Abstract
Acinetobacter baumannii is a leading cause of multidrug-resistant infections worldwide. This organism poses a particular challenge due to its ability to acquire resistance to new antibiotics through adaptation or mutation. This study was undertaken to determine the mechanisms governing the adaptability of A. baumannii to the antibiotic colistin. Screening of a transposon mutant library identified over 30 genes involved in inducible colistin resistance in A. baumannii. One of the genes identified was lpsB, which encodes a glycosyltransferase involved in lipopolysaccharide (LPS) synthesis. We demonstrate that loss of LpsB function results in increased sensitivity to both colistin and cationic antimicrobial peptides of the innate immune system. Moreover, LpsB is critical for pathogenesis in a pulmonary model of infection. Taken together, these data define bacterial processes required for intrinsic colistin tolerance in A. baumannii and underscore the importance of outer membrane structure in both antibiotic resistance and the pathogenesis of A. baumannii.
INTRODUCTION
Acinetobacter baumannii is a significant cause of nosocomial infections, accounting for up to 20% of infections in intensive care units worldwide (1). The burden of A. baumannii disease is particularly pronounced in the developing world, where this organism is among the top three causes of nosocomial pneumonia and bloodstream infections (2). With these infections, mortality rates can reach up to 100% in certain clinical settings (3, 4). Compounding these problems is the growing burden of A. baumannii disease caused by multidrug-resistant (MDR) strains. Worldwide surveillance data place rates of multidrug resistance in A. baumannii isolates at 48 to 85%, with the highest rates in Asia and Eastern Europe (3, 5–7). Although currently rare, pan-drug resistance has been reported for A. baumannii (8–12). The emergence of bacteria resistant to all clinically available antibiotics is a sentinel event signaling the dawn of a postantibiotic era.
Increasing rates of resistance to conventional antibiotics has necessitated the reintroduction of older drugs that were discontinued due to potential side effects. One example is colistin, or polymyxin E, a cationic peptide antibiotic that is increasingly used to treat MDR infections. In many cases, colistin is the only remaining antibiotic effective in treating MDR A. baumannii. Although uncommon in A. baumannii, heteroresistance and complete resistance to colistin have been reported clinically (7, 13–15). It is feared that increasing use of colistin coupled with the clonal spread of colistin-resistant isolates will lead to widespread resistance to this drug. As evidence of this possibility, in South Korea, rates of colistin resistance as high as 27.9% have been noted (15). These facts underscore the need to define the mechanisms mediating colistin resistance in A. baumannii. Colistin acts through disruption of the outer membrane, and this action requires binding to the lipid A portion of lipopolysaccharide (LPS). As a result, most mutations associated with colistin resistance disrupt the drug's interaction with LPS. For example, resistance can arise through mutations in the two-component system PmrAB, whose downstream target, PmrC, catalyzes phosphoethanolamine modification of lipid A (16–20). This modification reduces the net negative charge of the outer membrane, thus reducing the affinity of colistin for this subcellular target. Mutations or insertions in the genes encoding the lipid A biosynthesis machinery also mediate resistance by abolishing LPS production and thus eliminating colistin's target (13, 21, 22). Notably, the resistance mechanisms highlighted above are mediated by adaptive and occasionally reversible mutations rather than by acquisition of resistance determinants (16, 17). Moreover, evolution of resistant isolates through adaptive mutations during antibiotic therapy in human patients is well documented in A. baumannii (14, 23). Taken together, these facts highlight the adaptability of this organism and suggest that A. baumannii possesses intrinsic mechanisms to resist the initial onslaught of antibiotic therapy until adaptive mutations or resistance determinants can be acquired. The molecular basis for these intrinsic resistance mechanisms in A. baumannii is largely unknown.
We have previously demonstrated that A. baumannii displays increased tolerance to colistin in response to physiologic concentrations of monovalent cations (24). Here we describe the identification of over 30 genes involved in this inducible colistin tolerance in A. baumannii. A majority of these genes converge on pathways and systems involved in osmotolerance, including those involved in compatible solute and cell envelope biosynthesis as well as in protein folding. We further characterize one of these genes, lpsB, and define the role for LpsB in cationic antimicrobial peptide (AMP) resistance and pathogenesis in the lung.
MATERIALS AND METHODS
Bacterial strains and reagents.
A. baumannii strain ATCC 17978 (Ab17978) was obtained from the American Type Culture Collection and was used for all experiments unless otherwise noted. Primers and plasmids used in this study are listed in Table 1. Colistin sulfate was obtained from Sigma-Aldrich (St. Louis, MO). LL-37 was purchased from Phoenix Pharmaceuticals.
Table 1.
Primers and plasmids used in this work
| Primer or plasmid | Sequence or description |
|---|---|
| Primers | |
| lpsbKO1 | CCCGGATATCGTGATGCAATTTGGTATAGTCC |
| lpsbKO2 | CCCGCATATGACTGATGACCTTGTGCAACC |
| lpsbKO3 | CCCGCATATGCCTCAGCACAGTGGTTTAACC |
| lpsbKO4 | CCCGGATATCTAACGCGCTTGCTGTACTTG |
| lpsbKO6 | TTACCAATGCACAAGCTCAAG |
| lpsbKO7 | TAACCTTGACGGTTCTACGC |
| X430F | GCCCGAATTCGCTTCGTATCGCACCAACTC |
| X430R | CCCGGATATCTCAATTCAATACACTTTGATATAGCTC |
| 16SF | CTGTAGCGGGTCTGAGAGGAT |
| 16SR | CCATAAGGCCTTCTTCACAC |
| 0807F | CGCAGCTATTTTAGTTCTTCATCTTC |
| 0807R | CCCGACCACCAAATGC |
| 1008F | CTAAGTATACAGCTGCGATTATGC |
| 1008R | GCGATGAAACCATGCCAG |
| 3130F | GCATTTAGCGGAAGTGATTAGC |
| 3130R | ACGAATCGAAATTGCAACCTTG |
| Plasmids | |
| pKOlpsB | lpsB inactivation vector in pEX100T (this work) |
| plpsB | lpsB complementation vector (this work) |
| pMU125 | Derivative of pWH1266 containing gfp (40) |
| pEX100T | sacB-conjugative plasmid for gene replacement (26) |
Transposon library screen and mutant identification.
