42 to the GBD of N-WASP induces the release of the autoinhibited conformation, which allows binding of the VCA domain to the Arp2/3 complex and subsequent actin polymerization (13, 14). Cdc42 is essential for S. flexneri invasion into mammalian cells but not for actin-based motility (15, 16). Subsequent studies showed that the S. flexneri IcsA protein in fact mimics Cdc42 in vitro (6). It enhances the affinity of N-WASP for Arp2/3, leading to assembly of an IcsA–N-WASP–Arp2/3 complex which displays potent actin polymerization activity (6). Although the minimal complex required for actin polymerization contains IcsA, N-WASP, and Arp2/3 in vitro, in vivo studies revealed a requirement for type III secretion system-dependent recruitment of TOCA-1 to the bacterial surface in order to promote actin tail formation (17). That study indicated that unknown bacterial effectors are necessary for N-WASP recruitment to the bacterial surface in vivo.
Several tyrosine kinases have been shown to regulate N-WASP phosphorylation and activation during cell migration, neurite extension, and filopodium formation (18–20). The tyrosine residue targeted by these kinases for phosphorylation, tyrosine 256 (Y256) in N-WASP (Y291 in WASP), is located in the GBD of N-WASP (18, 21). It has been previously reported that phosphorylation of N-WASP/WASP by Src family kinases increases the basal activity of the protein toward Arp2/3 (18, 19). Moreover, the affinity of the GBD for the VCA domain was decreased when the GBD was phosphorylated (22). The role of N-WASP phosphorylation in Shigella actin tail formation is still unclear. An early report suggested that Abl kinases are required for S. flexneri actin tail formation and elongation (23). Additionally, mutations of the Abl phosphorylation sites on N-WASP (Y256F) impaired actin tail elongation. However, another report showed that S. flexneri actin tails remained largely unchanged when N-WASP phosphodefective or phosphomimic mutant proteins were expressed (24).
The vast majority of S. flexneri actin tail formation and intercellular motility studies have been conducted in nonintestinal cell lines such as HeLa cells and mouse fibroblasts (17, 23, 25). We sought to characterize the mechanism of actin tail formation in a relevant system for S. flexneri infection. Here, we used the HT-29 cell line as a powerful genetic system to investigate S. flexneri pathogenesis in intestinal cells. Our genetic studies conducted in intestinal cells uncovered a previously unappreciated role for Bruton's tyrosine kinase (Btk) in N-WASP activation and revealed N-WASP tyrosine phosphorylation and Btk expression as infection-regulated processes important for bacterial dissemination.
MATERIALS AND METHODS
Cell lines and bacterial strains.
HT-29 cells (ATCC) were cultured at 37°C with 5% CO2 in McCoy's 5A medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen). The wild-type (WT) Shigella flexneri strain and IcsA mutant strain used in this study are serotype 2a 2457T (26).
DNA constructs.
Stable cell lines were generated using pLB vector from Addgene (Addgene plasmid 11619) as described previously (27). N-WASP was originally cloned into the BclI and XhoI sites of pcDNA 4/TO 3X-Flag. 3X-Flag N-WASP was subsequently cloned into the AgeI and EcoRI sites of pLB vector. Btk was originally cloned into the BamHI sites of the pmTag red fluorescent protein (RFP) vector and subcloned into EcoRI and NheI sites of the pLB vector. Site-directed mutagenesis of N-WASP and Btk was performed using Pfu Turbo DNA polymerase (Stratagene) followed by DpnI (New England Biolabs [NEB]) digestion of the parental strain. The pcDNA 4/TO 3X-Flag vector was a gift from Craig Roy (Yale University). The pmTag RFP vector was previously described (28).
Bacterial infection.
S. flexneri strain 2457T was grown overnight in LB broth at 37°C with agitation. Twenty microliters of stationary-phase culture was used to inoculate 2 ml of LB, and the bacteria were grown to the exponential phase for approximately 3 h at 37°C. Cells were infected with S. flexneri 2457T expressing green fluorescent protein (GFP) under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter. Infection was initiated by centrifuging the plate at 1,000 rpm for 5 min, and internalization of the bacteria was allowed to proceed for 1 h at 37°C before gentamicin (50 μM final concentration) was added in order to kill the extracellular bacteria. Two hours before the infection was stopped, IPTG (4 mM final concentration) was added to the medium to induce GFP expression in intracellular bacteria. For actin tail and N-WASP recruitment quantification, infected cells were incubated at 37° for 4 h and fixed in phosphate-buffered saline (PBS)–4% formaldehyde at 4°C overnight before immunostaining was performed. For S. flexneri focus size analysis, infected cells were incubated at 37° for 8 h.
siRNA treatment and quantitative real-time PCR.
Cells were transfected by reverse transfection with DharmaFECT 1 and individual small interfering RNAs (siRNA) (D1, D2, D3, and D4; 50 nM final concentration) or a pool of the four silencing reagents (12.5 nM each; 50 nM total concentration) or with siRNA buffer alone (Mock) and incubated for 72 h in a 24-well plate format. Experiments for Btk kinase-depleted (KD) analysis were performed with a pool of silencing reagents, unless stated otherwise. For real-time PCR analysis, total RNA and first-strand cDNA synthesis was performed using SuperScript II reverse transcriptase (Invitrogen) and oligo(dT)12-18 primer (Invitrogen). mRNA levels were determined by quantitative real-time PCR using the Universal ProbeLibrary (Roche Biochemicals, Indianapolis, IN) and LightCycler 480 Probes Master (Roche Biochemicals, Indianapolis, IN). Thermal cycling was carried out using a Light Cycler 480 instrument (Roche Diagnostics) under the following conditions: 95°C for 5 min and 45 cycles of 95°C for 10 s and 60°C for 25 s.
Antibodies.
