Abstract
The capacity to form endospores is unique to certain members of the low-G+C group of Gram-positive bacteria (Firmicutes) and requires signature sporulation genes that are highly conserved across members of distantly related genera, such as Clostridium and Bacillus. Using gene conservation among endospore-forming bacteria, we identified eight previously uncharacterized genes that are enriched among endospore-forming species. The expression of five of these genes was dependent on sporulation-specific transcription factors. Mutants of none of the genes exhibited a conspicuous defect in sporulation, but mutants of two, ylxY and ylyA, were outcompeted by a wild-type strain under sporulation-inducing conditions, but not during growth. In contrast, a ylmC mutant displayed a slight competitive advantage over the wild type specific to sporulation-inducing conditions. The phenotype of a ylyA mutant was ascribed to a defect in spore germination efficiency. This work demonstrates the power of combining phylogenetic profiling with reverse genetics and gene-regulatory studies to identify unrecognized genes that contribute to a conserved developmental process.
INTRODUCTION
The formation of endospores is a distinctive developmental process wherein a dormant cell type (the endospore) is formed inside another cell (the mother cell) and ultimately released into the environment by lysis of the mother cell (1, 2). Endospores are metabolically inactive and highly resistant to environmental stresses, such as heat, radiation, chemicals, and desiccation (3). At the same time, these spores monitor the environment and are capable of rapidly resuming growth when conditions are favorable (4). Endospore formation is unique to the low-G+C group of Gram-positive bacteria (Firmicutes). For the most part, it is restricted to the family Bacillaceae and the class Clostridia, but members of the less well-studied family Veillonellaceae (e.g., Acetonema longum [5, 6]) also produce endospores. The last common ancestor of Clostridium and Bacillus predates the initial rise of oxygen in the atmosphere (7) approximately 2.3 billion years ago, and yet, remarkably, orthologs of signature sporulation genes are shared between the genomes of these distantly related bacteria (2, 8). We wondered whether gene conservation among endospore formers could be exploited to discover previously unrecognized genes involved in sporulation.
Sporulation has been most extensively studied in the model organism Bacillus subtilis. Entry into sporulation is governed by the master regulator Spo0A, which is activated by phosphorylation through a multicomponent signal transduction pathway (9). Phosphorylated Spo0A (Spo0A∼P) directly regulates (activates or represses) the expression of 121 genes (10) and significantly influences the expression of over 500 genes (11). Sporulation is initiated when Spo0A∼P levels reach a threshold (12). Sporulating cells undergo several successive morphological changes, a hallmark of which is the formation of a two-compartment sporangium consisting of forespore and mother cell compartments. As development proceeds, the forespore is wholly engulfed by the mother cell to create a cell within a cell. The inner cell becomes the dormant spore and is released from the mother cell by lysis (2). Upon release, the mature spore can remain dormant for long periods or, in response to germinants, give rise to a vegetative cell. The developmental program of sporulation is governed in part by the successive actions of four compartment-specific sigma factors (appearing in the order σF, σE, σG, and σK), whose activities are confined to the forespore (σF and σG) or the mother cell (σE and σK) (13).
Traditional approaches of forward genetics have identified many, if not all, genes that are essential for sporulation (spo). These approaches rely on conspicuous phenotypes for the identification of target genes. Complementary approaches, such as the identification of sporulation-specific proteins (e.g., SASP and coat proteins) and transcriptome analysis (14, 15), revealed additional genes under sporulation control, including genes that contribute to efficient sporulation and spore resistance properties. Here, we report the application of phylogenetic profiling in an effort to discover additional genes involved in sporulation that might have gone undetected in previous approaches. We therefore sought to identify previously unrecognized genes involved in sporulation in B. subtilis on the basis of their conservation among endospore-forming bacteria. Using phylogenetic profile analysis for the initial identification and transcriptional and mutational analyses, we discovered previously overlooked genes under sporulation control, including two genes whose mutants caused a small but detectable developmental defect.
MATERIALS AND METHODS
Bacterial strains and culturing conditions.
Escherichia coli strain DH5α was used for propagating plasmids and was grown and transformed using standard procedures (16). The B. subtilis strains used in this work are listed in Table 1. All strains were derived from the prototrophic laboratory strain PY79 (20). Transformation of Bacillus strains with double-stranded PCR fragments, or plasmid or genomic DNA, was done as previously described (21). Sporulation was induced by exhaustion in Difco sporulation (DS) medium or, in the case of β-galactosidase activity assays (see below), by resuspension in Sterlini-Mandelstam (SM) medium (22).
Table 1.
Strains used in this study
| Straina | Genotype | Source or reference |
|---|---|---|
| PY79 | Prototrophic derivative of B. subtilis subsp. subtilis 168 | 20 |
| RL5360 | amyE::Phyperspank-lacZ spc | This study |
| RL5361 | bkdR::spc | This study |
| RL5362 | bkdR::erm | This study |
| RL5363 | buk::erm | This study |
| RL5364 | ylmC::erm | This study |
| RL5365 | ylmC::erm amyE::ylmC spc | This study |
| RL5366 | ymxH::spc | This study |
| RL5367 | ylxY::spc | This study |
| RL5368 | ylxY::spc amyE::ylxY cat | This study |
| RL5369 | ymfB::spc | This study |
| RL5370 | yteA::erm | This study |
| RL5371 | ylyA::erm | This study |
| RL5372 | ylyA::erm sacA::ylyA kan | This study |
| RL5373 | yocK::tet | This study |
| RL5374 | amyE::PbkdR-lacZ cam | This study |
| RL5375 | amyE::Pbkd-lacZ cam | This study |
| RL5376 | spo0A::spc amyE::Pbkd-lacZ cam | This study |
| RL5377 | bkdR::erm amyE::Pbkd-lacZ cam | This study |
| RL5378 | spo0A::spc bkdR::erm amyE::Pbkd-lacZ cam | This study |
| RL5379 | amyE::PylmC-lacZ cam | This study |
| RL5380 | sigF::kan amyE::PylmC-lacZ cam | This study |
| RL5381 | sigE::erm amyE::PylmC-lacZ cam | This study |
| RL5382 | sigG::kan amyE::PylmC-lacZ cam | This study |
| RL5383 | spo0A::spc amyE::PymxH-lacZ cam | This study |
| RL5384 | sigF::kan amyE::PymxH-lacZ cam | This study |
| RL5385 | amyE::PylxY-lacZ cam | This study |
| RL5386 | sigF::kan amyE::PylxY-lacZ cam | This study |
| RL5387 | sigE::erm amyE::PylxY-lacZ cam | This study |
| RL5388 | sigG::kan amyE::PylxY-lacZ cam | This study |
| RL5389 | amyE::PylzJ-lacZ cam | This study |
| RL5390 | amyE::PymfB-lacZ cam | This study |
| RL5391 | sigF::kan amyE::PymfB-lacZ cam | This study |
| RL5392 | sigG::kan amyE::PymfB-lacZ cam | This study |
| RL5393 | spoVT::spc amyE::PymfB-lacZ cam | This study |
| RL5394 | amyE::PyteA-lacZ cam | This study |
| RL5395 | sigG::kan amyE::PyteA-lacZ cam | This study |
| RL5396 | spoVT::spc amyE::PyteA-lacZ cam | This study |
| RL5397 | amyE::PylyA-lacZ cam | This study |
| RL5398 | sigG::kan amyE::PylyA-lacZ cam | This study |
| RL5399 | spoVT::spc amyE::PylyA-lacZ cam | This study |
| RL2242 | spo0A::spc | 11 |
| RL1265 | sigF::kan | 17 |
| RL1061 | sigE::erm | 19 |
| RL4962 | sigG::kan | 18 |
| RL3873 | spoVT::spc | 15 |
All strains are isogenic with PY79 unless otherwise indicated.
