Abstract
The Rhodobacter capsulatus cbb3-type cytochrome c oxidase (cbb3-Cox) belongs to the heme-copper oxidase superfamily, and its subunits are encoded by the ccoNOQP operon. Biosynthesis of this enzyme is complex and needs dedicated biogenesis genes (ccoGHIS). It also relies on the c-type cytochrome maturation (Ccm) process, which requires the ccmABCDEFGHI genes, because two of the cbb3-Cox subunits (CcoO and CcoP) are c-type cytochromes. Recently, we reported that mutants lacking CcoA, a major facilitator superfamily type transporter, produce very small amounts of cbb3-Cox unless the growth medium is supplemented with copper. In this work, we isolated “Cu-unresponsive” derivatives of a ccoA deletion strain that exhibited no cbb3-Cox activity even upon Cu supplementation. Molecular characterization of these mutants revealed missense mutations in the ccmA or ccmF gene, required for the Ccm process. As expected, Cu-unresponsive mutants lacked the CcoO and CcoP subunits due to Ccm defects, but remarkably, they contained the CcoN subunit of cbb3-Cox. Subsequent construction and examination of single ccm knockout mutants demonstrated that membrane insertion and stability of CcoN occurred in the absence of the Ccm process. Moreover, while the ccm knockout mutants were completely incompetent for photosynthesis, the Cu-unresponsive mutants grew photosynthetically at lower rates and produced smaller amounts of cytochromes c1 and c2 than did a wild-type strain due to their restricted Ccm capabilities. These findings demonstrate that different levels of Ccm efficiency are required for the production of various c-type cytochromes and reveal for the first time that maturation of the heme-Cu-containing subunit CcoN of R. capsulatus cbb3-Cox proceeds independently of that of the c-type cytochromes during the biogenesis of this enzyme.
INTRODUCTION
Copper is an essential metal used as a cofactor of cuproproteins because of its ability to switch between oxidized (Cu2+) and reduced (Cu+) states. Cytochrome c oxidases (Cox), which belong to the heme-copper oxidase superfamily, are members of this group of enzymes and contain Cu at their heme-Cu catalytic center, where oxygen is reduced to water. Although this superfamily is diverse, the catalytic center is conserved among all members (1, 2).
Cox of the purple nonsulfur facultative phototrophic bacterium Rhodobacter capsulatus is a cbb3-type Cox (cbb3-Cox) that has four subunits (3). Two of them, CcoO and CcoP, are c-type cytochromes with a monoheme and a diheme, respectively. Both proteins are matured by the cytochrome c maturation (Ccm) process, which involves a dozen proteins with different functions (4, 5). The Ccm components are grouped into three functional modules: CcmABCDE for transport and preparation of the heme molecule; CcmG, CcdA, DsbA, and DsbB for thiol-oxidoreduction and preparation of apocytochrome; and CcmFHI for covalent heme ligation (4, 5).
In addition to the c-type cytochromes, the main catalytic subunit of cbb3-Cox is CcoN, which is a conserved 12-transmembrane-helix-containing membrane protein with a binuclear center composed of a high-spin heme b3 and a Cu atom (heme b3-CuB) (1, 2). Insertion of the cofactors during maturation of this subunit requires precise control of transport and trafficking of Cu and heme b. The fourth subunit of cbb3-Cox, CcoQ, does not contain any cofactor and is thought to enhance the stability of the enzyme (6). The complex structure of cbb3-Cox requires a multistep biogenesis pathway during which matured subunits are coordinately assembled (7, 8). In various CcoN mutants of R. capsulatus, the CcoO and CcoP subunits are not found in normal amounts in the membranes (9). Similarly, Ccm knockout mutants do not contain any CcoO or CcoP (10). However, the fate of CcoN in the absence of the Ccm process has not been examined, remaining hitherto unknown.
Previously, we identified a novel gene, ccoA, whose product (CcoA) is required for the presence of wild-type levels of active cbb3-Cox in R. capsulatus (11). CcoA is homologous to major facilitator superfamily (MFS)-type transporters. In mutants lacking CcoA, the cellular Cu content is lower than that in the wild-type strain in both normal and Cu-supplemented media, indicating a defect in Cu acquisition (11). In the absence of exogenous Cu supplementation, the activity of cbb3-Cox is extremely low in mutants lacking CcoA, but upon addition of 5 μM Cu2+ to the growth medium, this activity increases, exhibiting a Cu-dependent cbb3-Cox phenotype (11). In this work, in order to gain further insight into the CcoA-independent Cu uptake pathway, we isolated ΔccoA derivatives that were unresponsive to Cu supplementation with respect to cbb3-Cox activity. We found that these mutants were defective in the Ccm process and, interestingly, contained dissimilar levels of different c-type cytochromes. They contained sufficient amounts of cytochromes c1 and c2 to support slow photosynthetic (Ps) growth but completely lacked CcoO and CcoP, even though they had the CcoN subunit of cbb3-Cox. These findings indicate that different levels of Ccm efficiency are required to produce various c-type cytochromes and reveal that membrane insertion and stabilization of the CcoN subunit of cbb3-Cox proceed in a manner independent of that of the c-type cytochrome subunits during cbb3-Cox biogenesis in R. capsulatus.
MATERIALS AND METHODS
Strains, culture conditions, and phenotypes.
The bacterial strains and plasmids used in this work are described in Table 1. R. capsulatus strains were grown in enriched medium (MPYE) (12) or Sistrom's minimal medium A (MedA) (13) supplemented with appropriate antibiotics (at final concentrations of 10, 10, 70, and 2.5 μg/ml for spectinomycin [Spe], kanamycin [Kan], rifampin [Rif], and tetracycline [Tet], respectively). Growth was chemoheterotrophic (aerobic respiration) or photoheterotrophic (anaerobic Ps) in anaerobic jars with H2- and CO2-generating gas packs from BBL Microbiology Systems (Cockeysville, MD) at 35°C. CuSO4 (5 μM) was added as needed to MPYE to prepare MPYE+Cu medium. Escherichia coli strains were grown at 37°C in Luria-Bertani (LB) broth supplemented with appropriate antibiotics (at final concentrations of 100, 50, 50, and 12.5 μg/ml for ampicillin [Amp], Kan, Spe, and Tet, respectively), as described previously (14). The Cox activity of R. capsulatus colonies was revealed using NADI, made by mixing a 1:1 (vol/vol) ratio of 35 mM α-naphthol and 30 mM N,N,N′,N′-dimethyl-p-phenylenediamine (DMPD), dissolved in ethanol and water, respectively (15).
Table 1.