The primary antibodies used were anti-N-WASP (Cell Signaling) (1:500 for immunofluorescence assays [IF], 1:1,000 for Western blot analysis [WB]), anti-p-Tyr (Cell Signaling) (1:1,000 for WB), anti-Flag (Sigma) (1:1,000 for IF, 1:10,000 for WB), anti-p-Tyr-Btk (Cell Signaling) (1:1,000 for WB), anti-RFP (Evrogen) (1:1,000 for WB), Alexa Fluor 568 phalloidin (Invitrogen) (1:1,000 for IF), and Alexa Fluor 488 phalloidin (Invitrogen) (1:1,000 for IF) and were obtained commercially. For Western blots, horseradish peroxidase (HRP)-anti-mouse and -anti-rabbit (1:5,000) secondary antibodies were from Jackson Laboratories. For immunostaining, Alexa Fluor 594 anti-rabbit and Alexa Fluor 594 anti-mouse secondary antibodies were from Invitrogen.
Immunoprecipitation and immunoblotting.
HT-29 cells grown for 3 days after siRNA transfection were lysed in 200 μl lysis buffer (20 mM Tris-HCl [pH 7.6], 200 mM NaCl, 1% Triton X-100, 0.3 mM EDTA) with protease and phosphatase inhibitors. Cell extracts were centrifuged, and the detergent-soluble fraction was incubated with either anti-N-WASP or anti-Flag antibody and with protein G-Sepharose beads (GE Healthcare) overnight. These samples were analyzed by SDS-PAGE and immunoblotting with anti-phospho-Tyr antibody to detect and quantify phosphorylated N-WASP. The same membranes were blotted with an anti-N-WASP antibody or anti-Flag antibody for equal-pulldown confirmation.
Microscopy and data analysis.
Mock- and siRNA-treated HT-29 cells seeded onto glass coverslips were fixed in PBS containing 4% paraformaldehyde, and immunostaining was performed at room temperature. Coverslips were imaged using a TE 2000 microscope (Nikon) equipped with an Orca ER digital charge-coupled-device (CCD) camera (Hamamatsu), a motorized stage (Prior), motorized filter wheels (Sutter Instrument Company), and a 10× objective or a 60× objective (Nikon) mounted on a piezo focus drive system (Physik Instrumente). Image analysis for infectious focus size was conducted using the ImageJ software. For time-lapse video microscopy, mock- and siRNA-treated cells were grown on 35-mm-diameter imaging dishes (MatTek, Ashland, MA). At 3 h postinfection, Z-stacks were captured every 30 s on a Nikon TE2000E spinning disc confocal microscope. Bacteria were tracked individually in four dimensions using the tracking algorithm in the Volocity software package (PerkinElmer). The speed of bacteria undergoing motility (>0.01 μm/s) for at least 60 consecutive seconds was scored, and the period of sustained movement was recorded.
RESULTS
N-WASP is required for S. flexneri motility in HT-29 cells.
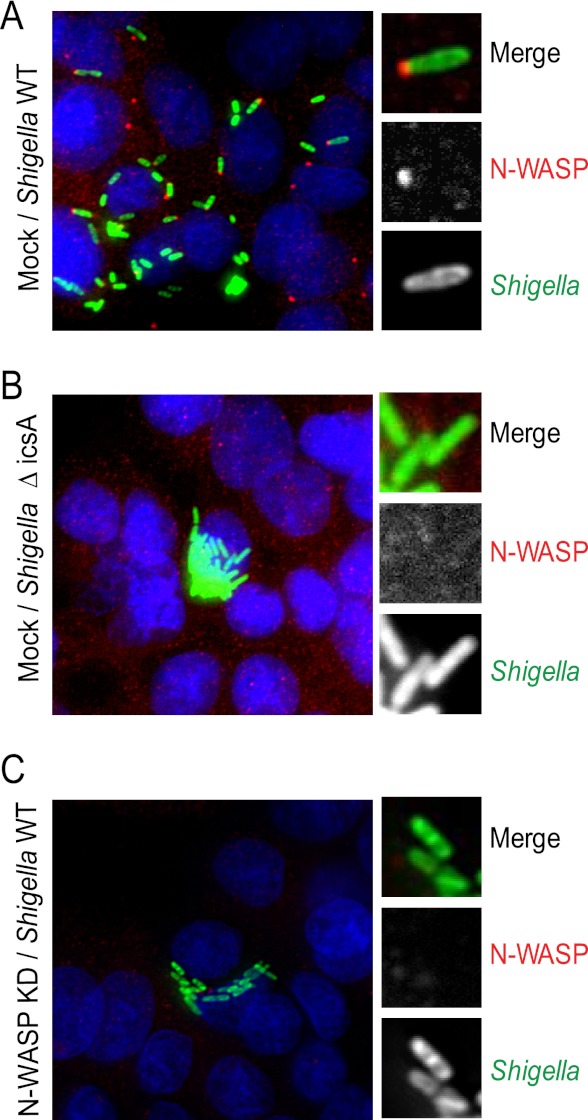
S. flexneri infects cells of the intestinal tract in vivo (29), but various nonintestinal cell lines have been shown to support S. flexneri infection in tissue culture systems (17, 23, 25). Thus, most studies on the cellular determinants supporting S. flexneri actin-based motility have been conducted in nonintestinal cell lines. We have recently screened various cell lines for their ability to support S. flexneri infection and identified the HT-29 cell line as a suitable model to support our genetic investigations (see below). HT-29 cells are human intestinal cells that form a epithelium-like monolayer of polarized cells in culture (see Fig. S1A, S1B, and S1C in the supplemental material). S. flexneri displayed actin-based motility in HT-29 cells, as demonstrated by the formation of actin tails (see Fig. S1D in the supplemental material), and spread from cell to cell, as demonstrated by the formation of characteristic infection foci (see Fig. S1D in the supplemental material). S. flexneri actin-based motility relies on the polar expression of the bacterial protein IcsA (5, 30). As expected, we found that the IcsA mutant strain did not form actin tails in HT-29 cells and that these nonmotile bacteria did not spread from cell to cell and grew as microcolonies (see Fig. S1E in the supplemental material). IcsA mediates the recruitment of N-WASP, which promotes the actin nucleation activity of the ARP2/3 complex at the bacterial surface and leads to actin tail formation at one pole of the bacteria (5, 31). Accordingly, we found that N-WASP was recruited to the pole of wild-type bacteria in HT-29 cells (Fig. 1A, Mock/Shigella WT). In contrast, N-WASP was not recruited to the bacterial surface in HT-29 cells infected with the IcsA mutant strain (Fig. 1B, Mock/Shigella ΔIcsA). To confirm the role of N-WASP in S. flexneri actin-based motility, we developed appropriate RNA interference (RNAi) procedures for silencing N-WASP expression in HT-29 cells (see Materials and Methods; see also Fig. S1G and S1H in the supplemental material). As expected, N-WASP was not detected at the bacterial pole in N-WASP-depleted cells (Fig. 1C, N-WASP KD/Shigella WT). Similar to the situation observed in mock-treated cells infected with the IcsA mutant (Fig. 1B, Mock/Shigella ΔIcsA), wild-type bacteria did not form actin tails and grew as microcolonies in N-WASP-depleted cells (see Fig. S1F in the supplemental material, N-WASP KD/Shigella WT). These results indicate that, as previously reported in various nonintestinal cell lines, S. flexneri actin-based motility relies on the IcsA-dependent recruitment of the nucleation-promoting factor N-WASP in the HT-29 intestinal cell line.
