Abstract
Objective
Saturated fatty acids, such as palmitic and stearic acid, cause detrimental effects in endothelial cells (ECs) and have been suggested to contribute to macrophage accumulation in adipose tissue and the vascular wall in states of obesity and insulin resistance. Long-chain fatty acids are believed to require conversion into acyl-CoA derivatives to exert most of their detrimental effects, a reaction catalyzed by acyl-CoA synthetases (ACSL). The objective of this study was to investigate the role of ACSL1, an ACSL isoform previously shown to mediate inflammatory effects in myeloid cells, in regulating EC responses to a saturated fatty acid-rich environment in vitro and in vivo.
Methods and Results
Saturated fatty acids caused increased inflammatory activation, ER stress, and apoptosis in mouse microvascular ECs. Forced ACSL1 overexpression exacerbated the effects of saturated fatty acids on apoptosis and ER stress. However, endothelial ACSL1-deficiency did not protect against the effects of saturated fatty acids in vitro, nor did it protect insulin resistant mice fed a saturated fatty acid-rich diet from macrophage adipose tissue accumulation or increased aortic adhesion molecule expression.
Conclusion
Endothelial ACSL1 is not required for inflammatory and apoptotic effects of a saturated fatty acid-rich environment.
Keywords: Acyl-CoA synthetase, Apoptosis, Endothelium, Inflammation
Introduction
The endothelium protects the organism from a number of diseases, including vascular disease and insulin resistance.1,2 However, diabetes and cardiovascular risk factors cause changes in the endothelium, leading to endothelial dysfunction. Elevated levels of fatty acids are generally believed to cause endothelial dysfunction.3 Palmitic acid (16:0) and stearic acid (18:0) are two common saturated fatty acids that cause endothelial dysfunction in vitro, including pro-inflammatory signaling and apoptosis.4-6 Increased activation of the ceramide pathway and toll-like receptor 4 (TLR4) have been implicated in the pro-inflammatory changes elicited by palmitate.5,7-10 However, the mechanism by which TLR4 is modulated by saturated fatty acids remains controversial.11 Saturated fatty acids also induce apoptosis by a pathway that appears to require endoplasmic reticulum (ER) membrane saturation and subsequent ER stress12 as well as activation of NF-κB signling.13 ER stress induces apoptosis through activation of the protein kinase JNK in many cells.14 These mechanisms require conversion of the free saturated fatty acids to their acyl-CoA derivatives.
Long-chain acyl-CoA synthetase (ACSL) isoforms ligate long-chain fatty acids to the CoA moiety. Conversion of free fatty acids into their acyl-CoA derivatives is required for channeling of fatty acids into cellular lipid pools and beta-oxidation, and most of the biological actions of fatty acids in cells.15 Of the five ACSL isoforms expressed in mammals, ACSL1, ACSL3, ACSL4, and ACSL5 are expressed in endothelial cells (ECs).16 Specific functions of these ACSL isoforms in endothelial cells are unknown.
Forced overexpression of ACSL1 in the heart causes cardiac lipotoxicity and increased apoptosis,17 whereas ACSL1-deficiency in myeloid cells protects against the inflammatory effects of diabetes in monocytes and macrophages, as well as diabetes-accelerated atherosclerosis.18 We therefore hypothesized that ACSL1-dependent acyl-CoA synthesis mediates the detrimental effects of saturated fatty acids in ECs in vitro and in vivo.
Our findings show that forced overexpression of ACSL1 in ECs indeed exacerbates saturated fatty acid-induced apoptosis and ER stress. However, endothelial ACSL1-deficiency does not protect ECs against the pro-inflammatory and pro-apoptotic effects of saturated fatty acids in vitro, nor does it protect against increased adipose tissue macrophage accumulation or aortic expression of vascular cell adhesion molecule 1 (Vcam1) in a saturated fat-fed mouse model of insulin resistance. These findings suggest that when overexpressed, ACSL1 is sufficient to exacerbate the effects of saturated fatty acids on ER stress and apoptosis, but that normally, ACSL1 does not contribute in major ways to these effects of saturated fatty acids, or to inflammatory changes associated with high fat diet-feeding.
Methods
Generation of conditional ACSL1 endothelial and hematopoietic-deficient mice
Acsl1flox/flox mice were generated as described previously,19 and backcrossed 10 generations into the C57BL/6 background. These mice were then crossed with Tie2-Cre mice (The Jackson Laboratory, Bar Harbor, ME) on a C57BL/6 background to obtain mice with endothelial and hematopoietic ACSL1-deficiency. Female breeders were Cre-negative to avoid germ-line transmission. The resulting Acsl1flox/floxTie2-Cre+ mice were termed ACSL1 endothelial and hematopoietic-deficient mice (ACSL1E/H−/− mice). Littermate controls carried the Cre transgene, but were wild-type (WT) for ACSL1 (Acsl1wt/wtTie2-Cre+). In addition, Acsl1flox/floxTie2-Cre− control mice were used for some experiments.
