Abstract
MIC assays with Paracoccidioides brasiliensis, the etiological agent of paracoccidioidomycosis, had been conducted with variable protocols, employing both macrodilution and microdilution tests and including differences in inoculum preparation, media used, incubation periods, and temperatures. Twenty-one clinical and environmental isolates of Paracoccidioides were tested using amphotericin B, itraconazole, ketoconazole, fluconazole, sulfamethoxazole, sulfamethoxazole-trimethoprim, and terbinafine, according to the National Committee for Clinical Laboratory Standards (National Committee for Clinical Laboratory Standards, document M27-A2, 2002), with modifications such as three medium formulations (RPMI 1640 medium, McVeigh and Morton [MVM] medium, and modified Mueller-Hinton [MMH] medium), two incubation temperatures (room temperature [25 to 28°C] and 37°C), and three incubation periods (7, 10, and 15 days). The antifungal activities were also classified as fungicidal or fungistatic. The best results were obtained after 15 days of incubation, which was chosen as the standard incubation time. The MICs for most individual isolates grown for the same length of time at the same temperature varied with the different media used (P < 0.05). Of the isolates, 81% showed transition from the yeast to the mycelial form in RPMI 1640 medium at 37°C, independent of the presence of antifungals. MMH medium appears to be a suitable medium for susceptibility testing of antifungal drugs with P. brasiliensis, except for sulfamethoxazole and the combination of sulfamethoxazole-trimethoprim, for which the MVM medium yielded better results. The incubation temperature influenced the MICs, with, in general, higher MICs at 25°C (mycelial form) than at 37°C (P < 0.05). Based on our results, we tentatively propose a microdilution assay protocol for susceptibility testing of antifungal drugs against Paracoccidioides.
INTRODUCTION
Paracoccidioides brasiliensis is a dimorphic fungus with filamentous structures (hyphae) containing infecting propagula or conidia in the natural environment; at 37°C, it presents yeast-like forms with multiple budding. Infection probably occurs as a result of inhalation of conidia, which subsequently transform into yeast cells (1, 2).
P. brasiliensis is the etiological agent of paracoccidioidomycosis (PCM), a systemic and endemic disease that affects at least 10 million people in Latin America (1). PCM is the most frequent primary cause of death among the systemic mycoses in Brazil (3). Two main clinical PCM presentations have been reported: acute/subacute (juvenile form), which progresses quickly and has a considerable mortality rate, and chronic (adult PCM), which progresses slowly and accounts for more than 90% of the cases. All of the data point to the lung epithelium as the primary site of infection, from whence it spreads to other organs and tissues. Secondary lesions can be found in the mucosae, skin, lymph nodes, liver, spleen, and adrenal glands (4).
Several drugs are available for the treatment of PCM. Sulfa drugs were the first to be employed for the treatment of this mycosis and continue to be active medications against this fungal infection, including the severe juvenile forms, although in vitro and clinical resistances have been reported (5–7). The sulfamethoxazole-trimethoprim combination is the most frequently employed treatment for these patients, in both its oral and intravenous formulations, and it is used for severe acute cases and for the neurological form of the disease. Amphotericin B is another therapeutic option used for severe forms of the disease, both in adults and in children. The advent of azoles (ketoconazole, fluconazole, and itraconazole) at the end of the 1970s revolutionized the treatment of the disease. These oral drugs present good activity against P. brasiliensis and are well tolerated, presenting few side effects (5, 7).
The currently available standardized procedures for antifungal susceptibility testing can be found in National Committee for Clinical Laboratory Standards (NCCLS; now called CLSI) document M27-A2 (8), which was proposed and adopted for certain yeast species. For filamentous fungi and the dimorphic fungus Sporothrix schenckii, testing procedures have been described in document M38-A (9). Standardized methods for antifungal susceptibility testing for other dimorphic fungi are absent from the literature. In the in vitro methods proposed by the NCCLS for testing filamentous and yeast fungi, P. brasiliensis is not included. The studies described in reports available for P. brasiliensis and other dimorphic fungi had been conducted with agar macrodilution, broth macrodilution, or microdilution tests. These studies have generally used modified versions of the NCCLS M27-A2 protocols or a Shadomy modified protocol (10). These modifications include differences in inoculum preparation, media used in MIC assays, incubation periods, and experimental temperatures (5, 6, 11–23). However, standardization of the methods used for in vitro susceptibility testing is important to facilitate the establishment of interpretative breakpoints and quality control parameters.
The purpose of this study was to evaluate the influence of different conditions (medium, incubation time, and temperature) on the execution of microdilution susceptibility tests to determine the MICs of seven antifungal drugs for 21 clinical and environmental isolates of the genus Paracoccidioides.
MATERIALS AND METHODS
Isolates.
We tested 19 clinical and 2 environmental isolates of the Paracoccidioides species complex (Table 1). The collection was maintained in yeast extract-peptone-dextrose (YPD; 1% yeast extract, 2% peptone, 2% dextrose, and 1.5% agar) agar in the filamentous form (M) at room temperature (25 to 28°C) with monthly subculture. The isolates were converted to the yeast-like (Y) phase by incubation at 37°C and maintained by weekly passages while testing was performed. The full transition of mycelium to the yeast form was confirmed by the observation of the macroscopic and microscopic aspects of the colonies. Candida parapsilosis (ATCC 22019) and Candida krusei (ATCC 6258) were included for quality control (8).
Table 1.
Paracoccidioides brasiliensis isolates used in this study
| Isolate | Origin | Country | Phylogenetic speciesb |
|---|---|---|---|
| Ed01 | Clinical | Brazil (Goiás) | Pb01- like (24) |
| 1578 | Clinical | Brazil (Goiás) | Pb01- like (24) |
| Pb01a | Clinical | Brazil (Goiás) | Pb01- like (25, 26) |
| Pb4 | Chronic PCM | Brazil (São Paulo) | PS2 (25–29) |
| Pb2 | Chronic PCM | Venezuela | PS2 (25–29) |
| Pb03a | Chronic PCM | Brazil (São Paulo) | PS2 (26–29) |
| Pb6 | Chronic PCM | Brazil (Paraná) | S1 (26–29) |
| Pb5 | Chronic PCM | Brazil (Paraná) | S1 (28, 29) |
| B339 | Chronic PCM | Brazil (São Paulo) | S1 (25–29) |
| Útero | Chronic PCM | Argentina | S1 (24) |
| Pb11 | Acute PCM | Brazil (Paraná) | S1 (26–29) |
| Pb13 | Acute PCM | Brazil (Goiás) | S1 (26–29) |
| Pb10 | Acute PCM | Peru | S1 (26–29) |
| Pb14 | Acute PCM | Brazil (São Paulo) | S1 (26–29) |
| 63265 | Acute PCM | Argentina | S1 (25, 26, 28) |
| Pb09 | Mucosal lesions | Venezuela | S1 (27) |
| Penguin | Penguin feces | Uruguay | S1 (26, 27) |
| Tatu | Armadillo | Brazil (Pará) | S1 (27) |
| Pb9 | Chronic PCM | Brazil (São Paulo) | S1 (26–29) |
| Pb8 | Chronic PCM | Brazil (São Paulo) | S1 (26–29) |
| Pb18a | Chronic PCM | Brazil (São Paulo) | S1 (25–28) |
P. brasiliensis isolate used in the genome sequencing project (Broad Institute MIT and Harvard).
Phylogenetic species are grouped in accordance with findings in the indicated references.
Study design.