Fig 1.
Role of N-WASP and IcsA in S. flexneri motility in HT-29 cells. (A) Images show infection of HT-29 cells with wild-type GFP-expressing S. flexneri and N-WASP recruitment at one bacterial pole in mock-treated HT-29 cells. (B) A bacterial mutant for cell surface protein IcsA displays deficient N-WASP recruitment and cytosolic motility. (C) Infection with wild-type bacteria in cells depleted for N-WASP shows abolishment of bacterial motility. Merged images: green, Shigella; red, N-WASP; blue, DNA.
Bruton's tyrosine kinase plays a role in S. flexneri actin tail formation in HT-29 cells.
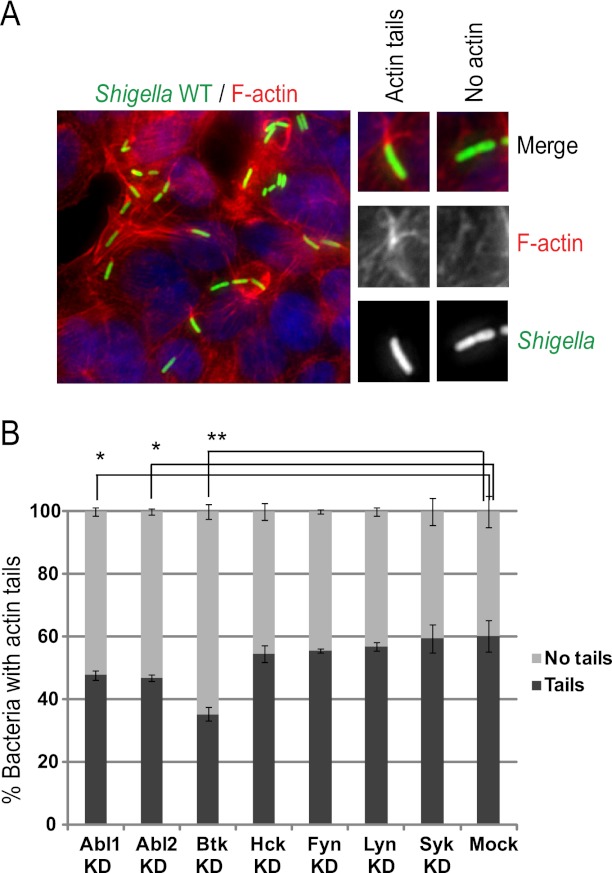
Multiple tyrosine kinases have been shown to regulate the nucleation-promoting activity of WASP/N-WASP family members through phosphorylation of the conserved tyrosine residue Y291 in WASP or Y256 in N-WASP (21, 32–34). A role for Abl1/Abl2-mediated N-WASP phosphorylation was previously reported for S. flexneri actin tail formation in cell lines of mouse embryonic fibroblasts (MEFs) derived from Abl1/Abl2-null mice (23). However, the role of N-WASP phosphorylation and the potential tyrosine kinase(s) involved in S. flexneri actin tail formation have not been investigated in intestinal cells. We addressed this question in HT-29 cells by silencing the expression of various tyrosine kinases formerly implicated in the phosphorylation of WASP/N-WASP, including Abl1, Abl2, Btk, Hck, Fyn, Lyn, and Syk. After 3 days of siRNA treatment, we infected the cells and quantified S. flexneri actin tail formation at 4 h postinfection (Fig. 2A). In mock-treated cells, 60% of the bacteria displayed actin tails in HT-29 cells (Fig. 2B, Mock). Of all the kinases tested, Btk depletion resulted in the most dramatic effect on Shigella actin tail formation, although the levels of knockdown efficiency were comparable for all kinases tested (see Fig. S2 in the supplemental material). We observed that only 35% of the bacteria formed actin tails in the Btk-depleted cells (Fig. 2B, Btk KD). The individual depletion of Abl1 or Abl2, previously reported to play a role in S. flexneri actin tail formation in MEFs, resulted in a marginal defect in actin tail formation in HT-29 cells. These results indicate that, in intestinal cells, Btk is a major tyrosine kinase involved in S. flexneri actin tail formation.
Fig 2.
Screen for tyrosine kinases involved in Shigella motility in HT-29 cells. (A) Images showing actin tails formed by S. flexneri in HT-29 cells. Merged images: green, Shigella; red, F-actin; blue, DNA. Right panels show representative images of scored bacteria with and without tails. (B) Quantification of scored actin tails as shown in panel A in mock-treated and tyrosine kinase-depleted cells. Values represent the means and standard deviations of the results of 3 independent experiments. The significance of the differences was confirmed by Student's t test. Asterisk (*), P < 0.05; double asterisks (**), P < 0.0025.
Validation of Btk-specific role in S. flexneri motility.
