Abstract
The sonication technique has been shown to be a promising tool for microbiological diagnosis of device-related infections. We evaluated the usefulness of the sonication method for pathogen detection in 80 explanted cardiac components collected from 40 patients, and the results were compared with those of conventional cultures. Forty subjects undergoing cardiac device removal were studied: 20 had cardiac device infection, and 20 subjects underwent elective generator replacement or revision in the absence of infection. Sonication of explanted devices was more sensitive than traditional culture for microbial detection (67% and 50%, respectively; P = 0.0005). The bacterial count detected in sonication fluid culture was significantly higher than that detected in traditional culture in both infected (P = 0.019) and uninfected (P = 0.029) devices. In the infected patients, sonication fluid culture yielded a significantly higher rate of pathogen detection in explanted electrodes than traditional culture (65% versus 45%; P = 0.02), while no differences were found in the generators. Ten strains were detected only through sonication fluid culture: 6 Staphylococcus epidermidis strains, 1 Staphylococcus hominis strain, 2 Corynebacterium striatum strains, and 1 Brevundimonas sp. Neither the type nor the duration of antimicrobial therapy before device removal had an effect on the diagnostic performance of sonication fluid culture (P = 0.75 and P = 0.56, respectively). In the patients without infection, sonication fluid culture was positive in 8 cases (40%), whereas conventional culture was positive in only 4 (20%). In summary, the sonication technique improves the microbiological diagnosis of explanted cardiac devices.
INTRODUCTION
Cardiac device infections (CDIs) are life-threatening conditions that occur as complications of cardiac device implantation and are associated with significant morbidity, mortality, and increased global health care system costs (1, 2). The estimated rate of CDIs ranges from 0.13% to 20% (3–5). Older age, device revision, renal dysfunction, and oral anticoagulation use are known to be risk factors for CDIs (6, 7). The identification of CDIs may be a challenge for physicians, due to the wide variety of presenting symptoms and the lack of a diagnostic “gold standard.” In addition, the management of CDIs is often difficult and complete device removal is required (3, 8).
A correct microbiological diagnosis of CDIs is of crucial importance for their appropriate treatment. Staphylococcal species, including both Staphylococcus aureus and coagulase-negative staphylococci, account for the majority of CDIs (7, 9); however, unusual organisms (Propionibacterium spp., Corynebacterium spp., Acinetobacter baumannii, Haemophilus influenzae) are also found, and antibiotic resistance is often detected (10–13). Moreover, there are data indicating that microorganisms can colonize cardiac devices without clinical signs of active infection (14–16).
Although conventional cultures of generator pocket site tissue and lead tips are useful in identifying the causative organisms of CDIs, no bacterial detection occurs in up to 30% of CDIs (1, 17, 18). The poor sensitivity of conventional microbiological methods is mainly due to the occurrence of adherent bacteria that are encased in biofilms on the surface of the implanted device (19). The sonication technique, which is based on the application of long-wave ultrasound, has been used in order to enhance bacterial detection by liberating sessile organisms embedded in biofilms on foreign bodies (20–24). In particular, the sonication of removed implants has been introduced in clinical practice and has shown good results for the microbiological diagnosis of device-related orthopedic infections (25–27), whereas its application in the setting of CDIs is still limited.
In the present study, the implant sonication method was compared with traditional culture (intraoperative pocket swab and device cultures) for the microbiological diagnosis of CDIs. Furthermore, the amount of bacteria detected by sonication was compared with that detected by traditional culture. We also evaluated the usefulness of the sonication method for the detection of bacterial colonization of devices in patients undergoing generator replacement or revision in the absence of signs of infection.
(This work was presented in part at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 17 to 20 September 2011.)
MATERIALS AND METHODS
Study population.
A total of 40 patients with a permanent pacemaker (PPM) or an implantable cardioverter-defibrillator (ICD) who underwent device explantation or elective surgery at the Electrophysiology Service at Azienda Policlinico Umberto I (Sapienza University of Rome) were included in the study. Twenty patients had cardiac device infection (18 with pocket infection, 2 with device-related endocarditis), and 20 subjects underwent elective generator replacement or revision in the absence of clinical signs of infection.
Clinical diagnosis of pocket infection was based on the local signs of inflammation as erythema, warmth, fluctuance, wound dehiscence, tenderness, and purulent drainage. Device-related endocarditis was diagnosed according to the modified Duke criteria (28, 29). A complete device removal, including removal of generators, pins, and atrial and/or ventricular leads, was performed in all patients with CDIs. Demographic, clinical, microbiological, and laboratory data were recorded for each patient. The study was approved by the institutional review board (Section of Infectious Diseases, Department of Public Health and Infectious Diseases, Sapienza University of Rome). All study participants gave informed written consent.
Device extraction procedure.