Plasmid construction.
The oligonucleotides used for PCR in this study are listed in Table 2. Fragments of the upstream regions of candidate genes, including the respective ribosome binding sites (RBS) and start codons, were amplified by PCR and cloned into pAH124 (23) using the appropriate restriction sites (Table 2). In this way, lacZ reporter constructs with the start codon of the gene fused directly to the lacZ gene were obtained. The constructs were introduced into PY79 by transformation.
Table 2.
Oligonucleotides used to construct reporter and complementation constructs
| Primera | Sequence (5′–3′) |
|---|---|
| bkdR −296E | CTGGAATTCGATGAATCCTGACAACCCTTG |
| bkdR +3H | CTGAAGCTTCATCCCGATACCCCTTTGTAT |
| Bkd −298E | CTGGAATTCGAAGGCGAAAAGCTGTCTGT |
| Bkd +3H | CTGAAGCTTCATCTGTTACCACCTTTCTTG |
| ylmC −300E | CTGGAATTCAAGTGAAACGGGAGTGTCCA |
| ylmC +3H | CTGAAGCTTCATTCCATCACGTCCTTTTTC |
| ylmC +362B | CTGAGGATCCCTTATTTTACCACATCTTACTG |
| ymxH −282E | CTGAGAATTCAATGCTGAGCTTAGAAAGCAC |
| ymxH +3H | CTGAAAGCTTCATGTCTGTCACCCCCTTG |
| ylxY −330E | CTGGAATTCTTCGGGGCTTTCGTTGAAATT |
| ylxY +3E | CTGGAATTCCATGTTCTGTCCCCCCCTCAC |
| ylxY +1076B | CTGAGGATCCATCGCAACAGAACGGACTGTC |
| ylzA −380E | CTGGAATTCAATCAAAGAATGGACTGAAGACG |
| ylzA +3E | CTGGAATTCCATCTTCTACGTTCCCCCTGT |
| ymfB −234E | CTGGAATTCAAACATCAAATGTCGAATGGTC |
| ymfB +3H | CTGAAGCTTCATAATGCTGTCCTTCGCATC |
| yteA −351E | CTGGAATTCTTGGCTTTATGTAATGCATGTAG |
| yteA +3E | CTGGAATTCCATTGTGATCGCCTCGTTTCT |
| ylyA −613E | CTGGAATTCGTGGTCTCATTTAACATTTGTTG |
| ylyA +3E | CTGGAATTCCATTCTTCACAACTCCTGCTC |
| ylyA +514B | CTGGGATCCCTGCAATAATAAGTAGTGCAATC |
The numbers refer to the 5′ nucleotide position relative to the first nucleotide of the start codon (+1) of the respective gene. B, BamHI; E, EcoRI; H, HindIII.
Complementation constructs for the ylmC, ylxY, and ylyA mutants were made by PCR amplification of fragments carrying the gene promoters and entire open reading frames (ORF). These PCR fragments were cloned, using the appropriate restriction enzymes, into pDG1662 (24), in the case of ylmC and ylxY, or pSac-Cam (25), in the case of ylyA. The constructs were introduced into the respective mutant strains by transformation.
Phylogenetic profile analysis.
For each predicted gene product in B. subtilis subsp. subtilis 168, its presence or absence in 626 complete archaeal and bacterial genomes that were available at the time of the initial phylogenetic analysis was determined by asking whether a putative ortholog was present (1) or absent (0) in that species, similar to previous descriptions (26). This analysis included 46 genomes of endospore-forming bacteria belonging to the family Bacillaceae and the class Clostridia, namely, Bacillus amyloliquefaciens FZB42, Bacillus anthracis strain Ames, B. anthracis strain Ames Ancestor, B. anthracis strain Sterne, Bacillus cereus ATCC 10987, B. cereus ATCC 14579, B. cereus E33L, B. cereus subsp. cytotoxis NVH 391-98, Bacillus clausii KSM-K16, Bacillus halodurans C-125, Bacillus licheniformis ATCC 14580, Bacillus pumilus SAFR-032, Bacillus thuringiensis serovar konkukian strain 97-27, B. thuringiensis strain Al Hakam, Bacillus weihenstephanensis KBAB4, Geobacillus kaustophilus HTA426, Geobacillus thermodenitrificans NG80-2, Oceanobacillus iheyensis HTE831, Alkaliphilus metalliredigens QYMF, Alkaliphilus oremlandii OhILAs, Caldicellulosiruptor saccharolyticus DSM 8903, Carboxydothermus hydrogenoformans Z-2901, Clostridium acetobutylicum ATCC 824, Clostridium beijerinckii NCIMB 8052, Clostridium botulinum A strain ATCC 3502, C. botulinum A strain ATCC 19397, C. botulinum A strain Hall, C. botulinum F strain Langeland, Clostridium difficile 630, Clostridium kluyveri DSM 555, Clostridium perfringens ATCC 13124, C. perfringens SM101, C. perfringens strain 13, Clostridium phytofermentans ISDg, Clostridium tetani E88, Clostridium thermocellum ATCC 27405, Desulfitobacterium hafniense Y51, Desulfotomaculum reducens MI-1, Moorella thermoacetica ATCC 39073, Symbiobacterium thermophilum IAM 14863, Syntrophomonas wolfei subsp. wolfei strain Goettingen, Thermoanaerobacter pseudethanolicus ATCC 33223, Thermoanaerobacter tengcongensis MB4, Pelotomaculum thermopropionicum SI, and Thermoanaerobacter sp. X514. Proteins were then grouped by their distribution patterns across species. In this way, a set of 58 genes, many of which are signature sporulation genes (see below) and all of which are highly enriched among endospore-forming species, was obtained.