Strains and plasmids used in this work
| Strain or plasmid | Description | Phenotype | Reference |
|---|---|---|---|
| Strains | |||
| E. colistrains | |||
| HB101 | F− Δ(gpt-proA)62 leuB6 supE44 ara-14 galK2 lacY1 Δ(mcrC-mrr) rpsL20 (Strr) xyl-5 mtl-1recA13 | Strr | 16 |
| XL1-Blue | F′::Tn10 proA+B+ lacIqZΔM15 recA1 endA1 gyrA96 (Nalr) thi hsdR17(rK− mK+) supE44 relA1 lac | Ampr | Stratagene |
| R. capsulatus strains | |||
| MT1131 | crtD121 | Wild type (NADI+), Rifr | 38 |
| Y262 | GTA overproducer | 18 | |
| MR1 | ccoO1 | NADI− on MPYE | 9 |
| GK2 | ccoO2 | NADI− on MPYE | 9 |
| SE8 | Δ(ccoA::spe) | NADI− on MPYE, NADIslow on MedA | 11 |
| GK32 | Δ(ccoNO::kan) | NADI− | 9 |
| MD12 | Δ(ccmF::spe) | Sper | 39 |
| MT-RBC1 | Δ(petABC::spe) | Sper | 12 |
| XJ3 | ccmA3 Δ(ccoA::spe) | Sper NADI− (+ or − Cu) Psslow | This work |
| XJ11 | ccmF11 Δ(ccoA::spe) | Sper NADI− (+ or − Cu) Psslow | This work |
| SE17 | Δ(ccmA::kan) Δ(ccoA::spe) | Kanr Sper NADI− Ps− | This work |
| SE18 | Δ(ccmB::kan) Δ(ccoA::spe) | Kanr Sper NADI− Ps− | This work |
| SE19 | Δ(ccmAB::kan) Δ(ccoA::spe) | Kanr Sper NADI− Ps− | This work |
| SE20 | Δ(ccmA::kan) | Kanr NADI− Ps− | This work |
| SE21 | Δ(ccmB::kan) | Kanr NADI− Ps− | This work |
| SE22 | Δ(ccmAB::kan) | Kanr NADI− Ps− | This work |
| Plasmids | |||
| pRK2013 | Conjugation helper | Kanr | 40 |
| pRK404 | Broad-host-range vector | Tetr | 40 |
| pRK415 | Broad-host-range vector | Tetr | 40 |
| pBluescript II KS(+) | Ampr | Stratagene | |
| pCW25 | ccoNOQP-ccoGHIS in pRK415 | Tetr | 23 |
| pMA117 | Carries nonpolar kan resistance cassette | Kanr | 17 |
| pSE3 | ccoA | Tetr | 11 |
| pSE10 | 7.6-kb chromosomal BamHI fragment on pRK415 | Tetr | This work |
| pSE11 | 4,912 bp deleted between XbaI and BstBI | Tetr | This work |
| pSE12 | 2,714 bp deleted between BstBI and KpnI | Tetr | This work |
| pSE14 | 2,389-bp XbaI-KpnI fragment containing ccmABcloned into XbaI and KpnI sites of pBluescript II KS(+) | Ampr | This work |
| pSE15 | kan cassette inserted at the BstEII site of ccmA | Ampr Kanr | This work |
| pSE16 | kan cassette inserted at the XhoI site of ccmB | Ampr Kanr | This work |
| pSE17 | 746 bp between BstEII and XhoI sites replaced by kan cassette | Ampr Kanr | This work |
| pSE21 | KpnI-XbaI fragment with Δ(ccmA::kan) in pRK415 | Kanr Tetr | This work |
| pSE22 | KpnI-XbaI fragment with Δ(ccmB::kan) in pRK415 | Kanr Tetr | This work |
| pSE23 | KpnI-XbaI fragment with Δ(ccmAB::kan) in pRK415 | Kanr Tetr | This work |
| pCS1582 | Strep-ccmF ccmH ccmI-FLAG+ | Tetr | 41 |
| pSVEN | ccmI | Tetr | 29 |
| pYZ4 | ccmF::spe ccmH | Sper Tetr | 39 |
| pYZ12 | ccmF ccmH::spe | Sper Tetr | 39 |
For testing of Cu2+, Mn2+, Fe3+, or Zn2+ sensitivity, appropriate strains were grown to an optical density at 630 nm (OD630) of ∼0.5 in MPYE medium. A total of 1.7 × 107 or 2.6 × 107 cells (estimated based on the following relationship: 1.0 OD630 unit = 7.5 × 108 R. capsulatus cells per ml), for respiratory or Ps growth conditions, respectively, was added to 4 ml of the same medium with 0.7% top agar and then poured on top of 10-ml MPYE medium-containing regular plates. Whatman 3MM paper discs (3-mm diameter), soaked with 8 μl per disc of the desired concentration of CuSO4, MnSO4, FeSO4, or ZnSO4 solution, were placed on top of the solidified top agar. Plates were incubated under the desired growth conditions, and the diameters of growth inhibition zones exhibited by different mutants were measured to estimate metal toxicity.
EMS mutagenesis.
Samples (1.5 ml) of two independent overnight cultures (10 ml each) of R. capsulatus strain SE8/pCW25 (Table 1) were centrifuged at 10,000 rpm for 2 min, and the pellets were resuspended together in 1 ml of 100 mM KH2PO4 buffer (pH 7.4). Cells were mutagenized by addition of 30 μl of ethyl methanesulfonate (EMS) from a 100 mM stock solution (Sigma-Aldrich, St. Louis, MO) and incubation at 35°C for 30 min and then diluted to yield ∼500 colonies per plate on MPYE medium containing 10 μM Cu2+. Plates were incubated for 2 days under respiratory growth conditions and screened using NADI staining, and colonies lacking cbb3-Cox were tested for Ps growth on MPYE medium at 35°C. NADI− Ps+ colonies were retained and cured of their plasmids (pCW25) (Table 1) by subculturing in the absence of the antibiotic selection (i.e., Tet) required for plasmid maintenance.
Molecular genetic techniques.
Standard molecular biological techniques were performed as previously described (16). All chromosomal insertion or insertion-deletion alleles were constructed by interposon mutagenesis using the Kanr cassette from pMA117 (17), which yields transcriptionally nonpolar insertions in the target genes (Table 1). Chromosomal knockout alleles of desired genes were constructed as described earlier (12), using the gene transfer agent (GTA) of R. capsulatus, which is a phage-like particle capable of transduction (18). Plasmid pSE10, which contained XbaI and KpnI restriction sites, was used to construct pSE11 by deleting the 4.9 kb between the XbaI and BstBI sites and pSE12 by deleting the 2.7 kb between the BstBI and KpnI sites. Plasmid pSE14 was obtained by amplification of a sequence from 334 bp 5′ (upstream) of ccmA to 448 bp 3′ (downstream) of ccmB, using pSE11 as the template and the primers CcmAB-Fwd (5′-AGC GCT CTA GAC GAT CGA CGG CTA CGG ACC C-3′) and CcmAB-Rev (5′-TTG GAG GGT ACT CCC ACC AGGTTC CCC ACAT-3′), containing 5′ XbaI and 3′ KpnI sites, respectively, with cloning of the amplified DNA fragment into the XbaI and KpnI sites of pBSII. The insertion alleles of ccmA and ccmB were obtained by ligating the Kanr cassette at XhoI and BstEII sites of pSE14 to yield pSE15 and pSE16, respectively. An insertion-deletion allele of ccmAB was also constructed by replacing the 746 bp between the XhoI and BstEII sites of pSE14 with the Kanr cassette, yielding pSE17. The XbaI-KpnI inserts of pSE15, pSE16, and pSE17 were subsequently cloned into the same sites of pRK415 to obtain pSE21, pSE22, and pSE23, respectively. The latter plasmids were used to construct the ccmA, ccmB, and ccmA ccmB knockout mutants SE17, SE18, and SE19, respectively, in the case of a ΔccoA (SE8) background, and SE20, SE21, and SE22, respectively, in the case of a wild-type (MT1131) background (Table 1). The DNA primers used for DNA sequence determination of ccmAB and ccmF are listed in Table S1 in the supplemental material.
SDS-PAGE, heme staining, and immunoblotting.
Intracytoplasmic membrane vesicles (chromatophore membranes) were prepared using a French pressure cell and 50 mM MOPS (morpholinepropanesulfonic acid; pH 7.0) containing 1 mM KCl and 1 mM phenylmethylsulfonyl fluoride (PMSF), as described earlier (19). Protein concentrations were determined using a bicinchoninic acid assay according to the supplier's recommendations (procedure TPRO-562; Sigma Inc.). For detection of the c-type cytochromes by their endogenous peroxidase activity, ∼50 μg of total membrane proteins was separated by 16.5% SDS-PAGE (20), and gels were stained with 3,3′,5,5′-tetramethylbenzidine (TMBZ) and H2O2 (21). For immunodetection, chromatophore membrane proteins were incubated at room temperature for 15 min in 62.5 mM Tris-HCl (pH 6.8), 2% (wt/vol) SDS, 25% (vol/vol) glycerol, 0.01% (wt/vol) bromophenol blue, and 5% β-mercaptoethanol prior to loading onto a 12% SDS-PAGE gel (22). The proteins were electroblotted onto Immobilon-P polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA) and probed with anti-R. capsulatus CcoN and CcoP rabbit polyclonal antibodies (23) and cytochrome c1 D42 monoclonal antibodies (12) and anti-Rubrivivax gelatinosus CcoO rabbit polyclonal antibody (a generous gift of A. Durand, CNRS, Orsay, France). Alkaline phosphatase-conjugated monoclonal anti-rabbit IgGs (Sigma-Aldrich, St. Louis, MO) were used as secondary antibodies with BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium) liquid substrate (Sigma-Aldrich, St. Louis, MO) for CcoN, CcoP, and CcoO immunoblots. Horseradish peroxidase-conjugated anti-mouse IgG antibodies (GE Healthcare) were used as secondary antibodies for cytochrome c1 immunoblots using SuperSignal West Pico chemiluminescence substrate from Thermo Scientific, Inc.