A transposon library was generated in Ab17978 using the EZ-Tn5 <R6Kγori-KAN-2> transposome system (Epicentre) as described previously (25). A total of 8,000 mutants were screened for loss of NaCl-induced colistin resistance by challenging them with 1.5 to 2 mg/liter colistin in Mueller-Hinton broth (MHB) with or without supplementation with 154 mM NaCl. Mutants that demonstrated no growth after 24 h in NaCl-supplemented media were selected for further analysis. Phenotypes were confirmed by growth curve analysis in MHB with or without NaCl, with or without colistin, as described previously (24). With selected mutants, MICs of colistin and LL-37 were determined by broth microdilution in MHB using established methods (24). Briefly, bacteria were cultured overnight in MHB, normalized, and then subcultured into MHB with or without NaCl, with or without colistin, in 96-well plates. The plates were incubated at 37°C with shaking at 180 rpm for approximately 24 h. For growth curve analyses, the optical densities of the cultures were determined at regular intervals throughout the incubation. In all cases, MICs and culture densities were determined after 20 to 24 h of growth. Data are graphed as averages of results from at least three biological replicates, and error bars represent the standard deviations (SDs) from the means. In some cases, error bars may be obscured by the symbols.
The locations of transposon insertions were determined by rescue cloning as described previously (26). Functional predictions for the disrupted genes were based on annotations in the NCBI database and the SEED (27). The SEED viewer was used to analyze genomic contexts surrounding the transposon integration sites. Predicted operons are based on proximity and orientations of predicted open reading frames as well as conservation of the genetic organization in multiple species.
Metabolic pathway analysis.
Metabolic pathway analysis was carried out using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway program (http://www.genome.jp/kegg/). The database was filtered for genes and pathways predicted to be present in Ab17978 based on gene annotations in the NCBI database, since there are limited experimental data available on this organism.
LPS analysis.
Bacteria were swabbed from LB agar plates into 154 mM NaCl, normalized to an optical density at 600 nm (OD600) of 1.5, pelleted, and resuspended in lysing buffer (2% SDS, 4% 2-mercaptoethanol, 10% glycerol, 0.1 M Tris-HCl, pH 6.8). Samples were then boiled for 10 min, cooled to 60°C, and treated with proteinase K for 1 h. These samples were electrophoresed through a 15% acrylamide gel using standard methods and stained with Pro-Q Emerald 300 LPS stain according to the manufacturer's recommendations (Invitrogen) (28).
Complementation of the LPS synthesis defect and colistin sensitivity of A. baumannii 5A7.
A 1,400-bp fragment including lpsB and 350 bp of flanking sequence was amplified from wild-type genomic DNA using primers X430F and X430R. The product was digested with EcoRI and EcoRV and cloned into pMU125, a derivative of pWH1266, which had been modified to remove green fluorescent protein (GFP). The resulting vector, plpsB, was introduced into 5A7 by electroporation as previously described (25). The empty vector, pWH1266, was used as a control. LPS from the resulting strains was analyzed as described above. The colistin sensitivity of 5A7 was compared to that of 5A7::plpsB with or without NaCl by kill curve analysis as described above.
Site-directed insertional mutagenesis of lpsB.
In order to assess the contribution of LpsB to pathogenesis in vivo, we first generated a strain in which the gene encoding LpsB was inactivated by allelic replacement. Approximately 1,000 bp of DNA sequence on each side of lpsB was amplified using primers lpsbKO1 and lpsbKO2 (lpsB-up) or lpsbKO3 and lpsbKO4 (lpsB-down). The flanking sequences were cloned separately into pCR2.1 (Invitrogen). lpsB-down was excised by digestion with NdeI and XhoI and cloned into the cognate sites of pCR2.1-lpsB-up. The combined flanking regions were then excised by digestion with EcoRV and cloned into SmaI-digested pEX100T, to yield pKO-lpsB-UD. The kanamycin resistance gene, aph, was amplified from pKAN-2 (Epicentre), digested with NdeI, and cloned into the NdeI sites of pKO-lpsB-UD. The resulting vector, pKO-lpsB, was introduced into Ab17978 by electroporation, and integration of the plasmid was selected for by plating the strain on LB agar supplemented with 40 μg/ml kanamycin. Kanamycin-resistant colonies were counterselected on LB agar supplemented with 40 μg/ml kanamycin and 2%, wt/vol, sucrose. Loss of the plasmid backbone was confirmed based on positive growth on sucrose-containing plates and failure to grow on LB agar supplemented with 500 μg/ml ampicillin. Integration of aph into lpsB was confirmed by PCR using primers that anneal outside the region contained in pKO-lpsB (lpsbKO6 and lpsbKO7). Disruption of lpsB function was confirmed by SDS-PAGE analysis of LPS from the ΔlpsB mutant.
Microarray analysis.
Bacterial cultures were grown overnight in LB medium supplemented with the appropriate antibiotic, diluted in fresh medium, and grown at 37°C with shaking at 180 rpm for 3.5 h. Cultures were then mixed with an equal volume of ice-cold ethanol-acetone (1:1) and stored at −80°C for RNA processing. For RNA extraction, samples were thawed on ice and total bacterial RNA was released by mechanical disruption and purified using Qiagen RNeasy columns (Qiagen, Valencia, CA), as previously described (24). For microarray analysis, 10 μg of each RNA sample was reverse transcribed, fragmented, and 3′-end biotinylated as previously reported (29). The resulting labeled cDNA (1.5 μg) was hybridized to a custom-made A. baumannii GeneChip (catalog no. PMDACBA1) according to the manufacturer's recommendations for antisense prokaryotic arrays (Affymetrix, Inc., Santa Clara, CA). Data from three independent biological replicates for each strain were analyzed as described previously (24). RNA species that exhibited a ≥2-fold change in expression, with titers above background, as determined by Affymetrix algorithms with the ΔlpsB mutant, and that were found to be statistically differentially expressed (t test; P ≤ 0.05) were reported. The data were filtered against microarray data from transposon mutant 5A7 and an unrelated mutant grown under identical conditions (data not shown) in order to select gene expression changes common to both lpsB mutants and eliminate changes resulting from antibiotic treatment.
qRT-PCR analysis of WT bacteria exposed to a subinhibitory concentration of colistin.
WT A. baumannii was grown in LB or LB supplemented with 1 mg/liter colistin for approximately 6 h at 37°C with shaking at 180 rpm. RNA was isolated as described above. Reverse transcription was carried out on 2 μg total RNA using Moloney murine leukemia virus (M-MLV) reverse transcriptase and 1 μg random hexamers according to the manufacturer's protocol (Promega, Madison, WI). Quantitative PCR (qPCR) was performed using 10 ng of the cDNA template (0.01 ng of the template for 16S rRNA). Sequences for the primers used in these analyses are shown in Table 1. Data were analyzed from three biological replicates after normalization to data for 16S rRNA. Data from colistin-treated samples are presented as levels of induction (n-fold) relative to those of untreated samples.