β-Galactosidase activity assays.
Samples were collected from shaking cultures in duplicate at various time points after sporulation was induced by resuspension in SM medium. β-Galactosidase activity was measured in a Synergy 2 plate reader (BioTek), as previously described (23). The experiment was repeated to ensure reproducibility. β-Galactosidase activity is reported in arbitrary units (AU) as the rate of o-nitrophenyl-β-d-galactopyranoside (ONPG) conversion (i.e., Vmax, with units of optical density at 420 nm [OD420] per minute) divided by the OD600 of the sample at the time of collection, as previously described (23).
Competition experiments.
A wild-type reference strain was competed against strains mutant for candidate genes, as previously described (27), with a few differences. Typically, starting from overnight LB cultures, the wild-type reference strain (RL5360), which carried an isopropyl β-d-1-thiogalactopyranoside (IPTG)-inducible lacZ, was mixed with an excess mutant strain in 5 ml DS medium. In competition assays using ylmC mutants, which displayed an advantage under sporulation-inducing conditions, we started with an excess of the wild-type strain. Cultures were grown and allowed to sporulate at 37°C in DS medium for 24 h. The cultures were then heat treated at 80°C for 20 min, briefly cooled at room temperature, and diluted in 5 ml fresh DS medium. At appropriate intervals, dilutions were plated on agar plates containing 0.008% (wt/vol) 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and 1 mM IPTG. The blue and white colonies, reflecting the ratio of wild-type reference to mutant strains, respectively, were counted. Similar competition experiments were done in LB to establish if observed phenotypes were specific to sporulation-inducing conditions. In these experiments, we grew the cocultures at 25°C to minimize the time the culture was in stationary phase. In short, wild-type reference and mutant strains were grown at 25°C in LB for 24 h and subsequently diluted in 5 ml fresh LB for another round of growth. The heat treatment step was omitted in these experiments.
Germination of purified spores.
Spores were purified essentially as previously described (22). In short, the strains were induced to sporulate by growing in DS medium for 48 to 72 h. Cells were pelleted by centrifugation, and the pellet was resuspended in ice-cold water. The cells were washed twice with ice-cold water and stored overnight at 4°C. The next day, the cells were pelleted by centrifugation and washed another 6 to 8 times with ice-cold water. In this way, preparations that were more than 95% phase-bright spores, as judged by phase-contrast microscopy, were obtained.
For germination assays, spores at an OD600 of 10 were activated by heat treatment at 80°C for 20 min and cooled on ice for 2 min. Activated spores were germinated at an OD600 of 0.5 in LB medium. Germination was recorded as a loss of optical density in a Synergy 2 plate reader (BioTekin).
RESULTS
Candidates for uncharacterized sporulation genes.
We used phylogenetic profile analysis to search for genes in the genome of B. subtilis subsp. subtilis 168 (here simply referred to as B. subtilis) that are specifically conserved among endospore-forming, low-G+C, Gram-positive bacteria. A similar method was previously used to identify signature sporulation genes in the thermophilic firmicute Carboxydothermus hydrogenoformans (26). Phylogenetic profiling works by grouping genes according to their distribution patterns in different species. For each predicted gene product in B. subtilis, its presence or absence in all complete archaeal and bacterial genomes available at the time of this analysis (626 genomes in total) was determined by asking whether an ortholog was present in that species. This analysis included 46 genomes of endospore-forming bacteria belonging to the family Bacillaceae and the class Clostridia. The orthologs were then grouped by their distribution patterns across species. In this way, 58 genes that were highly and specifically conserved among endospore-forming bacteria were identified (see Fig S1 in the supplemental material). Many of these are signature sporulation genes with well-studied roles in spore formation, such as spoIIR and spoIIGA, which mediate the activation of the mother-cell-specific transcription factor σE (28); spoIID, spoIIM, and spoIIP, required for forespore engulfment by the mother cell (29–31); and spoIVA, which encodes a morphogenetic protein required for coat assembly (32). Five genes (i.e., bkdR, ylmC, ymxH, ylzA, [formerly designated remA], and ymfB [formerly designated tepA]), however, had no previously documented role in spore formation, and an additional three genes (i.e., ylxY, yteA, and ylyA) had previously been shown to be under sporulation control, but no roles in sporulation had been described (15, 33). We hypothesized that these genes might play previously unrecognized or overlooked roles in sporulation.
Six of the eight genes were widely conserved among the 46 endospore-forming Bacillaceae and Clostridia genomes examined. In the cases of ymxH and ylyA, however, only 25 and 11 orthologs, respectively, were identified. ymxH orthologs are present in almost all endospore-forming Bacillaceae species but are missing from the majority of endospore-forming Clostridia species. Nonetheless, ymxH exhibits significant sequence similarity to one of the other genes on our list, ylmC, which is abundant among endospore-forming bacteria. Meanwhile, a close look at ylyA, which is homologous to yteA, revealed that several genes identified as yteA orthologs in endospore-forming bacteria share similar gene synteny with B. subtilis ylyA. B. subtilis ylyA is flanked by several well-characterized genes, including divIVA, encoding a cell division protein; ileS, encoding an isoleucyl-tRNA synthetase; lspA, encoding a type II signal peptidase; and rluD, encoding a pseudouridylate synthase, many of which are found in the vicinity of ylyA orthologs in Bacillaceae and Clostridia. This suggests that these genes are actually ylyA orthologs, raising the number of orthologs to 29 (Table 3). Thus, all eight genes are widely conserved among endospore-forming bacteria or are homologous to genes that are.
Table 3.
Candidates for uncharacterized sporulation genes among genes conserved in endospore-forming bacteria
| Gene | No. of orthologs in: |
Predicted product | |
|---|---|---|---|
| All bacteria/archaeaa | Bacillaceae/Clostridiab | ||
| bkdR | 87 | 43 | DNA-binding transcriptional regulator |
| buk | 62 | 34 | Butyrate kinase |
| ylmC | 45 | 45 | Hypothetical protein; PRC barrel domain |
| ymxH | 25 | 25 | Hypothetical protein; PRC barrel domain |
| ylxY | 42 | 38 | Polysaccharide deacetylase |
| ylzA | 73 | 43 | Hypothetical protein |
| ymfB | 44 | 44 | ClpP-like protease |
| yteA | 51 (33)c | 41 (23) | DksA-like regulator |
| ylyA | 11 (29) | 11 (29) | DksA-like regulator |
626 bacterial and archaeal genomes were considered.
46 Bacillus and Clostridia genomes were considered.
The numbers in parentheses are the numbers of orthologs for yteA and ylyA corrected for similarity in gene synteny with B. subtilis ylyA.