Device removal procedures were performed in the cardiac electrophysiology laboratory by interventional cardiac electrophysiologists under sterile conditions. The device pocket was opened, and pocket swabs were obtained. Leads were examined visually and by fluoroscopy in their intravascular segment. Lead extraction was performed manually with or without the assistance of traction devices, including stylets, locking stylets (lead locking devices 1, 2, and EZ LLD; Spectranetics, Colorado Springs, CO), snares, laser, or radiofrequency, in order to facilitate safe lead removal. When manual traction failed to remove the lead, various telescoping sheaths, such as 7F or 11F polypropylene or Teflon telescoping mechanical sheaths (Byrd, Cook Medical Inc., Bloomington, IN), were adopted. We used powered sheaths with an Excimer energy source (12-Fr, 14-Fr, or 16-Fr laser sheath SLS II; Spectranetics Corp.) to break down adhesions under fluoroscopic guidance (30, 31). Pacemaker-dependent patients received a temporary pacing system (32).
Sample collection.
In the subjects with CDIs, intraoperative pocket swab and blood cultures were performed and cardiac device components, including generators, pins, and atrial/ventricular lead tips, were collected. Each removed component underwent culture both with and without sonication of the device (sonication fluid and traditional culture, respectively). In the group undergoing device revision without evidence of a CDI, only the generators were collected and the removed samples were submitted to the same process (culture with and without sonication) used for the devices from subjects with infection.
All samples reached the microbiology laboratory within 3 h from the time of collection. For each specimen, traditional and sonication fluid cultures were performed in duplicate.
As negative controls, 7 sterile cardiac devices were included in the study and submitted to the same procedures used for the clinical collected samples.
Conventional microbiological cultures.
The devices explanted from subjects with and without CDIs were inoculated in Trypticase soy broth (TSB), incubated for 24 h at 37°C, and then cultured on aerobic and anaerobic sheep blood agar plates for 5 days. TSB (500 μl) was used to do the serial dilutions for bacterial counts. The detection limit was 2 CFU/ml. Strain identification and analysis of antibiotic susceptibility patterns were performed using a Vitek 2 system (bioMérieux, Marcy l'Etoile, France). Intraoperative pocket samples were obtained with a sterile polyester fiber-tipped swab that had been moistened with sterile saline (BBL culture swab; Becton, Dickinson France), and then the microorganisms were identified using conventional methods.
To avoid the possibility that the strains isolated from clinical specimens represented a contamination, at least two cultures positive for the same microorganism with an identical antibiotic susceptibility pattern from different samples were considered clinically significant. In particular, we took into account isolation of the same bacterium from at least two samples. Moreover, antimicrobial susceptibility testing (AST) was performed with all detected strains, using the Vitek 2 system (bioMérieux, Marcy l'Etoile, France). We considered the bacterial strains to be the same when the identification and the AST results were identical for both samples.
Sonication fluid cultures.
The collected samples were inoculated in TSB and incubated for 24 h at 37°C. After the removal of TSB, each device was further covered with Ringer's solution and vortexed for 30 s; then, samples were sonicated for 5 min at a frequency of >20 kHz and vortexed again for 30 s (20, 25, 30, 33). A BactoSonic apparatus (Bandelin Electronic GmbH & Co. KG, Berlin, Germany) was used for sonication. The resulting sonication fluid was centrifuged at 3,200 rpm for 20 min, and the sediment was plated onto aerobic Columbia sheep blood agar plates and on anaerobic Schaedler sheep blood agar for 5 days. In order to compare the number of bacteria grown in sonication fluid with that grown in traditional culture, 500 μl of sonication fluid was inoculated onto aerobic and anaerobic sheep blood agar, and the numbers of CFU/ml were counted after 24 h of incubation. The minimum detection level was 2 CFU/ml. Microorganisms were identified by standard automatic methods (Vitek 2 system; bioMérieux, Marcy l'Etoile, France). A 300-μl aliquot of sonication fluid and all the detected organisms were frozen at −20°C. Even if this method is not standardized to do a quantitative approach, we performed an overnight incubation before counting the number of bacteria in order to compare the results from the traditional culture with those from sonication fluid culture.
Statistical analysis.
Categorical variables were compared by using the χ2 test, Fisher's exact test, or the McNemar test, as appropriate. Continuous data were analyzed with Student's t test, whereas the nonparametric Mann-Whitney test was applied for values not normally distributed. Values of bacterial cell counts were given as means ± standard errors of the means (SEMs). A P value of <0.05 was considered statistically significant. Statistical analyses were performed using STATA (version 9) software (STATA Corp. LP, College Station, TX).
RESULTS
Characteristics of study population.
During the study period, a total of 40 subjects underwent cardiac device removal: 20 patients had cardiac device infection (18 with pocket infection, 2 with device-related endocarditis), and 20 subjects underwent elective generator replacement or revision in the absence of clinical signs of infection. All but one underwent transvenous lead extraction, whereas one patient underwent a two-step device removal process: first, the generator was removed through transvenous lead extraction in the Electrophysiology Service, and then the electrodes were retrieved via the femoral vein under fluoroscopic guidance in a different operating room. The mean age was 73.43 ± 12.7 years; 22 (55%) were males. Among the removed devices, 34 were PPM (2 single-chamber pacemakers, 32 dual-chamber pacemakers), 4 were ICDs, and 2 were implantable loop recorders (ILRs). All the patients with a clinical diagnosis of a CDI underwent complete device removal, with a total of 60 device components collected and further analyzed: 20 generators (PPMs, n = 17; ICDs, n = 3), 5 pins, 15 atrial electrodes, and 20 ventricular electrodes. Five generators were excluded from the microbiological analyses because of evident contamination. Because of the presence of fibrosis, the intracardiac electrodes of 2 patients remained at the insertion site. The median duration of symptoms was 31 days (range, 3 to 234 days); 16 out of 20 patients (80%) were on antibiotic therapy at the time of device removal. Subjects without CDI underwent device removal for battery exchange (12/20) or generator upgrade or revision (8/20). Antibiotic prophylaxis with cefazolin was performed in all the subjects. The patient characteristics are described in Table 1.