A. baumannii pneumonia model.
All animal experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee. We have previously established a murine Acinetobacter pneumonia model in our laboratory (25). Briefly, bacteria were harvested from log-phase cultures of Ab17978 or the ΔlpsB mutant, washed, and resuspended in phosphate-buffered saline (PBS) at 1 × 107 CFU/μl. Six- to 8-week-old, female C57BL/6 mice were anesthetized and infected intranasally with 30 μl of bacterial suspension. Mice were euthanized at 36 h postinfection (hpi), and lungs were aseptically removed, weighed, and homogenized in 1 ml sterile PBS. Serial dilutions were plated on LB agar and/or LB agar containing kanamycin (40 μg/ml). Data from two independent experiments with at least five mice per group in each experiment are included.
Nucleotide sequence accession number.
Data were deposited into the NCBI database with accession number GSE40771.
RESULTS
Identification of genes involved in NaCl-induced colistin tolerance.
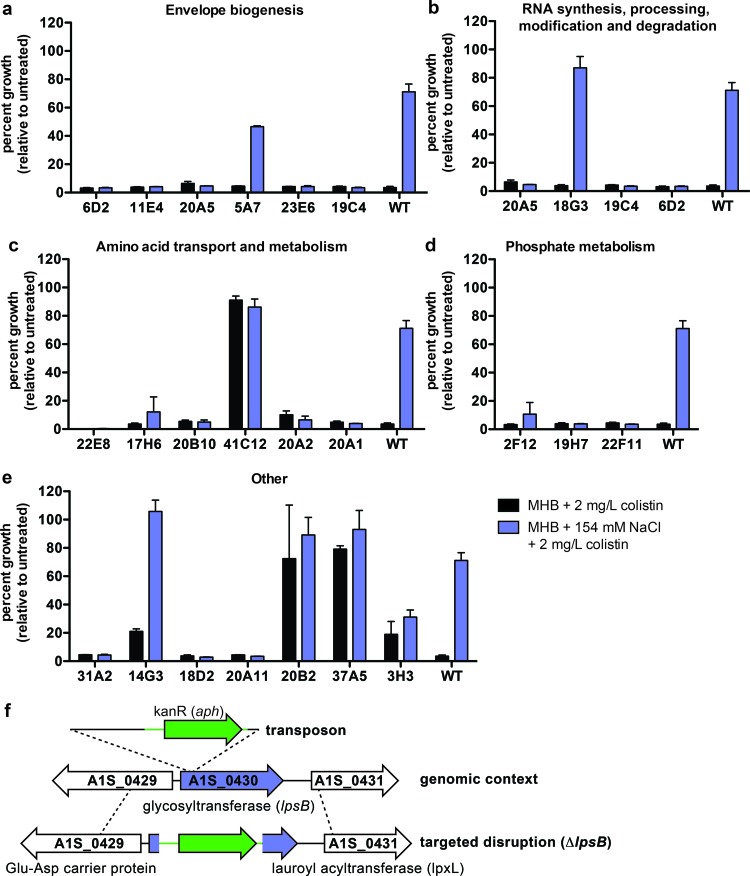
Based on our previous studies demonstrating that NaCl induces increased resistance to colistin (24), we designed a transposon mutant screen to identify genes involved in mediating this resistance. In the primary screen, approximately 8,000 mutants were tested for loss of NaCl-induced colistin resistance by challenging them with 1.5 to 2 mg/liter colistin in the presence or absence of 150 mM NaCl. Approximately 300 mutants that failed to grow in the presence of NaCl were identified, and their colistin sensitivities were reassessed by growth curve analyses. We determined the transposon integration sites for all of the mutants that consistently demonstrated increased sensitivity to colistin despite NaCl supplementation of media (Fig. 1a to e; Table 2). In addition, the integration sites were determined for several mutants that demonstrated increased resistance to colistin compared to that of the wild type (Fig. 1b to c and e; Table 2). The genomic loci were compared with data from the SEED to assess whether the disrupted genes were found within predicted operons, and putative functions were assigned based on annotations in the NCBI database and the SEED (Table 2). These analyses resulted in the identification of 23 mutants with altered NaCl-induced colistin resistance.
Fig 1.
(a to e) Schematic overview and results of the transposon library screen to identify genes involved in NaCl-induced colistin resistance. Representative growth analyses of mutants identified in the transposon screen. Bacteria were challenged with 2 mg/liter colistin in Mueller-Hinton broth (MHB) with or without 154 mM NaCl, and growth was measured after 20 to 24 h. Mutants are grouped according to the annotations of the disrupted genes, which can be found in Table 2. Data represent the averages of results of three biological replicates, and error bars indicate the SDs from the means. Statistical significance was determined by one-way analysis of variance (ANOVA), and only mutants whose growth differed significantly from that of the wild type are shown. (f) Schematic overview of the A1S_0430 genomic locus in the transposon mutant 5A7 and the targeted deletion mutant, the ΔlpsB strain.
Table 2.