Finally, two additional genes, yvjA and buk, that had orthologs both in endospore-forming bacteria and in all four species of Listeria included in this analysis were identified (see Fig S1 in the supplemental material). Listeria species are closely related to B. subtilis but do not form spores and lack almost all signature sporulation genes (34). Because of their presence in non-endospore-forming bacteria, we considered yvjA and buk unlikely candidates for unrecognized sporulation genes. Nonetheless, as a control, we retained one of these genes, buk, in our analysis. Thus, a total of nine genes were carried forward for further investigation.
Transcription under sporulation-inducing conditions.
We next asked if the nine candidate genes are transcribed under conditions that induce sporulation. For this purpose, we built transcriptional reporter constructs, typically cloning a 300- to 400-bp fragment directly upstream of the gene and fusing its start codon to that of the lacZ gene. In the case of buk, which is the third gene in a seven-gene (bkd) operon that is involved in branched-chain amino acid utilization (35), we instead cloned an approximately 300-bp fragment upstream of ptb, the first gene of the operon. B. subtilis strains carrying these constructs integrated at the amyE locus were induced to sporulate by resuspension in SM medium (22), and samples taken at various times were analyzed for β-galactosidase activity. Seven of the nine reporters were expressed during sporulation, six of which were induced at various times after the induction of sporulation (Fig. 1). We did not observe activity for the bkdR and ylzA reporters under the tested conditions (data not shown).
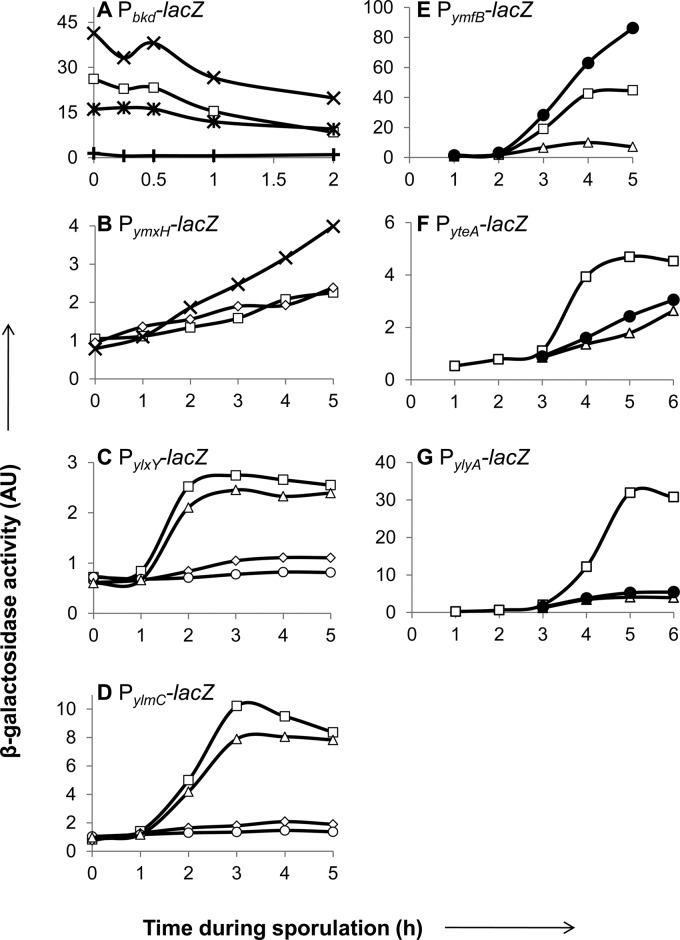
Fig 1.
Transcription and regulatory-gene dependence of candidate sporulation genes. Strains carrying lacZ transcriptional reporter constructs for bkd (operon including buk) (A), ymxH (B), ylxY (C), ylmC (D), ymfB (E), yteA (F), and ylyA (G) were induced to sporulate, and β-galactosidase activity was monitored for samples taken at the indicated time points after sporulation induction. Activity (AU) was assayed in the wild type (□) or strains mutant for spo0A (×), sigF (♢), sigE (○), sigG (△), spoVT (●), bkdR (+), or spo0A and bkdR (∗).
The bkd reporter was active from the time of the induction of sporulation, decreasing only slightly during the time it was monitored (Fig. 1A). As previously reported, expression of the bkd operon depends on the alternative sigma factor σL and BkdR (35). Indeed, in a bkdR mutant, expression was abolished (Fig. 1A). We next measured expression in a strain mutant for spo0A. The spo0A gene encodes the master regulator for entry into sporulation, Spo0A, which is active in its phosphorylated form, Spo0A∼P (2, 9). Activity was approximately 2-fold higher than that of the wild type at the times tested. Interestingly, in a strain doubly mutant for spo0A and bkdR, expression levels were similar to that of the wild type (Fig. 1A), showing that in the absence of Spo0A, BkdR is not required for expression. That is, a spo0A mutation is epistatic to a bkdR mutation. This suggests that BkdR antagonizes Spo0A∼P to activate transcription from the bkd operon promoter. We found a potential Spo0A binding site (GTCGAAA [see Fig S2 in the supplemental material]) with high similarity to the consensus binding sequence (TTTGTCGAAA [10]) located immediately downstream of the σL-dependent transcriptional start site (35). Just upstream of the promoter are tandem sequences previously shown to be important for BkdR-mediated activation (35). In toto, these observations suggest that the binding of BkdR upstream of the promoter overcomes the repressive effect of the binding of Spo0A∼P just downstream of the start site.
The ymxH reporter was expressed from an early time, increasing slightly but constantly during the first 5 h (Fig. 1B). We tested the activity in strains mutant for spo0A and sigF, which encodes the first forespore-specific sigma factor, σF (2). Expression in a spo0A mutant was upregulated, steadily increasing from 1 h after sporulation was induced. In contrast, activity in a sigF mutant was unchanged compared to that of the wild type (Fig. 1B). These findings suggest that ymxH is directly or indirectly under the negative control of Spo0A∼P but is not otherwise under sporulation control.
Expression from the ylxY and ylmC reporters was induced between hours 1 and 2 of sporulation (Fig. 1C and D). Previous work indicated that ylxY expression is σE dependent and under the negative control of the mother-cell-specific regulator SpoIIID (14, 33). We tested the activity of the ylxY and ylmC reporters in strains mutant for sigF, sigE (which encodes the mother-cell-specific sigma factor σE), or sigG (which encodes the late-appearing, forespore-specific sigma factor σG). Activity for both was abolished in the sigF and sigE mutants but reached wild-type levels in the sigG mutant (Fig. 1C and D). Thus, these genes are transcribed in a σE-dependent manner.