Determination of cellular Cu content by ICP-DRC-MS.
Total cellular Cu contents of various strains were determined using inductively coupled plasma-dynamic reaction cell mass spectrometry (ICP-DRC-MS) as described by Ekici et al. (11). For sample preparation, all containers, glassware, and tubes were washed with 2% nitric acid and rinsed with metal-free MilliQ water, at most 1 h prior to use, to prevent metal contamination. Metal-free water and buffers were prepared by stirring for 1 h at room temperature with 5 g Chelex 100 per liter. For each strain, a 1-liter culture was grown by respiration in MPYE medium to an OD630 of 0.8 to 0.9, and cells were harvested by centrifugation and washed three times with a metal-free buffer (20 mM Tris-HCl, pH 8.0) and once with metal-free MilliQ water. Cell pellets were lyophilized until complete dryness and shipped to Applied Speciation and Consulting, LLC, WA, for determination of total Cu and Mn contents, as described earlier (11). In each case, 50 mg of lyophilized cells was digested completely with aliquots of concentrated HNO3 and H2O2 at 95°C. The digests were diluted to a known final volume (50 ml) with metal-free reagent water and analyzed via ICP-DRC-MS according to a standard procedure of the company. The data were provided in μg of metal of interest per g of cells (ppm).
Chemicals.
All chemicals were reagent grade and were obtained from commercial sources.
RESULTS
“Cu-unresponsive” ΔccoA derivatives.
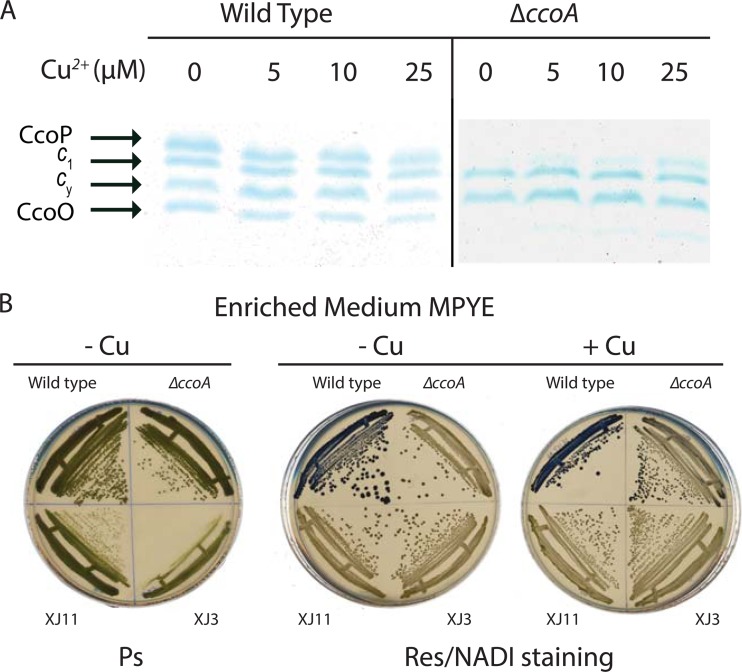
Mutants lacking CcoA (ΔccoA) displayed a NADI− phenotype and contained very small amounts of cbb3-Cox on growth media not supplemented with exogenous Cu2+ (e.g., enriched MPYE or MedA-Cu) (11). However, they exhibited a NADIslow phenotype when the growth medium contained a few micromolar Cu2+ (e.g., MPYE+Cu or MedA). For example, when cells were grown with a 5 μM Cu2+ supplement, the cbb3-Cox activity of a ΔccoA mutant (SE8) reached ∼30% of the wild-type activity. The amounts of CcoN (11) and the c-type cytochrome subunits CcoO and CcoP also increased gradually with increasing amounts (5, 10, or 25 μM) of Cu2+, whereas a slight decrease was seen with a wild-type strain under similar conditions (Fig. 1A). The increased cbb3-Cox activity observed in a ΔccoA mutant in response to Cu2+ supplementation does not derive from enhanced transcription or translation initiation of the structural genes (ccoNOQP) of this enzyme (9). Therefore, we hypothesized that another protein(s) can replace the function of CcoA when Cu is available at higher concentrations.
Fig 1.
cbb3-Cox subunit compositions and NADI phenotypes of the wild-type strain and the ccoA mutant and its derivatives. (A) Amounts of CcoP and CcoO subunits of cbb3-Cox in response to Cu2+ supplementation. Membrane-associated c-type cytochrome profiles are shown for the wild-type (MT1131) and ΔccoA (SE8) strains grown in MPYE medium supplemented with 0, 5, 10, or 25 μM Cu2+ under respiratory conditions at 35°C. Chromatophore membrane proteins (50 μg) were separated using 16.5% SDS-PAGE, and c-type cytochromes were visualized by TMBZ staining (see Materials and Methods). CcoO and CcoP are the c-type cytochrome subunits of cbb3-Cox, and c1 and cy refer to the cytochrome c1 subunit of cytochrome bc1 and the membrane-attached electron carrier cytochrome cy, respectively. (B) Ps growth and NADI phenotypes of Cu-unresponsive mutants XJ3 and XJ11. The wild type (MT1131), the ΔccoA mutant (SE8), and Cu-unresponsive ΔccoA derivatives XJ3 (ccmA3) and XJ11 (ccmF11) were grown on MPYE medium-containing plates under Ps and respiratory conditions, with 5 μM Cu2+ where indicated. The blue staining seen in the upper left quadrangle of each plate grown under respiratory conditions depicts the NADI+ phenotype of the wild-type strain.
To identify a protein(s) that can functionally substitute for CcoA in the presence of micromolar amounts of Cu supplementation, we chemically mutagenized the ΔccoA strain SE8 and screened for Cu-unresponsive derivatives (i.e., mutants that remained NADI− even in the presence of Cu2+ supplementation). In order to avoid mutating the cbb3-Cox structural genes (ccoNOQP) or the known assembly genes (ccoGHIS), we used strain SE8/pCW25, which carries those genes on a low-copy-number plasmid (23) (Table 1). Upon screening of ∼30,000 mutagenized R. capsulatus colonies on MPYE medium supplemented with 5 μM Cu2+, two completely NADI− (i.e., lacking cbb3-Cox activity) derivatives of SE8/pCW25 were found. These two Cu-unresponsive mutants were cured of pCW25 to yield the SE8 (ΔccoA) derivatives XJ3 and XJ11.
Under Ps growth conditions, the newly isolated Cu-unresponsive mutants formed colonies smaller than those of the wild-type strain after 2 days of incubation, although they could reach wild-type colony sizes upon longer incubations (Fig. 1B). The cbb3-Cox-negative phenotype of these mutants on Cu-containing media was not complemented with wild-type copies of olsAB (24), senC (25), or dsbA (26), genes known to affect cbb3-Cox biogenesis (data not shown), suggesting that the mutation(s) in XJ3 and XJ11 was located elsewhere. The Cu2+ tolerance (up to 1 mM) of XJ3 and XJ11 was comparable to that of SE8 (ΔccoA) or wild-type R. capsulatus, and thus different from those of previously identified ΔccoA suppressor mutants (11) showing enhanced Cu2+ sensitivity. The Cu-unresponsive mutants were also insensitive to Mn2+, Fe3+, or Zn2+ (tested like Cu2+, from 10 μM to 100 mM), and their cbb3-Cox phenotype was unaffected by addition of redox chemicals such as glutathione or cysteine/cystine to the growth medium (data not shown).