Insulin resistant obese mouse model
Male ACSL1E/H−/− mice and WT littermate controls (10-12 weeks of age) were fed chow diet or a diabetogenic diet with added cholesterol (DDC) rich in saturated fatty acids for 20 weeks, as previously described.20-21 Glucose tolerance tests were performed at week 18, as previously described.21
Isolation and culture of endothelial cells
Mouse microvascular endothelial cells (MMECs) were isolated by a fluorescence-activated cell sorting method from lungs or hearts22, or by a magnetic bead positive selection method from hearts using an ICAM-2 antibody, as previously described.23 Most of the experiments were performed on heart MMECs isolated by the magnetic bead separation method between passage 3 and 6. Bovine aortic endothelial cells (BAECs) were purchased from Cambrex Bioscience (Walkersville, MD).
Additional methods are described in the Supplemental Materials.
Results
Palmitate and stearate promote inflammatory changes and apoptosis in mouse microvascular endothelial cells
MMECs exposed to palmitate (16:0) or stearate (18:0) bound to BSA at indicated molar ratios for 24 h exhibited dose-dependent increases in secretion of the chemokines CCL2 (chemokine [C-C motif] ligand 2) and CXCL1 (chemokine [C-X-C motif] ligand 1), as well as increased shedding of soluble vascular cell adhesion molecule 1 (sVCAM-1) and soluble intracellular adhesion molecule 1 (sICAM-1), as shown in Figures 1A-D. Significant effects of 16:0 were observed at 1:2 and 1:3 BSA:fatty acid molar ratios, corresponding to 156 μmol/L and 234 μ 16:0, respectively, whereas 18:0 was more potent and induced significant changes at 1:0.5 to 1:1 BSA:fatty acid molar ratios, corresponding to 39 μmol/L and 78 μmol/L, respectively. These concentrations of 18:0 are in the physiological range for human plasma.24 Stearate and palmitate also induced increased caspase 3 activity in these MMECs (Figure 1E) and apoptosis (Figure 1F). Again, stearate was more potent than palmitate (Figure 1F). Thus, saturated fatty acids, and particularly stearate, induce an inflammatory response and apoptosis in MMECs, consistent with studies on ECs from other vascular beds and species.13,25-26
Figure 1. Saturated fatty acids exhibit pro-inflammatory and pro-apoptotic effects in mouse endothelial cells.
A-D, Heart MMECs (passage 3) were stimulated with 16:0 or 18:0 bound to BSA at indicated BSA:fatty acid molar ratios in the presence of 1% FBS for 24 h. The BSA concentration was 78 μmol/L. CCL2 secretion (A), CXCL1 secretion (B), sVCAM-1 (C) and sICAM-1 (D) shedding were analyzed by ELISAs. Caspase 3 activity was measured by a fluorometric assay in heart MMECs stimulated with 156 μmol/L 16:0 or 18:0 bound to BSA at a 1:2 molar ratio for 24 (E). Apoptosis was measured as DNA fragmentation (TUNEL) using the HT TiterTACSTM assay kit (F). N=3-6; mean ±SEM
Forced overexpression of ACSL1 results in increased palmitoyl-CoA and stearoyl-CoA levels and exacerbated apoptosis, ER stress and JNK activation in palmitate-stimulated bovine aortic endothelial cells
The pro-inflammatory and pro-apoptotic effects of saturated fatty acids are likely to be mediated by the acyl-CoA derivatives of these fatty acids, as discussed above. To investigate the effect of ACSL1 on saturated fatty acid-induced changes in ECs, we next stably overexpressed ACSL1 by using a retroviral vector in BAECs. BAECs were used for these experiments because of their greater proliferative capacity compared to MMECs. Both 16:0 and 18:0 increased chemokine (Ccl2) expression and cell death in these cells, like in MMECs, whereas oleate (18:1) prevented these effects (Figure S1A-B). The pro-inflammatory and pro-apoptotic effects of 16:0 were also prevented by triacsin C, an acyl-CoA synthetase inhibitor that inhibits most ACSL isoforms, consistent with previous studies13,26 (Figure S1C-E). BAECs infected with the ACSL1 retroviral vector exhibited clearly detectable myc-tagged ACSL1 protein compared to the empty vector (pBM)-infected controls (Figure 2A). Consistent with these observations, cell extracts from BAECs overexpressing ACSL1 exhibited a significant increase in palmitoyl-CoA synthesis (Figure 2B). Whereas there were no significant differences in acyl-CoA levels between ACSL1-overexpressing cells and controls under basal low-serum conditions, both palmitoyl-CoA and stearoyl-CoA levels increased in palmitate-stimulated cells overexpressing ACSL1 (Figure 2C). Forced ACSL1 overexpression exacerbated palmitate-induced BAEC cell death and caspase 3 activity (Figures 2D-E). Saturated fatty acids can promote JNK activation, thereby inducing apoptosis and pro-inflammatory changes in different cell types.27 To investigate if ACSL1 overexpression exacerbates the effects of 16:0 on JNK activation, BAECs were stimulated with 16:0 for 4 h. Cells stimulated with 16:0 demonstrated an increased JNK phosphorylation, and ACSL1 overexpression further increased 16:0-induced JNK phosphorylation (Figures 2F-G).