The Btk gene encodes the Bruton's tyrosine kinase, which was shown to play a crucial role in B-cell development (35, 36). To confirm that the Btk gene is also expressed in intestinal cells, we generated cDNA from total RNA extracted from HT-29 cells. We used specific Btk gene primers and successfully PCR amplified two intron-spanning DNA fragments corresponding to positions 1305 to 2025 and positions 482 to 1393 of Btk gene cDNA (see Fig. S3A and S3B in the supplemental material) and confirmed their identity by DNA sequencing (see Fig. S3C in the supplemental material). We next confirmed the functional relationship between the silencing of Btk expression and the observed actin tail formation defects. To this end, we tested four independent siRNA duplexes targeting Btk for their silencing efficiency and their ability to confer defects in S. flexneri actin tail formation. We observed a good correlation between silencing efficiency and the strength of the observed phenotypes. Strikingly, Btk-targeting duplexes D3 and D4 conferred the strongest actin tail formation defects (Fig. 3A, 37% bacteria with actin tails for D3 and 33% bacteria with actin tails for D4), which correlated with the strongest silencing efficiency (Fig. 3B, 25% Btk mRNA expression for D3 and 22% Btk mRNA expression for D4). To confirm the specific role of Btk, we engineered (Fig. 3C) and validated (see Fig. S4 in the supplemental material) stable HT-29 cell lines expressing an RFP-tagged version of wild-type Btk or an RFP-tagged version of Btk resistant to siRNA treatment. Quantification of actin tails revealed that overexpression of the siRNA-resistant form of Btk efficiently rescued the actin tail formation defect conferred by siRNA treatment (Fig. 3D). We also showed that treatment of HT-29 cells with ibrutinib (1 μM), a specific Btk inhibitor (37), reduced the autophosphorylation of Btk at tyrosine 223 (Y223) as previously reported (38), which correlated with a decrease in the size of S. flexneri infectious foci (see Fig. S5A, S5B, and S5C in the supplemental material). Finally, we showed that Btk depletion had no effect on the survival and growth of S. flexneri (see Fig. S5D in the supplemental material). These results unambiguously demonstrate that Btk is specifically involved in S. flexneri actin tail formation in HT-29 cells.
Fig 3.

Btk validation procedure. (A) Cells were transfected with four individual siRNA duplexes (D1, D2, D3, and D4) targeting Btk. Three days post-siRNA transfection, the cells were infected with S. flexneri and actin tail formation was scored. (B) Real-time PCR analysis showing silencing efficiency compared to mock-treated cell results (percent mRNA expression) for individual siRNA duplexes 3 days post-siRNA transfection. Values represent the means and standard deviations of the results of 3 independent experiments. The significance of the differences was confirmed by Student's t test for actin tail formation quantification (**, P < 0.0025) and silencing efficiency (**, P < 0.0025) for all siRNA duplexes. (C) HT-29 cells stably expressing an RFP-Btk siRNA D4-resistant mutant were infected with S. flexneri for quantification of formation of actin tails. Merged images: blue, Shigella; red, Btk; green, F-actin. (D) The presence of bacteria with actin tails was scored in mock-treated or siRNA (D4)-treated HT-29 cells overexpressing RFP-Btk WT or in the siRNA D4-resistant mutant (RFP-Btk mut). Values represent the means and standard deviations of the results of 3 independent experiments. The significance of the differences was confirmed by Student's t test for actin tail formation quantification (**, P < 0.0025).
Btk depletion affects cytosolic motility and dissemination.
To further investigate the role of Btk in actin tail formation, we analyzed S. flexneri cytosolic motility by time-lapse video microscopy. We found that the velocity of motile bacteria in the cytosol of Btk-depleted cells was comparable to the velocity recorded in mock-treated cells (Fig. 4A, top panel) and that the duration of movement for the motile bacteria was not affected in Btk-depleted cells (Fig. 4A, bottom panel). However, we observed a significant decrease in the number of motile bacteria in Btk-depleted cells (Fig. 4B, Mock versus Btk KD). These observations are consistent with our results showing a decrease in the number of bacteria displaying actin tails in Btk-depleted cells (Fig. 2B). These results suggest an important role for Btk in the actin tail formation process. We further determined the impact of Btk depletion on bacterial dissemination by quantifying the size of the infectious foci formed in mock-treated and Btk-depleted cells. We observed a 30% reduction in the size of foci formed in Btk-depleted cells compared to the foci formed in mock-transfected cells (Fig. 4C and 4D, average focus area, Mock versus Btk KD). We conclude that Btk contributes to an efficient actin tail formation process and is therefore important for S. flexneri overall dissemination in intestinal cells.
Fig 4.
S. flexneri cytosolic motility and spread from cell to cell in Btk-depleted cells. (A) Measurements of cytosolic velocity and duration of movement in mock-treated and Btk-depleted cells. The tracking features of Volocity software were used to score the motile bacteria and calculate their velocity. The Mann-Whitney test was performed to determine the statistical significance of the data determined for the velocity (P = 0.7323) and the duration of movement (P = 0.9181). (B) Scoring of motile GFP bacteria within the cytosol of mock-treated cells and Btk-depleted cells during 15 min of time-lapse microscopy. (C) Representative images of S. flexneri foci on a monolayer of mock-treated and Btk-depleted HT-29 cells after an 8 h infection. (D) Mean area of S. flexneri infectious foci as determined using the ImageJ software and normalized to the control transfected cells. Values represent the means and standard deviations of the results of 3 independent experiments. The significance of the differences was confirmed by Student's t test for motile bacteria (B) (**, P < 0.0025) and mean area of the foci (D) (**, P < 0.0025).
Overexpression of Btk positively modulates actin tail formation and bacterial dissemination.