Table 1.
Characteristics of study populationa
| Characteristic | Subjects with CDIs (n = 20) | Subjects without CDIs (n = 20) | P value |
|---|---|---|---|
| Mean (range) age (yr) | 74.1 (60–86) | 72.7 (30–93) | 0.74 |
| No. (%) male | 12 (60) | 10 (50) | 0.75 |
| No. (%) female | 8 (40) | 10 (50) | |
| No. (%) with the following type of implanted device: | |||
| PPM | 17 (85) | 17 (85) | 1.0 |
| ICD | 3 (15) | 1 (5) | 1.0 |
| ILR | 0 (0) | 2 (10) | 1.0 |
| No. (%) with the following reason for device implantation: | |||
| Sick sinus syndrome | 1 (5) | 5 (25) | 0.18 |
| Atrioventricular block type III | 6 (30) | 7 (35) | 1 |
| Chronic atrial fibrillation | 6 (30) | 1 (5) | 0.09 |
| Secondary prevention | 2 (10) | 0 (0) | 0.48 |
| Othera | 5 (25) | 7 (35) | 0.73 |
| Mean no. of days of implantation of device placement | 564 | 2,767 | <0.001 |
| No. (%) with a previous pocket revision | 16 (80) | 8 (40) | 0.02 |
| No. (%) with anticoagulant therapy | 12 (60) | 2 (10) | 0.06 |
CDIs, cardiac device infections; PPM, permanent pacemaker; ICD, implantable cardioverter-defibrillator; ILR, implantable loop recorder.
Other reasons for device implantation included neurocardiogenic syncope and sustained tachycardia.
Sensitivity of sonication fluid culture and conventional culture.
Overall, sonication fluid culture detected bacteria in 65% of the patients (26/40) and in 67% of the removed components (54/80), whereas standard culture (broth incubation method) was positive for 20/40 subjects and 40/80 device components (P = 0.04 and P = 0.0005, respectively). As negative controls, 7 sterile cardiac devices were analyzed and submitted to the same procedures as the clinical collected samples. Among them, no bacterial growth was observed.
Pathogen detection in sonication fluid culture and conventional culture of subjects with CDIs.
Out of the 20 patients with CDIs, sonication fluid culture was positive in 18 patients (90%), traditional culture of the device was positive in 16 cases (80%), and intraoperative pocket swab culture was positive in only 6 cases (33%). The microorganisms detected in the sonication fluid cultures and traditional cultures are shown in Table 2. Overall, culture after implant sonication yielded bacteria in 77% of the components (46/60), whereas standard culture yielded bacteria in 60% (36/60) (P = 0.001). Concordance between the sonication and traditional methods was found in 50 cases (83%). A total of 10 organisms were detected only through sonication fluid culture: 6 Staphylococcus epidermidis isolates, 1 Staphylococcus hominis isolate, 2 Corynebacterium striatum isolates, and 1 Brevundimonas sp. isolate.
Table 2.
Pathogen detection in sonication and traditional culture
| Subject group and culture resulta | No. of components | Microorganisms (no. of isolates)b |
|---|---|---|
| Subjects with CDIs (n = 20) | 60 | |
| Positive SC/negative TC | 10 | S. epidermidis (6), C. striatum (2), Brevundimonas sp. (1), S. hominis (1) |
| Positive SC/positive TC | 36 | S. epidermidis (n = 26), S. hominis (n = 3), P. aeruginosa (n = 3), S. aureus (n = 1), S. haemolyticus (n = 1), Bacillus sp. (n = 1), Klebsiella sp. (n = 1) |
| Negative SC/positive TC | 0 | No bacterial detection |
| Negative SC/negative TC | 14 | No bacterial detection |
| Subjects without CDIs (n = 20) | 20 | |
| Positive SC/negative TC | 4 | Coagulase-negative staphylococci |
| Positive SC/positive TC | 4 | Coagulase-positive staphylococcus (1), coagulase-negative staphylococci (3) |
| Negative SC/positive TC | 0 | No bacterial detection |
| Negative SC/negative TC | 12 | No bacterial detection |
CDI, cardiac device infection; SC, sonication culture; TC, traditional culture.
Polymicrobial cultures were found in 5 generators: S. epidermidis/S. hominis, n = 3; S. epidermidis/Bacillus sp. (n = 1), and S. epidermidis/Klebsiella sp. (n = 1).