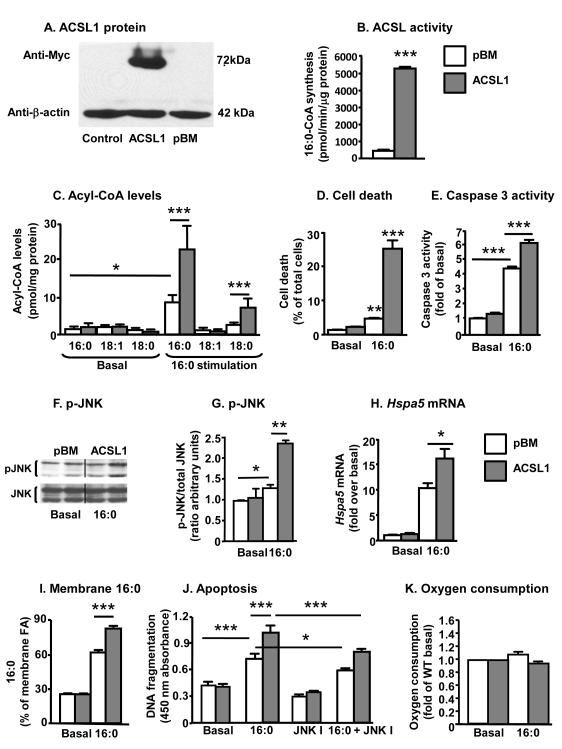
Figure 2. ACSL1 overexpression exacerbates effects of palmitate in bovine aortic endothelial cells.
BAECs were transduced with the empty pBM vector, a vector encoding ACSL1-myc, or no vector (control). A, Cell lysates (45 μg/lane) were separated on SDS-PAGE gels. Blots were probed with anti-myc and anti-β-actin antibodies. B, ACSL activity was analyzed in cell lysates as the rate of conversion of [3H]-16:0 to [3H]-palmitoyl-CoA. C, BAECs were treated with or without 156 μmol/L 16:0 bound to BSA at a 1:2 BSA:fatty acid ratio for 24 h. Acyl-CoA species were quantified by LC-ESI-MS/MS. D, BAECs overexpressing ACSL1 were treated with 16:0 for 2 days, and cell death was determined as the percentage of floating propidium iodide-positive cells. E, Caspase 3 activity was measured after 16:0 treatment for 24 h. F,G, Cells infected with pBM or ACSL1 were stimulated with 16:0 for 4 h. Total cell lysates (40 μg/lane) were separated on 12% SDS-PAGE gels. Blots were probed with anti-phospho-JNK and anti-JNK antibodies. H, Hspa5 mRNA was measured by real-time PCR after 24 h stimulation. I, Membrane fatty acid composition of BAECs was analyzed by GC-MS following a 24 h stimulation with 16:0 and expressed as % of total fatty acids analyzed. J, Apoptosis was detected using the HT TiterTACSTM assay in BAECs incubated in the presence or absence of a cell-permeable JNK inhibitor I (JNK I; 10 μmol/L). K, Oxygen consumption was measured in real time. Data represents the steady state values of kinetic data, after normalizing to the value obtained at 5 mmol/L glucose without palmitate (the oxygen consumption rate at 5 mmol/L glucose was 1.26 ± 0.35 nmol/min/105 cells for control BAECs, and 0.69 ± 0.28 for BAECs overexpressing ACSL1). N=3-6; mean±SEM; white bars, control cells; gray bars, ACSL1- overexpressing cells infected with the empty pBM vector
ER stress is often linked to JNK activation.28-29 Therefore, Hspa5 (Grp78) mRNA, an ER stress marker, was used to evaluate ER stress in control and ACSL1-overexpressing BAECs. Consistent with the JNK activation, Hspa5 mRNA levels were increased by palmitate-stimulation, and overexpression of ACSL1 resulted in a further elevation of Hspa5 mRNA levels (Figure 2H). Increased ER stress has been shown to be associated with an increased presence of saturated fatty acids in the membrane, and consistently, palmitate-stimulated BAECs exhibited a marked increase in membrane palmitate levels, which was exacerbated by ACSL1 overexpression (Figure 2I). Thus, ACSL1 overexpression results in membrane fatty acid saturation following palmitate-stimulation and accompanied ER stress, JNK activation, and increased apoptosis.
In order to investigate whether the increased JNK activation contributes to apoptosis in palmitate-stimulated BAECs, we used the cell-permeable JNK Inhibitor I (EMD Millipore). As shown by figure 2J, the JNK inhibitor partly but significantly protected palmitate-stimulated BAECs as well as ACSL1-overexpressing BAECs from apoptosis, as measured by reduced DNA fragmentation (TUNEL). Similar results were obtained using an unrelated JNK inhibitor (SP600125, Biomol; data not shown), suggesting that activation of the JNK pathway is in part responsible for the increased apoptosis in palmitate stimulated BAECs and BAECs overexpressing ACSL1.
