Abstract
We report herein the first case of acute acalculous cholecystitis caused by Lactococcus garvieae, which is known as a fish pathogen. A 69-year-old fisherman underwent laparoscopic cholecystectomy due to severe inflammation in the gallbladder. The isolate obtained from the gallbladder was identified as L. garvieae by 16S rRNA and manganese-dependent superoxide dismutase (sodA) gene sequence analysis.
CASE REPORT
A69-year-old man presented at Inje University Ilsan Paik Hospital (Goyang, Republic of Korea) with severe upper abdominal and postprandial pains for 2 days. His medical history included gastric ulcer perforation, fatty liver, and chronic alcoholism with tobacco use. The gastric ulcer perforation had been treated with exploratory laparotomy 19 years ago; however, the patient took no prescribed medications for fatty liver and alcoholism during that time. The patient was a fisherman by occupation, and during the middle period of his life worked at a fish culture farm located at the Han-tan and Nam-han rivers in the Republic of Korea. He occasionally ingested raw rainbow trout (Oncorhynchus mykiss) at that time and continued eating raw freshwater fish or shellfish frequently before this presentation. He had never traveled overseas.
Physical examination on admission showed crouching with a blood pressure of 130/80 mm Hg, a heart rate of 73 beats/min, a respiratory rate of 20/min, and a body temperature of 36.3°C. The patient exhibited no evidence of murmur, jugular vein engorgement, or liver cirrhosis. His abdomen was mildly distended and soft, with normoactive bowel sounds, tenderness in the right upper quadrant, positive Murphy's sign, and no rebound tenderness. The results of routine tests were as follows: white cell count, 17,000 cells/μl; polymorphoneutrophil count, 14,340 cells/μl; hemoglobin, 15.1 g/dl; erythrocyte sedimentation rate (ESR), 15 mm/h; electrolytes (Na, K, and Cl), 138, 4.8, and 104 mEq, respectively; blood urea nitrogen (BUN) and creatinine, 15 and 1.1 mg/dl, respectively; aspartate transferase (AST) and alanine transaminase (ALT), 94 and 73 IU/liter, respectively; total bilirubin, 2.6 mg/dl; and urine analysis, clear.
Abdominopelvic computed tomography (CT) findings were as follows: distension of the gallbladder, edematous wall thickening, and mild hyperemic change at the gallbladder bed. In addition, attenuation of parenchyma was lower in the liver than in the spleen. There were no remarkable findings in the pancreas, kidneys, spleen, or urinary bladder. No evidence of enlarged lymph nodes or ascites was found on CT scans. Therefore, the patient underwent laparoscopic cholecystectomy with closed drainage (Baro-vac), which showed a thickened, distended, hyperemic and edematous gallbladder. Obstructive stones were not observed in the gallbladder. There were no specific abnormalities in other organs, except that the liver angle was blunt. After the operation, the patient was treated with cefminox sodium (Meicelin) (2 g twice a day [b.i.d.]) for 3 days and thereafter cefaclor monohydrate (1 tablet [250 mg] three times a day [t.i.d.]) for 5 days. The patient recovered and was discharged from the hospital on the fourth postoperative day.
Three specimens (one specimen each from the patient's bile juice, gallbladder lumen tissue, and gallbladder mucosa) were obtained from the gallbladder. All cultures from the specimens yielded oxidase-negative, catalase-negative, alpha-hemolytic colonies of Gram-positive cocci in short chains on 5% sheep blood agar after 24 h of incubation at 37°C. The GP card of the Vitek2 system (bioMérieux, Marcy l'Etoile, France) was used for preliminary identification of bacterial isolates, and the results indicated that those isolates were Lactococcus garvieae with 99% probability. Based on these results, one of the bacterial isolates (L. garvieae LG-ilsanpaik-gs201105, isolated from bile juice) was subjected to further genetic identification based on 16S rRNA and manganese-dependent superoxide dismutase (sodA) gene sequence analysis. The partial 16S rRNA and sodA genes of L. garvieae LG-ilsanpaik-gs201105 were sequenced at Macrogen, Inc. (Republic of Korea), using universal primers (27F/1492R) and d1/d2 primers, respectively, as previously described (1). The obtained 1,343-bp 16S rRNA and 495-bp sodA sequences were compared with sequences in the NCBI GenBank database using a BLAST search (http://www.ncbi.nlm.nih.gov/BLAST). The 16S rRNA sequence of L. garvieae LG-ilsanpaik-gs201105 was a 99.4% match with the 16S rRNA gene of the L. garvieae strain NRIC 0612 (GenBank accession no. AB362690). In addition, the sodA sequence of the isolate was a 99.5% match with the sodA sequence of L. garvieae strain JIP 31-90 (GenBank accession no. AM490328). The sequences of the 16S rRNA and sodA genes were further compared with those of closely related Gram-positive species in GenBank by multiple sequence alignments using CLUSTAL X (version 1.83) (2). The phylogenetic relationships were determined by the neighbor-joining method using Molecular Evolutionary Genetics Analysis (MEGA) (version 5.0) software (3), which revealed that the patient's isolate was L. garvieae (Fig. 1).