Finally, three reporters were induced between 2 and 3 h after sporulation was induced, namely, those for ymfB, yteA, and ylyA (Fig. 1E to G). Previous transcriptome analyses indicated that yteA and ylyA are indeed part of the σG regulon (15), whereas ymfB was not known to be under sporulation control. The activities of all three reporters were abolished in a sigG mutant (Fig. 1E-G). In addition, ylyA was previously shown to be under the control of SpoVT, a modulator of σG-dependent transcription (15, 36). We tested the activities of all three reporters in a strain mutant for spoVT. The activities of the yteA and ylyA reporters were markedly reduced in a spoVT mutant, whereas the activity of the ymfB reporter increased in a spoVT mutant (Fig. 1E to G).
In summary, we conclude that five of the nine genes in our investigation are under sporulation control, with two, namely, ylxY and ylmC, under the control of σE and three, ymfB, yteA, and ylyA, under the control of σG.
Competition-based analysis of candidate gene mutants.
We constructed mutant strains for eight of the nine genes by deleting and replacing their ORF with antibiotic resistance cassettes. We were unable to obtain a mutant for the ninth gene, ylzA. Previously, others obtained transposon insertions directly upstream of ylzA; however, no report was made of transposon insertions internal to the ylzA coding sequence (37).
None of the eight mutants had a conspicuous phenotype, as judged by colony morphology or spore formation (data not shown). Thus, if any of these genes represent previously uncharacterized sporulation genes, their contributions to spore formation must be subtle. To test for such a subtle role, we carried out competition experiments in which mutant strains were competed for several rounds of sporulation against a wild-type reference strain marked by an IPTG-inducible lacZ gene. Typically, mutant cells were severalfold in excess of the wild type at the start of the experiment. Cocultures of the mutant and wild type were grown and allowed to sporulate at 37°C in DS medium for 24 h. The culture was then heat treated at 80°C and diluted in fresh DS medium for another round of sporulation. At appropriate intervals, dilutions were plated on agar plates containing X-Gal and IPTG, and blue (wild-type) and white (mutant) colonies were counted. Mutant strains that were outcompeted (or in one case slightly undercompeted) by the wild type were next subjected to competition experiments in growth medium to determine if the observed competition phenotype was an indirect consequence of a growth defect rather than a defect in sporulation. In these experiments, cocultures were grown at 25°C in LB medium for 24 h and subsequently diluted in fresh LB medium for another round of growth. The heat treatment step was omitted in these experiments.
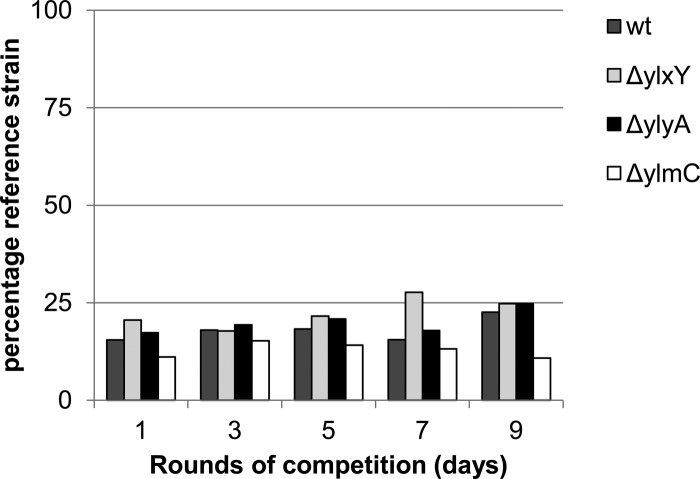
The mutant strains fell into three categories: those that did not have a competition phenotype under sporulation-inducing conditions, those that had a phenotype under sporulation-inducing but not under growth conditions, and lastly, those that had a phenotype under both conditions. The first category comprised the bkdR, ymxH, ymfB, and yteA mutants. In a competition experiment in which the wild-type reference strain was competed against an unmarked wild-type strain, the percentage of the wild-type reference strain remained constant during several rounds of competition (see Fig S3 in the supplemental material), indicating that the inducible lacZ construct did not affect the fitness of the reference strain. Similarly, in competition experiments with strains mutant for bkdR, ymxH, ymfB, or yteA, the percentage of the wild-type reference strain remained constant (see Fig S3 in the supplemental material).
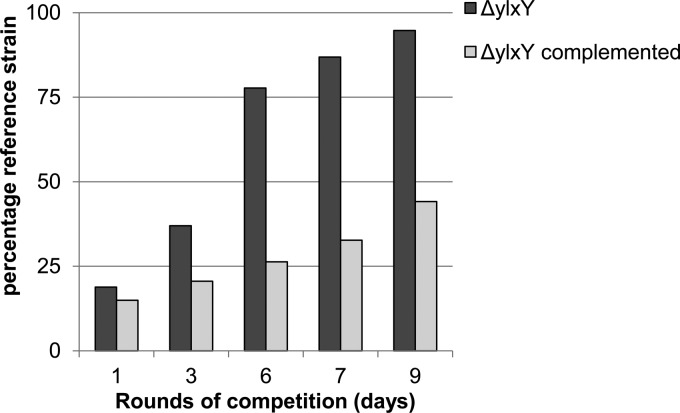
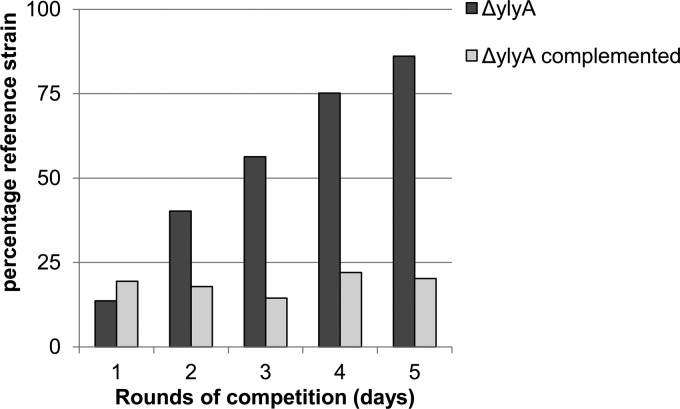
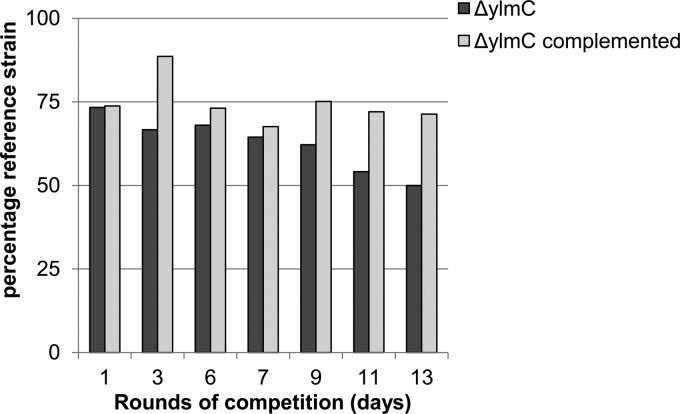
The second category comprised the ylxY, ylyA, and ylmC mutants. Strains mutant for ylxY and ylyA had clear competition deficits under sporulation-inducing conditions. Over the course of nine and five rounds of competition, respectively, the percentage of wild-type reference strain increased from approximately 20% to 90% of the population (Fig. 2 and 3). Genetic complementation by reintroducing a copy of the respective gene at an ectopic locus restored competitiveness to the mutant strains (Fig. 2 and 3), confirming that the observed phenotypes resulted from deletion of the genes. In contrast to the apparent deficit of ylxY and ylyA mutants, the ylmC mutant exhibited a slight competitive advantage under sporulation-inducing conditions (Fig. 4). As before, genetic complementation by reintroducing a copy of ylmC at the ectopic amyE locus reversed this phenotype (Fig. 4). All three competition phenotypes were found to be specific to competition experiments under sporulation-inducing conditions, because no changes from the starting ratio were observed in competition experiments during growth (Fig. 5).
