Abstract
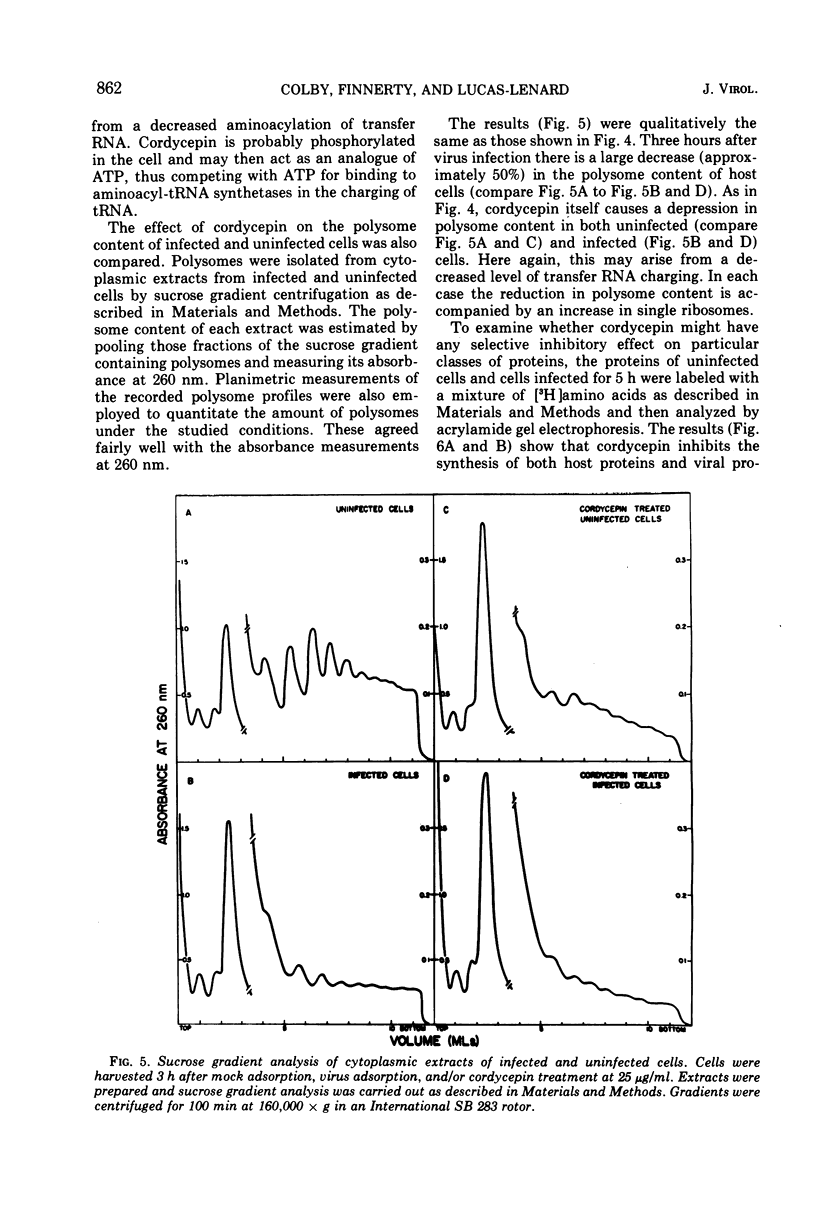
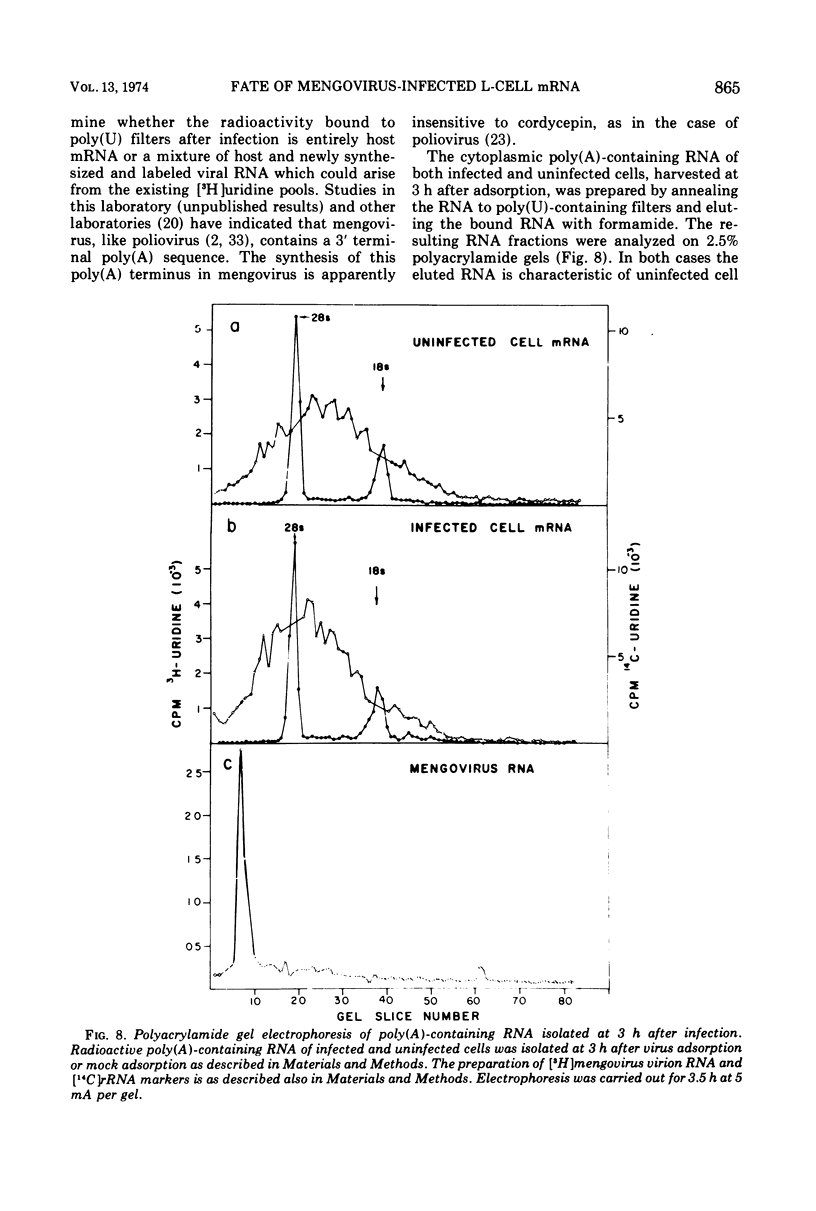
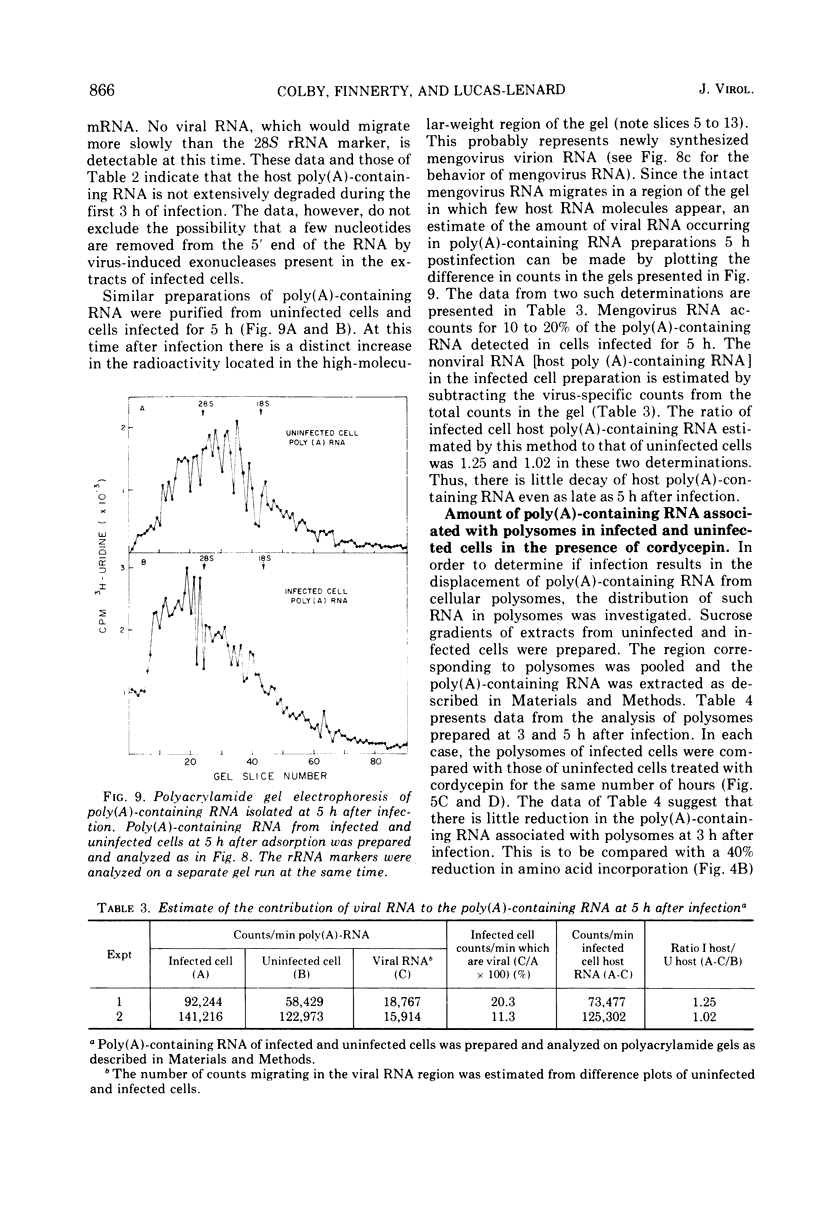
Mengovirus infection of L-cells results in an inhibition of host protein synthesis which is detectable in vivo by a decreased rate of incorporation of radioactive amino acids into acid-insoluble material and by a concomitant reduction in polysome content. The inhibition of host protein synthesis occurs early in the infection cycle, at a time when there is little synthesis of viral proteins. In this paper the stability of polyadenylic acid [poly(A)]-containing mRNA of uninfected L-cells and cells infected with mengovirus is compared. Our results suggest that there is no increase in the rate of degradation of cellular mRNA upon virus infection. The continued integrity of host mRNA throughout infection was verified by acrylamide gel electrophoresis.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Edmonds M., Nakazato H., Phillips B. A., Vaughn M. H. Polyadenylic acid sequences in the virion RNA of poliovirus and Eastern Equine Encephalitis virus. Science. 1972 May 5;176(4034):526–528. doi: 10.1126/science.176.4034.526. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras G., Cárdenas T., Grado C., Yudelevich S., Summers D. F. Modifications of polysome-associated RNA after poliovirus infection. Basic Life Sci. 1973;1:445–457. doi: 10.1007/978-1-4684-0877-5_34. [DOI] [PubMed] [Google Scholar]