Cu-unresponsive mutants lack the c-type cytochrome subunits of cbb3-Cox but contain the catalytic subunit CcoN.
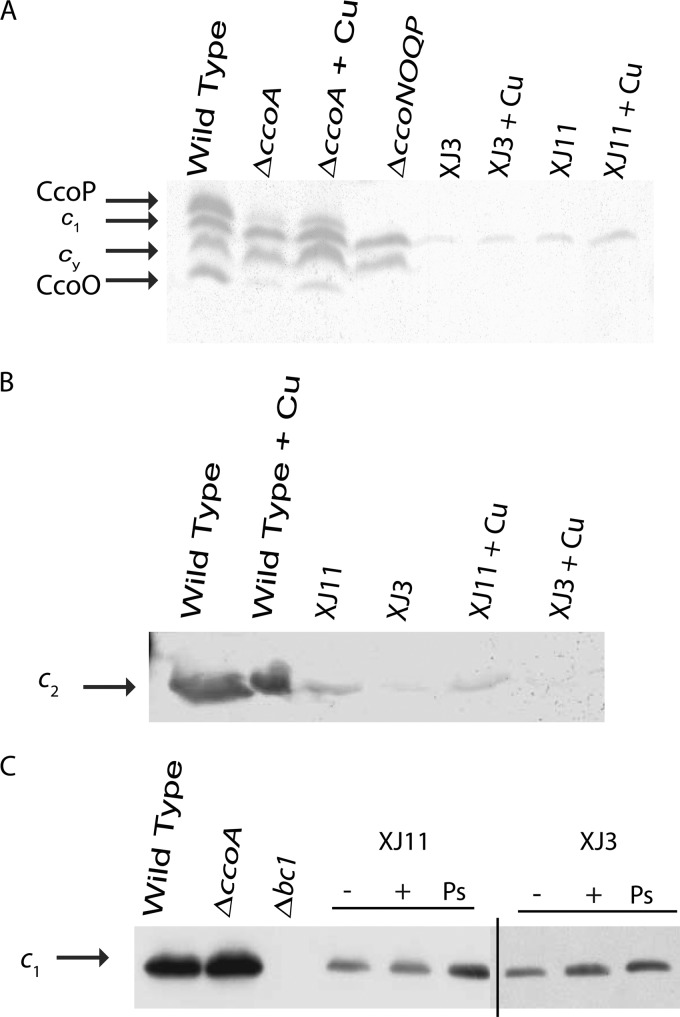
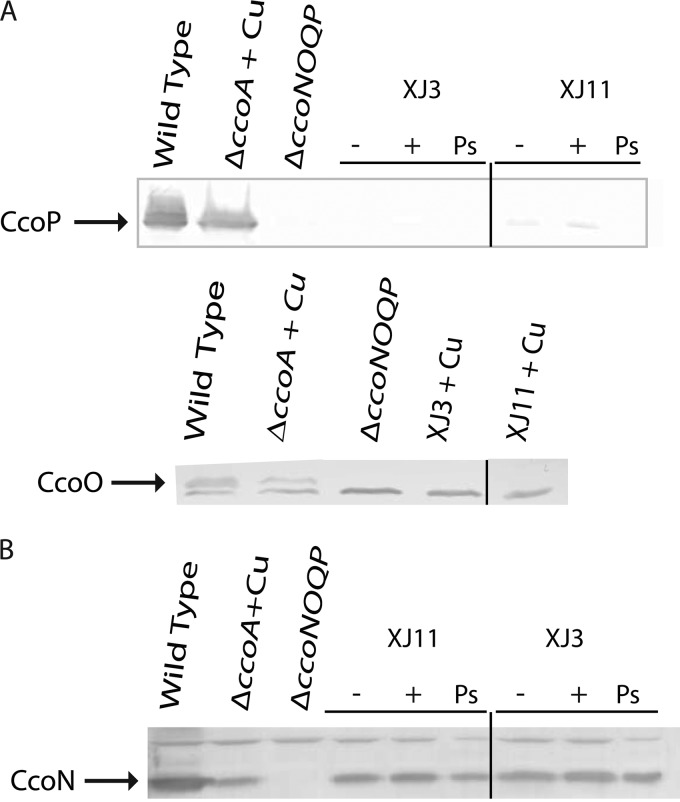
The c-type cytochrome profiles of the Cu-unresponsive mutants were determined using chromatophores prepared from cells grown in MPYE medium, with or without 5 μM Cu2+, under respiratory or Ps growth conditions. For membranes of a wild-type strain (MT1131), SDS-PAGE and TMBZ staining (see Materials and Methods) revealed four bands with molecular masses between 24 and 32 kDa, corresponding to the cytochromes cp (cbb3-Cox subunit), co (cbb3-Cox subunit), c1 (cytochrome bc1 subunit), and cy (membrane-anchored electron carrier) (Fig. 1A and 2A). In both XJ3 and XJ11, the cytochrome cp and co subunits were undetectable, but a faint band around 30 kDa, about the size of cytochrome c1, was present in cells grown with or without Cu2+. Similar TMBZ staining of the chromatophore supernatants, which contained the periplasmic contents of XJ3 and XJ11, indicated that the soluble electron carrier cytochrome c2 was present in reduced amounts compared to that in the wild-type strain (Fig. 2B). Immunoblot analyses with specific antibodies identified the 30-kDa band as cytochrome c1 (Fig. 2C) and confirmed the absence of cytochromes cp and co in both XJ3 and XJ11 (Fig. 3A). Thus, membranes of the Cu-unresponsive mutants XJ3 and XJ11 contained only small amounts of cytochromes c1 and c2, in agreement with their NADI− and slow-Ps-growth phenotypes. This finding indicates that in R. capsulatus, maturation of different c-type cytochromes requires different levels of Ccm efficiency under various physiological conditions. Remarkably, CcoN-specific antibodies revealed that, independent of Cu2+ supplementation, the catalytic subunit CcoN of cbb3-Cox was present in the chromatophore membranes of both the XJ3 and XJ11 mutants, despite the absence of the CcoP and CcoO subunits (Fig. 3B). In these mutants, the amounts of CcoN detected were comparable to those seen in a mutant lacking CcoA (SE8) and grown in the presence of Cu2+, which were lower than that seen in the wild-type strain.
Fig 2.
c-type cytochrome profiles of Cu-unresponsive mutants XJ3 and XJ11. (A) Membrane fractions (∼50 μg of total protein) of the wild-type (MT1131), ΔccoA (SE8), ΔccoNOQP (GK32), XJ3 (ccmA3), and XJ11 (ccmF11) strains grown under respiratory conditions in MPYE medium in the absence or presence (+ Cu) of 5 μM Cu2+ were analyzed by 16.5% SDS-PAGE, and the c-type cytochromes were revealed by TMBZ staining as described in Materials and Methods. (B) Chromatophore supernatants (∼100 μg of total protein) containing soluble cytochrome c2 of the wild-type (MT1131), XJ3 (ccmA3), and XJ11 (ccmF11) strains grown under respiratory conditions in MPYE medium in the absence (−) or presence (+) of 5 μM Cu2+ were analyzed by 18% SDS-PAGE, and the c-type cytochromes were revealed as described for panel A. (C) Membrane fractions (∼50 μg of total protein) of the wild-type (MT1131), ΔccoA (SE8), Δbc1 (MT-RBC1 lacking cytochrome bc1), XJ3 (ccmA3), and XJ11 (ccmF11) strains grown under photosynthetic (Ps) or respiratory conditions in MPYE medium in the absence (−) or presence (+) of 5 μM Cu2+ were analyzed by 12.5% SDS-PAGE, and the cytochrome c1 subunit of cytochrome bc1 was detected by specific antibodies as described in Materials and Methods.
Fig 3.