Each isolate was tested with amphotericin B, itraconazole, ketoconazole, fluconazole, sulfamethoxazole, a combination of sulfamethoxazole-trimethoprim, and terbinafine and by following modified versions of the NCCLS susceptibility testing guidelines for yeast fungi (document M27-A2 [8]) and procedures described by Hahn et al. (15, 17). The following conditions were evaluated: (i) three medium formulations, including RPMI 1640 medium (standard medium), McVeigh and Morton (MVM) medium (30), and modified Mueller-Hinton (MMH) medium (30); (ii) two incubation temperatures, room temperature (25 to 28°C) and 37°C; and (iii) three incubation periods, 7, 10, and 15 days. To evaluate the stability of the drugs among these incubation times, we performed a MIC assay with fluconazole and amphotericin B (drugs often used in candidiasis treatment) against C. parapsilosis (ATCC 22019) according to the NCCLS (document M27-A2 [8]). The fluconazole and amphotericin B were diluted as recommended and incubated at 37°C for 15 days without inoculating the microorganism. Forty-eight hours before performing the MIC readings at 7-, 10-, and 15-day intervals, C. parapsilosis was inoculated and MIC results were registered.
Media.
The standard RPMI 1640 medium at 34.54 g per liter was buffered with 0.165 M morpholinepropanesulfonic acid (MOPS) at pH 7.0. MVM medium was prepared as recommended by Restrepo and Jiménez (30), and MMH medium (Mueller-Hinton medium supplemented with 10 g/liter of glucose, 5 g/liter of ammonium sulfate, and 10 mg/liter of thiamine [Sigma-Aldrich]) was prepared as recommended by Restrepo and Arango (23). All drugs were tested in these three media for all of the isolates.
Antifungal drugs.
Antifungal drugs were obtained as follows: amphotericin B and sulfamethoxazole were from Sigma-Aldrich; itraconazole, ketoconazole, and fluconazole were obtained from the Pharmacy College of the Universidade Federal de Minas Gerais; sulfamethoxazole-trimethoprim was used in its commercial formulation (Bactrim); and terbinafine was purchased from Novartis. Fluconazole was dissolved in distilled water. The other drugs were dissolved in 100% dimethyl sulfoxide (DMSO) (Vetec) by following the NCCLS M27-A2 protocol. Except for sulfamethoxazole and the sulfamethoxazole-trimethoprim combination, which were prepared as 5.0-mg/liter stock solutions, the drugs were prepared as 1.0-mg/liter stock solutions.
Drug dilutions.
Serial 2-fold dilutions were prepared according to NCCLS document M27-A2 at 100 times the strength of the final concentration, followed by further dilutions (1:50) in RPMI 1640, MVM, or MMH medium to yield twice the final strength required for the test. Amphotericin B, fluconazole, and terbinafine were prepared in a range of concentrations from 0.0078 to 4.0 mg/liter. Itraconazole was prepared in a range of concentrations from 0.0039 to 2.0 mg/liter. Ketoconazole preparations ranged from 0.0019 to 1.0 mg/liter. Sulfamethoxazole and the sulfamethoxazole-trimethoprim combination preparations ranged from 1.17 to 600.0 mg/liter.
Preparation of inocula.
P. brasiliensis isolates were subcultured three times at 5-day intervals to achieve the exponential growth phase (31). Growth was collected aseptically with a platinum loop and resuspended in 3 ml of sterile saline (0.85%). Because large cell aggregates were common, the suspensions were allowed to settle for 3 to 5 min. Subsequently, the supernatants were collected, and their densities were adjusted by spectrophotometry at 530 nm to a transmittance of 70% (17, 22). The inoculum sizes ranged from 1 × 106 to 5 × 106 cells/ml. This range was confirmed by counting the yeast cells using a hematocytometer. The inoculum suspensions were diluted (1:10 and 1:50) in RPMI 1640, MVM, or MMH medium to obtain a cell number ranging from 0.5 × 105 to 2.5 × 105/ml.
Test procedure.
Flat-bottomed microdilution plates (96 wells) were set up in accordance with the NCCLS M27-A2 reference method (8, 22). Each microdilution well containing 0.1 ml of the 2-fold drug concentration was inoculated with 0.1 ml of the diluted inoculum suspension. For each test plate, two drug-free controls were included, one with the medium alone (sterile control) and the other with 0.1 ml of the medium plus 0.1 ml of inoculum suspension (growth control). The microdilution plates were incubated at room temperature (25 to 28°C) or at 37°C in a damp chamber and read after 7, 10, and 15 days of incubation.
Determination and interpretation of MICs.
Endpoint determination readings were performed visually based on the comparison of the growth in the wells containing the drug with that of the growth control. For terbinafine, ketoconazole, fluconazole, itraconazole, sulfamethoxazole, and the sulfamethoxazole-trimethoprim combination, the MIC was defined as the lowest concentration showing prominent growth inhibition (a drop in growth corresponding to approximately 80% of the growth control). For amphotericin B, the MIC was defined as the lowest concentration showing 100% growth inhibition. The MIC ranges of each drug were obtained to facilitate comparisons of the activities of the tested drugs, as were the MIC50 and MIC90.
Observation of P. brasiliensis morphology.
Inocula of 14 P. brasiliensis isolates (Ed01, Pb01, Pb2, Pb03, Pb5, B339, Útero, Pb13, Pb10, Pb09, Penguin, Tatu, Pb9, and Pb18) were prepared as described above and cultivated in flat-bottomed microdilution plates (96 wells) for 15 days with RPMI 1640 (with or without 2% glucose), MMH, or YPD (positive yeast control) medium at 37°C. At 2-, 5-, 10-, and 15-day intervals, 0.01-ml aliquots of the cultures were prepared with 0.001 ml of lactophenol solution and observed by optical microscopy at a magnification of ×400 for morphological analyses.
Fungicidal and fungistatic activities.
The in vitro fungicidal and fungistatic activities were determined for amphotericin B, terbinafine, fluconazole, itraconazole, sulfamethoxazole, and the sulfamethoxazole-trimethoprim combination exclusively in the RPMI 1640 and MMH media at a temperature of 37°C for each isolate, as recommended by Espinel-Ingroff et al. (14), with modifications. After 15 days, 0.01 ml from each well that was previously defined as the MIC, as well as 0.01 ml from each of two wells above the MIC reading and the growth control (drug-free medium), was subcultured onto YPD agar plates. The plates were incubated at 37°C in a damp chamber until growth was observed in the growth control subculture. The absence of growth of the isolates was indicative of fungicidal activity, and the residual and continuous growth of isolates on YPD agar was indicative of fungistatic activity.
Data analysis.
All MIC determinations were repeated twice. Comparisons of the influence of incubation temperatures, incubation times, and tested media were performed by the Wilcoxon (Mann-Whitney) and Kruskal-Wallis tests. A P value of <0.05 was considered significant (32).
RESULTS
Amphotericin B, terbinafine, ketoconazole, fluconazole, and itraconazole MICs for Candida parapsilosis (ATCC 22019) and Candida krusei (ATCC 6258) were in agreement with those in NCCLS document M27-A2 (data not shown). The assay performed with Candida parapsilosis (ATCC 22019) in order to evaluate drug stability at 7, 10, and 15 days of incubation showed that fluconazole and amphotericin B MICs were stable (data not shown) at 37°C among the incubation times.
Effect of incubation time on the MIC.
The fungal growth observed in the growth control wells (absent of any antifungal drugs) for a variable number of isolates at each drug microdilution was insufficient to allow easy MIC reading at 7 days of incubation, either when employing the RPMI 1640 medium or when employing the MMH and MVM media, although for some isolates and media it was possible to perform (when the reader was able to find 80% inhibition of growth in the presence of the drug compared to the growth control). Insufficient growth in the control wells occurred for at least 2 isolates in RPMI 1640 medium and for 5 isolates in MMH medium at room temperature when testing ketoconazole and amphotericin B. In MVM medium, insufficient growth was detected at 7 days of incubation for 5 isolates when determining the MICs for sulfamethoxazole, the sulfamethoxazole-trimethoprim combination, and fluconazole at room temperature and for 7 isolates when testing itraconazole and ketoconazole. Additionally, insufficient growth was detected for 4 isolates when testing terbinafine and for 9 isolates when testing amphotericin B at 37°C in MVM medium. At room temperature with MVM medium, insufficient growth was observed at 7 days of incubation for 18 isolates with terbinafine and for 19 isolates with sulfamethoxazole, the sulfamethoxazole-trimethoprim combination, and fluconazole; no isolate grew when amphotericin B was tested. Conversely, after 10 days of incubation, all of the isolates showed growth, and MIC readings were easier to perform. MIC readings were even clearer after 15 days of incubation for all of the isolates under each test condition. Table 2 summarizes the susceptibility data for the 21 P. brasiliensis isolates.