- Dalgarno L., Cox R. A., Martin E. M. Polyribosomes in normal Krebs 2 ascites tumor cells and in cells infected with encephalomyocarditis virus. Biochim Biophys Acta. 1967 Apr 18;138(2):316–328. doi: 10.1016/0005-2787(67)90492-3. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENWICK M. L. The influence of poliovirus infection of RNA synthesis in mammalian cells. Virology. 1963 Mar;19:241–249. doi: 10.1016/0042-6822(63)90061-8. [DOI] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Simple method for the isolation of encephalomyocarditis virus ribonucleic acid. J Virol. 1972 Mar;9(3):559–561. doi: 10.1128/jvi.9.3.559-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz R., Penman S. Regulation of protein synthesis in HeLa cells. 3. Inhibition during poliovirus infection. J Virol. 1971 Nov;8(5):661–668. doi: 10.1128/jvi.8.5.661-668.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Canellakis E. S. Adenine-rich polymer associated with rabbit reticulocyte messenger RNA. Nature. 1970 Aug 15;227(5259):710–712. doi: 10.1038/227710a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lust G. Alterations of protein synthesis in arbovirus-infected L cells. J Bacteriol. 1966 Apr;91(4):1612–1617. doi: 10.1128/jb.91.4.1612-1617.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Plagemann P. G. Purification of mengovirus and identification of an A-rich segment in its ribonucleic acid. J Gen Virol. 1972 Dec;17(3):349–353. doi: 10.1099/0022-1317-17-3-349. [DOI] [PubMed] [Google Scholar]