Cu-unresponsive mutants XJ3 and XJ11 contain CcoN in the absence of the CcoO and CcoP subunits of cbb3-Cox. Membrane fractions (∼50 μg of total protein) of the wild-type (MT1131), ΔccoA (SE8), ΔccoNOQP (GK32), XJ3 (ccmA3), and XJ11 (ccmF11) strains grown under Ps or respiratory conditions in MPYE medium in the absence (−) or presence (+ Cu) of 5 μM Cu2+ were analyzed by 12.5% SDS-PAGE, and the CcoP (A), CcoO (A), and CcoN (B) subunits of cbb3-Cox (indicated by arrows) were detected by respective antibodies against these subunits as described in Materials and Methods. The bands common to all lanes correspond to nonspecific proteins detected by the antibodies used.
Cu contents of Cu-unresponsive mutants.
R. capsulatus mutants lacking CcoA had lower cellular Cu contents as determined by ICP-DRC-MS measurements (11), which was also observed with GK32, a mutant lacking cbb3-Cox (Table 2 [data are normalized to Mn content]). For the Cu-unresponsive mutants XJ3 and XJ11, we expected lower cellular Cu contents, as suggested by their NADI− phenotype. Surprisingly, though, the total cellular Cu content of XJ3 was similar to that seen in a ΔccoA mutant grown in MPYE medium with or without a 5 μM Cu2+ supplement (Table 2), suggesting that its defect(s) is unrelated to Cu homeostasis. On the other hand, the total cellular Cu content of the XJ11 mutant in the absence of Cu2+ supplementation was comparable to that of a ΔccoA mutant, but it showed only a weak increase when Cu2+ was added (Table 2), a behavior which was clearly different from that of the wild-type, SE8, and XJ3 strains. In summary, the data suggest that cellular Cu homeostasis in XJ3 is not significantly altered in comparison to that of the parent strain, SE8, whereas the mutation in XJ11 somehow interferes with Cu2+ acquisition in the absence of CcoA.
Table 2.
Metal contents of wild-type strain (MT1131), ΔccoA strain (SE8), and Cu-unresponsive mutants XJ3 and XJ11 as determined by ICP-DRC-MS
| Strain | Presence of Cu2+ (5 μM)a | Metal content (%)b |
Cu/Mn ratio | |
|---|---|---|---|---|
| Cu | Mn | |||
| Wild type (MT1131) | − | 100 | 100 | 1.00 |
| + | 330 | 107 | 3.08 | |
| ΔccoA strain (SE8) | − | 77 | 110 | 0.70 |
| + | 292 | 112 | 2.60 | |
| XJ3 | − | 89 | 110 | 0.81 |
| + | 312 | 122 | 2.56 | |
| XJ11 | − | 92 | 129 | 0.71 |
| + | 134 | 126 | 1.06 | |
| Δcbb3 strain (GK32) | − | 94 | 140 | 0.67 |
| + | 347 | 140 | 2.47 | |
− and +, absence and presence, respectively, of 5 μM Cu2+ supplement during growth by respiration in MPYE medium as described in Materials and Methods.
See Materials and Methods for details on the determination of the amounts of Cu and Mn in cells. A content of 100% corresponds to 11.4 and 6.7 μg of Cu and Mn, respectively, per gram of lyophilized cells in all cases.
The mutation in the Cu-unresponsive XJ3 strain maps to the Ccm system.
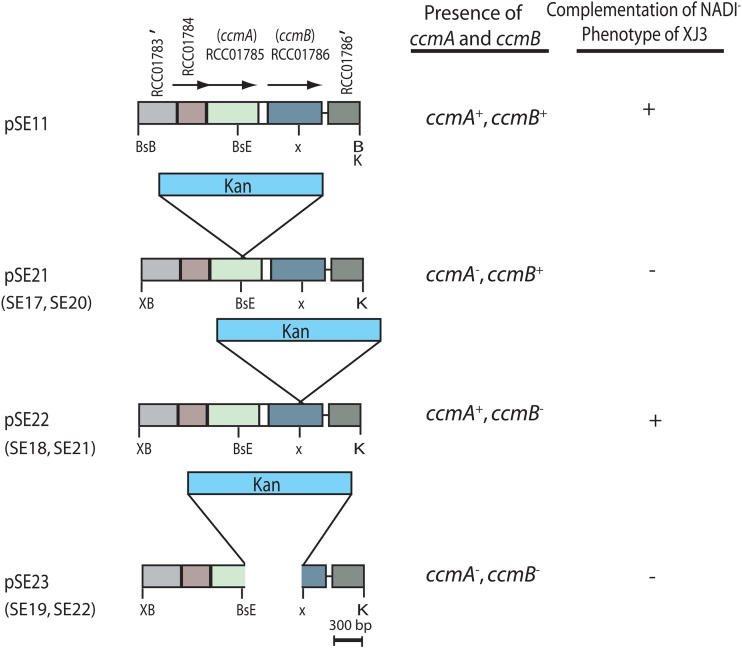
Conjugally transferable R. capsulatus genomic libraries were used to complement the NADI− phenotype and locate the gene(s) defective in the Cu-unresponsive XJ3 and XJ11 mutants. A plasmid called pSE10, carrying a single BamHI fragment of 7.6 kb, was obtained using the XJ3 strain as the recipient (see Fig. S1 in the supplemental material). This plasmid restored the NADI− phenotype of XJ3 but failed to do so for XJ11 on Cu-containing media, suggesting that the mutation(s) in XJ3 was different from that in XJ11. The DNA sequences determined from the ends of this insert and comparisons with the R. capsulatus genome (http://www.ncbi.nlm.nih.gov) indicated that pSE10 contained eight intact open reading frames (ORFs), annotated as follows: MazG family protein gene (RCC01779); peptidase, M20 family, amidohydrolase gene (RCC01780); protein translocase YajC subunit gene (RCC01781); protein export membrane protein gene (secD) (RCC01782); protein export membrane protein gene (secF) (RCC01783); gene for a protein of unknown function (DUF498) (RCC01784); heme exporter protein A gene (ccmA) (RCC01785); and heme exporter protein B gene (ccmB) (RCC01786). There was also a partial ORF corresponding to the heme exporter protein C gene (ccmC) (RCC01787) (see Fig. S1). Further subcloning of the DNA insert carried by pSE10 and subsequent conjugation experiments indicated that the mutation(s) in XJ3 was confined to ccmA and ccmB, carried by the pSE11 plasmid (Fig. 4). The ccmABCD genes are predicted to encode an ATP-binding cassette (ABC)-containing transporter which is essential for the Ccm process (4, 27, 28).
Fig 4.
Restriction map of plasmid pSE11 and its derivatives, used to complement XJ3 and to construct chromosomal knockout alleles. Plasmid pSE11 and its pSE21, pSE22, and pSE23 derivatives, constructed as described in Materials and Methods, are shown on the left, and their ability to complement the NADI phenotype of the Cu-unresponsive ΔccoA derivative XJ3 is shown on the right. When appropriate, R. capsulatus strains SE17, SE18, and SE19, as well as SE20, SE21, and SE22, carrying appropriate ccmA, ccmB, and ccmAB chromosomal knockout alleles, respectively, are indicated under the related plasmids. See the text for information on the ORFs annotated RCC01783, RCC01784, RCC01785, and RCC01786 in pSE11. BsE, BsB, X, XB, and K correspond to the restriction endonuclease sites for the BstEII, BstBI, XhoI, XbaI, and KpnI enzymes, respectively. A prime symbol indicates a partial gene.
