Abstract
Enteroviruses are recognized as important pathogens in pediatric patients; however, they are often overlooked as etiologic agents of disease in adults. Here, we report a case of echovirus 18-associated severe systemic infection and acute liver failure in an adult hematopoietic stem cell transplant recipient. Additionally, we illustrate the utility of molecular methods for the detection and typing of enteroviral infections.
CASE REPORT
A 48-year-old male with a history of non-Hodgkin lymphoma was admitted to our hospital with fever and multiorgan failure in the autumn, 4 months after receiving an allogeneic hematopoietic stem cell transplant (HSCT) (Fig. 1). The patient initially presented to an outside hospital (OSH) with fever, nausea, vomiting, and diarrhea. Though limited information was available from this hospitalization, by report he received an extensive infectious disease workup as well as upper and lower gastrointestinal endoscopy to evaluate for graft-versus-host disease (GVHD), all of which were negative. The patient's symptoms improved briefly while he was receiving nothing by mouth (NPO). He was discharged on broad-spectrum antimicrobials; however, he was readmitted 4 days later with fevers up to 39.5°C. Over the next 2 weeks, the patient developed hepatic failure with elevated liver function tests (LFTs), including alanine aminotransferase (ALT) of >9,000 U/liter, aspartate aminotransferase (AST) of >14,000 U/liter, alkaline phosphatase of 194 U/liter, and total bilirubin of 6.5 mg/dl. The patient also developed oliguria with creatinine of 2.65 mg/dl and blood urea nitrogen (BUN) of 43 mg/dl, as well as thrombocytopenia with platelet counts falling from 110,000 to 20,000/μl, accompanied by red cell fragments/schistocytes on peripheral blood smear. Extensive imaging of the chest, abdomen, pelvis, and brain was unremarkable. The patient also underwent a liver biopsy, which was notable for acute hepatitis with bile duct injury. The biopsy specimen was negative for hepatitis B virus (HBV) by immunostaining for surface and core antigens and for Epstein-Barr virus (EBV) by EBV-encoded RNA (EBER) in situ hybridization. The patient was treated with sirolimus and solumedrol for presumed GVHD and received four plasma exchanges due to concern for thrombotic thrombocytopenic purpura (TTP). Although his LFTs began to improve, he became acutely confused, requiring intubation for airway protection, and was transferred to our hospital for further evaluation and management.
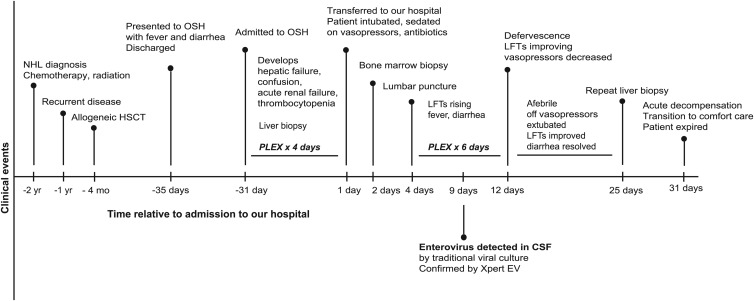
Fig 1.
Time course of the patient's presentation. Shown are major clinical events in the patient's clinical course both before and after he presented to our hospital. Timeline is not to scale. NHL, non-Hodgkin lymphoma; HSCT, hematopoietic stem cell transplant; OSH, outside hospital; PLEX, plasma exchange/plasmapheresis; LFTs, liver function tests; CSF, cerebrospinal fluid; IFA, indirect immunofluorescence antibody testing.
On admission, the patient was febrile, intubated, sedated, and on vasopressor support and broad-spectrum antibiotics. He continued to have evidence of liver failure, with LFTs progressively rising to AST of >10,000 U/liter, ALT of >6,000 U/liter, total bilirubin of 18.7 mg/dl, and alkaline phosphatase of >350 U/liter (Fig. 2). At that time, the differential diagnosis for his transaminitis included drug toxicity, ischemic hepatitis (also known as shock liver), and a viral etiology. Review of the patient's medication history did not reveal a potential toxin, while review of his hospital course indicated that transaminitis preceded hypotension. The initial workup for viral pathogens ruled out hepatitis virus infections. Hepatitis A virus IgM, HBV surface antigen, HBV core total and IgM antibodies, as well as serum HBV and hepatitis C virus nucleic acid tests were all negative. Nucleic acid testing for other potential viral pathogens was also negative, including plasma PCR for cytomegalovirus (CMV), EBV, varicella zoster virus (VZV), human herpesvirus 6 (HHV-6), human herpesvirus 8 (HHV-8), and adenovirus. Furthermore, stool immunoassays for adenovirus and norovirus antigens, as well as serology for adenovirus and parvovirus IgM antibodies, were negative. Additional negative infectious disease tests included multiple blood cultures for bacteria and fungi, nucleic acid testing for toxoplasmosis, stool examination for ova and parasites, and serology for Leptospira total antibodies.