Fig 2.
A ylxY mutant exhibits a competition deficit under sporulation-inducing conditions. A wild-type reference strain carrying an IPTG-inducible lacZ gene (RL5360) was competed in DS medium against a ylxY mutant, starting with approximately 20% wild-type strain. Cultures were incubated at 37°C for 24 h, heat treated at 80°C, and diluted in fresh DS medium. After the indicated rounds of competition, dilutions of the culture were plated on agar plates containing IPTG and X-Gal, and blue (wild type) and white (mutant) colonies were counted. The bars indicate the percentages of the wild-type reference strain. The wild-type strain was competed against a ylxY mutant and a complemented strain carrying a copy of ylxY at the ectopic amyE locus.
Fig 3.
A ylyA mutant exhibits a competition deficit under sporulation-inducing conditions. A wild-type reference strain carrying an IPTG-inducible lacZ gene (RL5360) was competed in DS medium against a ylyA mutant, starting with approximately 20% wild-type strain. Cultures were incubated at 37°C for 24 h, heat treated at 80°C, and diluted in fresh DS medium. After the indicated rounds of competition, dilutions of the culture were plated on agar plates containing IPTG and X-Gal, and blue (wild-type) and white (mutant) colonies were counted. The bars indicate the percentages of the wild-type reference strain. The wild-type strain was competed against a ylyA mutant and a complemented strain carrying a copy of ylyA at the ectopic sacA locus.
Fig 4.
A ylmC mutant exhibits a slight competitive advantage under sporulation-inducing conditions. A wild-type reference strain carrying an IPTG-inducible lacZ gene (RL5360) was competed in DS medium against a ylmC mutant, starting with approximately 75% wild-type strain. The cultures were incubated at 37°C for 24 h, heat treated at 80°C, and diluted in fresh DS medium. After the indicated rounds of competition, dilutions of the culture were plated on agar plates containing IPTG and X-Gal, and blue (wild-type) and white (mutant) colonies were counted. The bars indicate the percentages of the wild-type reference strain. The wild-type strain was competed against a ylmC mutant and a complemented strain carrying a copy of ylmC at the ectopic amyE locus.
Fig 5.
ylxY, ylyA, and ylmC mutants do not exhibit a significant competitive deficit during growth. A wild-type reference strain carrying an IPTG-inducible lacZ gene (RL5360) was competed in LB medium against an unmarked wild type (wt) and strains mutant for ylxY, ylyA, and ylmC, starting with approximately 20% wild-type strain. The cultures were incubated at 25°C for 24 h and diluted in fresh LB. After the indicated rounds of competition, dilutions of the culture were plated on agar plates containing IPTG and X-Gal, and blue and white colonies were counted. The bars indicate the percentages of the wild-type reference strain.
Finally, the third category contained only one strain, the buk mutant, which had a clear competition deficit under sporulation-inducing and growth conditions (see Fig S4 in the supplemental material).
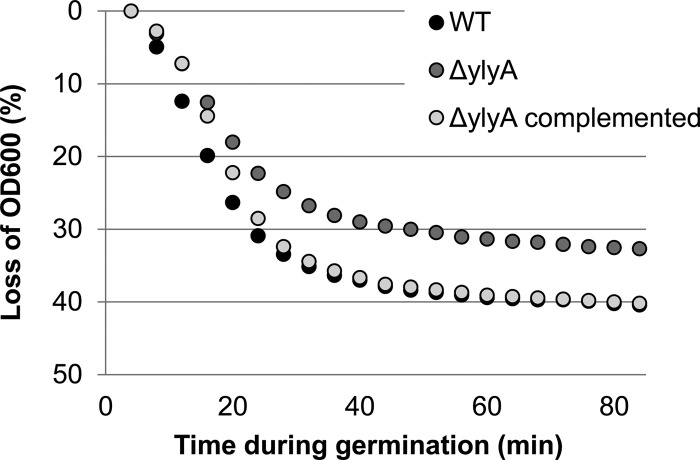
A ylyA mutant is defective in germination.
We do not know the precise step in sporulation at which the ylxY mutation impedes spore formation or the step at which the ylmC mutation confers a competitive advantage. However, in the case of ylyA, we can, at least in part, attribute the competitive disadvantage to impaired spore germination. As shown in Fig. 6, spores from a ylyA mutant are slower to germinate than either the wild-type parent or a ylyA mutant strain harboring a wild-type copy of the gene at the ectopic sacA locus.
Fig 6.
A ylyA mutant is delayed in germination. Purified spores were heat treated and cooled on ice. Activated spores were germinated at an OD600 of 0.5 by dilution in LB medium. Every 4 min after germination induction, the OD600 was measured, and germination is reported as the percent drop in optical density.
DISCUSSION
Using phylogenetic profiling, we identified eight genes (i.e., bkdR, ylmC, ymxH, ylxY, ylzA, ymfB, yteA, and ylyA) that are widely conserved among endospore-forming species of Bacillaceae and Clostridia but were not previously reported to be involved in sporulation or, in the cases of ylxY, yteA, and ylyA, not well characterized. ymfB, whose product is homologous to ClpP-like proteases (Table 3), was previously suggested to be involved in translocation and processing of the α-amylase AmyQ (reference38, where ymfB was named tepA). Researchers from the same laboratory, however, later reported that they were unable to replicate the initial results with a clean knockout of ymfB (39). ylzA, which encodes a hypothetical protein with no clear homology to known proteins (Table 3), was previously shown to be involved in the regulation of extracellular-matrix components during biofilm formation in B. subtilis (reference37, where ylzA is designated remA). Five of the eight genes were found to be under the control of sporulation-specific transcription factors, with ylmC and ylxY under the control of the mother-cell-specific sigma factor σE and ymfB, yteA, and ylyA under the control of the forespore-specific factors σG and SpoVT. Inactivation of ylxY, ylyA, and ylmC resulted in measurable changes in competitiveness under sporulation-inducing conditions, but not under growth conditions.