Coagulase-negative staphylococci accounted for 80.4% (37/46) of the strains, whereas Staphylococcus aureus and C. striatum accounted for 2.2% (1/46) and 4.3% (2/46) of the strains, respectively. Gram-negative bacilli (Pseudomonas aeruginosa, Klebsiella spp.) accounted for 8.8% (4/46). Uncommon pathogens such as Bacillus spp. and Brevundimonas spp. accounted for 4.3% (2/46) of the strains: these bacteria are usually not associated with CDIs, and it is likely that they were detected due to the oversensitivity of the sonication technique. No bacterial detection occurred in 8% of CDIs. In 5 subjects, cultures of generators were polymicrobial: S. epidermidis/S. hominis (n = 3), S. epidermidis/Bacillus sp. (n = 1), and S. epidermidis/Klebsiella sp. (n = 1). Among coagulase-negative staphylococci, S. epidermidis was detected in 86% (32/37) of cases, followed by S. hominis (4/37, 11%) and Staphylococcus haemolyticus (1/37, 3%). All the S. hominis and S. haemolyticus strains were oxacillin resistant, whereas 69% of the S. epidermidis strains were oxacillin resistant.
Comparison of sonication fluid culture and standard culture for generators and electrodes removed from subjects with CDIs.
Generator cultures detected bacteria in 86.6% (13/15) of the subjects, whereas electrode cultures were positive in only 57.9% (11/20). When we assessed the percentage of pathogen detection in generators, there was no significant difference between sonication fluid and conventional cultures (86.6% versus 80%) (P = 1). On the other hand, sonication fluid culture yielded a significantly higher rate of pathogen detection in explanted electrodes than traditional culture (65% versus 45%; P = 0.02) (Fig. 1). Patients with positive electrode cultures tended to have a longer duration of symptoms than those with negative electrode cultures, although the difference was not statistically significant (5 to 234 versus 3 to 89 days, P = 0.62).
Fig 1.
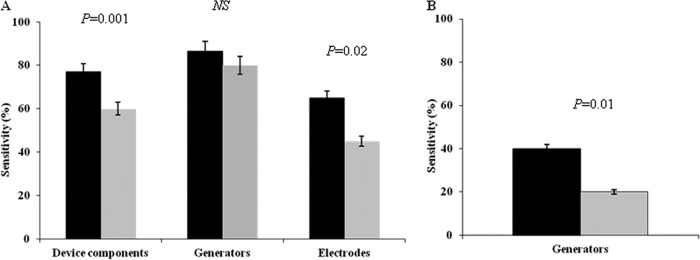
Sensitivity of sonication culture (black bars) and traditional culture (gray bars) for microbial detection in device components removed from infected (A) and uninfected (B) patients. Electrodes include atrial and ventricular electrodes. NS, not significant.
Effect of antimicrobial therapy on microbiological results for subjects with CDIs.
At the time of device removal, 16 patients (80%) were on antibiotic therapy: 9 with daptomycin, 5 with vancomycin, and 2 with other antibiotics (rifampin plus minocycline, ampicillin-sulbactam). The median length of therapy before device explantation was 6 days (range, 0 to 45 days). In 14 subjects, therapy was started ≤14 days before device removal, whereas 2 patients had received therapy for more than 14 days before device explantation. Among the 16 subjects on therapy, there was bacterial growth in 14 cases. The percentage of bacterial detection was higher in sonication fluid culture than in traditional culture, although the difference was not statistically significant (88% versus 75%, P = 0.65). In one of the two patients who received antibiotic therapy for more than 14 days before the explantation, bacterial detection was obtained only with sonication. Neither the type nor the duration (≤14 or >14 days) of antimicrobial therapy before device removal had an effect on the diagnostic performance of sonication fluid culture (P = 0.75 and P = 0.56, respectively).
Pathogen detection in sonication fluid culture and standard culture for subjects without CDIs.
Twenty patients undergoing generator replacement or revision in the absence of signs of infection were investigated with both traditional and sonication fluid cultures. Sonication fluid culture was positive in 8 patients (40%), whereas traditional culture of the device was positive in only 4 cases (20%) (P = 0.01) (Fig. 1). The microorganisms detected in sonication fluid culture and traditional culture of the explanted devices are illustrated in Table 2. Coagulase-negative and coagulase-positive staphylococci accounted for the organisms from 88% and 12% of the subjects, respectively. Concordance between the sonication and traditional methods was found in 16 cases (80%). No differences between colonized and noncolonized subjects in terms of previous pocket revision or the age of the implanted device were found.
Bacterial cell count in sonication fluid and standard cultures.