Finally, overexpression of ACSL1 did not significantly alter oxygen consumption, suggesting that the metabolic state of the ECs was not markedly different depending on ACSL1 levels (Figure 2K).
Endothelial ACSL1-deficiency does not provide protection from saturated fatty acid-induced pro-apoptotic or pro-inflammatory changes
To investigate if intrinsic ACSL1 promotes pro-apoptotic and pro-inflammatory changes in saturated fatty acid exposed ECs, we next generated mice with endothelium and hematopoietic-targeted ACSL1-deficiency. Acsl1flox/floxTie2-CreTg (ACSL1E/H−/−) mice and control littermate WT controls (Acsl1wt/wtTie2-CreTg) were genotyped (Figure 3A) and used for experiments. Relative Acsl1 mRNA levels were lower in heart MMECs than in heart tissue (Figure S2). Acsl1 mRNA levels were reduced by 60-75% in freshly isolated and early passage heart MMECs from ACSL1E/H−/− mice compared to littermate controls (Figure 3B), while mRNA levels of other ACSL isoforms was unchanged (Figure 3B). Furthermore, there was no detectable reduction of Acsl1 mRNA levels in livers from ACSL1EH−/− mice, demonstrating selective loss of ACSL1 (Figure 3B). ACSL1 protein was also reduced in MMECs from ACSL1EH−/− mice (Figure 3C).
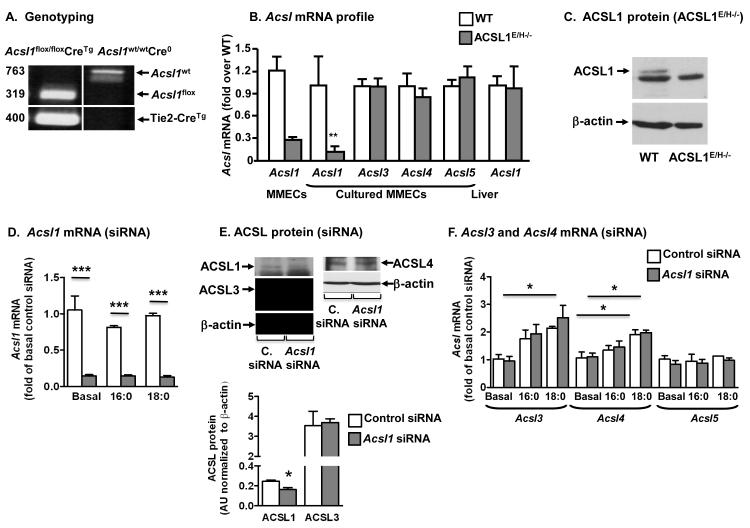
Figure 3. ACSL1-deficiency in MMECs induced by Tie2-Cre or siRNA.
A, PCR amplification of Acsl1 LoxP sites and Tie2-Cre recombinase. B, Levels of Acsl mRNA in heart MMECs and liver from ACSL1E/H−/− mice and wildtype (WT) littermates were measured by real-time qPCR. C, ACSL1 protein levels in lung MMECs from ACSL1E/H−/− mice and wildtype (WT) littermates. ACSL1 runs above a prominent non-specific band in MMECs. D, Acsl1 mRNA levels in heart MMECs treated with Acsl1 siRNA or control siRNA and then stimulated with 16:0 or 18:0 at a BSA:fatty acid ratio of 1:3 for 24 h. E, ACSL1, ACSL3 and ACSL4 protein levels in heart MMECs grown in 10% serum. Quantification of ACSL1 and ACSL3 protein levels (N=3) is shown below the representative blots. F, Acsl3, Acsl4 and Acsl5 mRNA levels in the samples analyzed in D. N=3-5; mean±SEM; white bars, WT MMECs (B) or MMECs expressing control siRNA (D-F); gray bars, MMECs from ACSL1E/H−/− mice (B) or MMECs expressing Acsl1 siRNA (D-F)
As a second approach, ACSL1 expression was knocked down by siRNA. Acsl1 siRNA reduced Acsl1 mRNA levels by 80-90% in MMECs, both under basal and fatty acid-stimulated conditions (Figure 3D). ACSL1 protein levels were also reduced, whereas no significant compensatory upregulation was found for ACSL3 or ACSL4 protein (Figure 3E). However, fatty acid stimulation, and especially stearate, upregulated Acsl3 and Acsl4 mRNA levels in MMECs (Figure 3F), suggesting that these ACSL isoforms might take on a more important role in saturated fatty acid-stimulated MMECs. No compensatory upregulation of Acsl3, Acsl4 or Acsl5 was observed in MMECs treated with Acsl1 siRNA under basal or fatty acid stimulated conditions (Figure 3F).
We next investigated fatty acid and glucose utilization in MMECs deficient in ACSL1. ACSL1 knockdown did not result in a measurable reduction in palmitate beta-oxidation (Figure S3A), no differences in glucose consumption and a very slight increase in lactate production (Figures S3B-C). These experiments also demonstrated that these MMECs are highly glycolytic cells.