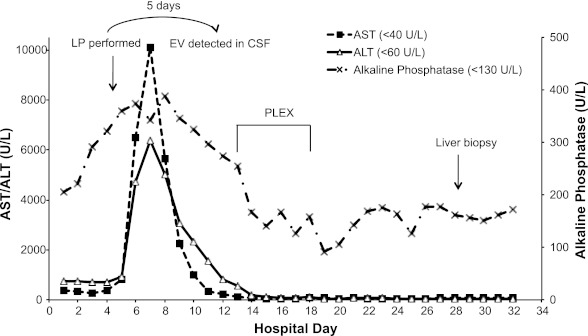
Fig 2.
Liver function tests during the patient's hospitalization. Levels of the patient's aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase are shown relative to the day of hospitalization. The values in parentheses represent the upper limit of normal for each analyte. Also indicated is the timing of lumbar puncture (LP), the second round of plasma exchange (PLEX), and the second liver biopsy, as well as the time at which enterovirus (EV) was detected in the patient's cerebrospinal fluid (CSF) by viral culture.
Given the patient's continued confusion, he also underwent a lumbar puncture, which was notable for borderline lymphocytic pleocytosis and slightly elevated protein levels (Table 1). The cerebrospinal fluid (CSF) Gram stain was negative, and bacterial cultures showed no growth. Further CSF testing was negative, including CMV shell vial culture, West Nile virus IgG and IgM antibodies, and PCR for herpes simplex viruses 1 and 2 and VZV. Traditional viral culture was also set up at 35°C, and 5 days later the rhesus monkey kidney (RMK) cell line demonstrated cytopathic effect (CPE) with rounded, refractile cells with an oil droplet appearance, whereas two human fibroblast cell lines, MRC-5 and foreskin fibroblasts, and the A549 human lung adenocarcinoma cell line showed no CPE. Indirect immunofluorescence antibody (IFA) testing using a pan-enterovirus antibody blend (EMD Millipore, Billerica, MA) identified the presence of enterovirus antigen in the cultured RMK cells. IFA typing reagents for a limited subset of enteroviruses (EMD Millipore, Billerica, MA), including antibodies against coxsackie A9 and coxsackie A24, as well as antibody blends to detect coxsackie B (1 to 6), poliovirus (1, 2, and 3), enteroviruses 70 and 71, and echovirus (4, 6, 9, 11, 30, and 34), were nonreactive. We therefore confirmed the presence of enterovirus in the CSF by performing pan-enterovirus real-time, reverse transcriptase PCR (rRT-PCR) (Xpert EV; Cepheid, Sunnyvale, CA) on the primary CSF specimen.
Table 1.
CSF profile
| CSF characteristica | Result | Reference |
|---|---|---|
| Chemistry profile | ||
| Glucose (mg/dl) | 71 | >40 |
| Protein (mg/dl) | 53 | <45 |
| Appearance | ||
| Color | Yellow | Colorless |
| Turbidity | Clear | Clear |
| Cell counts and differential | ||
| WBC (per μl) | 18 | 0–5 |
| RBC (per μl) | <1 | 0–5 |
| Neutrophils (%) | 0 | 0–6 |
| Lymphocytes (%) | 76 | 40–80 |
| Monocytoid cells (%) | 24 | 15–45 |
WBC, white blood cell count; RBC, red blood cell count.
The clinical team was unable to obtain pleconaril (1), a non-FDA-approved direct-acting antienterovirus agent that in the past has been used on a compassionate basis for severe enteroviral infections, particularly in neonates. The patient was managed supportively, and another trial of plasmapheresis was initiated. The following week, the patient defervesced, was extubated, and no longer required vasopressor support. His LFTs began to recover, although his mental status and renal function remained below baseline. Several days later, the patient became acutely hypoxic and hypotensive. According to the family's wishes, he was transitioned to comfort care and expired soon after.