In order to compare the number of bacteria grown in sonication fluid culture with that grown in traditional culture, bacterial cell counts in all infected and noninfected devices from both types of cultures were performed and expressed as the numbers of CFU/ml. The bacterial cell count was significantly higher in the subjects with CDIs than in the subject without CDIs through both traditional culture (211 × 103 ± 51.5 × 103 versus 11.6 ± 6.3 CFU/ml, P = 0.001) and sonication fluid culture (299 × 103 ± 51.8 × 103 versus 15.1 ± 9.7 CFU/ml, P < 0.001). The comparative analysis of the two methods in subjects with CDIs showed that the bacterial cell count detected in the sonication fluid culture was significantly higher than that detected in traditional culture (P = 0.019). Moreover, when we stratified patients according to the bacterial amount detected by standard culture, we found that the difference between the two methods was more evident for numbers of CFU/ml of less than 104 (P = 0.0002). With regard to the components of the removed devices (electrodes and generators), the difference in the bacterial amounts between cultures with and without sonication was significant only for the electrodes (194.1 × 103 ± 58.2 × 103 versus 66.9 × 103 ± 39.7 × 103 CFU/ml, P = 0.018) and not for the generators (477.7 × 103 ± 99.5 × 103 versus 465.5 × 103 ± 111 × 103 CFU/ml, P = 0.7) (Fig. 2). When we analyzed the bacterial cell count in subjects without CDIs, we found that, even in this group of patients, sonication fluid culture detected significantly more bacteria than standard culture (151.6 × 102 ± 97.6 × 102 versus 11.6 ± 6.3 CFU/ml) (P = 0.029).
Fig 2.
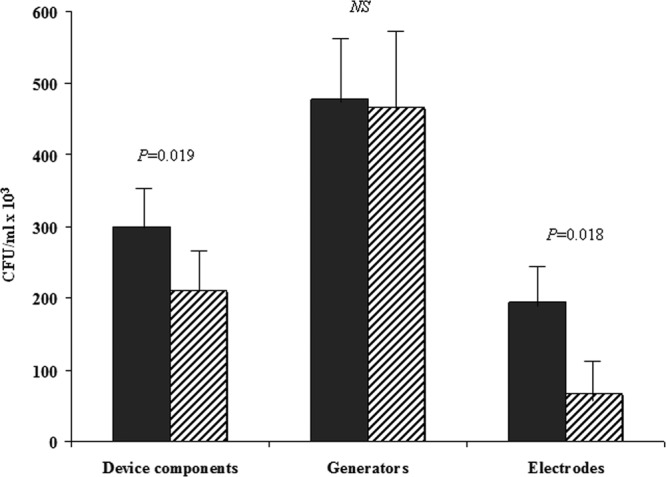
Sonication fluid culture (filled bars) and traditional culture (bars with diagonal lines) for pathogen quantification among subjects with cardiac device infections. Values are expressed as means ± SEMs. Device components include generators and atrial/ventricular electrodes. NS, not significant.
DISCUSSION
A correct microbiological diagnosis is of great importance for the appropriate management and antibiotic treatment of infections of pacemakers and implantable cardiovascular devices (3). Although both tissue and swab cultures are commonly performed in routine clinical practice, the identification of the causative organisms of CDIs is not achieved in up to one-third of cases (13, 17). Several factors may affect the results of conventional cultures in patients with CDIs. First of all, the causative organisms tend to have low virulence with a reduced replicative rate; in particular, infections with low-virulence organisms are likely to have negative tissue and swab cultures (34). Moreover, subjects with clinical infection often receive antibiotic therapy before device removal. Finally, bacteria can adhere to the prosthetic materials and survive on the surface of implanted devices, leading these infections to be resistant to both antibiotics and the host's immune defense system (19, 43).
Sonication is a reproducible and simple technique used to dislodge bacteria from infected devices (35–38). Several studies have shown that this technique improves the detection of causative organisms in device-related orthopedic infections, especially in patients already receiving antibiotic therapy (39–42). To date, little is known about the role of the sonication technique for pathogen detection in CDIs. A recent study comparing traditional swab cultures with sonication of intracardiac devices showed that bacterial detection through sonication was more sensitive than that through traditional cultures, especially in infected devices (33). Mason et al. demonstrated that ultrasonication of pacemaker and ICD generators increased the rate of diagnosis of pocket infection over that by tissue culture and swab culture alone (34).
In the present study, we demonstrated that the sensitivity of sonication fluid culture was higher than that of standard culture and pocket swab culture in both infected and noninfected cardiac devices. When we examined the different components of infected cardiac devices, we found that sonication was highly sensitive for pathogen recovery in electrodes rather than in generators. These findings could be explained by the intrinsic differences between generators and electrodes. In fact, generators are made of titanium alloy and their surface is smooth; instead, electrodes are coated with silicon and their surface is rough. In addition, generators are placed in a subcutaneous pocket, whereas electrodes are intracardiac, being in close contact with the host's protein and immune system. It is conceivable that the increased production of biofilms occurring on leads could be the main factor that affects the sensitivity of conventional culture results for electrodes: in such cases, the use of sonication, which dislodges bacteria embedded in the biofilm, may be crucial to improve bacterial detection in CDIs.
It is widely known that the results of conventional culture may be hampered by the administration of antibiotics prior to prosthesis removal (19, 27). In our study, antibiotic therapy had no effect on the diagnostic performance of sonication fluid culture, whereas the results of traditional culture were influenced by the duration of treatment. In the subjects receiving antibiotic therapy, culture of the device with sonication detected more organisms than culture of the device without sonication. Although the difference was not significant, it can be assumed that sonication may be useful in patients undergoing device removal while on antimicrobial therapy, because it led to a rate of pathogen detection higher than that by traditional culture. In such a situation, the high sensitivity of sonication fluid culture may be helpful in improving the microbiological diagnosis of CDIs.
