Abstract
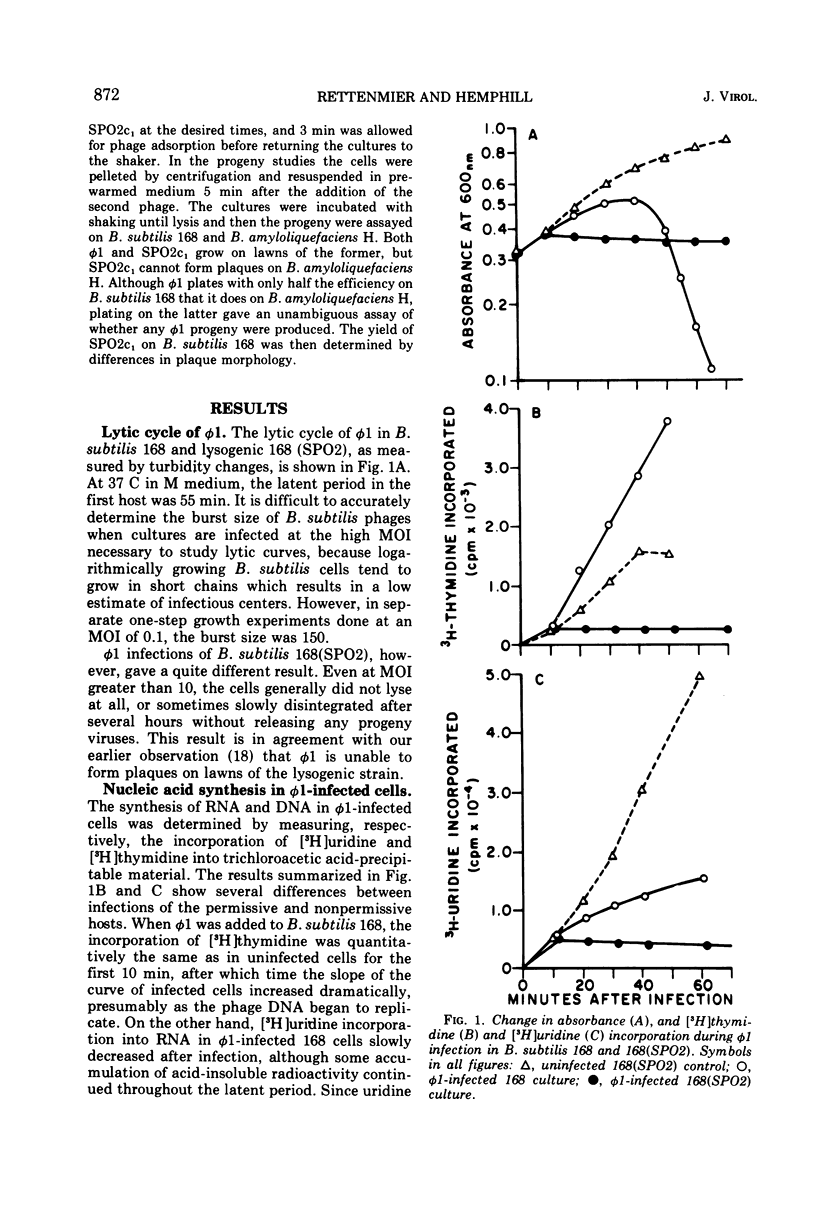
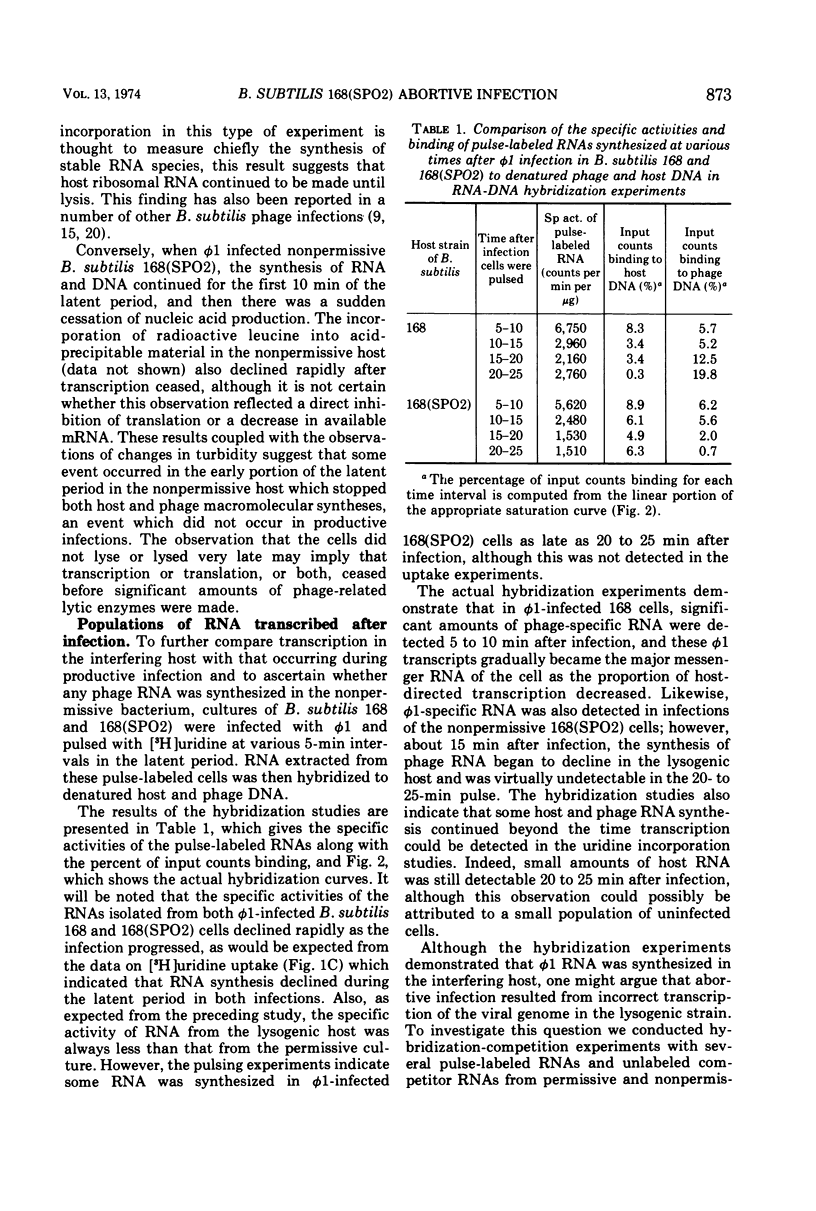
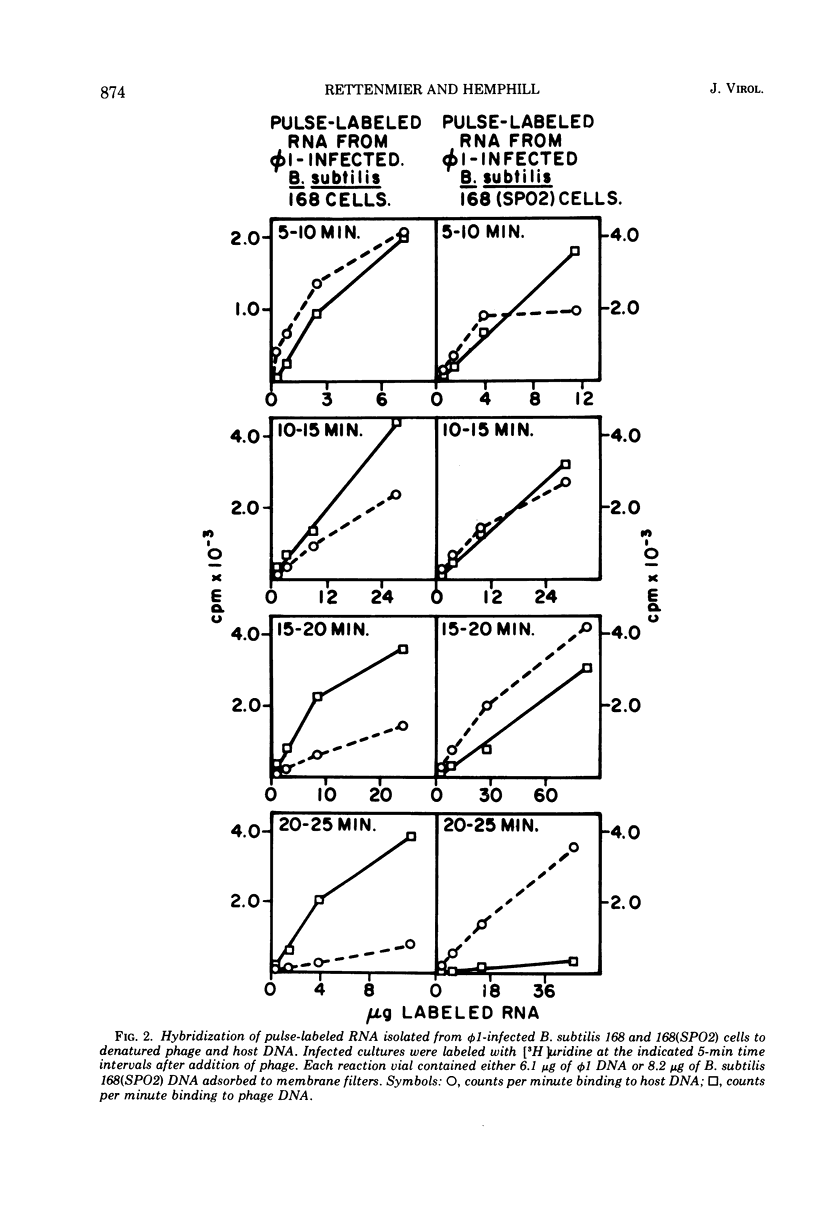
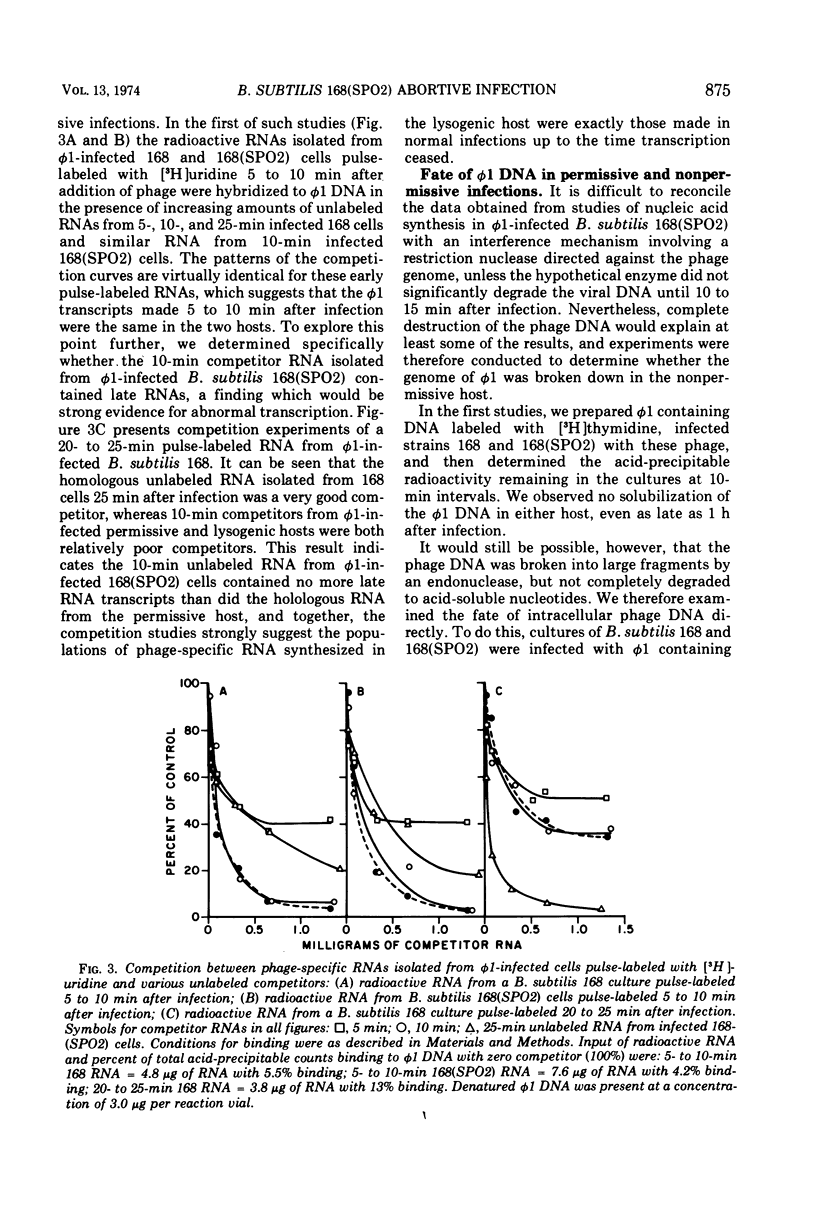
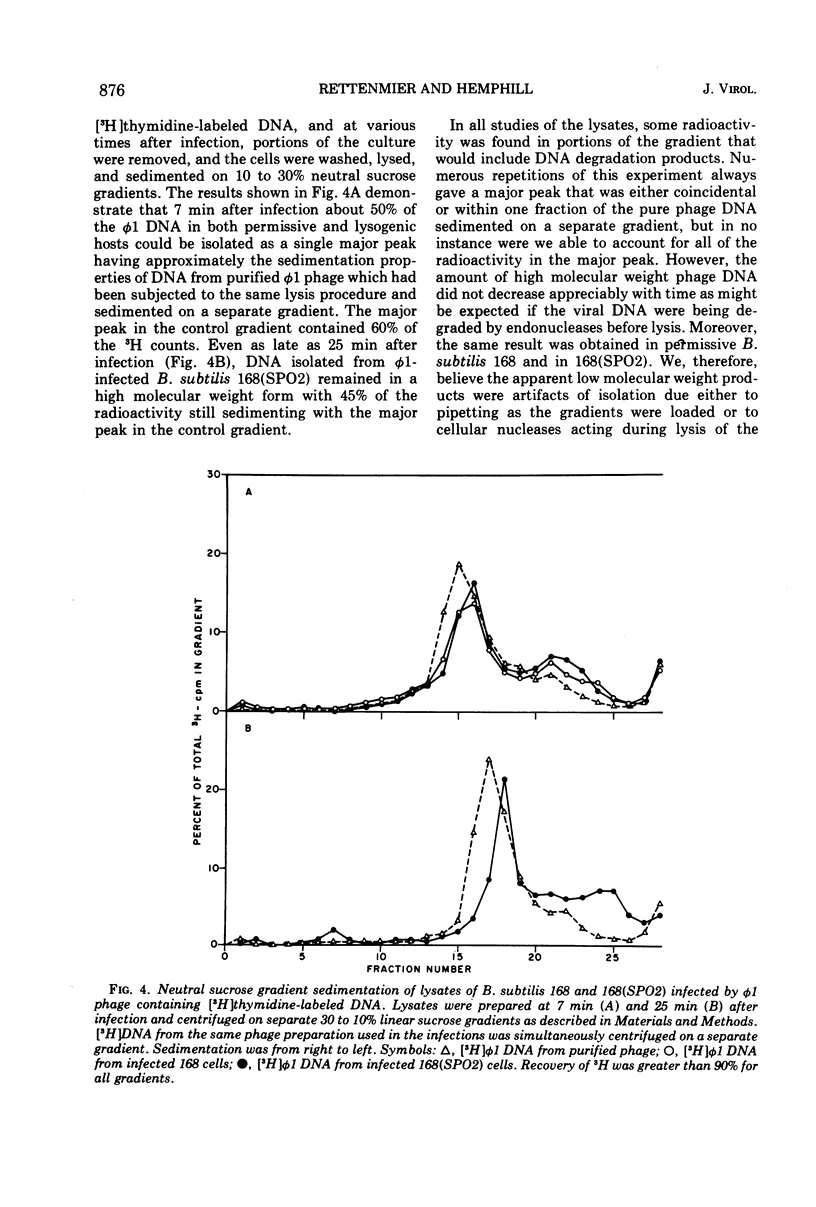
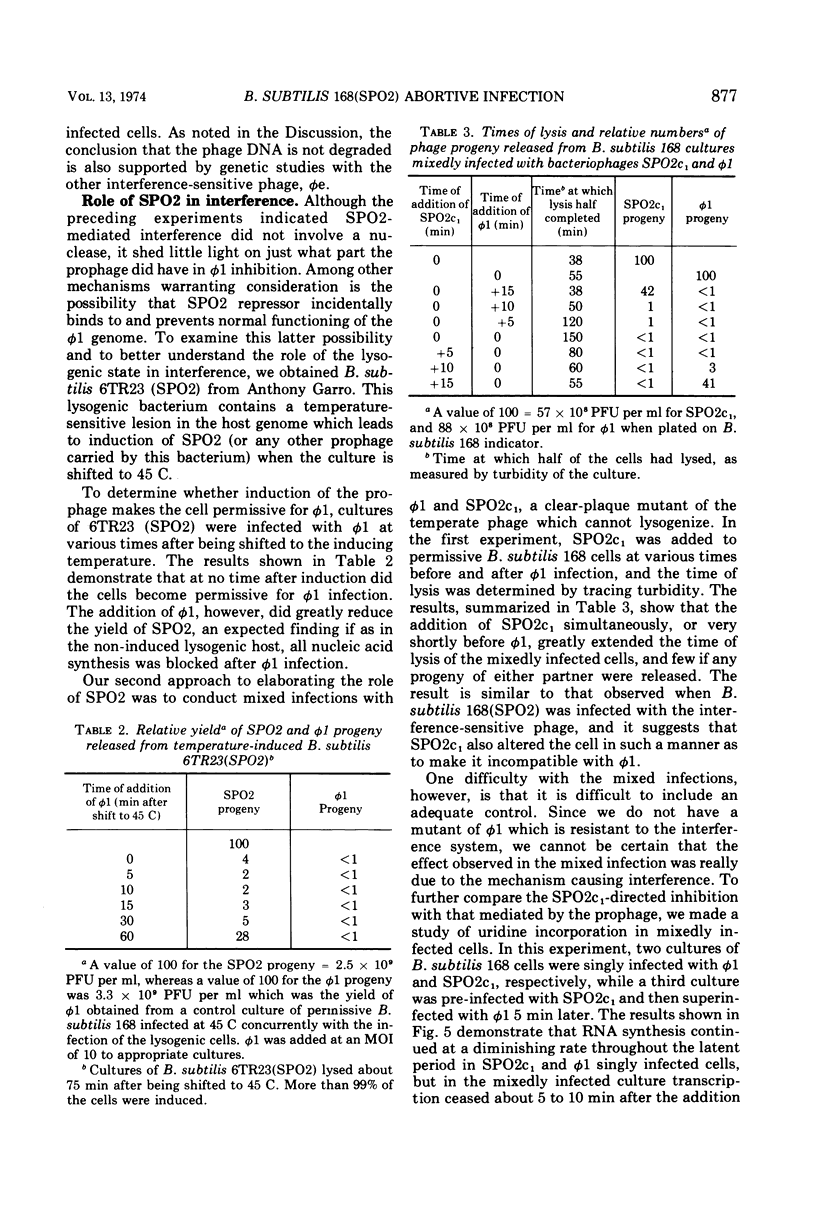
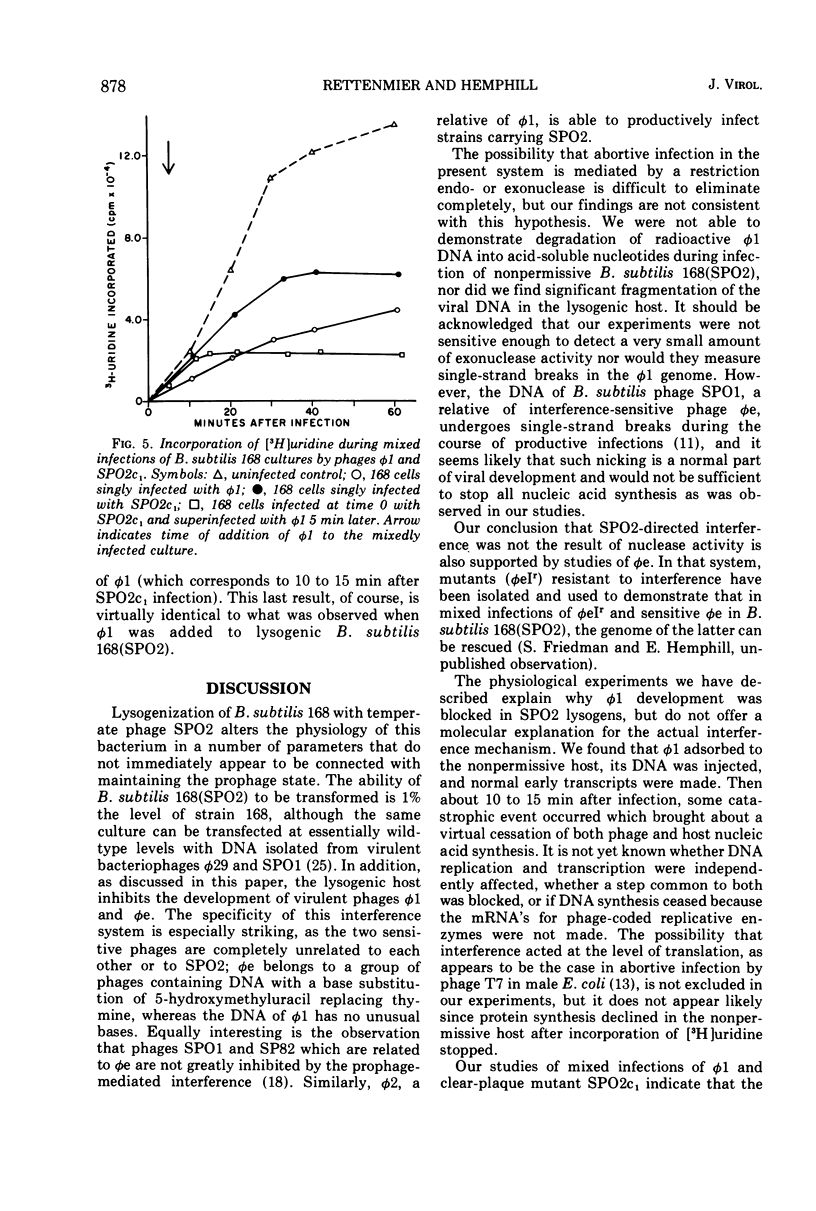
Virulent bacteriophage φ1 was not able to productively infect strains of Bacillus subtilis which were lysogenic for the temperate bacteriophage SPO2, although it adsorbed to, penetrated, and killed these bacteria. Studies of phage and host nucleic acid production in the nonpermissive host demonstrated that normal φ1 transcription was initiated early in the latent period, but this was followed by a general failure of host and phage nucleic acid synthesis about 10 to 15 min after infection. Mixed infections of φ1 and SPO2c1, a clear-plaque mutant of SPO2, indicated that a similar inhibition of φ1 