Abstract
Biofilm removal efficacy of vortexing alone was compared with the standard vortexing-sonication procedure. Among 135 removed prostheses, 35 were diagnosed with infection and 100 with aseptic failure. At a cutoff of ≥50 CFU/ml, sonication was more sensitive than vortexing (60% versus 40%, P = 0.151), while the specificity was 99% for both methods.
TEXT
Culture of periprosthetic tissue samples is the standard method for the microbiological diagnosis of prosthetic joint infections (PJI) but has limited sensitivity and specificity (1, 2). The vortexing-sonication procedure showed improved ability to diagnose implant-associated infection (3–8). However, the role of vortexing alone or its contributive role before sonication has not been determined. Therefore, we compared the utilities of vortexing alone and a vortexing-sonication procedure for the microbiological diagnosis of PJI and explored potential factors influencing their performance.
Patients from whom a joint prosthesis (or part of it) was removed for any reason between July 2010 and April 2012 were prospectively included in the participating institutions, Hospital del Mar (∼400 beds) and Hospital de l'Esperança (∼200 beds). The study protocol was approved by both institutional review boards. PJI was defined when at least one of the following criteria was present: (i) visible pus surrounding the prosthesis, (ii) presence of a sinus tract communicating with the prosthesis, (iii) acute inflammation in periprosthetic tissue, (iv) increased cell count in synovial fluid (i.e., leukocyte count > 1.7 g/liter or >65% neutrophils in knee prosthesis [9] or leukocyte count > 4.2 g/liter or >80% neutrophils in hip prosthesis [10], or (v) growth in synovial fluid or periprosthetic tissue culture (8, 11). Low-virulence microorganisms were considered pathogens when an additional PJI criterion (see above) was present. Acute PJI were defined as early postoperative infections (<3 months after implantation) and hematogenous infections, whereas chronic PJI were defined as low-grade infections manifesting 3 to 24 months after implantation (12). Aseptic failure (AF) was defined as prosthesis failure without any of the above criteria for PJI. Previous antimicrobial treatment was defined as the receiving of antibiotics ≥24 h before surgery.
Synovial fluid and periprosthetic tissue cultures were processed per routine practice. Blood agar plates (PoliVitex; bioMérieux, Marcy l'Etoile, France) were incubated at 37°C aerobically for 7 days with 5% CO2 and anaerobically for 14 days. In addition, 0.5 ml of synovial fluid and tissue homogenate was inoculated in thioglycolate broth and the residual amount in an anaerobic blood culture bottle (BacT/Alert; bioMérieux, Marcy l'Etoile, France). Distinct colony morphologies were identified using standard microbiological techniques.
Removed prosthesis components were placed in the operating room in solid polyethylene containers and sealed with an air-tight cover. Thioglycolate broth (50 to 200 ml, to cover the prosthesis) was added in the microbiology laboratory and subjected to vortexing for 1 min. Aliquots of 0.5 ml were plated onto agar plates and inoculated into thioglycolate broth, as described above (i.e., vortexing the fluid culture). Then, the container with the prosthesis was sonicated (model SM25E-MT; Branson Ultrasonics Corporation, Geneva, Switzerland) for 5 min at 40 ± 5 kHz and subjected to vortexing for 1 min. The resulting sonication fluid was plated onto agar plates and inoculated into thioglycolate broth as described above. For both the sonication and vortexing fluid procedures, a low cutoff (any growth on agar plate, i.e., ≥1 CFU/ml) and a high cutoff (≥50 CFU/ml) were evaluated. The latter cutoff was previously determined as the optimal tradeoff between sensitivity and specificity for sonication (8). The same cutoff values were used for both methods in order to compare their biofilm removal efficiencies. As a negative control, sterile prostheses were exposed for the duration of surgery in the operating room and processed as described for the removed prostheses. Comparisons between categorical variables were performed using χ2 or Fisher's exact tests, as appropriate. Comparisons of individual diagnostic tests were performed using the McNemar test. Differences were considered significant when the P value was <0.05.