Cultured MMECs from WT and ACSL1E/H−/− mice responded to saturated fatty acid stimulation with an increased secretion of CCL2 (data not shown), and increased shedding of sVCAM-1 and sICAM-1 (Figures 4A-B). ACSL1-deficiency did not protect against these effects, but rather tended to exacerbate the stearate-induced effects. Furthermore, palmitate and stearate induced ER stress, as measured by increased Hspa5 (Grp78) and Ddit3 (Chop) mRNA levels and Xbp1 splicing (Figures 4C-D and Figure S4). ACSL1-deficiency did not protect the cells from the stearate- or palmitate-induced ER stress response, but rather increased levels of the chaperone Hspa5 (Figure 4C). ACSL1-deficiency also did not protect against saturated fatty acid-induced apoptosis, measured as DNA fragmentation (Figure 4E). The lack of effects of ACSL1-deficiency was confirmed in MMECs in which ACSL1 had been downregulated by siRNA. Consistent with the results on MMECs from ACSL1E/H−/− mice, Acsl1 siRNA did not result in an inhibition of stearate-induced sVCAM-1 or sICAM-1 shedding, ER stress response genes or apoptosis, but rather an increase in was observed (Figures 4F-J). Thus, ACSL1 is not required for saturated fatty acid-induced pro-inflammatory and pro-apoptotic effects in cultured MMECs.
Figure 4. ACSL1-deficient MMECs are not protected from the pro-inflammatory or pro-apoptotic effects of saturated fatty acids.
sVCAM-1 and sICAM-1 shedding was measured by ELISA after a 24 h stimulation with 16:0 or 18:0 at a 1:2 BSA:fatty acid molar ratio in MMECs from ACSL1E/H−/− and WT mice (A-B) or heart MMECs treated with Acsl1 siRNA or control siRNA (F-G). The ER stress markers Hspa5 and Ddit3 were measured by real-time PCR in MMECs from ACSL1E/H−/− and WT mice (C-D) or MMECs treated with Acsl1 siRNA or control siRNA (H-I). Apoptosis was detected using the HT TiterTACSTM assay in MMECs from ACSL1E/H−/− and WT mice (E) or MMECs treated with Acsl1 siRNA or control siRNA (J). The effects of fatty acids were lower (F-J) in electroporated cells, and the palmitate effect was not significant and therefore not shown. N=3-5; mean±SEM; white bars, WT MMECs (A-E) or MMECs expressing control siRNA (F-J); gray bars, MMECs from ACSL1E/H−/− mice (A-E) or MMECs expressing Acsl1 siRNA (F-J)
Saturated fatty acids have been shown to mediate inflammatory effects through TLR4 in endothelial cells,5 and we therefore repeated some of these experiments in MMECs isolated from hearts of Tlr4+/+ and Tlr4−/− mice. In agreement with previous findings, TLR4-deficiency exerted a protective effect on stearate-induced CCL2 secretion and sVCAM-1 and sICAM-1 shedding (Figures 5A-C), whereas it did not significantly protect against saturated fatty acid-induced apoptosis (Figure 5D). Thus, in contrast to ACSL1-deficiency, TLR4-deficiency blunts the inflammatory effects of stearate. The effects of palmitate were weaker than those of stearate, and were not affected by TLR4-deficiency. It is possible that palmitate mediates its effects in part through other mechanisms, such as through activation of TLR2.30
Figure 5. TLR4-deficiency exerts protective effects in stearate-stimulated MMECs.

A-C, CCL2 secretion, sVCAM-1 shedding and sICAM-1 shedding were measured by ELISA in conditioned media from Tlr4+/+ or Tlr4−/− heart MMECs (passage 4) incubated in the presence or absence of 16:0 or 18:0 bound to BSA at a 1:3 molar ratio, or in the presence of BSA alone for 24 h (Basal). D, Apoptosis was detected using the HT TiterTACSTM assay. N=3-6; mean ± SEM; white bars, Tlr4+/+ MMECs; black bars, Tlr4−/− MMECs
Endothelial ACSL1-deficiency does not confer a protective effect in a mouse model of insulin resistance and dietary fatty acid saturation
Exposure of cells in vitro to single fatty acids bound to BSA can be regarded as a rather simple and artificial system,31 and we therefore next assessed the potential effects of endothelial ACSL1-deficiency in vivo. We investigated effects of endothelial ACSL1-deficiency in a mouse model of insulin resistance and obesity in which mice are fed a diet with increased fatty acid saturation, sucrose and cholesterol.20-21 Male WT and ACSL1E/H−/− mice were fed this diabetogenic diet (DDC) or chow for 20 weeks. At the end of the study, DDC-fed mice were significantly obese, showed increased fasting blood glucose levels, glucose intolerance, and increased plasma cholesterol levels, as compared to chow-fed mice (Figures 6A-D). Endothelial ACSL1-deficiency had no effect in chow-fed or DDC-fed mice on these parameters. Plasma levels of sICAM-1 and sVCAM-1 were not elevated by the DDC, and were not affected by ACSL1-deficiency (Figures 6E-F), but aortic levels of Vcam1 mRNA were significantly elevated by DDC-feeding (Figure 6G). Endothelial ACSL1-deficiency did not protect the aorta from DDC-induced Vcam1 induction (Figure 6G).