Although the pathophysiology of CDIs has been widely investigated, the source of device infection is still controversial (3). In the present study, cultures of the leads yielded bacteria in 57.9% of the subjects, thus suggesting that microorganisms could migrate from the generator to the intravascular portion of the lead and adhere to it.
Recent data showed a high rate of asymptomatic bacterial colonization of cardiac devices in subjects who had implants removed for elective reasons: the percentage of bacterial detection ranged from 33% when conventional cultures were performed to 47% when bacterial DNA was evaluated (15, 16). To date, there are only two studies that investigated the role of the sonication technique in detecting asymptomatic bacterial colonization (33, 34). Mason et al. showed that in 66 asymptomatic patients undergoing elective generator replacement, there was bacterial growth in 11 sonication fluid cultures, in 8 tissue cultures, and in only 2 swab sample cultures (34). A large study by Rohacek et al. analyzed 115 electrophysiological cardiac devices explanted without clinical signs of infection: they found that bacteria were detected in 38% of sonication fluid cultures and in 27% of conventional generator pocket swab cultures, with a concordance of 68% (33). Also in the present study, we found that sonication of explanted devices was more sensitive than conventional culture for bacterial detection in subjects undergoing generator replacement or revision in the absence of clinical signs of infection. Thus, the formation of a biofilm, which encases bacteria and protects them from the host's immune system, appears to be essential to initiate and to perpetuate the condition of asymptomatic bacterial colonization.
The clinical significance of asymptomatic bacterial colonization is still unclear. Kleeman et al. showed that 7.5% of the colonized subjects developed a subsequent CDI with the same bacterial strain (15). In the study of Rohacek et al., the incidence of subsequent CDIs among patients sonication fluid culture positive at the time of device removal was 4.5% (33). In our study, during a short-term follow-up of 6 months after device removal, none of the colonized patients developed active infection.
Whether the detection of bacteria on devices undergoing elective revision represents colonization or contamination remains an area of active investigation (14). In order to exclude the possibility of contamination during laboratory procedures, especially when sonication was added to traditional culture, we performed experiments in duplicate. All bacteria detected with sonication and traditional cultures shared the same biochemical and antibiotic susceptibility profiles. In addition, in order to exclude the possibility of contamination during laboratory procedures, we included in the study 7 sterile cardiac devices; among them, no bacterial growth was observed.
Previous studies of prosthetic joint infections found that quantification of bacteria could be helpful in discriminating between colonization and contamination. Methods utilizing sonication for improved detection of bacteria on explanted prosthetic joints (hips, knees, and shoulders) directly add Ringer's solution and sonicate within hours after removal. By using this approach, it has been shown that a cutoff of 10 CFU/ml bacteria in sonication fluid predicted infection (27). In our study, we tried to evaluate if the use of sonication could be useful not only for pathogen detection but also for comparison of the bacteria grown in sonication fluid with those grown in traditional culture. Thus, even if this method is not standardized to do a quantitative analysis, we performed an overnight incubation before counting the number of bacteria, and we found a higher number of bacterial CFU/ml in the sonication fluid than in broth. A possible limitation of this method is that the quantification of bacteria could have been influenced by the fact that the samples were incubated for 24 h, leading bacteria to multiply. However, in any case, we were able to demonstrate that bacteria were present in larger amounts through culture with sonication than through culture without sonication. It is conceivable that bacteria embedded in the biofilm could also have been detected in the sonication fluid. In this respect, our findings suggest that sonication not only may improve pathogen detection but also may be able to detect a higher number of bacteria. Further investigations exploring the role of sonication of device components immediately after removal, in the absence of overnight incubation, should be encouraged in order to introduce this technique into the routine clinical practice for the microbiological diagnosis of CDIs.
Another limitation of the present study was the nonblinded collection of patients and controls. Moreover, a mean follow-up of 6 months may not be sufficiently discriminative to exclude the possibility of infection in the colonized subjects. Thus, further research with a longer longitudinal follow-up is needed in order to identify the subset of patients with colonized devices who are at increased risk of subsequent infection.
In conclusion, we demonstrated the superiority of sonication fluid culture over traditional culture for both infected and noninfected cardiac devices. Sonication of explanted cardiac components may represent a useful tool in order to improve the microbiological diagnosis of CDIs, especially in patients receiving antibiotic treatment. In addition, the use of this technique may contribute to the study of bacterial colonization in patients undergoing device revision in the absence of clear signs of infection.
ACKNOWLEDGMENTS
This work was supported by a public grant from the Sapienza University of Rome, Rome, Italy.
We thank Giovanni Baglio (Agenzia di Sanità Pubblica, Lazio) for his support in statistical analysis.