Fig 1.
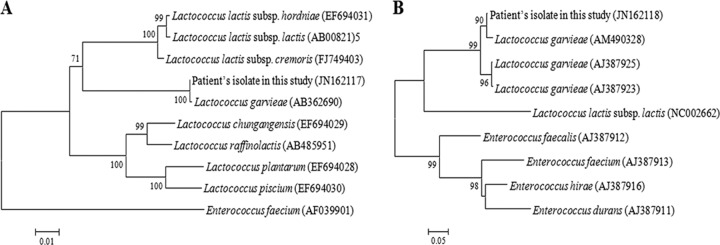
Phylogenetic analysis of the L. garvieae Lg-ilsanpaik-gs201105 isolate from the patient based on the partial nucleotide sequences of the 16S rRNA gene (A), including the 16S rRNA sequence of Enterococcus faecium (AF039901) as an outgroup, and the sodA gene (B). One thousand bootstrap replicates were subjected to nucleotide sequence distance analysis by neighbor-joining methods. All bootstrap values are displayed above the tree branches, and only bootstrap values of >70% are shown.
The antimicrobial susceptibility of L. garvieae LG-ilsanpaik-gs201105 was determined using the Etest (bioMérieux, Marcy l'Etoile, France) on Mueller-Hinton medium with 5% sheep blood. The resistance breakpoints for L. garvieae were followed according to the Clinical and Laboratory Standards Institute's (CLSI) guidelines (4). The L. garvieae isolates were susceptible to ampicillin (MIC, 0.25 μg/ml), ceftriaxone (MIC, 1 μg/ml), chloramphenicol (MIC, 2 μg/ml), linezolid (MIC, 2 μg/ml), ofloxacin (MIC, 2 μg/ml), tetracycline (MIC, 1 μg/ml), and vancomycin (MIC, 1 μg/ml) and intermediate to erythromycin (MIC, 0.5 μg/ml) but were resistant to clindamycin (MIC, 8 μg/ml). Therefore, to detect the potential erythromycin resistance methylase (erm) or macrolide efflux (mef) genes in L. garvieae LG-ilsanpaik-gs201105, PCR primers were used for the detection of erm(A)20, erm(B)17, erm(C)20, and mef16 (5). However, the erm or mef genes were not detected from the isolate.
We have described the identification by 16S rRNA and sodA gene sequencing of a strain of L. garvieae isolated from the gallbladder of a patient with acute acalculous cholecystitis. To the best of our knowledge, this is the first case of acute acalculous cholecystitis caused by this Lactococcus species. L. garvieae (formerly named Streptococcus garvieae or Enterococcus seriolicida) is a Gram-positive, nonmotile, and non-spore-forming coccus (6, 7) and is responsible for bacteremia in various fish species (8) and for mastitis in ruminants (9, 10). Since the number of cases of fatal human infections caused by L. garvieae has recently increased, this bacterium has received much attention as an emerging zoonotic pathogen; thus, the genomes of several L. garvieae clinical isolates from fish and humans have been sequenced (11–15). Although several potential virulence factors, such as capsule, NADH oxidase, and superoxide dismutase, have been reported on L. garvieae to explain these different clinical symptoms (13, 16), the bacterium is suspected to be an opportunistic pathogen in elderly immune-deficient subjects and individuals with prosthetic valves (17). In general, human infections caused by L. garvieae are known to be associated with significant morbidity and mortality, and most of the cases (10 of the 13 cases) exhibited bacteremia. Among the 13 L. garvieae infections reported in humans, the most common cases are infective endocarditis (n = 6; native valve, n = 5, and prosthetic valve, n = 1) (17–22), followed by spondylodiscitis (n = 2) (23, 24), liver abscess (n = 1) (25), secondary peritonitis (n = 1) (21), diverticulitis (n = 1) (21), septicemia with multiorgan failure (21), and prosthetic joint infection (n = 1) (26). However, this bacterium had never been reported as a causative agent of acute acalculous cholecystitis.