Table 2.
In vitro susceptibility data for amphotericin B, itraconazole, ketoconazole, fluconazole, sulfamethoxazole, sulfamethoxazole-trimethoprim, and terbinafine for 21 Paracoccidioides brasiliensis isolates under different microdilution test conditionsa
| Medium (temp) | Incubation time (days) | MIC (mg/liter) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphotericin B |
Itraconazole |
Ketoconazole |
Fluconazole |
Sulfamethoxazole |
Sulfamethoxazole-trimethoprim |
Terbinafine |
||||||||||||||||
| Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | ||
| RPMI (37°C) | 7 | 0.25–1.0 | 1.0 | 1.0 | 0.0039–0.062 | 0.0078 | 0.031 | 0.0019–0.015 | 0.0019 | 0.015 | 0.125–8.0 | 0.5 | 2.0 | 75.0–300.0 | 150.0 | 300.0 | 18.75–300.0 | 150.0 | 150.0 | 0.031–0.5 | 0.125 | 0.25 |
| 10 | 0.25–2.0 | 1.0 | 1.0 | 0.0039–0.062 | 0.0078 | 0.031 | 0.0019–0.031 | 0.0019 | 0.015 | 0.125–8.0 | 0.5 | 2.0 | 75.0–300.0 | 150.0 | 300.0 | 37.5–300.0 | 150.0 | 150.0 | 0.062–0.5 | 0.125 | 0.25 | |
| 15 | 0.25–2.0 | 1.0 | 1.0 | 0.0039–0.062 | 0.0078 | 0.062 | 0.0019–0.031 | 0.0019 | 0.015 | 0.25–8.0 | 1.0 | 2.0 | 150.0–300.0 | 300.0 | 300.0 | 75.0–300.0 | 150.0 | 300.0 | 0.125–0.5 | 0.25 | 0.5 | |
| MMH (37°C) | 7 | 0.0078–0.25 | 0.125 | 0.125 | 0.0039–0.0039 | 0.0039 | 0.0039 | 0.0019–0.0019 | 0.0019 | 0.0019 | 0.062–1.0 | 0.25 | 0.5 | 9.37–150.0 | 75.0 | 150.0 | 18.75–300.0 | 75.0 | 150.0 | 0.0078–0.25 | 0.015 | 0.125 |
| 10 | 0.015–0.5 | 0.125 | 0.125 | 0.0039–0.0039 | 0.0039 | 0.0039 | 0.0019–0.0019 | 0.0019 | 0.0019 | 0.062–1.0 | 0.25 | 1.0 | 9.37–150.0 | 75.0 | 150.0 | 18.75–300.0 | 75.0 | 150.0 | 0.0078–0.25 | 0.015 | 0.125 | |
| 15 | 0.125–1.0 | 0.25 | 0.25 | 0.0039–0.0039 | 0.0039 | 0.0039 | 0.0019–0.0019 | 0.0019 | 0.0019 | 0.062–1.0 | 0.25 | 1.0 | 9.37–150.0 | 75.0 | 150.0 | 18.75–300.0 | 75.0 | 150.0 | 0.0078–0.25 | 0.015 | 0.125 | |
| MVM (37°C) | 7 | 0.0078–1.0 | 1.0 | 1.0 | 0.062–0.25 | 0.25 | 0.25 | 0.0078–0.031 | 0.015 | 0.031 | 0.5–4.0 | 1.0 | 4.0 | 1.17–18.75 | 18.75 | 18.75 | 1.17–18.75 | 9.37 | 18.75 | 0.031–1.0 | 0.5 | 1.0 |
| 10 | 0.0078–2.0 | 0.5 | 2.0 | 0.0039–0.25 | 0.0078 | 0.25 | 0.0019–0.031 | 0.0078 | 0.031 | 0.0078–2.0 | 0.5 | 2.0 | 1.17–18.75 | 2.34 | 18.75 | 1.17–18.75 | 4.68 | 18.75 | 0.0078–1.0 | 0.5 | 1.0 | |
| 15 | 0.0078–2.0 | 0.5 | 2.0 | 0.0039–0.25 | 0.0078 | 0.25 | 0.0019–0.031 | 0.0078 | 0.031 | 0.0078–2.0 | 1.0 | 2.0 | 1.17–18.75 | 4.68 | 18.75 | 1.17–18.75 | 4.68 | 18.75 | 0.0078–1.0 | 0.5 | 1.0 | |
| RPMI (RT) | 7 | 0.031–0.5 | 0.125 | 0.25 | 0.0039–0.125 | 0.031 | 0.062 | 0.0019–0.25 | 0.031 | 0.25 | 0.5–8,0 | 1.0 | 4.0 | 75.0–300.0 | 150.0 | 300.0 | 75.0–300.0 | 150.0 | 300.0 | 0.0078–4.0 | 0.25 | 1.0 |
| 10 | 0.125–1.0 | 0.25 | 1.0 | 0.0039–0.125 | 0.062 | 0.125 | 0.0019–2.0 | 0.031 | 0.25 | 0.5–8.0 | 2.0 | 4.0 | 75.0–300.0 | 300.0 | 300.0 | 150.0–300.0 | 300.0 | 300.0 | 0.0078–4.0 | 0.5 | 1.0 | |
| 15 | 0.25–1.0 | 1.0 | 1.0 | 0.0078–0.125 | 0.062 | 0.125 | 0.0039–2.0 | 0.031 | 0.25 | 0.5–8.0 | 2.0 | 8.0 | 300.0–300.0 | 300.0 | 300.0 | 300.0–300.0 | 300.0 | 300.0 | 0.0078–4.0 | 1.0 | 2.0 | |
| MMH (RT) | 7 | 0.0078–0.062 | 0.0078 | 0.062 | 0.0039–0.125 | 0.0039 | 0.0078 | 0.0019–0.0078 | 0.0019 | 0.0078 | 0.031–2.0 | 0.25 | 2.0 | 9.37–150.0 | 150.0 | 150.0 | 18.75–300.0 | 150.0 | 300.0 | 0.0078–1.0 | 0.062 | 0.25 |
| 10 | 0.0078–0.125 | 0.031 | 0.062 | 0.0039–0.125 | 0.0039 | 0.015 | 0.0019–0.0078 | 0.0019 | 0.0019 | 0.031–2.0 | 0.25 | 2.0 | 18.75–300.0 | 150.0 | 300.0 | 18.75–300.0 | 150.0 | 300.0 | 0.0078–1.0 | 0.125 | 0.25 | |
| 15 | 0.015–0.25 | 0.031 | 0.25 | 0.0039–0.125 | 0.0039 | 0.031 | 0.0019–0.0078 | 0.0019 | 0.0039 | 0.062–2.0 | 0.25 | 2.0 | 37.5–300.0 | 150.0 | 300.0 | 75.0–300.0 | 150.0 | 300.0 | 0.062–1.0 | 0.125 | 0.25 | |
| MVM (RT) | 7 | — | — | — | 0.125–0.125 | 0.125 | 0.125 | 0.062–0.062 | 0.062 | 0.062 | 1.0–4.0 | 4.0 | 4.0 | 2.34 | 2.34 | 2.34 | 9.37–18.75 | 18.75 | 18.75 | 0.062–2.0 | 2.0 | 2.0 |
| 10 | 0.031–2.0 | 0.5 | 2.0 | 0.0039–0.125 | 0.015 | 0.125 | 0.0019–0.062 | 0.031 | 0.062 | 0.015–4.0 | 0.062 | 4.0 | 1.17–37.5 | 4.68 | 37.5 | 1.17–75.0 | 4.68 | 75.0 | 0.0078–2.0 | 0.062 | 1.0 | |
| 15 | 0.031–2.0 | 1.0 | 2.0 | 0.0039–0.125 | 0.031 | 0.125 | 0.0078–0.062 | 0.062 | 0.062 | 0.031–4.0 | 0.25 | 4.0 | 9.37–37.5 | 9.37 | 37.5 | 1.17–75.0 | 9.37 | 75.0 | 0.031–2.0 | 0.062 | 1.0 | |
—, no growth detected. Temperature is incubation temperature. RPMI, RPMI 1640 medium; MMH, MMH medium; MVM, MVM medium; RT, room temperature
With the exceptions of itraconazole at 37°C in RPMI 1640 medium, itraconazole and the sulfamethoxazole-trimethoprim combination at room temperature, and ketoconazole and sulfamethoxazole at 37°C in MMH medium, the MICs were significantly different for 7, 10, and 15 days of incubation under every test condition (P < 0.05).