Footnotes
Published ahead of print 28 November 2012
REFERENCES
- 1. Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Stoner S, Baddour LM. 2007. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J. Am. Coll. Cardiol. 49:1851–1859 [DOI] [PubMed] [Google Scholar]
- 2. Tarakji KG, Chan EJ, Cantillon DJ, Doonan AL, Hu T, Schmitt S, Fraser TG, Kim A, Gordon SM, Wilkoff BL. 2010. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 7:1043–1047 [DOI] [PubMed] [Google Scholar]
- 3. Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, Masoudi FA, Okum EJ, Wilson WR, Beerman LB, Bolger AF, Estes NA, III, Gewitz M, Newburger JW, Schron EB, Taubert KA. on behalf of the American Heart Association Rheumatic Fever Endocarditis and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young Council on Cardiovascular Surgery and Anesthesia Council on Cardiovascular Nursing Council on Clinical Cardiology and the Interdisciplinary Council on Quality of Care and Outcomes Research 2010. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 121:458–477 [DOI] [PubMed] [Google Scholar]
- 4. Johansen JB, Jørgensen OD, Møller M, Arnsbo P, Mortensen PT, Nielsen JC. 2011. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur. Heart J. 32:991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voigt A, Shalaby A, Saba S. 2010. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin. Electrophysiol. 33:414–419 [DOI] [PubMed] [Google Scholar]
- 6. Lekkerkerker JC, van Nieuwkoop C, Trines SA, van der Bom JG, Bernards A, van de Velde ET, Bootsma M, Zeppenfeld K, Jukema JW, Borleffs J-W, Schalij MJ, van Erven L. 2009. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart 95:715–720 [DOI] [PubMed] [Google Scholar]
- 7. Sohail MR, Hussain S, Le KY, Dib C, Lohse CM, Friedman PA, Hayes DL, Uslan DZ, Wilson WR, Steckelberg JM, Baddour LM, for the Mayo Cardiovascular Infections Study Group 2011. Risk factors associated with early- versus late-onset implantable cardioverter-defibrillator infections. J. Interv. Card. Electrophysiol. 31:171–183 [DOI] [PubMed] [Google Scholar]
- 8. Le KY, Sohail MR, Friedman PA, Uslan DZ, Cha SS, Hayes DL, Wilson WR, Steckelberg JM, Baddour LM, for the Mayo Cardiovascular Infections Study Group 2011. Clinical predictors of cardiovascular implantable electronic device-related infective endocarditis. Pacing Clin. Electrophysiol. 34:450–459 [DOI] [PubMed] [Google Scholar]
- 9. Nagpal A, Baddour LM, Sohail MR. 2012. Microbiology and pathogenesis of cardiovascular implantable electronic device infections. Circ. Arrhythm. Electrophysiol. 5:433–441 [DOI] [PubMed] [Google Scholar]
- 10. Anselmino M, Vinci M, Comoglio C, Rinaldi M, Bongiorni MG, Trevi GP, Golzio PG. 2009. Bacteriology of infected extracted pacemaker and ICD leads. J. Cardiovasc. Med. 10:693–698 [DOI] [PubMed] [Google Scholar]
- 11. Madhavan M, Sohail MR, Friedman PA, Hayes DL, Steckelberg JM, Wilson WR, Baddour LM, for the Mayo Cardiovascular Infections Study Group 2010. Outcomes in patients with cardiovascular implantable electronic devices and bacteremia caused by gram-positive cocci other than Staphylococcus aureus. Circ. Arrhythm. Electrophysiol. 3:639–645 [DOI] [PubMed] [Google Scholar]
- 12. Oliva A, Belvisi V, Iannetta M, Andreoni C, Mascellino MT, Lichtner M, Vullo V, Mastroianni CM. 2010. Pacemaker lead endocarditis due to multidrug-resistant Corynebacterium striatum detected with sonication of the device. J. Clin. Microbiol. 48:4669–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Viola GM, Awan LL, Darouiche RO. 2010. Nonstaphylococcal infections of cardiac implantable electronic devices. Circulation 121:2085–2091 [DOI] [PubMed] [Google Scholar]
- 14. Baddour LM. 2010. Cardiac device infection or not. Circulation 121:1686–1687 [DOI] [PubMed] [Google Scholar]
- 15. Kleemann T, Becker T, Strauss M, Dyck N, Weisse U, Saggau W, Burkhardt U, Seidl K. 2010. Prevalence of bacterial colonization of generator pockets in implantable cardioverter defibrillator patients without signs of infection undergoing generator replacement or lead revision. Europace 12:58–63 [DOI] [PubMed] [Google Scholar]
- 16. Pichlmaier M, Marwitz V, Kühn C, Niehaus M, Klein G, Bara C, Haverich A, Abraham W. 2008. High prevalence of asymptomatic bacterial colonization of rhythm management devices. Europace 10:1067–1072 [DOI] [PubMed] [Google Scholar]
- 17. Dy Chua J, Abdul-Karim AD, Mawhorter S, Procop GW, Tchou P, Niebauer M, Saliba W, Schweikert R, Wilkoff BL. 2005. The role of swab and tissue culture in the diagnosis of implantable cardiac device infection. Pacing Clin. Electrophysiol. 28:1276–1281 [DOI] [PubMed] [Google Scholar]
- 18. Viola GM, Mansouri MD, Nasir N, Darouiche RO. 2009. Incubation alone is adequate as a culturing technique for cardiac rhythm management devices. J. Clin. Microbiol. 47:4168–4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138 [DOI] [PubMed] [Google Scholar]
- 20. Bonkat G, Rieken M, Rentsch CA, Wyler S, Feike A, Schäfer J, Gasser T, Trampuz A, Bachmann A, Widmer AF. 2011. Improved detection of microbial ureteral stent colonisation by sonication. World J. Urol. 29:133–138 [DOI] [PubMed] [Google Scholar]