development occurred when this phage infected nonlysogenic B. subtilis cells committed to the SPO2c1 lytic cycle. It is proposed that the SPO2- and SPO2c1-mediated interference did not act directly on the φ1 genome, but rather these phages altered the host physiology in such a manner that some normal step in φ1 development triggered a collapse of vital metabolic activities.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrachan L., Miller J. F. Regulation of lambda rex expression after infection of Escherichia coli K by lambda bacteriophage. J Virol. 1972 Mar;9(3):510–518. doi: 10.1128/jvi.9.3.510-518.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. R., Geiman J. M. A new effect of the rex gene of phage lambda: premature lysis after infection by phage T1. Virology. 1973 Nov;56(1):285–290. doi: 10.1016/0042-6822(73)90306-1. [DOI] [PubMed] [Google Scholar]
- Dhillon E. K., Dhillon T. S. HK239: a P2 related temperate phage which excludes rII mutants of T4. Virology. 1973 Sep;55(1):136–142. doi: 10.1016/s0042-6822(73)81015-3. [DOI] [PubMed] [Google Scholar]
- Ennis H. L., Kievitt K. D. Association of the rIIA protein with the bacterial membrane. Proc Natl Acad Sci U S A. 1973 May;70(5):1468–1472. doi: 10.1073/pnas.70.5.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Gussin G. N., Peterson V. Isolation and properties of rex - mutants of bacteriophage lambda. J Virol. 1972 Oct;10(4):760–765. doi: 10.1128/jvi.10.4.760-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. D. Phage lambda mutants deficient in r-II exclusion. Science. 1967 Dec 22;158(3808):1588–1589. doi: 10.1126/science.158.3808.1588. [DOI] [PubMed] [Google Scholar]