Effects of the different media on the MICs.
The MICs obtained with the three different types of media for the same isolate at the same time and temperature of incubation differed significantly among the tested antifungal agents (P < 0.05), except for fluconazole at 37°C in MMH and MVM media. When using RPMI 1640 medium at both temperatures, the MICs for the antifungal agents were consistently higher than in MMH media. For instance, the MIC90 for amphotericin B using RPMI 1640 medium was 1.0 mg/liter, while the MIC90 was 0.25 mg/liter when using MMH medium at either temperature. The MIC90s for itraconazole using RPMI 1640 medium were 0.062 mg/liter at 37°C and 0.125 mg/liter at 25°C, while they corresponded to 0.0039 mg/liter and 0.031 mg/liter, respectively, in MMH medium. The MICs with MVM medium were similar to those found with RPMI 1640 medium, except for sulfamethoxazole and the combination of sulfamethoxazole-trimethoprim. For sulfamethoxazole, the MIC90 was 300 mg/liter when using RPMI 1640 medium and 150 mg/liter in MMH medium at 37°C. The MICs for sulfamethoxazole and for the sulfamethoxazole-trimethoprim combination in MVM medium were considerably lower than those found in RPMI 1640 or MMH medium. For instance, the MIC90 for sulfamethoxazole using RPMI 1640 medium was 300 mg/liter at both temperatures, compared to 37.5 mg/liter at 37°C and 18.75 mg/liter at 25°C using MVM medium. At the end of the MIC assays using RPMI 1640 medium at 37°C, transition of Paracoccidioides isolates from the yeast to the mycelial form was observed, independent of the presence of antifungals; this was true for 81% of the isolates. As expected, at room temperature, all of the isolates grew as mycelia in every tested medium.
P. brasiliensis morphology analysis at 37°C.
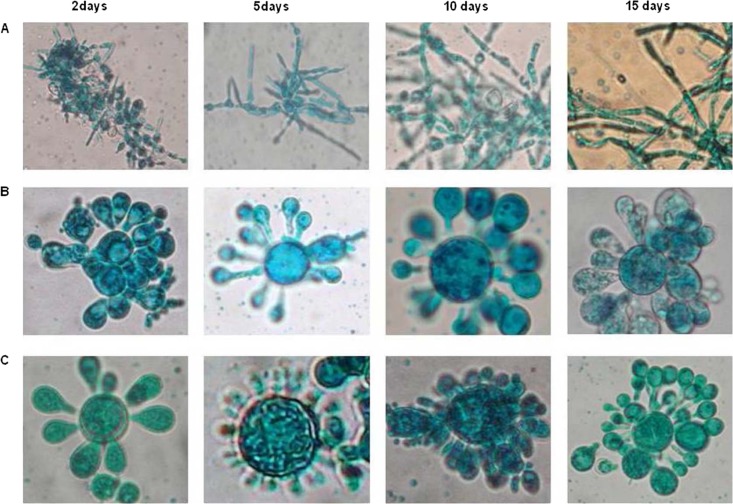
To observe the microscopic morphology in RPMI 1640 medium, 14 Paracoccidioides isolates were tested; 72% showed a yeast-mycelium transition. Representative photomicrographs of the yeast and mycelial forms of the Penguin, Pb10, and Pb9 isolates are shown in Fig. 1. With the exception of the Tatu isolate, RPMI 1640 medium supplemented with 2% glucose induced a morphological transition in all tested isolates, which started after an incubation period of 5 to 10 days, depending on the analyzed isolate. As a consequence, the variability of MICs in RPMI 1640 medium after 15 days would be associated with the filamentation that occurred since days 5 to 10. On the other hand, no yeast-to-mycelium transition was detected in MMH or YPD medium (positive control). Paracoccidioides morphology in MVM medium was not evaluated at these time intervals due to the slower growth in this medium than in other media. Microscopic analyses performed after MVM medium MIC assays only showed yeast forms at 37°C for all of the tested isolates.
Fig 1.
P. brasiliensis morphology at 37°C after 2, 5, 10, and 15 days of incubation. Cultures were prepared with 0.01 ml of the fungus and 0.001 ml of lactophenol solution and observed by optical microscopy at ×400. (A) Yeast-mycelium transition of the Penguin P. brasiliensis isolate cultivated in RPMI 1640 medium supplemented with 2% glucose; (B) yeast morphology of the Pb10 P. brasiliensis isolate cultivated in MMH medium; (C) morphology of the Pb9 P. brasiliensis isolate cultivated in YPD medium (yeast positive control).
Effect of incubation temperature on MIC.
The incubation temperature had a significant influence on the MICs (P < 0.05) (Table 2). This was true for all tested media and for all tested drugs, with the exception of fluconazole in RPMI 1640 and MMH media and sulfamethoxazole in MVM medium. At 37°C, higher MICs were obtained in RPMI 1640 and MMH media for only amphotericin B. In MVM medium, higher MICs were obtained at room temperature for amphotericin B, ketoconazole, itraconazole, fluconazole, and the sulfamethoxazole-trimethoprim combination, although similar values were obtained for sulfamethoxazole at room temperature. Higher MICs were obtained at room temperature in RPMI 1640 and MMH medium cultures for ketoconazole, itraconazole, terbinafine, sulfamethoxazole, and the sulfamethoxazole-trimethoprim combination. Terbinafine exhibited a larger MIC range at room temperature.
Table 3 allows assessment of the differences in MIC results for the seven drugs when tested in the three media for each P. brasiliensis isolate employing the following conditions: 105 yeast cells/ml, a temperature of 37°C, and incubation for 15 days. As previously stated, the MICs obtained with the three different media for the same isolate at the same time and temperature of incubation differed significantly among the tested antifungal agents (P < 0.05), except for fluconazole at 37°C in MMH and MVM media. When using RPMI 1640 medium at both temperatures, the MICs for the antifungal agents were consistently higher than in MMH media. The MICs with MVM medium were similar to those found with RPMI medium, except for sulfamethoxazole and the combination of sulfamethoxazole-trimethoprim. For sulfamethoxazole and the combination of sulfamethoxazole-trimethoprim, similar MICs were observed in RPMI 1640 and MHM media.
Table 3.