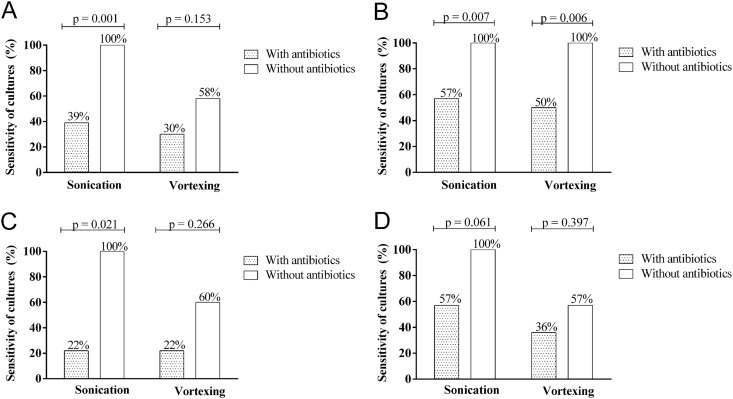
Table 1 shows characteristics of 135 included patients. Among 35 cases with PJI, 14 infections (40%) were acute and 21 (60%) chronic. Table 2 summarizes the diagnostic yields of the vortexing and sonication fluid culture procedures. By using a cutoff of ≥1 CFU/ml, the sensitivities of the sonication and vortexing fluid procedures were comparable (71% and 69%, respectively), whereas at a cutoff of ≥50 CFU/ml, the sensitivity of sonication fluid was superior to that of vortexing fluid (60% and 40%, respectively). The specificities of sonication fluid and vortexing fluid procedures were 92% to 93% at cutoff ≥ 1 CFU/ml and 99% at a cutoff of ≥50 CFU/ml. In patients previously receiving antibiotics (using a cutoff of ≥50 CFU/ml), the sensitivities of sonication and vortexing fluid culture were considerably reduced to 39% and 30%, respectively (Fig. 1A). However, using a cutoff of ≥1 CFU/ml fluid, the sensitivities of sonication and vortexing fluid culture decreased to 57% and 50%, respectively (Fig. 1B). Therefore, in patients who had previously received antibiotics, the cutoff of ≥1 CFU/ml should be used. The sensitivity of sonication fluid cultures was higher in chronic PJI (58%) than in acute PJI (33%), although the difference was not significant (P = 0.151). Sonication fluid culture results were positive in all patients without previous antibiotic therapy, whereas vortexing fluid culture results were positive in only 60% (in acute PJI; Fig. 1C) and 57% (in chronic PJI; Fig. 1D). Previous antimicrobial treatment reduced the culture sensitivities of sonication fluid and vortexing fluid more in acute PJI (22% and 22%, respectively) than in chronic PJI (57% and 36%, respectively).
Table 1.
Characteristics of 135 study patients with aseptic failure and prosthetic joint infectiona
| Parameter | Aseptic failure (n = 100) | Prosthetic joint infection (n = 35) | P value |
|---|---|---|---|
| Median patient age in yr (range) | 73 (27–89) | 73 (48–87) | 0.878 |
| Male patients | 36 (36) | 15 (43) | 0.545 |
| Type of joint prosthesis | |||
| Knee (n = 73) | 60 (60) | 25 (71) | 0.310 |
| Hip (n = 31) | 36 (36) | 5 (14) | 0.019 |
| Shoulder (n = 4) | 2 (2) | 3 (9) | 0.110 |
| Elbow (n = 4) | 2 (2) | 2 (6) | 0.276 |
| Type of revision surgery | |||
| Debridement and prosthesis retentionb | 0 | 14 (40) | <0.001 |
| One-stage exchange | 98 (98) | 1 (3) | <0.001 |
| Two-stage exchangec | 2 (2) | 20 (57) | <0.001 |
| Clinical signs of infection | |||
| Sinus tract | 0 | 7 (20) | <0.001 |
| Visible pus surrounding the prosthesis | 0 | 25 (71) | <0.001 |
| Mean synovial fluid cell countd | |||
| Leukocyte count in g/liter (range) | 0.3 (0.06–0.8) | 45 (1.4–76) | 0.005 |
| % neutrophils (range) | 19 (2–44) | 90 (54–98) | 0.017 |
| Received previous antibiotics | 0 | 23 (66) | <0.001 |
Values represent numbers (%) where not indicated otherwise. ND, not determined. Boldface P values represent significant values.
The polyethylene inlay and removable metal parts (i.e., femur head), where applicable, were investigated by a vortexing or sonication procedure.
The mean interval between explantation and reimplantation of the prosthesis was 118 days (range, 19 to 357 days).
Synovial fluid was available for 15 patients (11%).
Table 2.