Figure 6. Endothelial ACSL1-deficiency does not protect against the effects of obesity and insulin resistance on adipose tissue macrophage accumulation or aortic VCAM-1 expression.
Male WT and ACSL1E/H−/− mice were fed regular chow or DDC for 20 weeks. A, Body weights at the end of the study. B, Fasting (5 h) blood glucose levels at week 18. C, Glucose tolerance tests were performed at week 18 by injecting 1.5 g/kg dextrose into 5 h fasted mice and blood glucose was measured at indicated times. D, Plasma cholesterol levels at the end of the study. E-F, Plasma sICAM-1 and sVCAM-1 levels at the end of the study. G, Aortae were harvested in RNA-later (Qiagen, Valencia, CA), and levels of Vcam1 mRNA were measured by real-time PCR. H, Epididymal fat pads were isolated and sectioned. An anti-vWF antibody was used to detect ECs in microvessels. Scale bar indicates 20 μm. I-K, The macrophage markers Emr1 (F4/80) and Cd11b, and Ccl2 mRNA were measured in epididymal adipose tissue by real-time PCR. L, Fatty acid composition of the chow diet and DDC was analyzed by GC-MS and was expressed as saturated/unsaturated fatty acid ratio. M, Fatty acid composition of plasma samples was measured by GC-MS. N, sVCAM-1 secretion was measured by ELISA from MMECs incubated in the presence of fatty acid ratios reflecting plasma fatty acids in chow-fed and DDC-fed mice. N=5-8 per group; mean±SEM; white bars, WT mice; gray bars, ACSL1E/H−/− mice
Macrophages are known to accumulate in adipose tissue in mice fed high-fat diets, including the diet used in the present study.20-21 This macrophage accumulation requires monocytes to bind to and traverse microvessels. We therefore next investigated the effect of DDC and endothelial ACSL1-deficiency in epididymal fat from chow-fed and DDC-fed mice. ACSL1E/H−/− mice exhibited no significant differences in the number or morphology of microvessels in epididymal fat (Figure 6H). As expected, DDC-fed mice had significantly more macrophages in epididymal fat (measured as increased levels of the myeloid cell/macrophage markers Emr1 and Cd11b) and increased levels of Ccl2 mRNA (Figures 6I-K) as compared to chow-fed mice. Endothelial ACSL1-deficiency had no effect on these parameters (Figures 6I-K). Also, there were no differences in ER stress markers between the four groups of mice (data not shown).
To evaluate whether the mice had been exposed to elevated saturated fatty acid ratios, fatty acid composition of the chow diet and DDC as well as plasma fatty acid composition in the four groups of mice was analyzed by GC-MS. The DDC had higher relative levels of 16:1, 16:0, 18:1 and 18:0, and lower relative levels of 18:2 as compared to the chow diet, and the ratio of saturated fatty acids/unsaturated fatty acids was significantly higher (Figure 6L). Plasma levels of fatty acids in the four groups of mice generally reflected the fatty acid composition of the diet. Thus, mice fed DDC exhibited lower relative levels of 18:2 and higher relative levels of 18:1 and 18:0 as compared to chow-fed mice (Figure 6M). There were no differences in 16:0 plasma levels between the different groups. Endothelial ACSL1-deficiency did not alter plasma fatty acid composition (Figure 6M). Finally, cultured MMECs were exposed to fatty acid ratios mimicking those found in plasma of chow-fed and DDC-fed mice. The fatty acids were bound to BSA in a 1:2 BSA:fatty acid ratio. As shown by Figure 6N, sVCAM-1 shedding was the same in MMECs exposed to fatty acid ratios mimicking plasma fatty acid ratios in chow-fed mice and DDC-fed mice. Similarly, CCL2 secretion, sICAM-1 shedding and apoptosis were the same in both groups (data not shown).
These studies suggest that endothelial ACSL1-deficiency does not protect mice from a diet rich in saturated fatty acids on adipose tissue macrophage accumulation or aortic VCAM-1 expression, consistent with the in vitro studies.
Discussion
We demonstrate that whereas forced overexpression of ACSL1 in ECs results in exacerbation of apoptosis, ER stress and JNK activation induced by saturated fatty acids, ACSL1-deficiency does not protect ECs in vitro or in vivo from the detrimental effects of a saturated fatty acid-rich environment. Thus, overexpression of ACSL1 is likely to cause effects that are not normally conferred by ACSL1, perhaps because downstream enzymes that further process the generated palmitoyl-CoA and stearoyl-CoA are not able to efficiently deal with the increased acyl-CoA levels. In addition, overexpression of ACSL1 is likely to result in increased uptake and trapping of saturated fatty acids through vectorial acylation, especially under in vitro conditions in which only saturated fatty acids are provided. Consistent with these findings, overexpression of ACSL1 in the heart causes increased apoptosis and cardiomyopathy,17 and this can be prevented by also overexpressing diacylglycerol acyltransferase 1 (DGAT1), which promotes downstream triacylglycerol formation.32 Palmitate has previously been shown to cause ER stress and apoptosis by increasing ER membrane saturation.12 Our finding that ACSL1 overexpression exacerbates palmitate-induced apoptosis, ER stress and membrane palmitate accumulation suggests that ACSL1 has the ability to enhance this pathway in ECs.