MICs of amphotericin B, itraconazole, ketoconazole, fluconazole, sulfamethoxazole, sulfamethoxazole-trimethoprim, and terbinafine for 21 Paracoccidioides brasiliensis isolates in different culture media at 37°C after 15 days of incubation
| Isolate | MIC (mg/liter) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphotericin B |
Itraconazole |
Ketoconazole |
Fluconazole |
Sulfamethoxazole |
Sulfamethoxazole-trimethoprim |
Terbinafine |
|||||||||||||||
| RPMI | MMH | MVM | RPMI | MMH | MVM | RPMI | MMH | MVM | RPMI | MMH | MVM | RPMI | MMH | MVM | RPMI | MMH | MVM | RPMI | MMH | MVM | |
| Ed01 | 1.0 | 0.25 | 1.0 | 0.015 | 0.0039 | 0.062 | 0.0039 | 0.0019 | 0.015 | 2.0 | 0.25 | 1.0 | 300 | 75 | 2.34 | 300 | 150 | 4.68 | 0.25 | 0.0078 | 0.25 |
| 1578 | 0.5 | 0.125 | 0.0078 | 0.031 | 0.0039 | 0.0039 | 0.0019 | 0.0019 | 0.0019 | 0.5 | 0.5 | 0.0078 | 300 | 37.5 | 1.17 | 75 | 37.5 | 1.17 | 0.25 | 0.25 | 0.0078 |
| Pb01 | 1.0 | 0.25 | — | 0.0078 | 0.0039 | — | 0.0019 | 0.0019 | — | 0.5 | 0.25 | — | 150 | 75 | — | 150 | 75 | — | 0.125 | 0.015 | — |
| Pb4 | 1.0 | 0.125 | 1.0 | 0.062 | 0.0039 | 0.25 | 0.031 | 0.0019 | 0.031 | 8.0 | 1.0 | 1.0 | 300 | 150 | 4.68 | 300 | 150 | 4.68 | 0.5 | 0.125 | 1.0 |
| Pb2 | 0.25 | 0.125 | 0.031 | 0.0078 | 0.0039 | 0.0078 | 0.0019 | 0.0019 | 0.0039 | 0.5 | 0.25 | 0.5 | 150 | 150 | 2.34 | 150 | 75 | 4.68 | 0.5 | 0.015 | 0.5 |
| Pb03 | 1.0 | 1.0 | 0.5 | 0.015 | 0.0039 | 0.25 | 0.015 | 0.0019 | 0.031 | 2.0 | 1.0 | 2.0 | 300 | 150 | 4.68 | 300 | 300 | 9.37 | 0.5 | 0.062 | 1.0 |
| Pb6 | 1.0 | 0.25 | 0.062 | 0.0078 | 0.0039 | 0.0078 | 0.0019 | 0.0019 | 0.0078 | 0.5 | 0.25 | 1.0 | 300 | 75 | 4.68 | 300 | 150 | 2.34 | 0.25 | 0.015 | 0.5 |
| Pb5 | 1.0 | 0.25 | 0.125 | 0.015 | 0.0039 | 0.0078 | 0.0078 | 0.0019 | 0.0039 | 1.0 | 0.25 | 1.0 | 300 | 75 | 18.75 | 300 | 75 | 9.37 | 0.5 | 0.031 | 0.5 |
| B339 | 1.0 | 0.125 | 0.031 | 0.0039 | 0.0039 | 0.0078 | 0.0019 | 0.0019 | 0.0078 | 0.5 | 1.0 | 1.0 | 150 | 150 | 9.37 | 300 | 150 | 9.37 | 0.25 | 0.031 | 0.5 |
| Útero | 1.0 | 0.25 | 1.0 | 0.0039 | 0.0039 | 0.015 | 0.0019 | 0.0019 | 0.0078 | 0.5 | 0.25 | 0.125 | 300 | 75 | 1.17 | 150 | 75 | 1.17 | 0.25 | 0.015 | 0.125 |
| Pb11 | 0.5 | 0.25 | 0.5 | 0.062 | 0.0039 | 0.015 | 0.031 | 0.0019 | 0.0078 | 8.0 | 0.5 | 0.5 | 150 | 9.37 | 1.17 | 150 | 18.75 | 1.17 | 0.5 | 0.031 | 0.5 |
| Pb13 | 0.25 | 0.125 | 1 | 0.0039 | 0.0039 | 0.015 | 0.0019 | 0.0019 | 0.0039 | 0.25 | 0.125 | 0.5 | 150 | 75 | 4.68 | 300 | 150 | 4.68 | 0.25 | 0.015 | 1.0 |
| Pb10 | 1.0 | 0.25 | 0.5 | 0.0078 | 0.0039 | 0.062 | 0.0019 | 0.0019 | 0.0078 | 2.0 | 0.125 | 0.5 | 150 | 75 | 2.34 | 150 | 75 | 4.68 | 0.5 | 0.015 | 0.5 |
| Pb14 | 2.0 | 0.125 | 2.0 | 0.0078 | 0.0039 | 0.0039 | 0.0019 | 0.0019 | 0.0019 | 0.5 | 0.125 | 0.25 | 300 | 150 | 4.68 | 150 | 150 | 4.68 | 0.25 | 0.015 | 1.0 |
| 63265 | 1.0 | 0.25 | 0.5 | 0.0078 | 0.0039 | 0.0039 | 0.0019 | 0.0019 | 0.0039 | 0.5 | 0.25 | 0.25 | 300 | 37.5 | 1.17 | 150 | 75 | 1.17 | 0.125 | 0.015 | 0.25 |
| Pb09 | 1.0 | 0.25 | 0.062 | 0.062 | 0.0039 | 0.0078 | 0.0039 | 0.0019 | 0.0078 | 1.0 | 0.5 | 1.0 | 300 | 150 | 18.75 | 300 | 300 | 9.37 | 0.5 | 0.015 | 1.0 |
| Penguin | 1.0 | 0.25 | 0.062 | 0.0039 | 0.0039 | 0.0078 | 0.0078 | 0.0019 | 0.0078 | 2.0 | 1.0 | 1.0 | 300 | 150 | 18.75 | 150 | 150 | 18.75 | 0.5 | 0.125 | 0.5 |
| Tatu | 1.0 | 0.25 | 0.5 | 0.0078 | 0.0039 | 0.0039 | 0.015 | 0.0019 | 0.0039 | 1.0 | 0.125 | 0.25 | 300 | 75 | 2.34 | 300 | 75 | 4.68 | 0.25 | 0.0078 | 0.5 |
| Pb9 | 2.0 | 0.25 | 1.0 | 0.015 | 0.0039 | 0.015 | 0.0039 | 0.0019 | 0.0039 | 1.0 | 0.062 | 0.25 | 300 | 75 | 1.17 | 150 | 37.5 | 2.34 | 0.25 | 0.25 | 0.5 |
| Pb8 | 1.0 | 0.25 | — | 0.062 | 0.0039 | — | 0.0039 | 0.0019 | — | 1.0 | 0.25 | — | 300 | 75 | — | 150 | 75 | — | 0.5 | 0.031 | — |
| Pb18 | 1.0 | 0.25 | 0.031 | 0.0039 | 0.0039 | 0.0078 | 0.0019 | 0.0019 | 0.0078 | 0.25 | 0.25 | 1.0 | 300 | 150 | 18.75 | 150 | 150 | 4.68 | 0.25 | 0.031 | 1.0 |
—, no growth detected. RPMI, RPMI 1640 medium; MMH, MMH medium; MVM, MVM medium.
Fungicidal and fungistatic activities of antifungal drugs against P. brasiliensis.
