Abstract
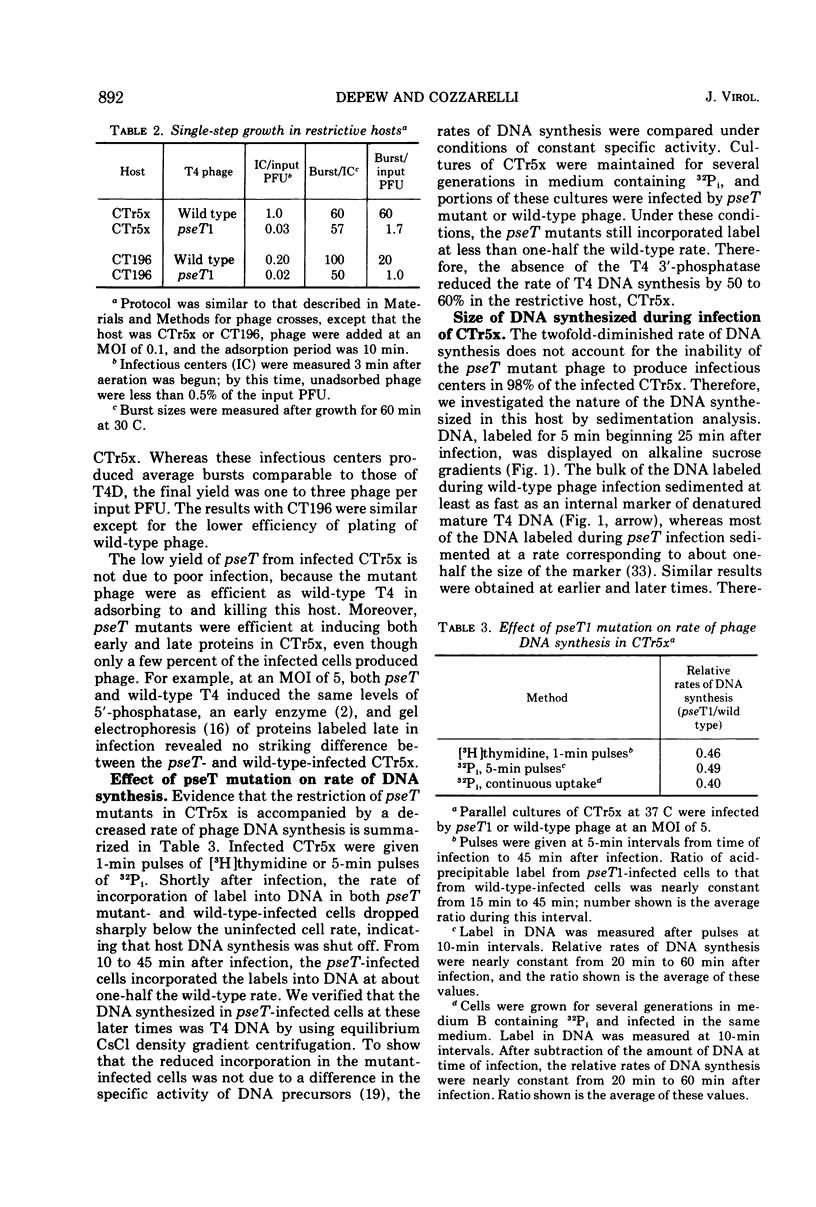
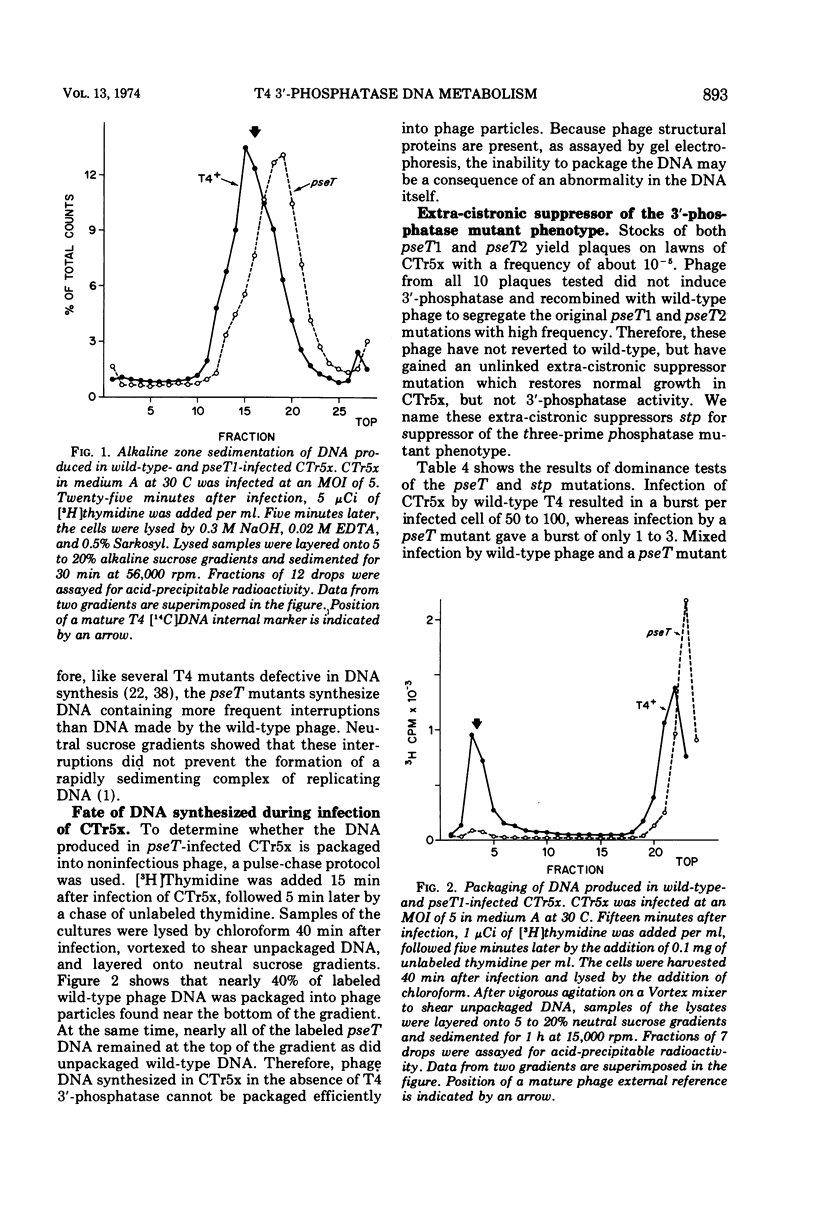
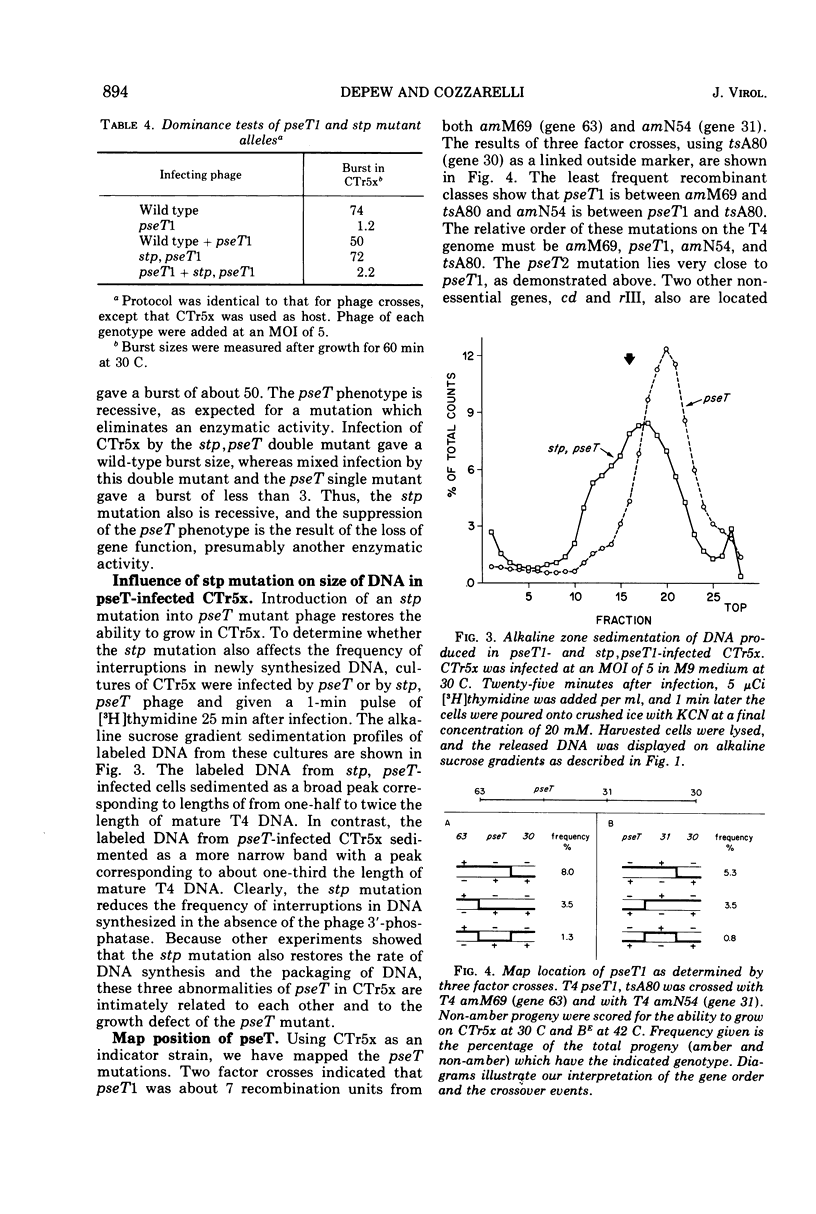
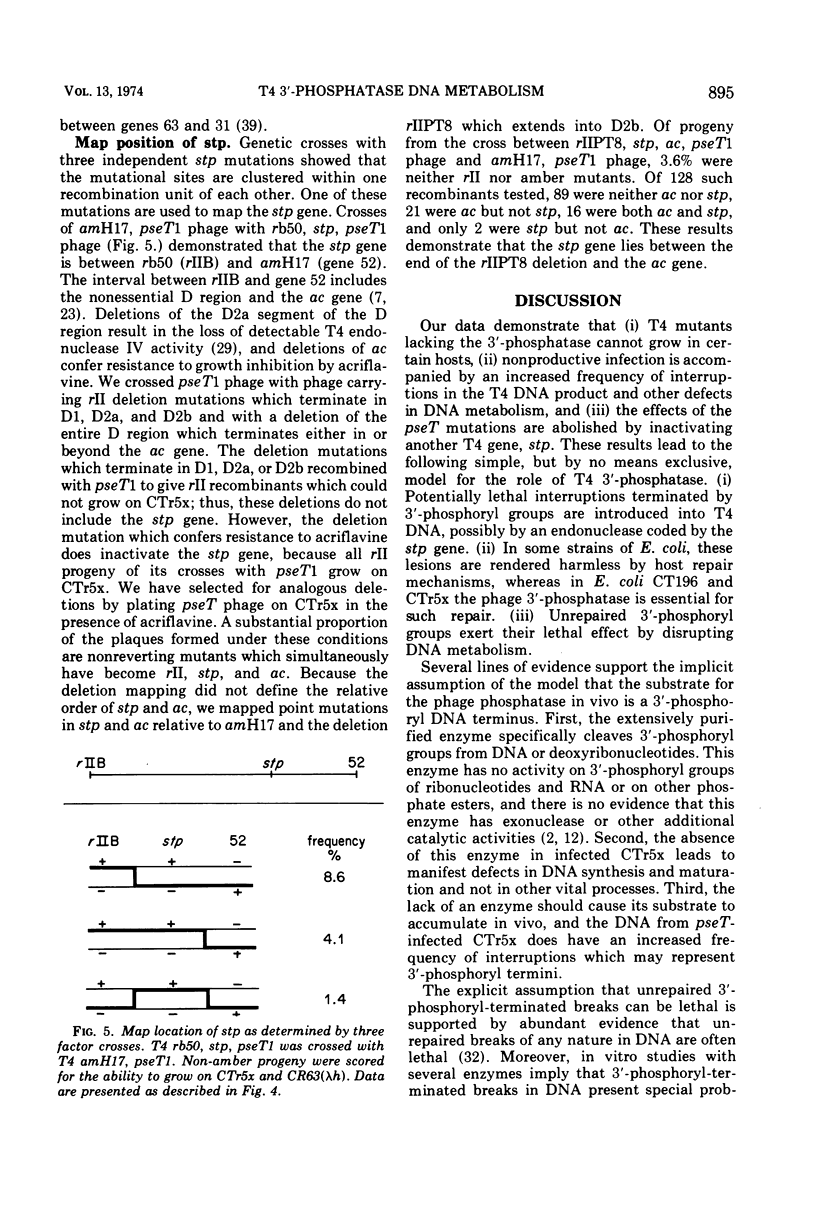
Mutants of bacteriophage T4D which fail to induce the deoxyribonucleotide-specific T4 3′-phosphatase have been isolated. These mutants (T4pseT) grow as well as wild-type T4 in most strains of Escherichia coli, but not in the T4-sensitive “Hospital Strain,” CT196, or in a derivative strain, CTr5x. Both the formation of infectious centers and the final yield of phage are reduced by 98% when CTr5x is infected by T4pseT mutants. The growth defects are accompanied by a 50% reduction in the rate of T4 DNA synthesis, a decrease in the single-strand length of the DNA product to about one-half the mature length, and greatly reduced packaging of DNA into phage particles. Introduction of an extra-cistronic suppressor mutation (stp) into T4pseT eliminates both the requirement for the T4 3′-phosphatase in infected CTr5x and the other observed effects of the pseT mutations. The pseT gene lies between genes 63 and 31. The stp gene lies in the nonessential region between rIIB and ac. Our results suggest that 3′-phosphoryl termini can disrupt T4 DNA replication to the extent that T4 3′-phosphatase becomes required for phage production.