We included buk as a control in our investigation. Like the other eight genes, orthologs of buk are enriched among endospore-forming bacteria but are also found in some non-endospore-forming species, most notably Listeria, a close relative of B. subtilis that is asporogenic. The buk gene, which codes for a butyrate kinase, is part of a seven-gene operon in B. subtilis that is involved in the utilization of branched-chain amino acids as a nitrogen source (35). Orthologs of other members of the operon are widespread among bacteria, much more so than buk itself. In B. subtilis the operon is under the control of the alternative sigma factor σL and the transcription activator BkdR (35), which, as we have shown, is itself highly conserved among endospore-forming bacteria. Inactivation of buk resulted in defects that were not specific to sporulation, and the bkd operon promoter was constitutively active under the tested conditions. Interestingly, we found a putative Spo0A binding site adjacent to the predicted transcriptional start site, and our results indicate that BkdR antagonizes Spo0A∼P to activate expression. The presence of BkdR orthologs in almost all endospore-forming species analyzed (Table 3), but not Listeria species, supports the idea that BkdR is conserved among endospore-forming bacteria to counteract the effect of Spo0A∼P on transcription.
ylmC and ymxH are paralogs that code for small hypothetical proteins that resemble a motif known as a photosynthetic reaction center (PRC) beta-barrel domain (Table 3). The PRC domain, which is itself widespread among photosynthetic and nonphotosynthetic bacteria, archaea, and plants, is thought to mediate protein-protein interactions (40). Deletion of ylmC resulted in a strain that had a slight competitive advantage over the wild type under sporulation-inducing conditions. It is unclear what causes this unexpected phenotype. A double mutant of ylmC and ymxH had essentially the same phenotype as the ylmC single mutant (data not shown). We infer that YlmC must confer some fitness advantage during spore formation under unknown environmental conditions (e.g., a spore resistance property) but that production of the protein evidently imposes a slight cost on spore formation that impedes development.
Deletion of ylxY and ylyA resulted in clear competitive deficits specific to sporulation-inducing conditions. ylxY encodes a probable polysaccharide deacetylase (Table 3) and exhibits some similarity to two B. subtilis genes under sporulation control, namely, pdaA and pdaB, mutants of which display defects in spore cortex maturation (41–43). The nature of the competition deficit of the ylxY mutant is currently unknown. In contrast, we have determined that the competition defect of the ylyA mutant stems, not from a defect in spore formation per se, but rather from impaired germination of the mutant spores. Krasny and Gourse previously reported that YlyA and YteA share some sequence similarity to the transcription factor DksA (44). DksA inhibits the transcription of rRNA genes by direct interaction with RNA polymerase (45). Mutation of ylyA or yteA, however, seemingly did not affect the activity of the P1 promoter of the rRNA gene rrnB under any of the tested growth conditions (44). It will be interesting to see whether YlyA similarly modifies RNA polymerase activity and, if so, whether this modification influences the expression of genes involved in spore germination.
Our results reinforce the view that phylogenetic profiling, in combination with reverse genetics and gene-regulatory studies, can be a powerful tool for the discovery of genes that play a subtle role in a complex developmental process and whose contributions might otherwise be overlooked by traditional approaches of forward genetics.
Supplementary Material
ACKNOWLEDGMENTS
We thank Martin Wu for help with phylogenetic profiling and Rick Gourse for bringing to our attention the similarity of yteA and ylyA to dksA. We thank members of the Losick laboratory for helpful discussion.
This work was supported by a Netherlands Organization for Scientific Research (NWO) Rubicon grant to B.A.T., Gordon and Betty Moore Foundation grant 1660 to J.A.E., and National Institutes of Health grant GM18568 to R.L.
Footnotes
Published ahead of print 2 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01778-12.
REFERENCES
- 1. Piggot PJ, Coote JG. 1976. Genetic aspects of bacterial endospore formation. Bacteriol. Rev. 40:908–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stragier P, Losick R. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 30:297–341 [DOI] [PubMed] [Google Scholar]
- 3. Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514–525 [DOI] [PubMed] [Google Scholar]
- 4. Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550–556 [DOI] [PubMed] [Google Scholar]
- 5. Kane MD, Breznak JA. 1991. Acetonema longum gen. nov. sp. nov., an H2/CO2 acetogenic bacterium from the termite, Pterotermes occidentis. Arch. Microbiol. 156:91–98 [DOI] [PubMed] [Google Scholar]
- 6. Tocheva EI, Matson EG, Morris DM, Moussavi F, Leadbetter JR, Jensen GJ. 2011. Peptidoglycan remodeling and conversion of an inner membrane into an outer membrane during sporulation. Cell 146:799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Battistuzzi FU, Feijao A, Hedges SB. 2004. A genomic timescale of prokaryote evolution: insights into the origin of methanogenesis, phototrophy, and the colonization of land. BMC Evol. Biol. 4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galperin MY, Mekhedov SL, Puigbo P, Smirnov S, Wolf YI, Rigden DJ. 13 August 2012. Genomic determinants of sporulation in bacilli and clostridia: towards the minimal set of sporulation-specific genes. Environ. Microbiol. doi:10.1111/j.1462–2920.2012.02841.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoch JA. 1993. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu. Rev. Microbiol. 47:441–465 [DOI] [PubMed] [Google Scholar]
- 10. Molle V, Fujita M, Jensen ST, Eichenberger P, Gonzalez-Pastor JE, Liu JS, Losick R. 2003. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 50:1683–1701 [DOI] [PubMed] [Google Scholar]
- 11. Fawcett P, Eichenberger P, Losick R, Youngman P. 2000. The transcriptional profile of early to middle sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 97:8063–8068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujita M, Gonzalez-Pastor JE, Losick R. 2005. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J. Bacteriol. 187:1357–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Losick R, Stragier P. 1992. Crisscross regulation of cell-type-specific gene expression during development in B. subtilis. Nature 355:601–604 [DOI] [PubMed] [Google Scholar]