In order to define the gene carrying the mutation(s) in the XJ3 mutant, three derivatives of pSE11 were constructed, yielding the plasmids pSE21 [Δ(ccmA::kan)], pSE22 [Δ(ccmB::kan)], and pSE23 [Δ(ccmAB::kan)], by using a nonpolar Kanr cassette. Of these plasmids, only pSE22 complemented the NADI− phenotype of XJ3, indicating that ccmA was the culprit (Fig. 4). On the R. capsulatus MT1131 genome, ccmA and ccmB are separated from each other by only 119 bp. Thus, it was necessary to assess whether the Δ(ccmA::kan) allele had a polar effect on ccmB. The plasmids pSE21 [Δ(ccmA::kan)] and pSE22 [Δ(ccmB::kan)] complemented the CcmB− (SE21) and CcmA− (SE20) mutants, respectively (Table 1), establishing that the Δ(ccmA::kan) allele did not have a polar effect on ccmB and that the mutation(s) in XJ3 was in ccmA. DNA sequencing of the ccmAB loci revealed that XJ3 carried a single base change (C to T) at position 211 of ccmA, changing alanine 71 to valine in a conserved region of CcmA (see Fig. S2A in the supplemental material). R. capsulatus mutants lacking CcmA are unable to produce any c-type cytochromes and hence are NADI− Ps− (27). The double mutant XJ3, carrying the ΔccoA mutation and the missense mutation (A71V) in ccmA, was NADI− but grew slowly by photosynthesis (Fig. 1B). Phenotypic comparisons (protoporphyrin secretion, NADI and Ps growth, and c-type cytochrome production) of null (SE17 ΔccmA::kan ΔccoA) and missense (XJ3 ccmA3 ΔccoA) alleles of ccmA indicated that the Ccm process was partly active in XJ3 and allowed limited production of cytochromes c1 and c2.
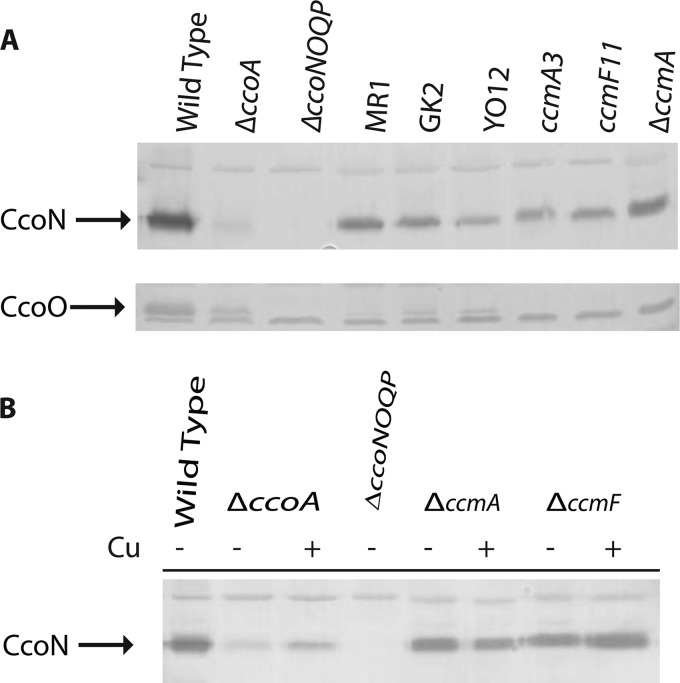
The identification of XJ3 as a ccmA mutant was unexpected because CcoN was detectable in both mutants, and earlier data had suggested that CcoN is not stable in the absence of the CcoO and CcoP subunits. Previously, we were unable to detect CcoN in a mutant (MR1) carrying a point mutation (encoding an Arg107Trp substitution) in ccoO, encoding CcoO (9). We therefore reexamined this strain by using CcoN antibodies in this work, together with another mutant (GK2) carrying an unknown ccoO allele and a double mutant (YO12) (Yavuz Ozturk and Fevzi Daldal, unpublished data) carrying both a deletion [Δ(petABC::spe)] of the cytochrome bc1 structural genes and an insertion (ccoP::kan) initially carried by MG1 (9). The CcoN contents of the two ccoO mutants (MR1 and GK2) and the ccoP mutant YO12 were comparable to that of XJ3 (Fig. 5A). Although the CcoN content was slightly lower than that in wild-type membranes, the data demonstrated that this catalytic subunit of cbb3-Cox was stable in the absence of the c-type cytochrome subunits CcoO and CcoP.
Fig 5.
Amounts of CcoN present in the membranes of various cbb3-Cox mutants and CcmA and CcmF knockout mutants of R. capsulatus. (A) Membrane fractions (∼50 μg of total protein) of the wild-type (MT1131), ΔccoA (SE8), ΔccoNOQP (GK32), MR1 (ccoO1), GK2 (ccoO2), YO12 (ΔpetABC::spe ccoP1), ccmA3 (XJ3), ccmF11 (XJ11), and ΔccoA ΔccmA (SE17) strains grown under respiratory conditions in MPYE medium in the absence of 5 μM Cu2+ were analyzed by 12.5% SDS-PAGE, and the CcoN and CcoO subunits of cbb3-Cox (indicated by arrows) were detected by specific antibodies as described in Materials and Methods. The band common to all lanes corresponds to a nonspecific protein detected by the antibodies used. (B) Membrane fractions (∼50 μg of total protein) of the wild-type (MT1131), ΔccoA (SE8), ΔccoNOQP (GK32), ΔccmA (SE20), and ΔccmF (MD12) strains grown under respiratory conditions in MPYE medium in the absence (−) or presence (+ Cu) of 5 μM Cu2+ were analyzed by 12.5% SDS-PAGE, and the CcoN subunit of cbb3-Cox (indicated by an arrow) was detected by subunit-specific antibodies as described in Materials and Methods. The bands common to all lanes correspond to nonspecific proteins detected by the antibodies used.
Defining the mutation(s) in the XJ11 mutant.
Knowing that the genetic defect in XJ11 was different from that in XJ3 (ccmA3) (Fig. 4 and Table 2), we tested whether any of the remaining ccm genes was responsible for its defect. Complementation assays using plasmids carrying different ccm genes (see Fig. S3 in the supplemental material) revealed that plasmid pYZ12, containing only ccmF, complemented XJ11 for production of active cbb3-Cox on Cu2+-supplemented media. DNA sequencing of the ccmF locus of XJ11 revealed a single G-to-A base pair substitution at position 803 of ccmF, resulting in the replacement of a highly conserved arginine with a histidine at position 268 of CcmF, located in a cytoplasmic loop between the fifth and sixth predicted transmembrane helices (see Fig. S2B). Thus, like that of ccmA, inactivation of ccmF also did not interfere with the steady-state presence of the CcoN subunit of cbb3-Cox in membranes (Fig. 3).
The CcoN subunit of cbb3-Cox is produced in the absence of the Ccm process.
The Cu-unresponsive mutants XJ3 and XJ11 revealed that CcoN was present in membranes in the absence of the CcoO and CcoP subunits of cbb3-Cox (Fig. 3). However, in addition to the missense ccmA3 and ccmF11 mutations, these double mutants contained a ΔccoA deletion known to interfere with cbb3-Cox biogenesis (11). Thus, whether the presence of the CcoN subunit of cbb3-Cox in these mutants was a consequence of a combined effect of these two mutations, i.e., with one affecting Cu transport due to the absence of CcoA and the other differentially decreasing the Ccm efficiency, could not be defined unambiguously. For direct comparisons, ccmA and ccmB knockout mutants were constructed in both ΔccoA (SE8) and wild-type (MT1131) backgrounds by interposon mutagenesis using the nonpolar Δ(ccmA::Kan), Δ(ccmB::Kan), and Δ(ccmAB::Kan) alleles (Fig. 4). These mutants were unable to produce any c-type cytochromes and were completely NADI− Ps− on both minimal and enriched media, indicating that the presence of a ΔccoA null mutation did not interfere with the growth phenotypes of the ccmA and ccmB knockout mutants. The steady-state amounts of CcoN were determined for SE20 (ccmA::kan) and MD12 (ccmF::spe), carrying only the ccmA and ccmF null alleles, respectively. The data indicated that, similar to XJ3 and XJ11 (Fig. 3), the single Ccm knockout mutants (SE20 and MD12) lacking all c-type cytochromes (i.e., cytochromes cp, c1, cy, and co) still produced quasi-wild-type amounts of CcoN (Fig. 5B). We therefore concluded that an absence of Ccm activity did not affect CcoN production, further confirming that two maturation systems (i.e., the Cco system for CcoN maturation and the Ccm system for CcoO and CcoP maturation) cooperate during cbb3-Cox biogenesis in R. capsulatus.