Diagnostic yield of vortexing and sonication fluid culture, depending on two cutoff values (≥1 CFU/ml and ≥50 CFU/ml fluid)a
| Cutoff value | No. (%) of PJI (n = 35) | No. (%) of AF (n = 100) | % sensitivity (95% CI) | % specificity (95% CI) | % PPV (95% CI) | % NPV (95% CI) | % PLR (95% CI) | % NLR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Cutoff ≥ 1 CFU/ml | ||||||||
| Sonication fluid | 25 (71)c | 7 (7)d | 71 (54–85) | 93 (86–97) | 78 (60–91) | 90 (83–95) | 10 (5–21) | 0 (0–1) |
| Vortexing fluid | 24 (69) | 8 (8)b | 69 (51–83) | 92 (85–96) | 75 (57–89) | 89 (82–95) | 9 (4–17) | 0 (0–1) |
| Cutoff ≥50 CFU/ml | ||||||||
| Sonication fluid | 21 (60) | 1 (1)e | 60 (42–76) | 99 (95–100) | 95 (77–99) | 88 (80–93) | 60 (8–430) | 0 (0–1) |
| Vortexing fluid | 14 (40) | 1 (1)e | 40 (24–58) | 99 (95–100) | 93 (68–99) | 83 (75–89) | 40 (5–293) | 1 (0–1) |
PJI, prosthetic joint infection; AF, aseptic failure; 95% CI, 95% confidence interval; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Propionibacterium acnes (n = 5), coagulase-negative staphylococci (n = 2), Corynebacterium spp. (n = 1).
In 4 of 25 (16%) cases, sonication cultures grew <50 CFU/ml. In all 4 cases, patients had previously received antimicrobial treatment (3 cases were caused by coagulase-negative staphylococci and 1 by Pasteurella multocida).
P. acnes (n = 5), coagulase-negative staphylococci (n = 2).
Corynebacterium spp. (n = 1).
Fig 1.
Effect of previous antimicrobial therapy on sensitivity of sonication and vortexing fluid cultures, using cutoffs of ≥50 CFU/ml (A) and ≥1 CFU/ml (B), or stratified according to patients with acute PJI (C) and chronic PJI (D) using a cutoff of ≥50 CFU/ml.
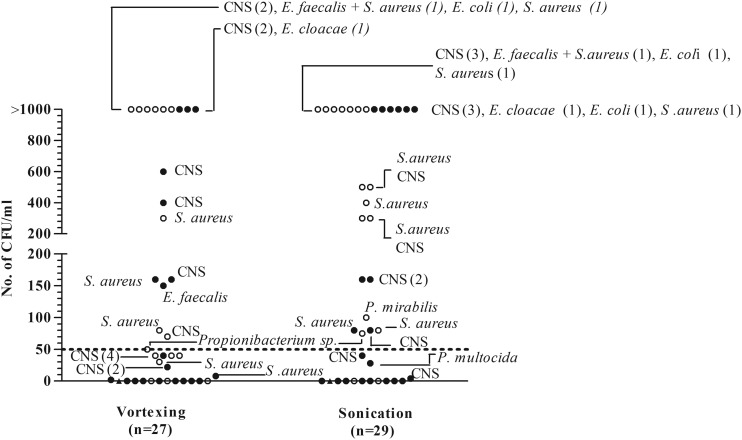
Figure 2 shows the types and numbers of microorganisms isolated from vortexing and sonication fluid cultures. Sonication allowed isolation of three additional pathogens in a high quantity (>1,000 CFU/ml) from patients with PJI who had previously received antibiotics. All cultures without growth after sonication and all four cultures in which microorganisms grew in low quantity (<50 CFU/ml; 3 coagulase-negative staphylococcus [CNS] and 1 Pasteurella multocida isolates) were sampled from patients previously receiving antibiotics. The concordance of sonication and vortexing fluid cultures is summarized in Fig. S1 in the supplemental material. Among 100 AF cases, 1 had positive sonication fluid and vortexing fluid cultures with Corynebacterium sp. This organism may have represented a contamination during surgery or sonication or may have represented asymptomatic colonization of the prosthesis (i.e., a “silent” biofilm), as was previously reported in cardiac electrophysiological devices (7) and breast implants (6).
Fig 2.
Microorganisms detected by vortexing fluid cultures (n = 27) and sonication fluid cultures (n = 29). The dashed line indicates a cutoff value of ≥50 CFU/ml of the same microorganism. Solid circles denote microorganisms isolated from patients who had received previous antimicrobial treatment; open circles denote microorganisms isolated from patients who had not received previous antimicrobial treatment. Triangles denote microorganisms isolated from thioglycolate broth only. CNS denotes coagulase-negative staphylococci. Numbers in parentheses represent numbers of patients.