To further confirm the involvement of Btk in S. flexneri actin tail formation, we infected HT-29 and HT-29–RFP-Btk cells with S. flexneri and analyzed actin tail formation at 4 h postinfection (Fig. 5A). We observed a significant increase in bacteria displaying actin tails in HT-29–RFP-Btk cells compared to HT-29 cells (Fig. 5B, 71% bacteria with actin tails in Btk RFP cells versus 58% of bacteria with actin tails in regular HT-29 cells). We also examined bacterial dissemination at 8 h postinfection (Fig. 5C, Btk RFP versus HT-29). We determined the sizes of the infection foci in both cell lines and observed a significant size increase for HT-29–RFP-Btk cells (Fig. 5D, Btk RFP versus HT-29). These results showing an increase in actin tail formation in cells overexpressing Btk are in agreement with our previous results showing a decrease in actin tail formation in Btk-depleted cells (Fig. 2B). Thus, the efficiency of actin tail formation and spread from cell to cell relies on Btk expression levels in intestinal cells.
Fig 5.
Btk overexpression enhances bacterial motility and cell-to-cell spread. (A) HT-29 cells stably expressing RFP-Btk were infected with S. flexneri for quantification of actin tail formation. Merged images: blue, Shigella; red, Btk; green, F-actin. (B) The presence of bacteria with actin tails was scored in regular HT-29 cells and HT-29 cells overexpressing Btk. (C) Representative images of S. flexneri foci in a monolayer of regular HT-29 cells and RFP-Btk-expressing HT-29 cells after an 8-h infection. (D) Mean area of S. flexneri infectious foci as determined using ImageJ software and compared to the regular HT-29 cells. Values represent the means and standard deviations of the results of 3 independent experiments. The significance of the differences was confirmed by Student's t test for bacteria with actin tails (B) (**, P < 0.0025) and mean area of the foci (D) (**, P < 0.0025).
Btk regulates N-WASP phosphorylation.
Since a role for Btk in WASP phosphorylation on tyrosine residue Y291 in hematopoietic cells was previously reported (21, 32, 33), we tested the hypothesis that, in intestinal cells, Btk may phosphorylate N-WASP on tyrosine residue Y256 (the functional equivalent of Y291 in WASP). To this end, we first analyzed the levels of endogenous N-WASP tyrosine phosphorylation in mock-treated and Btk-depleted cells. In agreement with the possibility that Btk may mediate N-WASP phosphorylation, we found that the level of N-WASP tyrosine phosphorylation was 40% lower in Btk-depleted cells than in mock-treated cells (Fig. 6A and B, IB: pTyr, Mock versus Btk KD). To confirm that N-WASP is phosphorylated on tyrosine residue Y256, we engineered HT-29 cell lines expressing a Flag-tagged version of N-WASP (3X-Flag N-WASP) or a Flag-tagged version of N-WASP displaying a mutation in tyrosine residue Y256 (3X-Flag N-WASP–Y256F). Pulldown experiments and Western blot analyses indicated that 3X-Flag N-WASP was tyrosine phosphorylated in HT-29 cells, whereas 3X-Flag N-WASP–Y256F was not (Fig. 6C, IP: p-Tyr, 3X-Flag N-WASP versus 3X-Flag N-WASP–Y256F). Altogether, these results show that Btk mediates N-WASP phosphorylation on tyrosine residue Y256 in HT-29 cells. Since Shigella cytosolic motility relies on the recruitment of N-WASP to the bacterial pole and since we observed fewer bacteria displaying actin tails in Btk-depleted cells, we tested the hypothesis that N-WASP phosphorylation modulates N-WASP recruitment to the bacteria. To this end, we quantified N-WASP recruitment to the bacterial pole in cells expressing 3X-Flag N-WASP or 3X-Flag N-WASP Y256F (Fig. 6D, 3X-Flag N-WASP). In agreement with our results showing that 60% of the bacteria displayed an actin tail (Fig. 2B), we found that 60% of the bacteria were positive for 3X-Flag N-WASP in HT-29 cells (Fig. 6E). In contrast, we observed that only 36% of the bacteria were positive for 3X-Flag N-WASP–Y256F, indicating that N-WASP phosphorylation modulates N-WASP recruitment to the bacterial pole. We also analyzed endogenous N-WASP recruitment in mock-treated and Btk-depleted cells (see Fig. S6A and S6B in the supplemental material). We found that 62% of the bacteria were positive for N-WASP recruitment in mock-treated cells whereas only 39% of the bacteria were positive for N-WASP recruitment in Btk-depleted cells (see Fig. S6A and S6B in the supplemental material, Mock versus Btk KD). We finally explored the correlation between N-WASP recruitment and the ability to form actin tails for two individual siRNA duplexes targeting Btk (see Fig. S6C in the supplemental material). We found that the percentage of bacteria recruiting N-WASP and forming an actin tail was decreased for both siRNA duplexes targeting Btk. Altogether, these results support the notion that, in intestinal cells, Btk-mediated phosphorylation of N-WASP modulates its recruitment to the bacterial pole, which is required for initiating actin tail formation.
Fig 6.
Role of Btk in N-WASP phosphorylation in HT-29 cells. (A) N-WASP phosphorylation in mock-treated and Btk-depleted cells. Cell lysates were immunoprecipitated (IP) with an antibody specific for N-WASP, and levels of phospho–N-WASP and total N-WASP were determined by immunoblotting (IB) with anti-phosphotyrosine antibody (p-Tyr-100) and anti-N-WASP antibody, respectively. N-WASP total levels in the whole-cell lysate (Input) were determined with anti-N-WASP antibody. (B) N-WASP phosphorylation levels were quantified in mock-treated and Btk-depleted cells using ImageJ software analysis and normalized to the total N-WASP levels. The N-WASP phosphorylation level in Btk-depleted cells was calculated as a percentage of the level of mock-treated cells. Values represent the means and standard deviations of the results of 3 independent experiments. The significance of the differences was confirmed by Student's t test (*, P < 0.05). (C) 3X-Flag N-WASP and 3X-Flag N-WASP Y256F phosphorylation levels were determined by immunoprecipitation. Lysates from stable 3X-Flag N-WASP-expressing HT29 cells were immunoprecipitated with an antibody specific for Flag, and total levels of phospho-3X-Flag N-WASP were determined by immunoblotting with anti-phosphotyrosine antibody (p-Tyr-100). Flag-tagged N-WASP total levels in the whole-cell lysate (Input) were determined with anti-Flag antibody. (D) HT-29 cells overexpressing 3X-Flag-tagged N-WASP were infected with wild-type GFP-expressing S. flexneri, and 3X-Flag N-WASP recruitment at one bacterial pole was analyzed 4 h postinfection with an anti-Flag antibody. Merged images: green, Shigella; red, 3X-Flag N-WASP. Right panels show representative images of scored bacteria with and without N-WASP. (E) Quantification of bacteria that recruited 3X-Flag N-WASP or 3X-Flag N-WASP Y256F mutant as shown in panel D. Values represent the means and standard deviations of the results of 3 independent experiments. The significance of the differences was confirmed by Student's t test for 3X-Flag N-WASP recruitment (E) (**, P < 0.0025).