This is a case of a 48-year-old male with a history of lymphoma and HSCT, who presented with fulminant hepatitis and mental status changes associated with an enterovirus infection that was nontypeable by IFA. To identify the enterovirus serotype, a reverse transcriptase-seminested PCR (RT-snPCR) assay was employed (2). This approach consists of an amplification step targeting consensus regions of the VP1 and VP3 genes, followed by a seminested PCR step targeting variable VP1 regions and then sequencing of the products to identify specific enteroviruses. PCR products were visualized by agarose gel electrophoresis and sequenced, and the sequences were queried against reference enterovirus strains in the NCBI database. Using this strategy, the virus isolated by viral culture was identified as echovirus 18. The presence of echovirus 18 was also demonstrated by RT-snPCR in plasma collected on the same day as the CSF and in a repeat liver biopsy specimen collected later in the hospitalization; there was insufficient tissue for RNA recovery from the initial paraffin-embedded liver biopsy specimen. These results confirm previous findings which show that commercial enteroviral IFA typing reagents are limited by the absence of serotype-specific antibodies for many serotypes, including echovirus 18 (3, 4). In contrast, molecular methods involving partial sequencing of VP1 have improved sensitivity compared to serologic methods and the capacity to identify all known enterovirus serotypes, while simultaneously reducing nonspecific results (2, 4).
To our knowledge, this is the first report of echovirus 18-associated severe systemic infection and acute liver failure. Echovirus 18 is a picornavirus from the genus Enterovirus, which includes the polioviruses, group A coxsackieviruses, group B coxsackieviruses, echoviruses, and the numbered enteroviruses. Enteroviruses have a single-stranded, positive-sense RNA genome and are transmitted through the fecal-oral route. Enterovirus infections typically peak in the summer and fall, consistent with the timing of disease in this case. Enteroviruses can cause a wide range of clinical syndromes, from a mild, nonspecific illness to severe disseminated disease (5). Nonpoliovirus enteroviruses have a known tropism for the central nervous system, which explains their propensity to cause meningitis and encephalitis (6). Indeed, the majority of severe echovirus 18 cases in the literature report central nervous system (CNS) disease, although sepsis has also been described in neonates (7, 8). Hepatitis caused by other enterovirus serotypes has been reported in neonates and is frequently associated with thrombocytopenia and coagulopathy, which were also present in our patient (5, 9, 10).
Humoral immunity is particularly important for the ability to clear enteroviruses, as agammaglobulinemic patients have persistent enteroviral infections, and neonates whose immune systems are immature are more susceptible to severe enteroviral disease (6). Additionally, several reports have demonstrated that patients with lymphoma who have undergone treatment with the anti-B lymphocyte agent rituximab are susceptible to severe enteroviral CNS infections (11, 12). In our case, the patient was severely lymphopenic throughout his hospitalization and had been treated with rituximab in the previous year, which likely resulted in a severely compromised ability to mount a neutralizing antibody response to echovirus 18.
Disseminated enterovirus infections in patients with hematolymphoid malignancies and bone marrow transplants have been reported for other echovirus serotypes, including 6, 11, and 13 (13–15). A number of antienterovirus interventions have been attempted in these and other immunocompromised patients, including plasmapheresis, intravenous immunoglobulin, and antiviral agents such as ribavirin, alpha interferon, and pleconaril (13–15). These approaches are nonspecific, their success in treating disseminated enterovirus infections is variable, and none is currently FDA approved. Our patient received two courses of plasmapheresis and appeared to show clinical improvement after each course, particularly in regard to his transaminitis.
This case underscores that enteroviruses should be considered in the differential diagnosis of hepatitis in adult transplant recipients, particularly those with impaired humoral immunity and mental status changes. Enterovirus testing in this patient population may be especially important during the annual peak of enteroviral infections, which typically occurs in summer and fall in temperate climates but may vary in a given community. Since enterovirus had not been considered part of this patient's differential diagnosis, the virus was not identified in his CSF until the traditional viral culture became positive, which delayed diagnosis by nearly 1 week. While traditional viral culture proved useful in this case, detection of enteroviruses by rRT-PCR is currently the laboratory standard for enterovirus diagnosis. Enterovirus rRT-PCR testing of the CSF on the day of collection or possibly earlier in plasma may have allowed more timely initiation of plasmapheresis or other potentially therapeutic interventions. Additionally, early detection of enterovirus may have limited unnecessary and costly diagnostic testing. However, given the apparent severity of this patient's underlying immune compromise, it is uncertain whether earlier diagnosis would have significantly altered the patient's clinical course. This case also illustrates how molecular methods can be employed to definitively identify viral pathogens to the genotype/serotype level, including enteroviruses that are nontypeable by IFA. While enterovirus typing was of limited clinical utility in this case, molecular typing is critical for enterovirus surveillance programs, may provide type-specific prognostic information, and could be important as type-specific antienteroviral agents are developed.