ACSL1 normally expressed in ECs is likely to act in different subcellular compartments or on different fatty acids as compared to overexpressed ACSL1 because endothelial ACSL1-deficiency did not protect ECs from saturated fatty acid-induced apoptosis, ER stress, or release of inflammatory mediators, nor did ACSL1E/H−/− mice show a clear phenotype in a mouse model of insulin resistance associated with saturated fatty acid feeding. Instead, other ACSL isoforms or other enzymes with acyl-CoA synthetase activity are likely to mediate at least part of the effects of saturated fatty acids in ECs. This conclusion is based on the lack of effect of ACSL1-deficiency on saturated fatty acid-mediated effects, and also on the finding that triacsin C, a pharmacologic general ACSL inhibitor, prevents saturated fatty acid-induced apoptosis13,26, indicating that a triacsin C-inhibitable enzyme(s) is in part responsible for the saturated fatty acid effects in ECs, consistent with results from the present study, which demonstrated that triacsin C prevents the pro-inflammatory and pro-apoptotic effects of palmitate in BAECs. ACSL1 acts on a broad range of fatty acids, but is not specific to saturated fatty acids. For example, in hepatocytes oleoyl-CoA levels are most reduced by ACSL1-deficiecy,19 whereas in thioglycollate-elicited macrophages arachidonoyl-CoA levels are preferentially reduced by ACSL1-deficiency.18 Since ECs do not express significant levels of ACSL6, it is possible that the effects of palmitate and stearate are mediated by ACSL4, ACSL3 and/or ACSL5 in ECs. Consistently, our results suggest an increase in ACSL3 and ACSL4 expression in fatty acid-stimulated ECs. However, part of the effect of saturated fatty acids on release if inflammatory markers and apoptosis might be mediated by the free fatty acids, for example by altering membrane biophysical properties. The tendency of ACSL1-deficiency to exacerbate the effects of stearate supports this interpretation. Contrary to ACSL1-deficiency, TLR4-deficiency conferred protective effects against the inflammatory effects of stearate, consistent with published studies.5 Thus, ACSL1-deficiency and TLR4-deficiency exert the opposite effects in stearate-stimulated ECs.
Rather than mediating effects of saturated fatty acids, ACSL1 appears to have other biological functions in ECs. ACSL1 has a prominent role in mediating beta-oxidation in many tissues depending on fatty acids as an energy source, such as heart, liver, and adipose tissue.19,33-34 Conversely, we have recently demonstrated that in macrophages, ACSL1 does not contribute to beta-oxidation, but has a prominent role in mediating inflammatory changes in response to diabetes, most likely by acting on arachidonic acid.18 ACSL1 also does not appear to contribute to beta-oxidation in endothelial cells, at least not under the conditions tested. Overall, these studies support the hypothesis that ACSL isoforms may have distinct biological functions that are context- and cell type-dependent, and perhaps dependent on the availability of different fatty acid substrates under different conditions as well as the metabolism of the cell. The current work is the first study to investigate the biological role of any of the ACSL isoforms in endothelial cells in vivo. Future studies on endothelial ACSL1 include its potential role in atherosclerosis, vasoreactivity and blood pressure regulation, as well as evaluation of potential effects of ACSL1 on eNOS activity. The study also raises more complex questions. For example, to what extent do plasma levels of saturated fatty acids contribute to inflammatory reactions in vivo? Ratios of fatty acids mimicking those found in plasma of mice fed the high fat diet used in the present study did not promote inflammatory changes or apoptosis, as compared to ratios of fatty acids reflecting plasma levels in chow fed mice. It is possible that the inflammatory effects of diets rich in saturated fatty acids are due to associated systemic factors, such as cholesterol,20 or to increased tissue levels of saturated fatty acids or acyl-CoAs. In this context, it is interesting to speculate that upregulation of ACSL1 or other ACSL isoforms under inflammatory conditions18 might lead to increased trapping of fatty acids as acyl-CoAs in tissues regardless of plasma fatty acid levels.
In conclusion, this study shows that although overexpressed ACSL1 can act to exacerbate detrimental effects of saturated fatty acids in ECs, normally, ACSL1 does not mediate the inflammatory or apoptotic effects of a saturated fatty acid-rich environment.
Supplementary Material
Acknowledgments
We are grateful to Nicholas Ieronimakis for help and advice isolating MMECs by FACS.
Sources of Funding
This study was supported by NIH grants HL062887, HL092969, and HL097365 to KEB, DK59935 and P30 DK056350 to RAC, DK082841 to SP, DK073878 to FK, and DK064989 and Burroughs Wellcome 1005935 to JES. GO and XS was supported by the Cardiovascular Training grant T32 HL07828. The study was also supported by the Diabetes Research Center at the University of Washington (P30 DK017047) and a Seeding Collaborative Interdisciplinary Team Science in Diabetes, Endocrinology and Metabolic Diseases R24 grant. Acyl-CoAs were analyzed through the Molecular Phenotyping Core, Michigan Nutrition and Obesity Center (P30 DK089503).