Transposon mutants with reduced NaCl-induced colistin resistance
| Function(s) and strain | Insertion site | Locus tagb | Gene annotation |
|---|---|---|---|
| Phosphate metabolism | |||
| 2F12 | Intergenic | A1S_2445 | High-affinity phosphate transport protein (PstB) |
| 2F12 | Intergenic | A1S_2444 | Putative periplasmic protease |
| 19H7 | Gene | A1S_3030 | Phosphate-inducible protein, PhoH-like |
| 22F11 | Gene | A1S_0462 | Putative phosphatase |
| Envelope biogenesis | |||
| 6D2 | Gene | A1S_2982 | YidD |
| 11E4 | Gene | A1S_2250 | Zn-dependent protease with chaperone function |
| 20A5 | Intergenic | A1S_3424 | Putative lipoprotein-34 precursor (NlpB) |
| 5A7 | Gene | A1S_0430 | Putative glycosyltransferase (LpsB) |
| 23E5 | Gene | A1S_1030 | DNA-binding ATP-dependent protease La |
| 19C4 | Gene | A1S_0499 | Putative Fe-S cluster redox enzyme (rRNA large-subunit methyltransferase N) |
| RNA synthesis, processing, modification, and degradation | |||
| 20A5 | Intergenic | A1S_3425 | Phosphoribosylaminoimidazole-succinocarboxamide |
| 18G3a | Intergenic | A1S_0531 | Putative GTPase |
| 18G3a | Intergenic | A1S_0532 | Oligoribonuclease |
| 19C4 | Gene | A1S_0499 | Putative Fe-S cluster redox enzyme (rRNA large-subunit methyltransferase N) |
| 6D2 | Gene | A1S_2982 | YidD |
| Amino acid transport and metabolism (glutamate, aspartate, alanine), urea cycle | |||
| 22E8 | Gene | A1S_3185 | Glutamate synthase subunit alpha |
| 17H6 | Intergenic | A1S_1142 | Aspartate kinase |
| 17H6 | Intergenic | A1S_1143 | Hypothetical protein |
| 20B10 | Gene | A1S_2793 | Putative amino acid transport protein |
| 41C12a | Gene | A1S_2454 | Diaminobutyrate-2-oxoglutarate transaminase |
| 20A2 | Intergenic | A1S_2023 | Hypothetical protein |
| 20A2 | Intergenic | A1S_2024 | Glutamate 5-kinase |
| 20A1 | Gene | A1S_3025 | Malate dehydrogenase |
| TCA cycle | |||
| 31A2 | Gene | A1S_2710 | Citrate synthase |
| Cofactor biosynthesis | |||
| 14G3a | Gene | A1S_0807 | 8-Amino-7-oxononanoate synthase |
| Multidrug efflux system | |||
| 18D2 | Gene | A1S_0909 | Multidrug resistance protein B |
| 20A11 | Intergenic | A1S_1231 | Major facilitator superfamily transporter |
| 20A11 | Intergenic | A1S_1232 | EsvB |
| Lipid metabolism | |||
| 3H3 | Intergenic | A1S_2842 | Putative acetyl-CoA acetyltransferase |
| 20B2a | Intergenic | A1S_3159 | Lipase chaperone |
| 20B2a | Intergenic | A1S_3160 | Lipase |
| Phage | |||
| 37A5a | Intergenic | A1S_1585 | Putative replicative DNA helicase |
| 37A5a | Intergenic | A1S_1586 | EsvKI |
Mutant that is more resistant to colistin.
Locus tags refer to the NCBI reference sequence NC_009085.
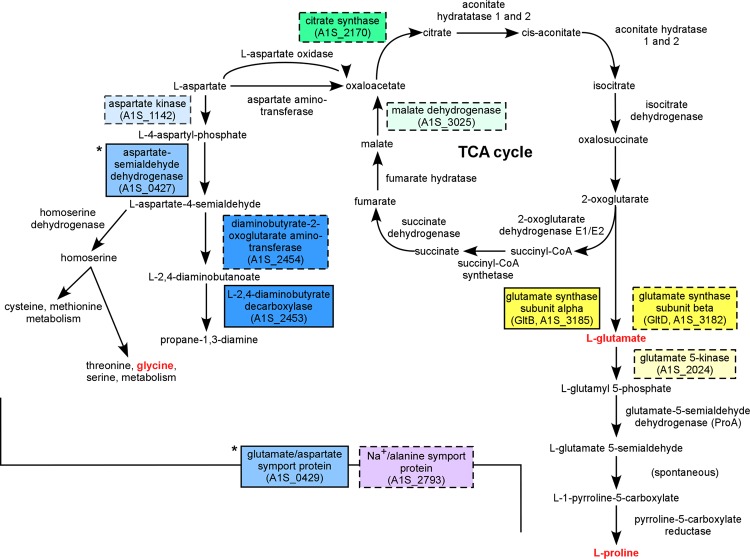
Overall, the predicted functions fell into several broad categories (Table 2). In particular, genes encoding proteins involved in amino acid transport and metabolism, protein folding, phosphate metabolism, and cell envelope biogenesis were overrepresented. Interestingly, those with predicted roles in amino acid biosynthesis cluster into two interconnected pathways (Fig. 2). These pathways lead to production of osmolytes that mediate osmotic tolerance through membrane and protein stabilization (30–32). These genes include those involved in proline biosynthesis from glutamate and in aspartate metabolism. We also identified genes involved in the tricarboxylic acid (TCA) cycle which can mediate interconversion of aspartate and glutamate. Taken together, these analyses highlight critical contributions to the intrinsic colistin resistance of genes encoding proteins involved directly in the maintenance of membrane integrity and protein folding as well as those indirectly involved in these processes through compatible solute synthesis.
Fig 2.
Metabolic pathways disrupted in colistin-sensitive mutants of A. baumannii. Colored boxes indicate enzymes or transporters whose gene or predicted operon was disrupted in one of the colistin-sensitive transposon mutants. Dashed borders indicate genes that were directly disrupted by the transposon insertion or the immediate downstream genes if the insertion was intergenic. Boxes of the same color indicate genes in the same predicted operon. Asterisks indicate genes in a predicted operon upstream of a transposon insertion site. Names of amino acids or compatible solutes that contribute to osmotic protection are indicated with red lettering.
LpsB contributes to colistin resistance.
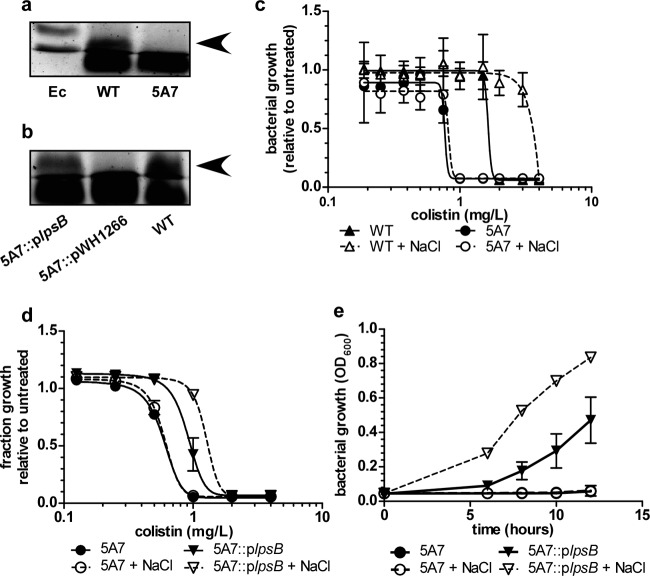
Given that the subcellular target of colistin is LPS, we focused our attention on mutant 5A7, in which the transposon disrupts lpsB (Fig. 1f). In A. baumannii strain AB307, lpsB encodes a glycosyltransferase involved in the synthesis of the LPS core (33). SDS-PAGE analyses of LPS from the wild type and 5A7 confirmed that in Ab17978, disruption of lpsB results in production of a truncated LPS, which is complemented by providing a wild-type copy of lpsB in trans (Fig. 3a and b) (33). We also observed some differences in the higher-molecular-weight banding patterns, but these were not consistently observed (data not shown). There are conflicting data in the literature regarding the expression of O antigen in A. baumannii. It is possible that some of the higher-molecular-weight bands that we have observed were actually exopolysaccharide. It is also possible that these bands represented glycosylated proteins (34). The core region of LPS is important for maintaining the structure and integrity of the outer membrane (35). We therefore hypothesized that, unlike other mutations affecting LPS, loss of LpsB function increases susceptibility to colistin through destabilization of the outer membrane. To confirm the role of A. baumannii LPS core in mediating resistance to colistin, we performed kill curve analyses in the presence of increasing concentrations of colistin with or without NaCl. These analyses demonstrate that 5A7 is more susceptible to colistin than the wild type and that 5A7 lacks NaCl-induced colistin resistance (Fig. 3c). Moreover, providing lpsB in trans rescues the sensitivity of 5A7 to colistin and partially restores NaCl-induced resistance to colistin (Fig. 3d and e).