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Lerman L. S. Kinetics and intermediates in the intracellular synthesis of bacteriophage T4 deoxyribonucleic acid. J Mol Biol. 1970 Jun 14;50(2):235–261. doi: 10.1016/0022-2836(70)90190-7. [DOI] [PubMed] [Google Scholar]
- Becker A., Hurwitz J. The enzymatic cleavage of phosphate termini from polynucleotides. J Biol Chem. 1967 Mar 10;242(5):936–950. [PubMed] [Google Scholar]
- Chan V. L., Ebisuzaki K. Polynucleotide kinase mutant of bacteriophage T4. Mol Gen Genet. 1970;109(2):162–168. doi: 10.1007/BF00269652. [DOI] [PubMed] [Google Scholar]
- Doermann A. H., Boehner L. The identification of complex genotypes in bacteriophage T4. I. Methods. Genetics. 1970 Nov;66(3):417–428. doi: 10.1093/genetics/66.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove W. The extent of rII deletions in phage T4. Genet Res. 1968 Apr;11(2):215–219. doi: 10.1017/s001667230001140x. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- Hurwitz J., Becker A., Gefter M. L., Gold M. Enzymatic reactions at termini of DNA. J Cell Physiol. 1967 Oct;70(2 Suppl):181–199. doi: 10.1002/jcp.1040700413. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Effect of infection with T-even phage on the inducible synthesis of beta-glactosidase in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):453–468. doi: 10.1016/0022-2836(67)90051-4. [DOI] [PubMed] [Google Scholar]
- Koerner J. F. Enzymes of nucleic acid metabolism. Annu Rev Biochem. 1970;39:291–322. doi: 10.1146/annurev.bi.39.070170.001451. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Kaplan J. C., Ono H., Grossman L. Enzymatic repair of deoxyribonucleic acid. IV. Mechanism of photoproduct excision. Biochemistry. 1971 Aug 31;10(18):3325–3334. doi: 10.1021/bi00794a002. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., NUSSBAUM A. L. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. V. ON THE SPECIFICITY OF EXONUCLEASE I (PHOSPHODIESTERASE). J Biol Chem. 1964 Aug;239:2628–2636. [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mathews C. K. Biochemistry of deoxyribonucleic acid-defective amber mutants of bacteriophage T4. 3. Nucleotide pools. J Biol Chem. 1972 Nov 25;247(22):7430–7438. [PubMed] [Google Scholar]
- Milcarek C., Weiss B. Mutants of Escherichia coli with altered deoxyribonucleases. I. Isolation and characterization of mutants for exonuclease 3. J Mol Biol. 1972 Jul 21;68(2):303–318. doi: 10.1016/0022-2836(72)90215-x. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Shalitin C. Defective concatemer formation in cells infected with deoxyribonucleic acid-delay mutants of bacteriophage T4. J Virol. 1972 Oct;10(4):858–862. doi: 10.1128/jvi.10.4.858-862.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma D. H. Acriflavin resistant rII deletions of bacteriophage T4. Genet Res. 1969 Jun;13(3):329–331. doi: 10.1017/s0016672300003037. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. I. PURIFICATION OF THE ENZYME AND CHARACTERIZATION OF THE PHOSPHATASE ACTIVITY. J Biol Chem. 1964 Jan;239:242–250. [PubMed] [Google Scholar]
- Richardson C. C. Enzymes in DNA metabolism. Annu Rev Biochem. 1969;38:795–840. doi: 10.1146/annurev.bi.38.070169.004051. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C. The 5'-terminal nucleotides of T7 bacteriophage deoxyribonucleic acid. J Mol Biol. 1966 Jan;15(1):49–61. doi: 10.1016/s0022-2836(66)80208-5. [DOI] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D., Vetter D. Control of T4 endonuclease IV by the D2a region of bacteriophage T4. Virology. 1973 Aug;54(2):544–546. doi: 10.1016/0042-6822(73)90165-7. [DOI] [PubMed] [Google Scholar]
- Short E. C., Jr, Koerner J. F. Separation and characterization of deoxyribonucleases of Escherichia coli B. II. Further purification and properties of an exonuclease induced by infection with bacteriophage T2. J Biol Chem. 1969 Mar 25;244(6):1487–1496. [PubMed] [Google Scholar]
- Strauss B. S. DNA repair mechanisms and their relation to mutation and recombination. Curr Top Microbiol Immunol. 1968;44:1–85. [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I. Mutagenic treatment of double- and single-stranded DNA phages T4 ans S13 with hydroxylamine. Virology. 1968 Jun;35(2):330–333. doi: 10.1016/0042-6822(68)90275-4. [DOI] [PubMed] [Google Scholar]
- Warner H. R. Partial suppression of bacteriophage T4 ligase mutations by T4 endonuclease II deficiency: role of host ligase. J Virol. 1971 Apr;7(4):534–536. doi: 10.1128/jvi.7.4.534-536.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegian C. D., Mueller M., Selzer G., Russo V., Stahl F. W. Properties of the DNA-delay mutants of bacteriophage T4. Virology. 1971 Dec;46(3):900–919. doi: 10.1016/0042-6822(71)90090-0. [DOI] [PubMed] [Google Scholar]
- Yeh Y. C., Tessman I. Control of pyrimidine biosynthesis by phage T4. II. In vitro complementation between ribonucleotide reductase mutants. Virology. 1972 Mar;47(3):767–772. doi: 10.1016/0042-6822(72)90567-3. [DOI] [PubMed] [Google Scholar]