N-WASP phosphorylation and Btk expression are modulated in response to S. flexneri infection.
S. flexneri infection in various cell types leads to profound reprogramming of infected cells, including induction of production of proinflammatory factors (39). As expected, such reprogramming also occurs in HT-29 cells in response to S. flexneri infection, as exemplified by strong induction of interleukin-8 (IL-8) expression (Fig. 7A). We tested the possibility that reprogramming of infected HT-29 cells may modulate the activity of cellular factors involved in S. flexneri cytosolic motility, such as N-WASP. To this end, we compared the levels of N-WASP phosphorylation in uninfected and infected cells. Interestingly, we observed a significant increase in N-WASP phosphorylation in mock-treated cells in response to infection (Fig. 7B and C, Mock, infected versus uninfected). In contrast, this increase in N-WASP phosphorylation was not observed in Btk-depleted cells (Fig. 7B and C, Btk KD, uninfected versus infected). This observation suggested that the increase in N-WASP phosphorylation could reflect an increase in Btk activity in response to infection. Accordingly, we found that Btk transcription was significantly increased in HT-29 cells in response to S. flexneri infection (Fig. 7D). Thus, in addition to the expression of proinflammatory factors, reprogramming of infected cells modulates the activity of factors involved S. flexneri cytosolic motility, including Btk expression and Btk-dependent N-WASP phosphorylation.
Fig 7.
N-WASP phosphorylation and Btk expression are modulated in response to S. flexneri infection. (A) IL-8 mRNA levels were determined by real-time PCR and showed a more than 80-fold increase in infected HT-29 cells (8 h) compared to uninfected cells. (B) N-WASP phosphorylation in mock-treated and Btk-depleted cells in S. flexneri-infected and uninfected cells. Cell lysates were immunoprecipitated with an antibody specific for N-WASP, and levels of phospho–N-WASP and total N-WASP were determined by immunoblotting with anti-phosphotyrosine antibody (p-Tyr-100) and anti-N-WASP antibody. N-WASP total levels in the whole-cell lysate (Input) were determined with anti-N-WASP antibody. (C) N-WASP phosphorylation levels were quantified using ImageJ software analysis and normalized to the total N-WASP levels. N-WASP phosphorylation levels were calculated as a percentage of mock-treated and infected-cell levels. Values represent the means and standard deviations of the results of 3 independent experiments. The significance of the differences was confirmed by Student's t test (*, P < 0.05). (D) Btk mRNA levels were determined by real-time PCR and showed a 3-fold increase in HT-29 infected cells (8 h) compared to uninfected-cell levels.
DISCUSSION
The mechanisms underlying S. flexneri actin tail formation have been extensively studied in various cell lines, including HeLa cells and mouse fibroblasts (17, 23, 25). However, studies in cell lines that recapitulate the properties of the intestinal epithelium are limited. Here we used the HT-29 cell line to characterize N-WASP-dependent actin-based motility of S. flexneri in polarized intestinal cells. Since the activity of N-WASP is regulated by tyrosine phosphorylation, we sought to determine the tyrosine kinase(s) potentially involved in regulating N-WASP activity in HT-29 cells. We found that Btk depletion led to a striking decrease in the number of bacteria displaying actin-based motility in HT-29 cells. The effect on actin tail formation was matched by a decrease in the number of bacteria displaying N-WASP recruitment. We also showed that the level of N-WASP phosphorylation was modulated in response to infection in a Btk-dependent manner. Altogether, our results support the notion that, in HT-29 intestinal cells, S. flexneri actin-based motility is facilitated by the Btk-mediated phosphorylation of N-WASP on Y256, which supports N-WASP recruitment to the bacterial surface and the efficiency of actin tail formation. We discuss the implications of these findings below.
Previous studies conducted in mouse embryonic fibroblasts (MEFs) had established a role for Abl1/Abl2 in S. flexneri actin-based motility (23). Our results in HT-29 intestinal cells differ from those previous results and instead point to a critical role for Btk. Whereas earlier studies conducted in MEFs proposed a role for N-WASP phosphorylation not only in regulating the initiation of actin-based motility but also in modulating the velocity of motile bacteria, our studies showed that the velocity or the duration of movement of motile bacteria in the cytosol of Btk-depleted cells was comparable to the velocity recorded in mock-treated cells. Our findings are, however, in agreement with those of another study reporting wild-type velocity of motile bacteria in fibroblasts expressing phosphomimic (Y256E) or phosphodefective (Y256F) N-WASP mutants (24). Together with the latter study, our work thus indicates that N-WASP phosphorylation facilitates the efficiency of actin-based motility but plays little role in the velocity of motile bacteria.