The fungicidal and fungistatic activities of the antifungal agents against 21 clinical and environmental P. brasiliensis isolates are summarized in Table 4. Amphotericin B was demonstrated to have fungicidal activity against all 21 isolates in both RPMI 1640 and MMH media (Table 4). The other antifungal agents, at the concentrations used in this study, presented either predominantly fungicidal or fungistatic profiles in RPMI 1640 or MMH medium. In RPMI 1640 cultures, terbinafine had fungicidal activity toward 57% of the isolates, fluconazole was fungistatic toward 57% of the isolates, and sulfamethoxazole had fungicidal activity toward 62% of the isolates. Itraconazole and the sulfamethoxazole-trimethoprim combination showed fungicidal activity toward 57% and 52% of the isolates, respectively. Itraconazole, sulfamethoxazole, and the sulfamethoxazole-trimethoprim combination were also fungicidal toward cultures from the MMH medium for 100%, 66%, and 71% of the isolates, respectively. Divergent activity profiles were verified for terbinafine and fluconazole when they were tested in RPMI 1640 and MMH media. Terbinafine was predominantly found to be fungicidal in RPMI 1640 medium and fungistatic in MMH medium (52%), while fluconazole, which was predominantly fungistatic in RPMI 1640 medium, was fungicidal in MMH medium (52%).
Table 4.
Fungicidal activities of amphotericin B, itraconazole, fluconazole, sulfamethoxazole, sulfamethoxazole-trimethoprim, and terbinafine against 21 Paracoccidioides brasiliensis isolates in the RPMI and MMH MIC assays at 37°Ca
| % fungicidal activity in indicated medium | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphotericin B |
Itraconazole |
Fluconazole |
Sulfamethoxazole |
Sulfamethoxazole-trimethoprim |
Terbinafine |
||||||
| RPMI | MMH | RPMI | MMH | RPMI | MMH | RPMI | MMH | RPMI | MMH | RPMI | MMH |
| 100 | 100 | 57 | 100 | 43 | 52 | 62 | 66 | 52 | 71 | 57 | 48 |
RPMI, RPMI 1640 medium; MMH, MMH medium.
DISCUSSION
The absence of standardized working protocols is directly related to the variety of MICs that are found in the literature for a specific fungal pathogen, as different researchers have adopted distinct ways of performing these tests. In our study, we followed the recommendations of the NCCLS document M27-A2 microdilution protocols, with certain modifications, for testing isolates of the Paracoccidioides species complex. On the basis of our results, we have discussed the influence of variable conditions on the MIC readings for the seven drugs and suggest a protocol for the in vitro susceptibility testing of P. brasiliensis (Table 5). Since this work was a single-center study, it is interesting to highlight the relevance of subsequent efforts in developing multicenter validation and comparison of the accuracy of the test method and results.
Table 5.
Conditions employed in previous studies and in the present work for determination of MIC against Paracoccidioides species complex: a tentative protocol based on our present resultsa
| Conditions | Previous references | This work | Proposed protocol |
|---|---|---|---|
| Inoculum concn | 1 × 103 to 1 × 105 cells/ml (9, 15, 16, 18, 20, 21) | 1 × 103, 1 ×104, and 1 ×105 cells/ml | 1 × 105 cells/ml |
| Incubation temp | 35–37°C, room temp (6, 11, 12, 15–21, 23) | 35–37°C, room temp | 35–37°C |
| Media | RPMI, MMH, MVM (9, 23, 30) | RPMI,c MMH, MVM | MMH and MVMb |
| Reading time | 72 h to 8 days (15–19) | 7, 10, and 15 days | 15 days |
| Protocol | Macro- and microdilution (6, 15–18) | Microdilution | Microdilution |
RPMI, RPMI 1640 medium; MMH, MMH medium; MVM, MVM medium.
MVM is to be used when necessary to test sulfamethoxazole and the combination sulfamethoxazole-trimethoprim.
When employed at 37°C, mycelial morphology may be found.
First, a previous testing assay with three different inoculum concentrations (103, 104, and 105 cells/ml) in RPMI 1640 medium was performed with 13 isolates. The use of 103 cells/ml is suggested by the NCCLS M27-A2 protocol, though concentrations of 104 and 105 cells/ml were used in previous P. brasiliensis MIC assay studies (15, 16, 18, 20, 21, 33). Our results, which are in agreement with the results of these authors, indicated that the obtained growth and MIC readings were easier to perform when a concentration of 105 cells/ml was used (data not shown), and this is the inoculum size in the proposed protocol (Table 5).
A number of authors have proposed different incubation times ranging from 72 h to 8 days (15–19) or starting the MIC reading after the growth of the positive control (22) to test P. brasiliensis. There are indications that the exponential growth of P. brasiliensis occurs at between 5 and 7 days of incubation in complete medium (1, 2). Our results showed that the incubation time directly influenced the MICs under all tested conditions for most of the isolates (P < 0.05). Regarding the incubation times, the highest MIC variations were found with RPMI 1640 medium, and the lowest variations were observed with MMH medium. Our results showed that an incubation time of 7 days was not sufficient for the satisfactory growth of all the isolates, which may indicate a probability of false MIC readings. After 10 days of incubation, the growth was still poor for the isolates that were, apparently, more fastidious. On the other hand, all of the tested isolates grew better after 15 days of incubation, a result that supports a previous suggestion of starting the MIC reading after the growth of the positive controls (22) and indicative that 15 days would be the most reliable time for MIC readings, as we propose in a tentative protocol (Table 5).
The ideal incubation temperature for dimorphic fungi can be questionable. Several authors used a small range (35°C to 37°C) for the MIC assays (6, 11–13, 15–21, 23), probably because in this temperature range, the dimorphic fungus is found in the parasitic tissue form when antifungal therapy is necessary. However, it would also be interesting to compare the results from both conditions and the environmental studies at room temperature. Our results showed that the incubation temperature influenced the MICs (P < 0.05) and that in general, higher MICs were obtained at 25°C than at 37°C. Few studies have presented MICs at both temperatures. Nakai et al. (22) found higher MICs at 25°C than at 37°C for fluconazole, while higher MICs were observed at 37°C for amphotericin B, and similar MICs were found at both temperatures for itraconazole in RPMI 1640 medium. Our results also showed higher MICs at 37°C for amphotericin B and similar MICs for fluconazole at both temperatures when RPMI 1640 medium was tested.
All of the tested media (standard RPMI 1640 broth, MVM medium, and MMH medium) yielded significantly different results (P < 0.05). Higher MICs were obtained in RPMI 1640 medium than in the other tested media. The medium proposed by the NCCLS allowed adequate growth for all isolates, confirming reports that it allows suitable visible growth of P. brasiliensis. However, the majority of the P. brasiliensis isolates showed either complete or incomplete yeast-mycelium transition in this medium during incubation at 37°C. This transition phenotype was also observed by other authors (22) in dimorphic fungi when MIC assays with RPMI 1640 medium were conducted. This transition phenomenon may influence the MIC results, as distinct structures of the fungi (yeast-like form, hypha, and chlamydospore) may be present in variable proportions in the wells at different time points. We evaluated this transition phenotype in 14 of the P. brasiliensis isolates used in this study and observed that the yeast-mycelium transition started at 5 to 10 days of incubation. The NCCLS M27-A2 protocol and some authors have suggested supplementation of the RPMI1640 medium with 2% glucose for Sporothrix schenckii dimorphic fungus (22, 34). We also tested this increased glucose concentration in RPMI 1640 medium in the MIC assays, but no differences in the yeast-mycelium transition were observed. Interestingly, there was no yeast-mycelium transition in MMH or MVM medium. These observations suggest that the composition of the medium used in the MIC assay probably influences the changes in the morphology of this dimorphic fungus at 37°C.