DISCUSSION
We previously reported that CcoA is a novel transporter involved in cbb3-Cox biogenesis and demonstrated that mutants lacking it had low cellular Cu contents and very small amounts of cbb3-Cox activity (11). In such strains, CcoA-independent Cu acquisition and delivery to cbb3-Cox still occurred upon addition of exogenous Cu2+. In order to gain insight into this pathway, we isolated mutants that were unresponsive to Cu2+ supplementation. Interestingly, molecular characterizations of two such mutants, XJ3 and XJ11, revealed that they were defective for c-type cytochrome maturation and led to unexpected findings.
First, the Cu-unresponsive mutants contained a missense allele of ccmA or ccmF and exhibited slow Ps growth but no Cox activity. They produced small amounts of cytochromes c1 and c2 but completely lacked the cytochrome co and cp subunits of cbb3-Cox, even though they produced its catalytic subunit, CcoN. These findings indicated that different degrees of Ccm efficiency are required for the production of various c-type cytochromes. R. capsulatus Ccm mutants usually abolish the biosynthesis of all c-type cytochromes (except for CcmI [29]), unlike the Cu-unresponsive mutants studied here. Why the production of different c-type cytochromes requires different degrees of Ccm efficiency is unknown. A possibility is that the Ccm machinery shows different affinities for different apocytochromes and that this preference becomes more pronounced upon compromised Ccm efficiency. A related possibility is that different apocytochromes also use different membrane insertion pathways. Upon decreased Ccm efficiency, the c-type apocytochromes that follow posttranslational (i.e., the carboxyl-terminally anchored cytochrome c1) or cotranslational (i.e., the amino-terminally anchored cytochromes cp, co, and cy) membrane insertion might exhibit different degrees of proteolytic susceptibilities if they are not matured effectively (30, 31), leading to different steady-state levels under distinct physiological conditions.
The second, and perhaps most striking, finding of this work was the observation, for the first time, that the production, membrane insertion, and stability of the R. capsulatus cbb3-Cox CcoN subunit are completely independent of the Ccm process. Both the Cu-unresponsive mutants carrying a ccmA or ccmF missense allele in a ΔccoA background and the ΔccmA or ΔccmF single knockout mutant carrying a wild-type allele of ccoA completely abolished the production of the c-type cytochrome subunits CcoO and CcoP, without affecting the CcoN subunit of cbb3-Cox. Clearly, the transcription, translation, and membrane insertion of CcoN proceeded in the absence of the Ccm process, without being affected by the absence of CcoA, required for normal production of cbb3-Cox (11). It is not known whether the CcoN thus produced interacts with other assembly components (e.g., CcoH) (32) or contains its heme b and Cu cofactors, as these topics were beyond the scope of this work.
Upon reexamination of previously isolated ccoO and ccoP mutants with the CcoN antibodies used in this work, we found that they all contained some amount of CcoN, like the Cu-unresponsive mutants. Thus, mutations affecting biosynthesis of the ccoO or ccoP gene product also behave like the ccm mutations that abolish their subsequent modification(s). We note that these two processes are distinct and, conceivably, that different steady-state amounts of CcoN might be found in the membranes, depending on the nature of the mutation(s). Because CcoN together with CcoO forms a stable CcoNO subcomplex (8, 32), it appears that CcoN awaits the completion of its own maturation process when CcoO is absent. Conversely, when a mature CcoO protein is produced, its association with immature CcoN (as occurs in the absence of CcoA [11] or CcoI [23]) presumably leads to its degradation.
Biogenesis of cbb3-Cox is a complex process (7, 8), and independent maturation pathways need to be coordinated spatially and temporally to produce an active enzyme. The CcoO and CcoP subunits are matured via the Ccm pathway, while the heme b3-CuB center of the catalytic subunit CcoN relies on a less-well-understood pathway for insertion of heme b and Cu cofactors (7). The similarities of the cbb3-Cox phenotypes of CcoA (11), SenC (33), and CcoI (23) mutants and their plausible link(s) to cellular Cu traffic suggest that mutants lacking the Cu atom of CcoN induce degradation of the c-type cytochrome subunits of the enzyme, unlike CcoS mutants that lack both the heme b and the Cu atom of CcoN (23).
Finally, it is intriguing that the Cu-unresponsive mutants affected several of the heme-handling components of Ccm. CcmA is part of the ABC transporter CcmAB, which releases heme-loaded CcmE from its heme ligation partners, CcmCD (34), and CcmF is involved in the transfer of heme from CcmE to an apocytochrome (35). Earlier, using a Pseudomonas fluorescens strain isolated from Cu-contaminated soil (36), CcmI and CcmF were found to be required for resistance to Cu. The lack of an increase of cellular Cu content in the presence of an exogenous Cu supplement, as seen here with XJ11 carrying the ccmF11 mutation, also raises the question of whether CcmF interferes with cellular Cu homeostasis. Moreover, we observed that upon Cu2+ supplementation of the growth medium, excretion of porphyrin derivatives seen with the Ccm knockout mutants became less pronounced (unpublished data). Clearly, these observations suggest some link(s) between Cu, heme, and Ccm (37) and deserve future investigations.
In summary, a search for Cu-unresponsive derivatives of a mutant lacking CcoA, undertaken to isolate an additional protein(s) involved in Cu homeostasis and delivery to cbb3-Cox, yielded Ccm missense mutants. These mutants displayed a cbb3-Cox-negative phenotype but contained small amounts of cytochromes c1 and c2 and significant amounts of the CcoN subunit of cbb3-Cox in the membranes. These findings indicate that in R. capsulatus, the available Ccm capacity exerts a differential control over the production of various c-type cytochromes under physiological conditions, and that membrane insertion and stability of the CcoN subunit of cbb3-Cox occur independently of the Ccm process. The availability of strains producing only the CcoN subunit of cbb3-Cox in the absence of its c-type cytochrome partners might be invaluable for future studies addressing how, when, and in which order the heme and Cu cofactors are inserted into CcoN during cbb3-Cox biogenesis. Similarly, identification of the alternative Cu acquisition pathway(s) that operates in the absence of CcoA should be informative about Cu homeostasis and toxicity in R. capsulatus and related species.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant GM 38237 and by the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences, U.S. Department of Energy, through grant DE-FG02-91ER20052 (for isolation and study of the mutants and related proteins) to F.D. It was also supported by the Deutsche Forschungsgemeinschaft (grants DFG-GRK1478 and DFG-FOR 929 to H.-G.K.) and by grants from the German-French-University (DFH) Ph.D. College on Membranes and Membrane Proteins to H.-G.K.
Footnotes
Published ahead of print 2 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01415-12.