- Jacquemin-Sablon A., Lanni Y. T. Lambda-repressed mutants of bacteriophage T5. I. Isolation and genetical characterization. Virology. 1973 Nov;56(1):230–237. doi: 10.1016/0042-6822(73)90302-4. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Hemphill H. E., Whiteley H. R. Nucleic acid synthesis in bacteriophage SPO2c 1 -infected Bacillus subtilis. J Virol. 1972 May;9(5):776–784. doi: 10.1128/jvi.9.5.776-784.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG S. Suppression of the multiplication of heterologous bacteriophages in lysogenic bacteria. Virology. 1957 Jun;3(3):496–513. doi: 10.1016/0042-6822(57)90006-5. [DOI] [PubMed] [Google Scholar]
- Levner M. H. Eclipse of viral DNA infectivity in SPO1-infected Bacillus subtilis. Virology. 1972 Oct;50(1):267–272. doi: 10.1016/0042-6822(72)90369-8. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Sironi G., Bialy H., Calendar R. Bacteriophage lambda; abortive infection of bacteria lysogenic for phage P2. Proc Natl Acad Sci U S A. 1970 Jul;66(3):587–594. doi: 10.1073/pnas.66.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- Palefski S., Hemphill H. E., Kolenbrander P. E., Whiteley H. R. Dominance relationships in mixedly infected Bacillus subtilis. J Virol. 1972 Apr;9(4):594–601. doi: 10.1128/jvi.9.4.594-601.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. F., Kievitt K. D., Ennis H. L. Membrane protein synthesis after infection of Escherichia coli B with phage T4: the rIIB protein. Virology. 1972 Nov;50(2):520–527. doi: 10.1016/0042-6822(72)90403-5. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Smith H. S., Miovic M., Pylkas L. Effect of prophage W on the propagation of bacteriophages T2 and T4. J Virol. 1968 Nov;2(11):1339–1345. doi: 10.1128/jvi.2.11.1339-1345.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pène J. J. Host macromolecular synthesis in bacteriophage-infected Bacillus subtilis. Bacteriol Rev. 1968 