The exact role of tyrosine phosphorylation in the activation of WASP/N-WASP family members has been controversial. N-WASP activation requires unfolding of its autoinhibited conformation in which the autoinhibitory N-terminal GBD binds the C-terminal VCA domain displaying nucleation-promoting activity. Since the tyrosine residue targeted for phosphorylation is masked in the autoinhibited conformation of N-WASP, it was suggested that binding of Cdc42 to the GBD stabilizes the unfolded conformation and allows for optimal tyrosine phosphorylation (22, 40). However, other studies showed that the introduction of a negative charge into the hydrophobic core of the GBD through phosphorylation of Y291 in WASP, or Y256 in N-WASP, destabilizes the autoinhibited conformation and causes a conformational change that activates WASP/N-WASP, independently of Cdc42 (19). The N-WASP-dependent actin-based motility of S. flexneri does not rely on Cdc42 (15, 16). In addition, experiments using N-WASP-WASP chimeras showed that interfering with the autoinhibited conformation of N-WASP increased its affinity for IcsA in vitro and in vivo (31). Altogether, those experiments as well as the evidence presented here support a model in which Y256 phosphorylation stabilizes the unfolded conformation of N-WASP in the cytosol of infected cells, which facilitates its recruitment to the bacterial surface through interaction with IcsA. Incomplete depletion of Btk may explain why only a fraction of cytosolic bacteria was affected by Btk silencing. This might also be the case for the absence of an effect on bacteria velocity. Alternatively, we cannot exclude the possibility that other kinases, including Abl1 and Abl2, might play a role in actin tail formation in Btk-depleted cells.
The role of Btk in the regulation of WASP family members has been essentially documented in hematopoietic cells. Btk is central to B-cell development and differentiation, as well as B-cell receptor signaling and NF-κB activation (41). Here, we demonstrate that Bkt is also expressed in intestinal HT-29 cells and is required for N-WASP phosphorylation. To our knowledge, this is the first report of a role for Btk-mediated phosphorylation of N-WASP in a nonhematopoietic cell type. Interestingly, we observed a striking increase in Btk-dependent N-WASP phosphorylation upon S. flexneri infection, suggesting that the infection process modulates Btk activity. In agreement with this assumption, we observed a significant increase in Btk transcription in response to infection. Since S. flexneri infection leads to NOD1/NOD2-dependent activation of NF-κB signaling (42) and since Btk transcription has been shown to rely on NF-κB in B cells (43), we speculate that S. flexneri infection modulates Btk transcription through NF-κB signaling in HT-29 intestinal cells. We note, however, that, in addition to this transcriptional level of regulation, we cannot exclude the possibility of a potential modulation of Btk activity at the posttranscriptional level, as previously shown (38, 44). Altogether, our data suggest that the infection process not only leads to a cellular response geared toward production of immune factors but also leads to modulation of cellular processes essential to bacterial dissemination, including N-WASP phosphorylation and Btk expression.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Agaisse laboratory for critical discussion and comments on the manuscript.
This work was supported by the Anna Fuller fellowship agency (A.-M.D.) and National Institutes of Health grants R21-AI094228 and R01-AI073904 (H.A.).
Footnotes
Published ahead of print 10 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00853-12.
REFERENCES
- 1. Sansonetti PJ. 1998. Molecular and cellular mechanisms of invasion of the intestinal barrier by enteric pathogens. The paradigm of Shigella. Folia Microbiol. (Praha) 43:239–246 [DOI] [PubMed] [Google Scholar]
- 2. Sansonetti PJ. 2006. The bacterial weaponry: lessons from Shigella. Ann. N. Y. Acad. Sci. 1072:307–312 [DOI] [PubMed] [Google Scholar]
- 3. Gouin E, Welch MD, Cossart P. 2005. Actin-based motility of intracellular pathogens. Curr. Opin. Microbiol. 8:35–45 [DOI] [PubMed] [Google Scholar]
- 4. Lommel S, Benesch S, Rottner K, Franz T, Wehland J, Kuhn R. 2001. Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep. 2:850–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suzuki T, Miki H, Takenawa T, Sasakawa C. 1998. Neural Wiskott-Aldrich syndrome protein is implicated in the actin-based motility of Shigella flexneri. EMBO J. 17:2767–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, Carlier MF. 1999. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146:1319–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldberg MB, Theriot JA. 1995. Shigella flexneri surface protein IcsA is sufficient to direct actin-based motility. Proc. Natl. Acad. Sci. U. S. A. 92:6572–6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldberg MB, Theriot JA, Sansonetti PJ. 1994. Regulation of surface presentation of IcsA, a Shigella protein essential to intracellular movement and spread, is growth phase dependent. Infect. Immun. 62:5664–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldberg MB, Barzu O, Parsot C, Sansonetti PJ. 1993. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. Infect. Agents Dis. 2:210–211 [PubMed] [Google Scholar]
- 10. Cossart P. 2000. Actin-based motility of pathogens: the Arp2/3 complex is a central player. Cell Microbiol. 2:195–205 [DOI] [PubMed] [Google Scholar]
- 11. Pollitt AY, Insall RH. 2009. WASP and SCAR/WAVE proteins: the drivers of actin assembly. J. Cell Sci. 122:2575–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. 2000. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 404:151–158 [DOI] [PubMed] [Google Scholar]
- 13. Rohatgi R, Ho HY, Kirschner MW. 2000. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J. Cell Biol. 150:1299–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. 1999. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97:221–231 [DOI] [PubMed] [Google Scholar]