REFERENCES
- 1. Garcia-Horsman JA, Barquera B, Rumbley J, Ma J, Gennis RB. 1994. The superfamily of heme-copper respiratory oxidases. J. Bacteriol. 176:5587–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pereira MM, Santana M, Teixeira M. 2001. A novel scenario for the evolution of haem-copper oxygen reductases. Biochim. Biophys. Acta 1505:185–208 [DOI] [PubMed] [Google Scholar]
- 3. Gray KA, Grooms M, Myllykallio H, Moomaw C, Slaughter C, Daldal F. 1994. Rhodobacter capsulatus contains a novel cb-type cytochrome c oxidase without a CuA center. Biochemistry 33:3120–3127 [DOI] [PubMed] [Google Scholar]
- 4. Kranz RG, Richard-Fogal C, Taylor JS, Frawley ER. 2009. Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol. Mol. Biol. Rev. 73:510–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanders C, Turkarslan S, Lee DW, Daldal F. 2010. Cytochrome c biogenesis: the Ccm system. Trends Microbiol. 18:266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters A, Kulajta C, Pawlik G, Daldal F, Koch HG. 2008. Stability of the cbb3-type cytochrome oxidase requires specific CcoQ-CcoP interactions. J. Bacteriol. 190:5576–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ekici S, Pawlik G, Lohmeyer E, Koch HG, Daldal F. 2012. Biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. Biochim. Biophys. Acta 1817:898–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kulajta C, Thumfart JO, Haid S, Daldal F, Koch HG. 2006. Multi-step assembly pathway of the cbb3-type cytochrome c oxidase complex. J. Mol. Biol. 355:989–1004 [DOI] [PubMed] [Google Scholar]
- 9. Koch HG, Hwang O, Daldal F. 1998. Isolation and characterization of Rhodobacter capsulatus mutants affected in cytochrome cbb3 oxidase activity. J. Bacteriol. 180:969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanders C, Deshmukh M, Astor D, Kranz RG, Daldal F. 2005. Overproduction of CcmG and CcmFH(Rc) fully suppresses the c-type cytochrome biogenesis defect of Rhodobacter capsulatus CcmI-null mutants. J. Bacteriol. 187:4245–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ekici S, Yang H, Koch HG, Daldal F. 2012. Novel transporter required for biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. mBio 3:293–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atta-Asafo-Adjei E, Daldal F. 1991. Size of the amino acid side chain at position 158 of cytochrome b is critical for an active cytochrome bc1 complex and for photosynthetic growth of Rhodobacter capsulatus. Proc. Natl. Acad. Sci. U. S. A. 88:492–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sistrom WR. 1960. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J. Gen. Microbiol. 22:778–785 [DOI] [PubMed] [Google Scholar]
- 14. Myllykallio H, Zannoni D, Daldal F. 1999. The membrane-attached electron carrier cytochrome cy from Rhodobacter sphaeroides is functional in respiratory but not in photosynthetic electron transfer. Proc. Natl. Acad. Sci. U. S. A. 96:4348–4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marrs B, Gest H. 1973. Genetic mutations affecting the respiratory electron-transport system of the photosynthetic bacterium Rhodopseudomonas capsulata. J. Bacteriol. 114:1045–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 17. Daldal F, Cheng S, Applebaum J, Davidson E, Prince RC. 1986. Cytochrome c2 is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc. Natl. Acad. Sci. U. S. A. 83:2012–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yen HC, Hu NT, Marrs BL. 1979. Characterization of the gene transfer agent made by an overproducer mutant of Rhodopseudomonas capsulata. J. Mol. Biol. 131:157–168 [DOI] [PubMed] [Google Scholar]
- 19. Valkova-Valchanova M, Darrouzet E, Moomaw CR, Slaughter CA, Daldal F. 2000. Proteolytic cleavage of the Fe-S subunit hinge region of Rhodobacter capsulatus bc1 complex: effects of inhibitors and mutations. Biochemistry 39:15484–15492 [DOI] [PubMed] [Google Scholar]
- 20. Schagger H, von Jagow G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368–379 [DOI] [PubMed] [Google Scholar]
- 21. Thomas PE, Ryan D, Levin W. 1976. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal. Biochem. 75:168–176 [DOI] [PubMed] [Google Scholar]
- 22. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 23. Koch HG, Winterstein C, Saribas AS, Alben JO, Daldal F. 2000. Roles of the ccoGHIS gene products in the biogenesis of the cbb3-type cytochrome c oxidase. J. Mol. Biol. 297:49–65 [DOI] [PubMed] [Google Scholar]
- 24. Aygun-Sunar S, Mandaci S, Koch HG, Murray IV, Goldfine H, Daldal F. 2006. Ornithine lipid is required for optimal steady-state amounts of c-type cytochromes in Rhodobacter capsulatus. Mol. Microbiol. 61:418–435 [DOI] [PubMed] [Google Scholar]
- 25. Swem DL, Swem LR, Setterdahl A, Bauer CE. 2005. Involvement of SenC in assembly of cytochrome c oxidase in Rhodobacter capsulatus. J. Bacteriol. 187:8081–8087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Onder O, Turkarslan S, Sun D, Daldal F. 2008. Overproduction or absence of the periplasmic protease DegP severely compromises bacterial growth in the absence of the dithiol:disulfide oxidoreductase DsbA. Mol. Cell. Proteomics 7:875–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beckman DL, Trawick DR, Kranz RG. 1992. Bacterial cytochromes c biogenesis. Genes Dev. 6:268–283 [DOI] [PubMed] [Google Scholar]
- 28. Goldman BS, Beckman DL, Bali A, Monika EM, Gabbert KK, Kranz RG. 1997. Molecular and immunological analysis of an ABC transporter complex required for cytochrome c biogenesis. J. Mol. Biol. 268:724–738 [DOI] [PubMed] [Google Scholar]
- 29. Lang SE, Jenney FE, Jr, Daldal F. 1996. Rhodobacter capsulatus CycH: a bipartite gene product with pleiotropic effects on the biogenesis of structurally different c-type cytochromes. J. Bacteriol. 178:5279–5290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koch HG, Schneider D. 2007. Folding, assembly, and stability of transmembrane cytochromes. Curr. Chem. Biol. 1:59–74 [Google Scholar]
- 31. Luirink J, Yu Z, Wagner S, de Gier JW. 2012. Biogenesis of inner membrane proteins in Escherichia coli. Biochim. Biophys. Acta 1817:965–976 [DOI] [PubMed] [Google Scholar]
- 32. Pawlik G, Kulajta C, Sachelaru I, Schroder S, Waidner B, Hellwig P, Daldal F, Koch HG. 2010. The putative assembly factor CcoH is stably associated with the cbb3-type cytochrome oxidase. J. Bacteriol. 192:6378–6389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lohmeyer E, Schroder S, Pawlik G, Trasnea PI, Peters A, Daldal F, Koch HG. 2012. The ScoI homologue SenC is a copper binding protein that interacts directly with the cbb3-type cytochrome oxidase in Rhodobacter capsulatus. Biochim. Biophys. Acta 1817:2005–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feissner RE, Richard-Fogal CL, Frawley ER, Kranz RG. 2006. ABC transporter-mediated release of a haem chaperone allows cytochrome c biogenesis. Mol. Microbiol. 61:219–231 [DOI] [PubMed] [Google Scholar]
- 35. Ren Q, Ahuja U, Thony-Meyer L. 2002. A bacterial cytochrome c heme lyase. CcmF forms a complex with the heme chaperone CcmE and CcmH but not with apocytochrome c. J. Biol. Chem. 277:7657–7663 [DOI] [PubMed] [Google Scholar]
- 36. Yang CH, Azad HR, Cooksey DA. 1996. A chromosomal locus required for copper resistance, competitive fitness, and cytochrome c biogenesis in Pseudomonas fluorescens. Proc. Natl. Acad. Sci. U. S. A. 93:7315–7320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cianciotto NP, Cornelis P, Baysse C. 2005. Impact of the bacterial type I cytochrome c maturation system on different biological processes. Mol. Microbiol. 56:1408–1415 [DOI] [PubMed] [Google Scholar]
- 38. Scolnik PA, Walker MA, Marrs BL. 1980. Biosynthesis of carotenoids derived from neurosporene in Rhodopseudomonas capsulata. J. Biol. Chem. 255:2427–2432 [PubMed] [Google Scholar]
- 39. Deshmukh M, May M, Zhang Y, Gabbert KK, Karberg KA, Kranz RG, Daldal F. 2002. Overexpression of ccl1-2 can bypass the need for the putative apocytochrome chaperone CycH during the biogenesis of c-type cytochromes. Mol. Microbiol. 46:1069–1080 [DOI] [PubMed] [Google Scholar]
- 40. Ditta G, Schmidhauser T, Yakobson E, Lu P, Liang XW, Finlay DR, Guiney D, Helinski DR. 1985. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid 13:149–153 [DOI] [PubMed] [Google Scholar]
- 41. Sanders C, Turkarslan S, Lee DW, Onder O, Kranz RG, Daldal F. 2008. The cytochrome c maturation components CcmF, CcmH, and CcmI form a membrane-integral multisubunit heme ligation complex. J. Biol. Chem. 283:29715–29722 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.