The MICs with MVM medium were similar to those found with RPMI 1640 medium, except for sulfamethoxazole and the combination of sulfamethoxazole-trimethoprim. In this particular case, the MICs were considerably lower than in RPMI 1640 or MMH medium. These results are in disagreement with those of other authors (6, 12) who utilized macrodilution techniques, although only one isolate was analyzed in the studies by these authors. The influence of different media upon MICs for sulfamide derivatives was studied by other authors for Aspergillus spp. and Cryptococcus spp. This influence, as found in this work, was observed when these authors compared the results obtained in complexes or synthetic media in the presence or absence of folic acid supplementation (33). This may be due to the mechanism of action of these drugs. In bacteria, sulfamide derivatives interfere with the synthesis of folic acid by competing with p-aminobenzoic acid (PABA) in a reaction catalyzed by dihydropteroate synthase. As a consequence, there is a depletion of intracellular folate, which is essential for growth (26). This mechanism of action differs from that of the other antifungal agents tested in this study. Sulfamide derivatives are used frequently in Brazil for the treatment of PCM. There are no reports in the literature on its mechanism of action for P. brasiliensis. However, the complete genome of P. brasiliensis (www.broadinstitute.org) shows the existence of sequences that are similar to those for dihydropteroate synthase and dihydrofolate reductase. Although we do not know whether there is antagonism or synergism between sulfamide derivatives or the components of MVM (35), our results suggest that this medium influenced the MICs for all of the tested isolates. RPMI 1640 medium is supplemented with PABA (0.001 g/liter) and folic acid (0.001 g/liter). MVM medium is often used in MIC determinations for P. brasiliensis by the broth macrodilution method (6, 15–18). MVM medium is a chemically defined medium that is supplemented only with folic acid (0.0001 g/liter) at a quantity much lower than that found in RPMI 1640 medium (30). We tested the MVM medium in the microdilution assay. Reading the MIC in plates was experimentally more difficult, mainly because of the poor growth of some of the isolates. This poor growth was also reported by other authors (36) that used the MIC assay with MVM medium in other fungi.
MMH medium is not a chemically defined medium, and microorganisms can obtain folic acid or its precursors from different sources. MMH medium was tested in our study because it was previously used in MIC assays with agar dilution for P. brasiliensis isolates (23), yielding values similar to those obtained in RPMI 1640 medium, and because yeast morphology was maintained at 37°C in this medium until the end of the protocol. Our results showed that the MICs with MMH medium were similar to those found with RPMI 1640 medium when sulfamethoxazole and the sulfamethoxazole-trimethoprim combination were tested. However, the use of the sulfamide derivatives is unusual for the treatment of other mycoses, and it became difficult to compare the MICs of sulfamethoxazole and the sulfamethoxazole-trimethoprim combination.
Amphotericin B was found to have fungicidal properties against all tested isolates in RPMI 1640 or MMH medium cultures. Amphotericin B binds to the ergosterol and disrupts the osmotic integrity of the fungal membrane, compromising its barrier function (37). Hamdan and Resende (18) demonstrated that the viability of P. brasiliensis progressively decreases as the time of exposure to the drug increases. These authors also showed that K+ ions were liberated in a few minutes when the cells were incubated with amphotericin B and that the liberation of proteins and nucleic acids required a longer incubation period (18). The other antifungal agents used in this study, such as azole derivates (itraconazole and fluconazole) and sulfamide derivatives (sulfamethoxazole and the combination of sulfamethoxazole-trimethoprim), are fungistatic and act by competition in the ergosterol biosynthesis pathway and the folic acid biosynthesis pathway, respectively (37). The fungicidal or fungistatic activity was related to the tested concentration and the time of exposure. This fact may result in different activities of P. brasiliensis yeast cells (16). Our results suggest that incubation in RPMI 1640 or MMH medium may have influenced the activity of these drugs, probably by promoting different levels of growth of the isolates, as the fungicidal activity of primary fungistatic agents was more evident in the MMH medium. The terbinafine activity against P. brasiliensis is poorly understood because this drug is not frequently used for PCM treatment. Our results demonstrated that terbinafine has fungicidal activity mainly in RPMI 1640 medium. Hahn et al. (17) found similar MICs for terbinafine and itraconazole against P. brasiliensis by a macrodilution method. These authors attributed this fact to the similar mechanisms of action of these antifungals. To our knowledge, this is the first report of the classification of antifungals as fungicidal or fungistatic against this dimorphic fungus.
The absence of interpretative breakpoints for the antifungal agents against P. brasiliensis makes it difficult to classify the isolates as susceptible or resistant. These breakpoints could be achieved by clinical laboratory studies, which are, unfortunately, scarce. Based on other in vitro studies (6, 11–13, 15–19, 22, 23), all of the isolates used in this work were susceptible to all of the tested antifungals under all experimental conditions. In the literature, there is a single reference to an isolate that was considered resistant to trimethoprim-sulfamethoxazole (MIC = 320 mg/liter) (6). However, in the present work, MICs around 300 mg/liter for sulfamethoxazole and for the combination of sulfamethoxazole-trimethoprim were registered for various isolates when RPMI 1640 and/or MMH medium was used.
In conclusion, this investigation demonstrated that the microdilution assay for the Paracoccidioides species complex can be affected by different factors. There was not sufficient growth after 7 days of incubation for a number of isolates, and better results were obtained after 15 days of incubation for all of the experimental conditions when initial inocula of 105 cells/ml were used (Table 5). Our results demonstrated that the MICs may change depending on the medium used. We found higher MICs with RPMI 1640 medium for all antifungal agents and all tested isolates, which correlated with the transition morphology of yeast to mycelium during the test. Indeed, the variations of MICs were more prominent in RPMI 1640 medium than in MMH or MVM medium when the time of incubation was evaluated. The temperature directly influenced the MICs, with higher MICs found at room temperature. More studies are still necessary to better understand the physiology of this fungus, as our RPMI 1640 MIC assays demonstrated that temperature does not appear to be the only factor responsible for morphology transition in the Paracoccidioides species complex. This fact may compromise the reliability of the MIC results when RPMI 1640 medium is used. On the other hand, MMH medium appears to be a suitable test medium for susceptibility testing of antifungal drugs with P. brasiliensis, except for sulfametoxazole and the combination of sulfamethoxazole-trimethoprim, with which the MVM medium gave better results, as indicated in the tentative protocols presented in Table 5.
ACKNOWLEDGMENTS
This work was supported by the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq, process number 480616/2007-2008), by a fellowship during Ph.D. studies, and by the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG).