Footnotes
Disclosures
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32(Suppl 2):S314–321. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, Michel T, Plutzky J. PPARγ in the endothelium regulates metabolic responses to high-fat diet in mice. J Clin Invest. 2009;119:110–124. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100:1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–1845. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- 5.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 6.Harvey KA, Walker CL, Pavlina TM, Xu Z, Zaloga GP, Siddiqui RA. Long-chain saturated fatty acids induce pro-inflammatory responses and impact endothelial cell growth. Clin Nutr. 2010;29:492–500. doi: 10.1016/j.clnu.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-κB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233–245. doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab. 2011;300:E145–E154. doi: 10.1152/ajpendo.00490.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz EA, Zhang WY, Karnik SK, Borwege S, Anand VR, Laine PS, Su Y, Reaven PD. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol. 2010;30:802–808. doi: 10.1161/ATVBAHA.109.201681. [DOI] [PubMed] [Google Scholar]
- 11.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29:1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 12.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Staiger K, Staiger H, Weigert C, Haas C, Häring HU, Kellerer M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 2006;55:3121–3126. doi: 10.2337/db06-0188. [DOI] [PubMed] [Google Scholar]
- 14.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 15.Li LO, Klett EL, Coleman RA. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim Biophys Acta. 2010;1801:246–251. doi: 10.1016/j.bbalip.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandoval A, Fraisl P, Arias-Barrau E, Dirusso CC, Singer D, Sealls W, Black PN. Fatty acid transport and activation and the expression patterns of genes involved in fatty acid trafficking. Arch Biochem Biophys. 2008;477:363–371. doi: 10.1016/j.abb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanter JE, Kramer F, Barnhart S, Averill MM, Vivekanandan-Giri A, Vickery T, Li LO, Becker L, Yuan W, Chait A, Braun KR, Potter-Perigo S, Sanda S, Wight TN, Pennathur S, Serhan CN, Heinecke JW, Coleman RA, Bornfeldt KE. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A. 2012;109:E715–E724. doi: 10.1073/pnas.1111600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li LO, Ellis JM, Paich HA, Wang S, Gong N, Altshuller G, Thresher RJ, Koves TR, Watkins SM, Muoio DM, Cline GW, Shulman GI, Coleman RA. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J Biol Chem. 2009;284:27816–27826. doi: 10.1074/jbc.M109.022467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A, 3rd, Kirk EA, O’Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Averill MM, Barnhart S, Becker L, Li X, Heinecke JW, Leboeuf RC, Hamerman JA, Sorg C, Kerkhoff C, Bornfeldt KE. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation. 2011;123:1216–1226. doi: 10.1161/CIRCULATIONAHA.110.985523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ieronimakis N, Balasundaram G, Reyes M. Direct isolation, culture and transplant of mouse skeletal muscle derived endothelial cells with angiogenic potential. PLoS One. 2008;3:e0001753. doi: 10.1371/journal.pone.0001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15:372–281. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamagishi S, Okamoto T, Amano S, Inagaki Y, Koga K, Koga M, Choei H, Sasaki N, Kikuchi S, Takeuchi M, Makita Z. Palmitate-induced apoptosis of microvascular endothelial cells and pericytes. Mol Med. 2002;8:179–184. [PMC free article] [PubMed] [Google Scholar]
- 26.Ciapaite J, van Bezu J, van Eikenhorst G, Bakker SJ, Teerlink T, Diamant M, Heine RJ, Krab K, Westerhoff HV, Schalkwijk CG. Palmitate and oleate have distinct effects on the inflammatory phenotype of human endothelial cells. Biochim Biophys Acta. 2007;1771:147–154. doi: 10.1016/j.bbalip.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 28.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298:E1027–E1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:2002–2013. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watt MJ, Hoy AJ, Muoio DM, Coleman RA. Distinct roles of specific fatty acids in cellular processes: implications for interpreting and reporting experiments. Am J Physiol Endocrinol Metab. 2012;302:E1–3. doi: 10.1152/ajpendo.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, Shi X, Bharadwaj KG, Ikeda S, Yamashita H, Yagyu H, Schaffer JE, Yu YH, Goldberg IJ. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–36323. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis JM, Li LO, Wu PC, Koves TR, Ilkayeva O, Stevens RD, Watkins SM, Muoio DM, Coleman RA. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab. 2010;12:53–64. doi: 10.1016/j.cmet.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellis JM, Mentock SM, Depetrillo MA, Koves TR, Sen S, Watkins SM, Muoio DM, Cline GW, Taegtmeyer H, Shulman GI, Willis MS, Coleman RA. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs fatty acid oxidation and induces cardiac hypertrophy. Mol Cell Biol. 2011;31:1252–1262. doi: 10.1128/MCB.01085-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.