Although the clinical symptoms of the previously reported L. garvieae infections varied, manipulation and consumption of raw fish have been suspected as the most probable sources of the infections in humans (21). In addition, skin wounds are suspected to be the portal of entry for asymptomatic bacteremia caused by L. garvieae, as in the case which occurred in an immune-suppressed woman fishmonger who lived near the sea (26). Likewise, our patient was a fisherman and frequently ingested raw fish or shellfish before this presentation. Based on these results, even though the source of infection is still uncertain in this case, the consumption of raw fish (especially rainbow trout) is suspected to be the most probable cause of the infection.
L. garvieae LG-ilsanpaik-gs201105, which was isolated from the patient's gallbladder, was almost 100% identical to previously reported bacterial isolates based on their 16S rRNA and sodA gene sequences. Moreover, the resistance of the isolates in this case to clindamycin is consistent with that of a previous study which reported intrinsic resistance of L. garvieae to clindamycin and proposed to use this resistance as a criterion for distinguishing between L. garvieae and L. lactis (27). Therefore, based on these results, the bacterial isolates from the patient's gallbladder were confirmed as L. garvieae.
Laparoscopic cholecystectomy successfully treated our patient with acute acalculous cholecytitis caused by L. garvieae. Our patient also received antibiotic therapy after surgery and was discharged from the hospital on the second postoperative day. However, in most of the laparoscopic cholecystectomies, bacteriologic diagnoses are not established through cultures. Therefore, physicians and clinical microbiologists should be aware of the need to take a meticulous medical history, and bacteriologic studies could help in the discovery of cases of acute acalculous cholecystitis in patients at high risk of L. garvieae infection. Indeed, due to the similarities in the phenotypic characteristics of L. garvieae and its clinical symptoms of acute acalculous cholecystitis to those of other genera, especially Enterococcus spp., culture-based molecular analysis using the 16S rRNA or sodA gene will help to make accurate identification of L. garvieae infection. Further studies are currently in preparation to investigate the genome sequence of L. garvieae isolate LG-ilsanpaik-gs201105.
Nucleotide sequence accession numbers.
The nucleotide sequences for the 16S rRNA and sodA genes of L. garvieae LG-ilsanpaik-gs201105 have been deposited in GenBank under accession no. JN162117 and JN162118, respectively.
Footnotes
Published ahead of print 12 December 2012
REFERENCES
- 1. Poyart C, Quesne G, Coulon S, Berche P, Trieu-Cuot P. 1998. Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J. Clin. Microbiol. 36:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2009. Performance standards for antimicrobial susceptibility testing; nineteenth informational supplement. CLSI document M100-S19. CLSI, Wayne, PA [Google Scholar]
- 5. Lim JA, Kwon AR, Kim SK, Chong Y, Lee K, Choi EC. 2002. Prevalence of resistance to macrolide, lincosamide and streptogramin antibiotics in Gram-positive cocci isolated in a Korean hospital. J. Antimicrob. Chemother. 49:489–495 [DOI] [PubMed] [Google Scholar]
- 6. Collins MD, Farrow JA, Phillips BA, Kandler O. 1983. Streptococcus garvieae sp. nov. and Streptococcus plantarum sp. nov. J. Gen. Microbiol. 129:3427–3431 [DOI] [PubMed] [Google Scholar]
- 7. Garvie EI, Farrow JA, Phillips BA. 1981. A taxonomic study of some strains of streptococci which grow at 10°C but not at 45°C including Streptococcus lactis and Streptococcus cremoris. Zentralbl. Bakteriol. Parasitenkd. Infektionskrankh. Hyg. C 2:151–165 [Google Scholar]
- 8. Vendrell D, Balcázar JL, Ruiz-Zarzuela I, de Blas I, Gironés O, Múzquiz JL. 2006. Lactococcus garvieae in fish: a review. Comp. Immunol. Microbiol. Infect. Dis. 29:177–198 [DOI] [PubMed] [Google Scholar]
- 9. Teixeira LM, Merquior VL, Vianni MC, Carvalho MG, Fracalanzza SE, Steigerwalt AG, Brenner DJ, Facklam RR. 1996. Phenotypic and genotypic characterization of atypical Lactococcus garvieae strains isolated from water buffalos with subclinical mastitis and confirmation of L. garvieae as a senior subjective synonym of Enterococcus seriolicida. Int. J. Syst. Bacteriol. 46:664–668 [DOI] [PubMed] [Google Scholar]