We thank Gilvânia Ferreira da Silva Santos for technical support.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 21 November 2012
REFERENCES
- 1. Brummer E, Castaneda E, Restrepo A. 1993. Paracoccidioidomycosis: an update. Clin. Microbiol. Rev. 6:89–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lacaz CS. 1994. Paracoccidioides brasiliensis: morphology, evolutionary cycle; maintenance during saprophytic life; biology, virulence, taxonomy, p 13–25 In Franco M, Lacaz CS, Restrepo-Moreno A, Del-Negro G. (ed), Paracoccidioidomycosis. CRC Press, Boca Raton, FL [Google Scholar]
- 3. Prado M, Da Silva MB, Laurenti R, Travassos LR, Taborda CP. 2009. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brasil: a review from 1996 to 2006. Mem. Inst. Oswaldo Cruz 104:513–521 [DOI] [PubMed] [Google Scholar]
- 4. Borges-Walmsley MIB, Chen D, Shu X, Walmsley AR. 2002. The pathobiology of Paracoccioides brasiliensis. Trends Microbiol. 10:80–85 [DOI] [PubMed] [Google Scholar]
- 5. Ferreira MS. 2009. Paracoccidioidomycosis. Paediatr. Respir. Rev. 10:161–165 [DOI] [PubMed] [Google Scholar]
- 6. Hahn RC, Yvelise TMC, Santos NL, Ferreira JF, Hamdan JS. 2003. Disseminated paracoccidioidomycosis: correlation between clinical and in vitro resistance to ketoconazole and trimethoprim sulphamethoxazole. Mycoses 46:324–329 [DOI] [PubMed] [Google Scholar]
- 7. Ramos-e-Silva M, Saraiva LES. 2008. Paracoccidioidomycosis. Dermatol. Clin. 26:257–269 [DOI] [PubMed] [Google Scholar]
- 8. National Committee for Clinical Laboratory Standards 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 9. National Committee for Clinical Laboratory Standards 2002. Reference method for broth dilution antifungal susceptibility testing of yeast fungi. Approved standard M38-A2. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 10. Shadomy S, Espinel-Ingroff A, Cartwright RY. 1987. Estudios de laboratório com agentes antifúngicos: pruebas de susceptibilidade y bioensayos, p 1229–1238 In Lenette EH, Ballows A, Hausler WJ, Shadomy HJ. (ed), Manual de microbiologia clinica, 4th ed Editorial Medica Panamericana, Buenos Aires, Argentina [Google Scholar]
- 11. Campos FF, Johann S, Cota BB, Alves TMA, Rosa LH, Caligiorne RB, Cisalpino PS, Rosa CA, Zani CL. 2011. Antifungal activity of trichothecenes from Fusarium sp. against clinical isolates of Paracoccidioides brasiliensis. Mycoses 54:122–129 [DOI] [PubMed] [Google Scholar]
- 12. Da Silva MB, Marques AF, Nosanchuk JD, Casadevall A, Travassos LR, Taborda CP. 2006. Melanin in the dimorphic fungal pathogen Paracoccidioides brasiliensis; effects on phagocytosis, intracellular resistance and drug susceptibility. Microbes Infect. 8:197–205 [DOI] [PubMed] [Google Scholar]
- 13. Espinel-Ingroff A, Boyle K, Sheehan DJ. 2001. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia 150:101–115 [DOI] [PubMed] [Google Scholar]
- 14. Espinel-Ingroff A, Chaturvedi V, Fothergill A, Rinaldi MG. 2002. Optimal testing conditions for determining MICs and minimum fungicidal concentration of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J. Clin. Microbiol. 40:3776–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hahn RC, Hamdan JS. 2000. In vitro susceptibilities of Paracoccidioides brasiliensis yeast form to antifungal drugs. Mycoses 43:403–407 [PubMed] [Google Scholar]
- 16. Hahn RC, Hamdan JS. 2000. Effects of amphotericin B and three azole derivatives on the lipids of yeast cells of Paracoccidioides brasiliensis. Antimicrob. Agents Chemother. 44:1997–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hahn RC, Fontes CJF, Batista RD, Hamdan JS. 2002. In vitro comparison of activities of terbinafine and itraconazole against Paracoccidioides brasiliensis. J. Clin. Microbiol. 40:2828–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamdan JS, Resende MA. 1988. Lipid composition and the effect of amphotericin B on yeast cells of Paracoccidioides brasiliensis. Mycopathologia 102:97–105 [DOI] [PubMed] [Google Scholar]
- 19. Johann S, Sá NP, Lima LARS, Cisalpino PS, Cota BB, Alves TMA, Siqueira EP, Zani CL. 2010. Antifungal activity of schinol and a new biphenyl compound isolated from Schinus terebinthifolius against the pathogenic fungus Paracoccidioides brasiliensis. Ann. Clin. Microbiol. Antimicrob. 9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johann S, Cisalpino PS, Watanabe GA, Cota BB, Siqueira EP, Pizzolati MG, Zani CL, Rezende MA. 2010. Antifungal activity of extracts of some plants used in Brazilian traditional medicine against pathogenic fungus Paracoccidioides brasiliensis. Pharm. Biol. 48(Suppl 4):388–396 [DOI] [PubMed] [Google Scholar]
- 21. Lima LARS, Johann S, Cisalpino PS, Pimenta LPS, Boaventura MAD. 2011. Antifungal activity of 9-hydroxy-folianin and sucrose octaacetate from the seeds of Annona cornifolia A.St.-Hil (Annonaceae). Rev. Soc. Bras. Med. Trop. 44:777–780 [DOI] [PubMed] [Google Scholar]
- 22. Nakai T, Uno J, Ikeda F, Tawara S, Nishimura K, Miyaji M. 2003. In vitro antifungal activity of micafungin (FK463) against dimorphic fungi: comparison of yeast-like and mycelial forms. Antimicrob. Agents Chemother. 47:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Restrepo AM, Arango MD. 1980. In vitro susceptibility testing of Paracocidioides brasiliensis to sulfonamides. Antimicrob. Agents Chemother. 18:190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marini MM, Zanforlin T, Santos PC, Barros RRM, Guerra ACP, Puccia R, Felipe MSS, Brigido M, Soares CMA, Ruiz JC, Silveira JF, Cisalpino PS. 2010. Identification and characterization of TC1/mariner-like DNA transposons in genome of the pathogenic fungi of the Paracoccidioides species complex. BMC Genomics 11:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carrero LL, Niño-Vega G, Teixeira MM, Carvalho MJA, Soares CMA, Pereira M, Jesuino RSA, McEwen JG, Mendoza L, Taylor JW, Felipe MS, San-Blas G. 2008. New Paracoccidioides brasiliensis isolate reveals unexpected genomic variability in this human pathogen. Fungal Genet. Biol. 45:605–612 [DOI] [PubMed] [Google Scholar]
- 26. Teixeira MM, Theodoro RC, Carvalho MJ, Fernandes L, Paes HC, Hahn RC, Mendoza L, Bagagli E., San-Blas G, Felipe MSS. 2009. Phylogenetic analisys reveals a high level of speciation in the Paracoccidioides genus. Mol. Phylogenet. Evol. 52:273–283 [DOI] [PubMed] [Google Scholar]
- 27. Matute DR, McEween JG, Puccia R, Montes BA, San-Blas G, Bagagli E, Rauscher JT, Restrepo A, Morais F, Niño-Vega G, Taylor JW. 2006. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol. Biol. Evol. 23:65–73 [DOI] [PubMed] [Google Scholar]
- 28. Morais FV, Barros TF, Fukada MK, Cisalpino PS, Puccia R. 2000. Polymorphism in the gene coding for the immunodominant antigen gp43 from the pathogenic fungus Paracoccidioides brasiliensis. J. Clin. Microbiol. 38:3960–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Puccia R, McEwen JG, Mendoza L. 2008. Diversity in Paracoccidioides brasiliensis: the PbGP43 gene as a genetic marker. Mycopathologia 165:275–287 [DOI] [PubMed] [Google Scholar]
- 30. Restrepo A, Jiménez B. 1980. Growth of Paracoccidioides brasiliensis yeast phase in a chemically defined culture medium. J. Clin. Microbiol. 12:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. San-Blas G, San-Blas F. 1994. Biochemistry of Paracoccidioides brasiliensis dimorphism, p 49–66 In Franco M, Lacaz CS, Restrepo-Moreno A, Del-Negro G. (ed), Paracoccidioidomycosis. CRC Press, Boca Raton, FL [Google Scholar]
- 32. Sampaio IBM. 2002. Estatística aplicada a experimentação animal, 2nd edition FEPMVZ, Belo Horizonte, Brazil [Google Scholar]
- 33. Hanafy A, Uno J, Meshnick S, Kang Y, Mikami Y. 2001. In vitro antifungal activities of sulfa drugs against clinical isolates of Aspergillus and Cryptococcus species. J. Med. Mycol. 47:47–50 [DOI] [PubMed] [Google Scholar]
- 34. Kohler LM, Monteiro PCF, Hahn RC, Hamdan JS. 2004. In vitro susceptibilities of isolates of Sporothrix schenckii to itraconazole and terbinafine. J. Clin. Microbiol. 42:4319–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stevens DA, Vo PT. 1982. Synergistic interaction of trimethoprim and sulfamethoxazole on Paracoccidioides brasiliensis. Antimicrob. Agents Chemother. 21:852–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santos DA, Hamdan JS. 2005. Evaluation of broth microdilution antifungal susceptibility testing conditions for Trichophyton rubrum. J. Clin. Microbiol. 43:1917–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Visbal G, San-Blas G, Murgich J, Franco H. 2005. Paracoccidioides brasiliensis paracoccidioidomycosis, and antifungal antibiotics. Curr. Drug Targets Infect. Disord. 5:211–226 [DOI] [PubMed] [Google Scholar]