Fig 3.
Demonstration of the LPS synthesis defect and colistin sensitivity of 5A7. (a) SDS-PAGE analysis of LPS purified from the WT and 5A7. LPS isolated from E. coli (Ec), WT A. baumannii, and 5A7 was electrophoresed in a 15% polyacrylamide gel and stained with Pro-Q Emerald 300 LPS stain. A higher-molecular-weight band is present in the WT lane (arrow), but this band is absent in 5A7. (b) Complementation of the LPS synthesis defect by providing lpsB in trans. LPS samples were electrophoresed and stained as described for panel a. (c to d) Kill curves comparing colistin sensitivities of the WT and 5A7 (c) or of 5A7 and 5A7::plpsB (d) in media with or without NaCl supplementation (150 mM). The data are presented as means ± 1 SD from the means of results from at least three biological replicates. In some cases, error bars may be obscured by the symbols. Curves were generated by nonlinear regression analysis using a least-squares fitting method. (e) Growth curve comparing the growth of 5A7 with that of 5A7::plpsB in media with colistin (1 mg/liter) and with or without NaCl (150 mM). The data are presented as means ± 1 SD from the means of results from at least three biological replicates.
Adaptation to chronic membrane instability.
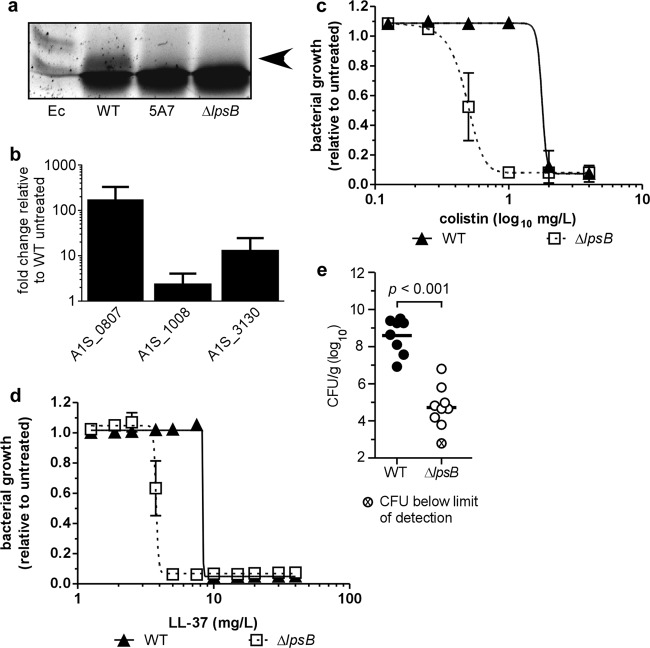
Disruption of LPS core biosynthesis in A. baumannii leads to increased sensitivity to human serum and detergents, which suggests that inactivation of lpsB compromises outer membrane integrity (33). Given that colistin acts through disruption of the bacterial membrane, an lpsB mutant can serve as a tool to probe the adaptations that allow A. baumannii to survive in the presence of chronic membrane perturbation. In order to determine how A. baumannii responds to the chronic membrane instability resulting from loss of LpsB function, we first generated a targeted deletion of lpsB in Ab17978 (ΔlpsB mutant) and confirmed that this mutant produces a truncated LPS molecule similar to that of 5A7 (Fig. 1f and 4a). We next performed microarray analyses comparing wild-type Ab17978 with the ΔlpsB mutant during exponential growth.
Fig 4.
A. baumannii strains lacking LpsB are attenuated for virulence in the lung. (a) SDS-PAGE analysis of LPS from the WT, 5A7, and an lpsB deletion strain (ΔlpsB mutant). (b) Expression analysis of the response to colistin treatment on select genes identified in the microarray (1 mg/liter) on WT A. baumannii. The data are presented as means ± 1 SD from the means of results of at least three biological replicates. (c to d) Kill curves comparing the colistin (c) or LL-37 (d) sensitivities of the WT and the ΔlpsB mutant. The data are presented as means ± 1 SD from the means of results of at least three biological replicates. In some cases, error bars may be obscured by the symbols. Curves were generated by nonlinear regression analysis using a least-squares fitting method. (e) Bacterial burdens in lungs of mice infected with the WT or the ΔlpsB mutant harvested at 36 hpi. Data are combined from two independent experiments, with 4 to 5 mice per group used in each experiment. Means for each data set are indicated by horizontal bars. Statistical significance was determined by a two-tailed, unpaired Student t test.
A significant proportion of the genes whose expression is altered in the ΔlpsB mutant are involved in metabolic processes and energy generation within the cell (see Table S1 in the supplemental material). For example, we observed downregulation of genes encoding enzymes involved in lipid transport and metabolism as well as downregulation of genes involved in the transport and metabolism of numerous amino acids. We also observed upregulation of a number of genes involved in energy generation and the TCA cycle. Overall, these changes suggest a decrease in anabolic processes and an overall increase in catabolism and energy generation. These data demonstrate that disruption of lpsB profoundly impacts critical cellular processes.