The vortexing-sonication procedure demonstrated higher biofilm removal efficiency than vortexing alone (sensitivity of 60% versus 40% using a cutoff of ≥50 CFU/ml, with the same specificity of 99%). The sensitivity of sonication fluid culture in our study was lower (60%) than in published results (4, 8, 11), which may reflect the high proportion of patients previously receiving antibiotics (66%) and the large proportion of sonicated mobile parts only (40%), which have a smaller surface than total prostheses. Previous antibiotic therapy reduced the culture sensitivity of both methods (using a cutoff of ≥50 CFU/ml). However, the sensitivity of sonication and vortexing fluid cultures was higher with a cutoff of ≥1 CFU/ml. Therefore, any growth (≥1 CFU/ml) should be considered to represent a positive result in patients who previously received antimicrobials.
Interestingly, vortexing alone demonstrated acceptable sensitivity and specificity, especially in acute PJI, and may be used for the diagnosis of PJI in laboratories where sonication is not available. In addition, vortexing fluid represents a single clinical sample, with such analyses reaching sensitivity comparable to that of analyses using multiple periprosthetic tissue cultures (∼70%). Furthermore, sonication may kill bacteria, especially Gram-negative bacilli and anaerobes, whereas vortexing has not been shown to be harmful to bacteria. In addition, vortexing is an easy and simple procedure which can be performed in most laboratories without additional costs. Despite vortexing having originally been introduced as a preparatory step before sonication to generate microbubbles, which increase the cavitation effect (8), it seems to be a powerful removal method. High shear forces generated on the interface between the prosthesis and the vortexing fluid may explain the biofilm removal effect. These shear forces could be increased by a pulsatile change of the fluid movement direction (as used in Stomacher analysis). The removal efficiency may be also increased by addition of detergents (e.g., polysorbate 80) or anticoagulants (e.g., EDTA) to the vortexing fluid (13). Another possibility is the addition of beads to vortexing fluid, as is used for processing tissue samples in a bead mill (14). However, the contamination risk, increased workload, and costs need to be considered. In summary, using a cutoff of ≥50 CFU/ml, sonication showed higher sensitivity than vortexing (60% versus 40%), while the specificities remained equal (99%). Using the lower cutoff value (≥1 CFU/ml), the sensitivities of vortexing and sonication fluid were similar (69% to 71%); however, the specificities decreased to 92% to 93%.
Supplementary Material
Footnotes
Published ahead of print 7 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02482-12.
REFERENCES
- 1. Spangehl MJ, Masri BA, O'Connell JX, Duncan CP. 1999. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J. Bone Joint Surg. Am. 81:672–683 [DOI] [PubMed] [Google Scholar]
- 2. Tsukayama DT, Goldberg VM, Kyle R. 2003. Diagnosis and management of infection after total knee arthroplasty. J. Bone Joint Surg. Am. 85(A Suppl 1):S75–S80 [DOI] [PubMed] [Google Scholar]
- 3. Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R, Presterl E. 2011. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J. Orthop. Res. 29:617–622 [DOI] [PubMed] [Google Scholar]
- 4. Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. 2009. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J. Clin. Microbiol. 47:1878–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Portillo ME, Salvado M, Sorli L, Alier A, Martinez S, Trampuz A, Gomez J, Puig L, Horcajada JP. 2012. Multiplex PCR of sonication fluid accurately differentiates between prosthetic joint infection and aseptic failure. J. Infect. 65:541–548 [DOI] [PubMed] [Google Scholar]
- 6. Rieger UM, Pierer G, Luscher NJ, Trampuz A. 2009. Sonication of removed breast implants for improved detection of subclinical infection. Aesthetic Plast. Surg. 33:404–408 [DOI] [PubMed] [Google Scholar]
- 7. Rohacek M, Weisser M, Kobza R, Schoenenberger AW, Pfyffer GE, Frei R, Erne P, Trampuz A. 2010. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation 121:1691–1697 [DOI] [PubMed] [Google Scholar]
- 8. Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N. Engl. J. Med. 357:654–663 [DOI] [PubMed] [Google Scholar]
- 9. Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. 2004. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am. J. Med. 117:556–562 [DOI] [PubMed] [Google Scholar]
- 10. Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. 2008. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J. Bone Joint Surg. Am. 90:1869–1875 [DOI] [PubMed] [Google Scholar]
- 11. Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. 2010. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J. Clin. Microbiol. 48:1208–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645–1654 [DOI] [PubMed] [Google Scholar]
- 13. Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. 2012. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int. J. Artif. Organs 35:923–934 [DOI] [PubMed] [Google Scholar]
- 14. Roux AL, Sivadon-Tardy V, Bauer T, Lortat-Jacob A, Herrmann JL, Gaillard JL, Rottman M. 2011. Diagnosis of prosthetic joint infection by beadmill processing of a periprosthetic specimen. Clin. Microbiol. Infect. 17:447–450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.