ACKNOWLEDGMENTS
We thank the staff of the Stanford Clinical Virology Laboratory for their hard work, technical expertise, and enthusiasm for both traditional and molecular virology.
Footnotes
Published ahead of print 21 November 2012
REFERENCES
- 1. Pevear DC, Tull TM, Seipel ME, Groarke JM. 1999. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 43:2109–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nix WA, Oberste MS, Pallansch MA. 2006. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 44:2698–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bendig J, Earl P. 2005. The Lim Benyesh-Melnick antiserum pools for serotyping human enterovirus cell culture isolates—still useful, but may fail to identify current strains of echovirus 18. J. Virol. Methods 127:96–99 [DOI] [PubMed] [Google Scholar]
- 4. She RC, Hymas WC, Taggart EW, Petti CA, Hillyard DR. 2010. Performance of enterovirus genotyping targeting the VP1 and VP2 regions on nontypeable isolates and patient specimens. J. Virol. Methods 165:46–50 [DOI] [PubMed] [Google Scholar]
- 5. Abzug MJ. 2004. Presentation, diagnosis, and management of enterovirus infections in neonates. Paediatr. Drugs 6:1–10 [DOI] [PubMed] [Google Scholar]
- 6. Rhoades RE, Tabor-Godwin JM, Tsueng G, Feuer R. 2011. Enterovirus infections of the central nervous system. Virology 411:288–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maus MV, Posencheg MA, Geddes K, Elkan M, Penaranda S, Oberste MS, Hodinka RL. 2008. Detection of echovirus 18 in human breast milk. J. Clin. Microbiol. 46:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah SS, Gallagher PG. 1997. Neonatal sepsis due to echovirus 18 infection. J. Perinat. Med. 25:381–384 [DOI] [PubMed] [Google Scholar]
- 9. Imai T, Itoh S, Okada H, Onishi S. 2002. Aplastic anemia following hepatitis associated with echovirus 3. Pediatr. Int. 44:522–524 [DOI] [PubMed] [Google Scholar]
- 10. Pino-Ramirez RM, Pertierra-Cortada A, Iriondo-Sanz M, Krauel-Vidal X, Munoz-Almagro C. 2008. Neonatal echovirus 30 infection associated with severe hepatitis in twin neonates. Pediatr. Infect. Dis. J. 27:88. [DOI] [PubMed] [Google Scholar]
- 11. Schilthuizen C, Berenschot HW, Levin MD. 2010. Enteroviral encephalitis in a patient with a marginal zone lymphomatreated with rituximab. Neth. J. Med. 68:221–223 [PubMed] [Google Scholar]
- 12. Servais S, Caers J, Warling O, Frusch N, Baron F, De Prijck B, Beguin Y. 2010. Enteroviral meningoencephalitis as complication of rituximab therapy in a patient treated for diffuse large B-cell lymphoma. Br. J. Haematol. 150:379–381 [DOI] [PubMed] [Google Scholar]
- 13. Biggs DD, Toorkey BC, Carrigan DR, Hanson GA, Ash RC. 1990. Disseminated echovirus infection complicating bone marrow transplantation. Am. J. Med. 88:421–425 [DOI] [PubMed] [Google Scholar]
- 14. Schwarer AP, Opat SS, Watson AM, Spelman D, Firkin F, Lee N. 1997. Disseminated echovirus infection after allogeneic bone marrow transplantation. Pathology 29:424–425 [DOI] [PubMed] [Google Scholar]
- 15. Tan PL, Verneris MR, Charnas LR, Reck SJ, van Burik JA, Blazar BR. 2005. Outcome of CNS and pulmonary enteroviral infections after hematopoietic cell transplantation. Pediatr. Blood Cancer 45:74–75 [DOI] [PubMed] [Google Scholar]