Notably, a number of the pathways altered in the ΔlpsB mutant either overlap or connect with those identified in the transposon library screen. These findings support the hypothesis that membrane perturbation through colistin treatment has an effect on the cell similar to that of membrane perturbation through disruption of LPS core biosynthesis. To further validate this comparison, we selected three genes whose expression was either induced or repressed in the ΔlpsB mutant compared to that in the WT. The expression of these genes was determined in WT bacteria exposed to subinhibitory concentrations of colistin using qPCR analyses. In the ΔlpsB mutant, the gene encoding isocitrate lyase (locus tag A1S_1008 from the A. baumannii 17978 reference genome; NCBI reference sequence NC_009085) was significantly upregulated compared to its expression in WT bacteria (2.2-fold). Colistin treatment likewise induced a similar magnitude of upregulation in wild-type bacteria (Fig. 4b). In contrast, A1S_3130 was downregulated in the ΔlpsB mutant compared to in WT bacteria, yet this gene was upregulated approximately 10-fold in WT bacteria treated with colistin (Fig. 4b). The fact that colistin alters the expression of A1S_3130 suggests that this gene has a role in the response to colistin. Whether this response is adaptive or detrimental to the bacterium is not yet clear. Interestingly, A1S_0807, which encodes a gene necessary for biotin biosynthesis, was downregulated 3-fold in the ΔlpsB mutant, while this gene was upregulated over 150-fold in WT bacteria treated with colistin (Fig. 4b). In addition, disruption of this gene by transposon mutagenesis results in increased resistance to colistin (Fig. 1 and Table 2). Biotin is a cofactor required by a number of enzymes, including acetyl coenzyme A (acetyl-CoA) carboxylase (ACCase) and propionyl-CoA carboxylase, both of which are involved in lipid metabolism. The results of the transposon screen, microarray analysis, and qPCR analysis all suggest that biotin plays a key role in the response to membrane perturbation, perhaps through its role in lipid metabolism. Taken together, these data suggest that inactivation of lpsB and treatment with colistin have similar yet distinct effects on gene expression in A. baumannii.
Involvement of LpsB in the pathogenesis of pneumonia.
Colistin shares a mechanism of action similar to that of antimicrobial peptides of the innate immune system. We therefore hypothesized that the mechanisms involved in colistin resistance likewise confer resistance to antimicrobial peptides and contribute to virulence. As expected, the ΔlpsB mutant exhibits an increase in sensitivity to colistin similar to that of 5A7 (Fig. 4c). Likewise, kill curve analyses revealed a reduction in the MIC of the human antimicrobial peptide LL-37 for the ΔlpsB mutant compared to that for the wild type (Fig. 4d). These data establish that LpsB contributes to protection against cationic AMPs of the innate immune system.
Cationic AMPs are known to be important mediators of host defense at mucosal surfaces. A. baumannii LPS stimulates the release of AMPs from respiratory epithelial cells in vitro, suggesting that AMPs may be an important component of host defenses that A. baumannii must overcome during infection of the lung (36). Given the role for LpsB in resistance to AMPs, we hypothesized that loss of LpsB function reduces A. baumannii pathogenesis within the lung. Furthermore, our microarray analyses demonstrate that disruption of lpsB significantly alters critical cellular processes, which may also impact A. baumannii growth within the host. To elucidate the contribution of LpsB to pulmonary infection, we intranasally infected mice with either the wild-type strain or the ΔlpsB mutant and allowed the infection to proceed for 36 h. Quantification of bacteria in lungs revealed a nearly 4-log reduction in bacterial burden in ΔlpsB mutant-infected mice compared to wild-type-infected mice (Fig. 4c). These data establish that LpsB plays a critical role in the pathogenesis of A. baumannii pulmonary infections.
DISCUSSION
A. baumannii is emblematic of the looming public health crisis threatening nearly all facets of medical practice. Specifically, A. baumannii represents the growing burden of infections caused by organisms resistant to most, if not all, conventional antibiotics. This organism has proven particularly challenging due to its ability to persist in the hospital environment, its resistance to both antibiotics and common disinfectants, and its propensity to acquire resistance to new antimicrobial agents. The last fact underscores the adaptability of this organism to the hospital environment and suggests that A. baumannii possesses intrinsic mechanisms to respond and adapt to antibiotic treatment.
Colistin resistance in A. baumannii is currently rare and typically arises through adaptation of a previously susceptible isolate, often during treatment with colistin in vivo. The fact that colistin resistance arises without the acquisition of horizontally transferred resistance determinants suggests that A. baumannii possesses intrinsic mechanisms to resist colistin (16–18). Furthermore, recent evidence suggests that strains that adapt to colistin treatment must undergo significant changes in physiologic processes in order to maintain the resistance phenotype (14, 21). Taken together, these facts underscore the need to better understand the inherent mechanisms that contribute to colistin resistance in order to identify possible targets for therapeutic intervention. Toward this end, we have identified over 20 genes involved in this adaptation in A. baumannii. Colistin, like other polymyxins, acts by disrupting the cell envelope, leading to osmotic lysis of the bacterium by dysregulating the permeability of the bacterial membrane (37–39). Given the action of colistin, it is not surprising that many of the genes identified in our screen are involved in processes that protect the bacterium from osmotic stress. For example, we identified several genes with roles in the synthesis of compatible solutes, such as proline (Fig. 2). Importantly, certain compatible solutes not only act as neutral osmolytes but also exert protective effects by preventing protein misfolding (30–32). Proline, in particular, is known to protect protein structure at physiologically attainable concentrations. Furthermore, proline synthesis and uptake are induced by NaCl (30). Taken together, the results of the transposon mutagenesis screen suggest that bacteria exposed to colistin experience osmotic stress, which can be alleviated through synthesis of compatible solutes and expression of proteases that presumably degrade misfolded proteins. When these systems are inactivated, A. baumannii is more susceptible to colistin and NaCl no longer exerts a protective effect.
Loss of LpsB function leads to significant changes in gene expression, particularly with regard to genes involved in metabolic processes within the cell (see Table S1 in the supplemental material). Interestingly, some expression changes observed in the ΔlpsB mutant are complementary to the presumed effect of gene disruption in the transposon screen. One pathway where this is particularly notable is in lipid metabolism. Given that colistin acts through membrane disruption and that the ΔlpsB mutant is defective in production of the main constituent of the outer membrane, it is not surprising that we would observe significant overlap in the effects of colistin and lpsB mutation on lipid metabolism. As noted above, several genes required for lipid metabolism are downregulated in the ΔlpsB mutant (A1S_0806-7, which is involved in biotin cofactor biosynthesis, and the biotin-dependent enzymes ACCase and propionyl carboxylase, which are involved directly in the metabolism of fatty acids). These data suggest that resistance to membrane disruption by colistin or through loss of LpsB function requires downregulation of lipid metabolism. Consistent with this idea, disruption of A1S_0807 leads to increased resistance to colistin, presumably by reducing biotin biosynthesis and therefore reducing the activity of biotin-dependent enzymes involved in lipid metabolism. Notably, A1S_0807 is markedly upregulated in WT bacteria exposed to subinhibitory concentrations of colistin. Although this may seem contradictory, it is possible that colistin induces A1S_0807 and that this gene product or downstream metabolite contributes to colistin toxicity, consistent with the results of the transposon screen. Since the ΔlpsB mutant must survive in the presence of chronic membrane perturbation, A1S_0807 is relatively downregulated in order to improve survival. Finally, we have previously observed that the biotin synthase carboxyl carrier proteins of ACCase (BCCP) and ACCase genes are significantly downregulated in response to NaCl and that NaCl increases resistance to colistin (24). Taken together, these data suggest that disrupting lipid metabolism by decreasing biotin synthesis protects A. baumannii against colistin-mediated membrane damage.