Dec;32(4 Pt 1):379–386. [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Hemphill H. E. Prophage-mediated interference affecting the development of Bacillus subtilis bacteriophage phi e. J Virol. 1973 Mar;11(3):372–377. doi: 10.1128/jvi.11.3.372-377.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y., Karu A. E., Linn S., Echols H. Purification and properties of the gamma-protein specified by bacteriophage lambda: an inhibitor of the host RecBC recombination enzyme. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2215–2219. doi: 10.1073/pnas.70.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., De Sain C. V., Hawley L. A., Anderson D. L. Transcription during the development of bacteriophage phi 29: production of host- and phi 29-specific ribonucleic acid. J Virol. 1972 Dec;10(6):1170–1178. doi: 10.1128/jvi.10.6.1170-1178.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi G., Bialy H., Lozeron H. A., Calendar R. Bacteriophage P2: interaction with phage lambda and with recombination-deficient bacteria. Virology. 1971 Nov;46(2):387–396. doi: 10.1016/0042-6822(71)90040-7. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Pizer L. I. Abortive infection of Escherichia coli strain W by T2 bacteriophage. J Mol Biol. 1968 Oct 14;37(1):131–149. doi: 10.1016/0022-2836(68)90078-8. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Pizer L. I., Pylkas L., Lederberg S. Abortive infection of Shigella dysenteriae P2 by T2 bacteriophage. J Virol. 1969 Aug;4(2):162–168. doi: 10.1128/jvi.4.2.162-168.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S. B., Frankel F. R. Identification of the T4rIIB gene product as a membrane protein. J Mol Biol. 1972 Oct 14;70(3):589–615. doi: 10.1016/0022-2836(72)90561-x. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