- 14. Eichenberger P, Jensen ST, Conlon EM, van Ooij C, Silvaggi J, Gonzalez-Pastor JE, Fujita M, Ben-Yehuda S, Stragier P, Liu JS, Losick R. 2003. The sigmaE regulon and the identification of additional sporulation genes in Bacillus subtilis. J. Mol. Biol. 327:945–972 [DOI] [PubMed] [Google Scholar]
- 15. Wang ST, Setlow B, Conlon EM, Lyon JL, Imamura D, Sato T, Setlow P, Losick R, Eichenberger P. 2006. The forespore line of gene expression in Bacillus subtilis. J. Mol. Biol. 358:16–37 [DOI] [PubMed] [Google Scholar]
- 16. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 17. Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 98:11621–11626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Camp AH, Losick R. 2008. A novel pathway of intercellular signalling in Bacillus subtilis involves a protein with similarity to a component of type III secretion channels. Mol. Microbiol. 69:402–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kenney TJ, Moran CP., Jr 1987. Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J. Bacteriol. 169:3329–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Youngman P, Perkins JB, Losick R. 1984. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid 12:1–9 [DOI] [PubMed] [Google Scholar]
- 21. Wilson GA, Bott KF. 1968. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J. Bacteriol. 95:1439–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450 In Harwood CR, Cutting SM. (ed), Molecular biological methods for Bacillus. Wiley-Interscience, Hoboken, NJ [Google Scholar]
- 23. Camp AH, Losick R. 2009. A feeding tube model for activation of a cell-specific transcription factor during sporulation in Bacillus subtilis. Genes Dev. 23:1014–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guerout-Fleury AM, Frandsen N, Stragier P. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57–61 [DOI] [PubMed] [Google Scholar]
- 25. Middleton R, Hofmeister A. 2004. New shuttle vectors for ectopic insertion of genes into Bacillus subtilis. Plasmid 51:238–245 [DOI] [PubMed] [Google Scholar]
- 26. Wu M, Ren Q, Durkin AS, Daugherty SC, Brinkac LM, Dodson RJ, Madupu R, Sullivan SA, Kolonay JF, Haft DH, Nelson WC, Tallon LJ, Jones KM, Ulrich LE, Gonzalez JM, Zhulin IB, Robb FT, Eisen JA. 2005. Life in hot carbon monoxide: the complete genome sequence of Carboxydothermus hydrogenoformans Z-2901. PLoS Genet. 1:e65 doi:10.1371/journal.pgen.0010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmalisch M, Maiques E, Nikolov L, Camp AH, Chevreux B, Muffler A, Rodriguez S, Perkins J, Losick R. 2010. Small genes under sporulation control in the Bacillus subtilis genome. J. Bacteriol. 192:5402–5412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hofmeister AE, Londono-Vallejo A, Harry E, Stragier P, Losick R. 1995. Extracellular signal protein triggering the proteolytic activation of a developmental transcription factor in B. subtilis. Cell 83:219–226 [DOI] [PubMed] [Google Scholar]
- 29. Coote JG. 1972. Sporulation in Bacillus subtilis. Characterization of oligosporogenous mutants and comparison of their phenotypes with those of asporogenous mutants. J. Gen. Microbiol. 71:1–15 [DOI] [PubMed] [Google Scholar]
- 30. Frandsen N, Stragier P. 1995. Identification and characterization of the Bacillus subtilis spoIIP locus. J. Bacteriol. 177:716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith K, Bayer ME, Youngman P. 1993. Physical and functional characterization of the Bacillus subtilis spoIIM gene. J. Bacteriol. 175:3607–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roels S, Driks A, Losick R. 1992. Characterization of spoIVA, a sporulation gene involved in coat morphogenesis in Bacillus subtilis. J. Bacteriol. 174:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eichenberger P, Fujita M, Jensen ST, Conlon EM, Rudner DZ, Wang ST, Ferguson C, Haga K, Sato T, Liu JS, Losick R. 2004. The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis. PLoS Biol. 2:e328 doi:10.1371/journal.pbio.0020328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stragier P. 2002. A gene odyssey: exploring the genomes of endospore-forming bacteria, p 519–526 In Sonenshein AL, Hoch JA, Losick R. (ed), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 35. Debarbouille M, Gardan R, Arnaud M, Rapoport G. 1999. Role of bkdR, a transcriptional activator of the sigL-dependent isoleucine and valine degradation pathway in Bacillus subtilis. J. Bacteriol. 181:2059–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bagyan I, Hobot J, Cutting S. 1996. A compartmentalized regulator of developmental gene expression in Bacillus subtilis. J. Bacteriol. 178:4500–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winkelman JT, Blair KM, Kearns DB. 2009. RemA (YlzA) and RemB (YaaB) regulate extracellular matrix operon expression and biofilm formation in Bacillus subtilis. J. Bacteriol. 191:3981–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bolhuis A, Matzen A, Hyyrylainen HL, Kontinen VP, Meima R, Chapuis J, Venema G, Bron S, Freudl R, van Dijl JM. 1999. Signal peptide peptidase- and ClpP-like proteins of Bacillus subtilis required for efficient translocation and processing of secretory proteins. J. Biol. Chem. 274:24585–24592 [DOI] [PubMed] [Google Scholar]
- 39. Westers H, Darmon E, Zanen G, Veening JW, Kuipers OP, Bron S, Quax WJ, van Dijl JM. 2004. The Bacillus secretion stress response is an indicator for alpha-amylase production levels. Lett. Appl. Microbiol. 39:65–73 [DOI] [PubMed] [Google Scholar]
- 40. Anantharaman V, Aravind L. 2002. The PRC-barrel: a widespread, conserved domain shared by photosynthetic reaction center subunits and proteins of RNA metabolism. Genome Biol. 3:RESEARCH0061 doi:10.1186/gb-2002-3-11-research0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fukushima T, Tanabe T, Yamamoto H, Hosoya S, Sato T, Yoshikawa H, Sekiguchi J. 2004. Characterization of a polysaccharide deacetylase gene homologue (pdaB) on sporulation of Bacillus subtilis. J. Biochem. 136:283–291 [DOI] [PubMed] [Google Scholar]
- 42. Fukushima T, Yamamoto H, Atrih A, Foster SJ, Sekiguchi J. 2002. A polysaccharide deacetylase gene (pdaA) is required for germination and for production of muramic delta-lactam residues in the spore cortex of Bacillus subtilis. J. Bacteriol. 184:6007–6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Silvaggi JM, Popham DL, Driks A, Eichenberger P, Losick R. 2004. Unmasking novel sporulation genes in Bacillus subtilis. J. Bacteriol. 186:8089–8095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krasny L, Gourse RL. 2004. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 23:4473–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paul BJ, Barker MM, Ross W, Schneider DA, Webb C, Foster JW, Gourse RL. 2004. DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell 118:311–322 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.