Another response that complements the results of the transposon screen is the observed upregulation of genes involved in cold shock responses. Although these genes were not identified in the transposon screen, several genes involved in protection against osmotic stress were identified. Moreover, we have previously shown that some genes involved in cold shock responses are upregulated in response to NaCl, which induces protection against colistin treatment (24). Adaptation to cold shock requires protection of membrane integrity and protein stability, and these adaptations are likewise important for resisting osmotic stress. Taking the results of the transposon screen and the microarray together, it is possible to identify genes and physiologic processes that are important both for resistance to acute membrane disruption as induced by colistin and for chronic membrane perturbation as experienced by the ΔlpsB mutant. Moreover, the overlap in physiologic processes observed in the transposon screen and the microarray suggests that some of the genes involved in adaptation to the loss of lpsB may also be important in adapting to colistin treatment.
It has been demonstrated previously that an lpsB transposon mutant of A. baumannii exhibits decreased competitive fitness during coinfection with wild-type bacteria in a soft tissue model of infection (33). However, in the soft tissue model, the mutant does not exhibit a virulence defect in a single-strain infection. This stands in contrast to our data in which the ΔlpsB mutant is significantly attenuated for virulence in the lung. The striking difference between the phenotypes of the lpsB mutants in pulmonary and soft tissue models of infection suggests that A. baumannii has different virulence requirements depending on the infection site. These differences may stem from physical characteristics of the infection site, variation in innate immune factors, and differing nutrient availability. It has been established, for example, that A. baumannii induces production of antimicrobial peptides at mucosal sites like the lung (36). The increased sensitivity of the ΔlpsB mutant to these defense peptides likely contributes to this strain's reduced virulence in the lung. Moreover, we have defined significant transcriptional changes in genes involved in nutrient acquisition and metabolism in the ΔlpsB mutant. It is possible that nutritional requirements and availability in the lung also restrict the growth of the ΔlpsB mutant, particularly if this strain lacks the flexibility to adapt to the host environment. The possibility that nutrient availability impacts both pathogenesis and antibiotic resistance in A. baumannii is particularly intriguing, as further work in this area may elucidate novel targets for therapeutic intervention.
The rise of extensively drug resistant bacteria that are capable of causing lethal infections is creating a public health crisis, yet therapeutic development is not keeping pace. A. baumannii poses a particular challenge due to the ability of this bacterium to readily acquire resistance to new antibiotics. This fact underscores the adaptability of A. baumannii within the hospital and host environments. Targeting mechanisms that mediate the intrinsic antibiotic resistance of A. baumannii is therefore a viable strategy for developing novel inhibitors that could serve as adjuncts to our current antibiotic armamentarium.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Skaar laboratory for critical reading of the manuscript, Luis Actis (Miami University) for providing the Escherichia coli-A. baumannii shuttle vectors used in this work, and Herbert Schweizer (College of Veterinary Medicine and Biomedical Science, Colorado State University) for providing pEX100T.
This work was supported by the National Institutes of Health (NIAID grant AI091771 to E.P.S.), a Howard Hughes Medical Institute International Student Research Fellowship to M.I.H., a Burroughs Wellcome Fund grant to E.P.S., and the National Institute of General Medical Studies (grant T32 GM07347 to Vanderbilt University Medical Scientist Training Program).
Footnotes
Published ahead of print 10 December 2012
REFERENCES
- 1. Vincent J-L, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, EPIC II Group of Investigators. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329 [DOI] [PubMed] [Google Scholar]
- 2. Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, Pittet D. 2011. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 377:228–241 [DOI] [PubMed] [Google Scholar]
- 3. Gaynes R, Edwards JR, National Nosocomial Infections Surveillance System. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848–854 [DOI] [PubMed] [Google Scholar]
- 4. Srivastava S, Shetty N. 2007. Healthcare-associated infections in neonatal units: lessons from contrasting worlds. J. Hosp. Infect. 65:292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erdem I, Ozgultekin A, Sengoz Inan A, Dincer E, Turan G, Ceran N, Ozturk Engin D, Senbayrak Akcay S, Akgun N, Goktas P. 2008. Incidence, etiology, and antibiotic resistance patterns of gram-negative microorganisms isolated from patients with ventilator-associated pneumonia in a medical-surgical intensive care unit of a teaching hospital in Istanbul, Turkey (2004–2006). Jpn. J. Infect. Dis. 61:339–342 [PubMed] [Google Scholar]
- 6. Garza-González E, Llaca-Díaz JM, Bosques-Padilla FJ, González GM. 2010. Prevalence of multidrug-resistant bacteria at a tertiary-care teaching hospital in Mexico: special focus on Acinetobacter baumannii. Chemotherapy 56:275–279 [DOI] [PubMed] [Google Scholar]
- 7. Jean S-S, Hsueh P-R. 2011. High burden of antimicrobial resistance in Asia. Int. J. Antimicrob. Agents 37:291–295 [DOI] [PubMed] [Google Scholar]
- 8. Apisarnthanarak A, Mundy LM. 2009. Mortality associated with pandrug-resistant Acinetobacter baumannii infections in Thailand. Am. J. Infect. Control 37:519–520 [DOI] [PubMed] [Google Scholar]
- 9. Chan P-C, Huang L-M, Lin H-C, Chang L-Y, Chen M-L, Lu C-Y, Lee P-I, Chen J-M, Lee C-Y, Pan H-J, Wang J-T, Chang S-C, Chen Y-C. 2007. Control of an outbreak of pandrug-resistant Acinetobacter baumannii colonization and infection in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 28:423–429 [DOI] [PubMed] [Google Scholar]
- 10. Falagas ME, Bliziotis IA. 2007. Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int. J. Antimicrob. Agents 29:630–636 [DOI] [PubMed] [Google Scholar]
- 11. Falagas ME, Rafailidis PI, Matthaiou DK, Virtzili S, Nikita D, Michalopoulos A. 2008. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: characteristics and outcome in a series of 28 patients. Int. J. Antimicrob. Agents 32:450–454 [DOI] [PubMed] [Google Scholar]
- 12. Souli M, Galani I, Giamarellou H. 2008. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro Surveill. 13(47):pii=19045. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19045 [PubMed] [Google Scholar]