- Moss B. Inhibition of HeLa cell protein synthesis by the vaccinia virion. J Virol. 1968 Oct;2(10):1028–1037. doi: 10.1128/jvi.2.10.1028-1037.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W., Attardi G. Stability of cytoplasmic messenger RNA in HeLa cels. Proc Natl Acad Sci U S A. 1973 Jan;70(1):115–119. doi: 10.1073/pnas.70.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Summers D. Effects on host cell metabolism following synchronous infection with poliovirus. Virology. 1965 Dec;27(4):614–620. doi: 10.1016/0042-6822(65)90187-x. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. ACTINOMYCIN D AND VACCINIA VIRUS INFECTION OF HELA CELLS. Nature. 1963 Jul 27;199:357–358. doi: 10.1038/199357a0. [DOI] [PubMed] [Google Scholar]
- Scraba D. G., Hostvedt P., Colter J. S. Physical and chemical studies of Mengo virus variants. 3. Absorbance--temperature profiles, sedimentation in dextran sulfate gradients, and total-infectious particle ratios. Can J Biochem. 1970 Apr;48(4):412–417. doi: 10.1139/o70-067. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Disaggregation of HeLa cell polysomes after infection with poliovirus. Virology. 1967 Mar;31(3):550–552. doi: 10.1016/0042-6822(67)90237-1. [DOI] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]
- Willems M., Penman S. The mechanism of host cell protein synthesis inhibition by poliovirus. Virology. 1966 Nov;30(3):355–367. doi: 10.1016/0042-6822(66)90114-0. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Polyadenylic acid at the 3'-terminus of poliovirus RNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1877–1882. doi: 10.1073/pnas.69.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]