- 21. Klug D, Wallet F, Kacet S, Courcol RJ. 2003. Involvement of adherence and adhesion Staphylococcus epidermidis genes in pacemaker lead-associated infections. J. Clin. Microbiol. 41:3348–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen LL, Nelson CL, Saccente M, Smeltzer MS, Wassell DL, McLaren SG. 2002. Detecting bacterial colonization of implanted orthopaedic devices by ultrasonication. Clin. Orthop. Relat. Res. 403:29–37 [DOI] [PubMed] [Google Scholar]
- 23. Rieger UM, Pierer G, Lüscher NJ, Trampuz A. 2009. Sonication of removed breast implants for improved detection of subclinical infection. Aesthetic Plast. Surg. 33:404–408 [DOI] [PubMed] [Google Scholar]
- 24. Sampedro MF, Huddleston PM, Piper KE, Karau MJ, Dekutoski MB, Yaszemski MJ, Currier BL, Mandrekar JN, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Patel R. 2010. A biofilm approach to detect bacteria on removed spinal implants. Spine 35:1218–1224 [DOI] [PubMed] [Google Scholar]
- 25. Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R, Presterl E. 2011. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J. Orthop. Res. 29:617–622 [DOI] [PubMed] [Google Scholar]
- 26. Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. 2009. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J. Clin. Microbiol. 47:1878–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N. Engl. J. Med. 357:654–663 [DOI] [PubMed] [Google Scholar]
- 28. Durack DT, Lukes AS, Bright DK. 1994. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am. J. Med. 96:200–209 [DOI] [PubMed] [Google Scholar]
- 29. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Ryan T, Bashore T, Corey GR. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 30:633–638 [DOI] [PubMed] [Google Scholar]
- 30. Love CJ, Wilkoff BL, Byrd CL, Belott PH, Brinker JA, Fearnot NE, Friedman RA, Furman S, Goode LB, Hayes DL, Kawanishi DT, Parsonnet V, Reiser C, Van Zandt HJ. 2000. Recommendations for extraction of chronically implanted transvenous pacing and defibrillator leads: indications, facilities, training. North American Society of Pacing and Electrophysiology Lead Extraction Conference Faculty. Pacing Clin. Electrophysiol. 23:544–551 [DOI] [PubMed] [Google Scholar]
- 31. Neuzil P, Taborsky M, Rezek Z, Vopalka R, Sediva L, Niederle P, Reddy V. 2007. Pacemaker and ICD lead extraction with electrosurgical dissection sheaths and standard transvenous extraction systems: results of a randomized trial. Europace 9:98–104 [DOI] [PubMed] [Google Scholar]
- 32. Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, American College of Cardiology, American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, Society of Thoracic Surgeons 2008. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary. Heart Rhythm 5:934–955 [DOI] [PubMed] [Google Scholar]
- 33. Rohacek M, Weisser M, Kobza R, Schoenenberger AW, Pfyffer GE, Frei R, Erne P, Trampuz A. 2010. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation 121:1691–1697 [DOI] [PubMed] [Google Scholar]
- 34. Mason PK, Dimarco JP, Ferguson JD, Mahapatra S, Mangrum JM, Bilchick KC, Moorman JR, Lake DE, Bergin JD. 2011. Sonication of explanted cardiac rhythm management devices for the diagnosis of pocket infections and asymptomatic bacterial colonization. Pacing Clin. Electrophysiol. 34:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bjerkan G, Witsø E, Bergh K. 2009. Sonication is superior to scraping for retrieval of bacteria in biofilm on titanium and steel surfaces in vitro. Acta Orthop. 80:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carmen JC, Roeder BL, Nelson JL, Ogilvie RL, Robison RA, Schaalje GB, Pitt WG. 2005. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am. J. Infect. Control 33:78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pitt WG, Ross SA. 2003. Ultrasound increases the rate of bacterial cell growth. Biotechnol. Prog. 19:1038–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trampuz A, Osmon DR, Hanssen AD, Steckelberg JM, Patel R. 2003. Molecular and antibiofilm approaches to prosthetic joint infection. Clin. Orthop. Relat. Res. 414:69–88 [DOI] [PubMed] [Google Scholar]
- 39. Achermann Y, Vogt M, Leunig M, Wüst J, Trampuz A. 2010. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J. Clin. Microbiol. 48:1208–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trampuz A, Zimmerli W. 2005. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med. Wkly. 30:243–251 [DOI] [PubMed] [Google Scholar]
- 41. Trampuz A, Zimmerli W. 2008. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr. Infect. Dis. Rep. 10:394–403 [DOI] [PubMed] [Google Scholar]
- 42. Trampuz A, Piper KE, Hanssen AD, Osmon DR, Cockerill FR, Steckelberg JM, Patel R. 2006. Sonication of explanted prosthetic components in bags for diagnosis of prosthetic joint infection is associated with risk of contamination. J. Clin. Microbiol. 44:628–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Bbeachey EC. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]