- 13. Moffatt JH, Harper M, Adler B, Nation RL, Li J, Boyce JD. 2011. Insertion sequence ISAba11 is involved in colistin resistance and loss of lipopolysaccharide in Acinetobacter baumannii. Antimicrob. Agents Chemother. 55:3022–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. López-Rojas R, Jiménez-Mejías ME, Lepe JA, Pachón J. 2011. Acinetobacter baumannii resistant to colistin alters its antibiotic resistance profile: a case report from Spain. J. Infect. Dis. 204:1147–1148 [DOI] [PubMed] [Google Scholar]
- 15. Ko KS, Suh JY, Kwon KT, Jung S-I, Park Kang K-HCI, Chung DR, Peck KR, Song J-H. 2007. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J. Antimicrob. Chemother. 60:1163–1167 [DOI] [PubMed] [Google Scholar]
- 16. Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, Bonomo RA. 2009. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob. Agents Chemother. 53:3628–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, Dhanji H, Chart H, Bou G, Livermore DM, Woodford N. 2011. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob. Agents Chemother. 55:3370–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park YK, Choi JY, Shin D, Ko KS. 2011. Correlation between overexpression and amino acid substitution of the PmrAB locus and colistin resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents 37:525–530 [DOI] [PubMed] [Google Scholar]
- 19. López-Rojas R, Domínguez-Herrera J, McConnell MJ, Docobo-Peréz F, Smani Y, Fernández-Reyes M, Rivas L, Pachón J. 2011. Impaired virulence and in vivo fitness of colistin-resistant Acinetobacter baumannii. J. Infect. Dis. 203:545–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock REW. 2011. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob. Agents Chemother. 55:3743–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henry R, Vithanage N, Harrison P, Seemann T, Coutts S, Moffatt JH, Nation RL, Li J, Harper M, Adler B, Boyce JD. 2012. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-β-1,6-N-acetylglucosamine. Antimicrob. Agents Chemother. 56:59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moffatt JH, Harper M, Harrison P, Hale JDF, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 54:4971–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hornsey M, Loman N, Wareham DW, Ellington MJ, Pallen MJ, Turton JF, Underwood A, Gaulton T, Thomas CP, Doumith M, Livermore DM, Woodford N. 2011. Whole-genome comparison of two Acinetobacter baumannii isolates from a single patient, where resistance developed during tigecycline therapy. J. Antimicrob. Chemother. 66:1499–1503 [DOI] [PubMed] [Google Scholar]
- 24. Hood MI, Jacobs AC, Sayood K, Dunman PM, Skaar EP. 2010. Acinetobacter baumannii increases tolerance to antibiotics in response to monovalent cations. Antimicrob. Agents Chemother. 54:1029–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobs AC, Hood I, Boyd KL, Olson PD, Morrison J, Carson S, Sayood K, Iwen PC, Skaar EP, Dunman PM. 2010. Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect. Immun. 78:1952–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dorsey CW, Tomaras AP, Connerly PL, Tolmasky ME, Crosa JH, Actis LA. 2004. The siderophore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology 150:3657–3667 [DOI] [PubMed] [Google Scholar]
- 27. Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang Cohoon H-YM, de Crécy-Lagard V, Diaz N, Disz T, Edwards R, Fonstein M, Frank ED, Gerdes S, Glass EM, Goesmann A, Hanson A, Iwata-Reuyl D, Jensen R, Jamshidi N, Krause L, Kubal M, Larsen N, Linke B, McHardy AC, Meyer F, Neuweger H, Olsen G, Olson R, Osterman A, Portnoy V, Pusch GD, Rodionov DA, Rückert C, Steiner J, Stevens R, Thiele I, Vassieva O, Ye Y, Zagnitko O, Vonstein V. 2005. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 33:5691–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fomsgaard A, Freudenberg MA, Galanos C. 1990. Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J. Clin. Microbiol. 28:2627–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, Blevins JS, Smeltzer MS. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 186:4665–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diamant S, Eliahu N, Rosenthal D, Goloubinoff P. 2001. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J. Biol. Chem. 276:39586–39591 [DOI] [PubMed] [Google Scholar]
- 31. Ignatova Z, Gierasch LM. 2006. Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proc. Natl. Acad. Sci. U. S. A. 103:13357–13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yancey PH. 2005. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 208:2819–2830 [DOI] [PubMed] [Google Scholar]
- 33. Luke NR, Sauberan SL, Russo TA, Beanan JM, Olson R, Loehfelm TW, Cox AD, St Michael F, Vinogradov EV, Campagnari AA. 2010. Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect. Immun. 78:2017–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iwashkiw JA, Seper A, Weber BS, Scott NE, Vinogradov E, Stratilo C, Reiz B, Cordwell SJ, Whittal R, Schild S, Feldman MF. 2012. Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation. PLoS Pathog. 8:e1002758 doi:10.1371/journal.ppat.1002758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyce JD, Harper M, St Michael F, John M, Aubry A, Parnas H, Logan SM, Wilkie IW, Ford M, Cox AD, Adler B. 2009. Identification of novel glycosyltransferases required for assembly of the Pasteurella multocida A:1 lipopolysaccharide and their involvement in virulence. Infect. Immun. 77:1532–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. March C, Regueiro V, Llobet E, Moranta D, Morey P, Garmendia J, Bengoechea JA. 2010. Dissection of host cell signal transduction during Acinetobacter baumannii-triggered inflammatory response. PLoS One 5:e10033 doi:10.1371/journal.pone.0010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liechty A, Chen J, Jain MK. 2000. Origin of antibacterial stasis by polymyxin B in Escherichia coli. Biochim. Biophys. Acta 1463:55–64 [DOI] [PubMed] [Google Scholar]
- 38. Oh JT, Cajal Y, Skowronska EM, Belkin S, Chen J, Van Dyk TK, Sasser M, Jain MK. 2000. Cationic peptide antimicrobials induce selective transcription of micF and osmY in Escherichia coli. Biochim. Biophys. Acta 1463:43–54 [DOI] [PubMed] [Google Scholar]
- 39. Oh JT, Van Dyk TK, Cajal Y, Dhurjati PS, Sasser M, Jain MK. 1998. Osmotic stress in viable Escherichia coli as the basis for the antibiotic response by polymyxin B. Biochem. Biophys. Res. Commun. 246:619–623 [DOI] [PubMed] [Google Scholar]
- 40. Dorsey CW, Tomaras AP, Actis LA. 2002. Genetic and phenotypic analysis of Acinetobacter baumannii insertion derivatives generated with a transposome system. Appl. Environ. Microbiol. 68:6353–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.