- 10. Vela AI, Vázquez J, Gibello A, Blanco MM, Moreno MA, Liébana P, Albendea C, Alcalá B, Mendez A, Domínguez L, Fernández-Garayzábal JF. 2000. Phenotypic and genetic characterization of Lactococcus garvieae isolated in Spain from lactococcosis outbreaks and comparison with isolates of other countries and sources. J. Clin. Microbiol. 38:3791–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aguado-Urda M, López-Campos GH, Blanco MM, Fernández-Garayzábal JF, Cutuli MT, Aspiroz C, López-Alonso V, Gibello A. 2011. Genome sequence of Lactococcus garvieae 21881, isolated in a case of human septicemia. J. Bacteriol. 193:4033–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aguado-Urda M, López-Campos GH, Gibello A, Cutuli MT, López-Alonso V, Fernández-Garayzábal JF, Blanco MM. 2011. Genome sequence of Lactococcus garvieae 8831, isolated from rainbow trout lactococcosis outbreaks in Spain. J. Bacteriol. 193:4263–4264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morita H, Toh H, Oshima K, Yoshizaki M, Kawanishi M, Nakaya K, Suzuki T, Miyauchi E, Ishii Y, Tanabe S, Murakami M, Hattori M. 2011. Complete genome sequence and comparative analysis of the fish pathogen Lactococcus garvieae. PLoS One 6:e23184 doi:10.1371/journal.pone.0023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reimundo P, Pignatelli M, Alcaraz LD, D'Auria G, Moya A, Guijarro JA. 2011. Genome sequence of Lactococcus garvieae UNIUD074, isolated in Italy from a lactococcosis outbreak. J. Bacteriol. 193:3684–3685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ricci G, Ferrario C, Borgo F, Rollando A, Fortina MG. 2012. Genome sequences of Lactococcus garvieae TB25, isolated from Italian cheese, and Lactococcus garvieae LG9, isolated from Italian rainbow trout. J. Bacteriol. 194:1249–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitchell TJ. 2003. The pathogenesis of streptococcal infections: from tooth decay to meningitis. Nat. Rev. Microbiol. 1:219–230 [DOI] [PubMed] [Google Scholar]
- 17. Fihman V, Raskine L, Barrou Z, Kiffel C, Riahi J, Berçot B, Sanson-Le Pors MJ. 2006. Lactococcus garvieae endocarditis: identification by 16S rRNA and sodA sequence analysis. J. Infect. 52:e3–e6 [DOI] [PubMed] [Google Scholar]
- 18. Fefer JJ, Ratzan KR, Sharp SE, Saiz E. 1998. Lactococcus garvieae endocarditis: report of a case and review of the literature. Diagn. Microbiol. Infect. Dis. 32:127–130 [DOI] [PubMed] [Google Scholar]
- 19. Li WK, Chen YS, Wann SR, Liu YC, Tsai HC. 2008. Lactococcus garvieae endocarditis with initial presentation of acute cerebral infarction in a healthy immunocompetent man. Intern. Med. 47:1143–1146 [DOI] [PubMed] [Google Scholar]
- 20. Vinh DC, Nichol KA, Rand F, Embil JM. 2006. Native-valve bacterial endocarditis caused by Lactococcus garvieae. Diagn. Microbiol. Infect. Dis. 56:91–94 [DOI] [PubMed] [Google Scholar]
- 21. Wang CY, Shie HS, Chen SC, Huang JP, Hsieh IC, Wen MS, Lin FC, Wu D. 2007. Lactococcus garvieae infections in humans: possible association with aquaculture outbreaks. Int. J. Clin. Pract. 61:68–73 [DOI] [PubMed] [Google Scholar]
- 22. Yiu KH, Siu CW, To KK, Jim MH, Lee KL, Lau CP, Tse HF. 2007. A rare cause of infective endocarditis; Lactococcus garvieae. Int. J. Cardiol. 114:286–287 [DOI] [PubMed] [Google Scholar]
- 23. Chan JF, Woo PC, Teng JL, Lau SK, Leung SS, Tam FC, Yuen KY. 2011. Primary infective spondylodiscitis caused by Lactococcus garvieae and a review of human L. garvieae infections. Infection 39:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. James PR, Hardman SMC, Patterson DLH. 2000. Osteomyelitis and possible endocarditis secondary to Lactococcus garvieae: a first case report. Postgrad. Med. J. 76:301–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mofredj A, Baraka D, Kloeti G, Dumont JL. 2000. Lactococcus garvieae septicemia with liver abscess in an immunosuppressed patient. Am. J. Med. 109:513–514 [DOI] [PubMed] [Google Scholar]
- 26. Aubin GG, Bémer P, Guillouzouic A, Crémet L, Touchais S, Fraquet N, Boutoille D, Reynaud A, Lepelletier D, Corvec S. 2011. First report of a hip prosthetic and joint infection caused by Lactococcus garvieae in a woman fishmonger. J. Clin. Microbiol. 49:2074–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elliott JA, Facklam RR. 1996. Antimicrobial susceptibilities of Lactococcus lactis and Lactococcus garvieae and a proposed method to discriminate between them. J. Clin. Microbiol. 34:1296–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]