- 15. Mounier J, Laurent V, Hall A, Fort P, Carlier MF, Sansonetti PJ, Egile C. 1999. Rho family GTPases control entry of Shigella flexneri into epithelial cells but not intracellular motility. J. Cell Sci. 112(Pt 13):2069–2080 [DOI] [PubMed] [Google Scholar]
- 16. Shibata T, Takeshima F, Chen F, Alt FW, Snapper SB. 2002. Cdc42 facilitates invasion but not the actin-based motility of Shigella. Curr. Biol. 12:341–345 [DOI] [PubMed] [Google Scholar]
- 17. Leung Y, Ally S, Goldberg MB. 2008. Bacterial actin assembly requires toca-1 to relieve N-wasp autoinhibition. Cell Host Microbe 3:39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cory GO, Garg R, Cramer R, Ridley AJ. 2002. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott-Aldrich Syndrome protein. J. Biol. Chem. 277:45115–45121 [DOI] [PubMed] [Google Scholar]
- 19. Suetsugu S, Hattori M, Miki H, Tezuka T, Yamamoto T, Mikoshiba K, Takenawa T. 2002. Sustained activation of N-WASP through phosphorylation is essential for neurite extension. Dev. Cell 3:645–658 [DOI] [PubMed] [Google Scholar]
- 20. Wu X, Suetsugu S, Cooper LA, Takenawa T, Guan JL. 2004. Focal adhesion kinase regulation of N-WASP subcellular localization and function. J. Biol. Chem. 279:9565–9576 [DOI] [PubMed] [Google Scholar]
- 21. Baba Y, Nonoyama S, Matsushita M, Yamadori T, Hashimoto S, Imai K, Arai S, Kunikata T, Kurimoto M, Kurosaki T, Ochs HD, Yata J, Kishimoto T, Tsukada S. 1999. Involvement of Wiskott-Aldrich syndrome protein in B-cell cytoplasmic tyrosine kinase pathway. Blood 93:2003–2012 [PubMed] [Google Scholar]
- 22. Torres E, Rosen MK. 2003. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol. Cell 11:1215–1227 [DOI] [PubMed] [Google Scholar]
- 23. Burton EA, Oliver TN, Pendergast AM. 2005. Abl kinases regulate actin comet tail elongation via an N-WASP-dependent pathway. Mol. Cell. Biol. 25:8834–8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adamovich DA, Nakamura F, Worth A, Burns S, Thrasher AJ, Hartwig JH, Snapper SB. 2009. Activating mutations of N-WASP alter Shigella pathogenesis. Biochem. Biophys. Res. Commun. 384:284–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heindl JE, Saran I, Yi CR, Lesser CF, Goldberg MB. 2010. Requirement for formin-induced actin polymerization during spread of Shigella flexneri. Infect. Immun. 78:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Labrec EH, Schneider H, Magnani TJ, Formal SB. 1964. Epithelial cell penetration as an essential step in the pathogenesis of bacillary dysentery. J. Bacteriol. 88:1503–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kissler S, Stern P, Takahashi K, Hunter K, Peterson LB, Wicker LS. 2006. In vivo RNA interference demonstrates a role for Nramp1 in modifying susceptibility to type 1 diabetes. Nat. Genet. 38:479–483 [DOI] [PubMed] [Google Scholar]
- 28. Counihan NA, Rawlinson SM, Lindenbach BD. 2011. Trafficking of hepatitis C virus core protein during virus particle assembly. PLoS Pathog. 7:e1002302 doi:10.1371/journal.ppat.1002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashida H, Ogawa M, Mimuro H, Kobayashi T, Sanada T, Sasakawa C. 2011. Shigella are versatile mucosal pathogens that circumvent the host innate immune system. Curr. Opin. Immunol. 23:448–455 [DOI] [PubMed] [Google Scholar]
- 30. Bernardini ML, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti PJ. 1989. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. U. S. A. 86:3867–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzuki T, Mimuro H, Suetsugu S, Miki H, Takenawa T, Sasakawa C. 2002. Neural Wiskott-Aldrich syndrome protein (N-WASP) is the specific ligand for Shigella VirG among the WASP family and determines the host cell type allowing actin-based spreading. Cell Microbiol. 4:223–233 [DOI] [PubMed] [Google Scholar]
- 32. Guinamard R, Aspenstrom P, Fougereau M, Chavrier P, Guillemot JC. 1998. Tyrosine phosphorylation of the Wiskott-Aldrich syndrome protein by Lyn and Btk is regulated by CDC42. FEBS Lett. 434:431–436 [DOI] [PubMed] [Google Scholar]
- 33. Oda A, Ochs HD, Druker BJ, Ozaki K, Watanabe C, Handa M, Miyakawa Y, Ikeda Y. 1998. Collagen induces tyrosine phosphorylation of Wiskott-Aldrich syndrome protein in human platelets. Blood 92:1852–1858 [PubMed] [Google Scholar]
- 34. Badour K, Zhang J, Shi F, Leng Y, Collins M, Siminovitch KA. 2004. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J. Exp. Med. 199:99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohamed AJ, Yu L, Backesjo CM, Vargas L, Faryal R, Aints A, Christensson B, Berglof A, Vihinen M, Nore BF, Smith CI. 2009. Bruton's tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol. Rev. 228:58–73 [DOI] [PubMed] [Google Scholar]
- 36. Maas A, Hendriks RW. 2001. Role of Bruton's tyrosine kinase in B cell development. Dev. Immunol. 8:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. 2010. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. U. S. A. 107:13075–13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rawlings DJ, Scharenberg AM, Park H, Wahl MI, Lin S, Kato RM, Fluckiger AC, Witte ON, Kinet JP. 1996. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science 271:822–825 [DOI] [PubMed] [Google Scholar]
- 39. Ogawa M, Handa Y, Ashida H, Suzuki M, Sasakawa C. 2008. The versatility of Shigella effectors. Nat. Rev. Microbiol. 6:11–16 [DOI] [PubMed] [Google Scholar]
- 40. Torres E, Rosen MK. 2006. Protein-tyrosine kinase and GTPase signals cooperate to phosphorylate and activate Wiskott-Aldrich syndrome protein (WASP)/neuronal WASP. J. Biol. Chem. 281:3513–3520 [DOI] [PubMed] [Google Scholar]
- 41. Khan WN. 2001. Regulation of B lymphocyte development and activation by Bruton's tyrosine kinase. Immunol. Res. 23:147–156 [DOI] [PubMed] [Google Scholar]
- 42. Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, Philpott DJ. 2001. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu L, Mohamed AJ, Simonson OE, Vargas L, Blomberg KE, Bjorkstrand B, Arteaga HJ, Nore BF, Smith CI. 2008. Proteasome-dependent autoregulation of Bruton tyrosine kinase (Btk) promoter via NF-kappaB. Blood 111:4617–4626 [DOI] [PubMed] [Google Scholar]
- 44. Park H, Wahl MI, Afar DE, Turck CW, Rawlings DJ, Tam C, Scharenberg AM, Kinet JP, Witte ON. 1996. Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity 4:515–525 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.