Abstract
The filamentous basidiomycete Ceriporia lacerata, an agent of white rot on wood, has never been reported in human disease and its clinical significance is not yet known. We describe 4 patients with respiratory diseases where C. lacerata was implicated in a wide spectrum of clinical manifestations ranging from saprobic colonization to fungal pneumonia. The isolates did not show the morphological characteristics that facilitate recognition of filamentous basidiomycetes, such as the presence of clamp connections, spicules along hyphae, or fruiting bodies. The identity of the mold was confirmed by sequencing the internal transcribed spacer 1 and 4 (ITS-1 and ITS-4) and D1/D2 regions of the rRNA gene. All of the isolates exhibited the lowest MICs of posaconazole and isavuconazole (MIC range, 0.06 to 0.125 μg/ml), followed by itraconazole (MIC range, 0.06 to 0.5 μg/ml), voriconazole (MIC range, 0.125 to 0.5 μg/ml), and amphotericin B (MIC range, 0.25 to 1 μg/ml). The infections reported here occurred in patients with preexisting lung damage induced by tuberculosis or chronic obstructive pulmonary disease. Chronic, sometimes fatal infections by the ascomycete Aspergillus fumigatus and the basidiomycete Schizophyllum commune are well established in the presence of an anatomical pulmonary defect or in the background of immunodeficiency. It is postulated that C. lacerata, a novel opportunist basidiomycete, may be involved in similar pathological processes.
INTRODUCTION
Recent developments in molecular identification of fungi have provided an opportunity to evaluate the clinical significance of molds that were previously discarded as unidentifiable contaminants because of a lack of phenotypic characters and an absence of sporulation. Among these are several filamentous basidiomycetes, which produce snow-white, cottony, rapidly growing colonies, in culture consisting entirely of hyphae. Occasionally, special features such as clamp connections, hyphal pegs, chlamydospores, cystidia, or conidia may be present, but morphological identification alone fails to identify with certainty as to which (out of the nearly 20,000 described species prevalent in the environment) species it could be. Although the number of these species reported from clinical and environmental sources is growing rapidly, molecular data are available for only a fraction of these species. Sequencing of the ribosomal DNA (rDNA) internal transcribed spacer (ITS) region may offer a match in GenBank; therefore, an increasing number of taxa are being introduced in medical literature (1–3). Classically recognized basidiomycetes, such as Schizophyllum commune, Coprinopsis spp. (anamorph, Hormographiella spp.), and Phanerochaete chrysosporium (anamorph, Sporotrichum pruinosum), possess some morphological characteristics specific for their identification (4–10). Lately, lesser known members from this class of molds, such as Bjerkandera adusta (11), Cyclomyces tabacinus (12), Irpex lacteus (13), Inonotus (Phellinus) tropicalis (14, 15), Phellinus undulatus (16), Oxyporus corticola (17), Volvariella volvacea (18), and Perenniporia spp. (4), have been incriminated as agents of human disease. In the present communication, another filamentous basidiomycete, Ceriporia lacerata, isolated from 4 human cases, is presented with a discussion on its possible clinical significance.
CASE REPORTS
Case 1.
A 55-year-old male farmer from the suburbs of Delhi, India, on antituberculous therapy (ATT) for 1 week, presented to the hospital in June 2011 with complaints of loss of appetite for 2 years, left-sided nonanginal, nonpleuritic chest pain for 1 year, and productive cough associated with hemoptysis for 6 months. The patient never smoked and had no history of diabetes mellitus or of intravenous (i.v.) drug abuse. However, he mentioned to have received treatment for pulmonary tuberculosis twice in the past. The first episode, occurring 6 months previously for sputum smear-negative pulmonary tuberculosis, was treated with oral drugs, which he discontinued on his own after 2 weeks; while in the present episode, oral isoniazid, rifampin, pyrazinamide, and ethambutol were started 1 week prior to presentation of sputum smear-negative pulmonary tuberculosis. His hemogram, liver, and kidney functions were within normal limits. However, the radiograph of his chest showed the presence of cystic shadows and an inhomogeneous opacity in the paracardiac area of the left lower zone. A contrast-enhanced computerized tomogram (CECT) of his thorax done a week later showed centrilobular infiltrates in the superior segment of the left lower lobe and cylindrical bronchiectasis in the inferior segment of the lingula. A diagnosis of left lower-lobe consolidation with lingular segment bronchiectasis as a sequel of the previous pulmonary tuberculosis was made. Pulmonary tuberculosis was considered the primary diagnosis for his present symptoms, given his history of tuberculosis and the high endemicity of the disease in this part of the world. However, he did not show any induration to the Mantoux test done with 1 tuberculin unit of purified-protein derivative (PPD) of Mycobacterium spp. (Span Diagnostics, Surat, Gujarat, India) and sputum samples tested negative for the presence of acid-fast bacilli (AFB) or aerobic pathogens. Direct microscopy of KOH wet mounts of three consecutive sputum specimens and a bronchoalveolar lavage (BAL) specimen revealed hyaline, septate hyphae and isolation of multiple colonies of white mold in culture on Sabouraud's glucose agar (SGA) at 28°C and 37°C after 7 days of incubation. The white mold was later identified as Ceriporia lacerata, and the isolate was assigned the accession no. VPCI 1873/11 (CBS 133710) for morphological characterization, molecular identification, and antifungal susceptibility testing. The diagnosis of fungal pneumonia due to C. lacerata was considered. The patient discontinued follow-up and could not be further evaluated.
Case 2.
A 60-year-old male farmer from Delhi, India, presented to our institute in August 2011 with complaints of productive cough associated with occasional hemoptysis for 4 years, progressive, exertional dyspnea for 6 months, and low-grade fever for 1 month. The patient consumed a nonvegetarian diet and had no history of high-risk sexual behavior, i.v. drug abuse, or diabetes mellitus. Nevertheless, the patient was a smoker who had been smoking about 10 beedies (Nicotiana tabacum coiled in a leaf of Diospyros melanoxylon) per day for the past 35 to 40 years. The patient was emaciated and had poor general health. The hemogram, liver, and kidney function tests were within normal limits. The X ray of the patient's chest showed consolidation in the right lower zone with ipsilateral pleural effusion. A CECT of his thorax showed consolidation of the posterior basal segment of the right lower lobe with ipsilateral pleural effusion. A diagnosis of chronic obstructive pulmonary disease (COPD) with right lower-lobe consolidation with ipsilateral pleural effusion, pending etiologic confirmation, was made. The patient was initiated on empirical antibiotic therapy comprising a combination of amoxicillin-clavulanic acid (625 mg three times a day [t.i.d.]) and doxycycline (100 mg twice a day [b.i.d.]) for 2 weeks. However, he failed to respond to medical management and was further investigated as described hereunder.
Three weeks later, the patient underwent a diagnostic flexible, fiberoptic bronchoscopy. The endobronchial biopsy was suggestive of chronic bronchitis on histopathology. The bronchial aspirate was negative for the presence of AFB, aerobic pathogens, or malignant cells. Direct microscopy of KOH wet mounts of the BAL specimen revealed hyaline septate hyphae. Cultures yielded multiple, white, cottony colonies of a mold on SGA plates incubated for 7 days at 28°C and 37°C, which was subsequently identified as C. lacerata (VPCI 1921/11; CBS 133711). Three consecutive sputum specimens from the patient also showed the presence of hyaline septate hyphae and growth of the same fungus. He was diagnosed with a case of fungal pneumonia with pleural effusion. Therefore, the patient was administered itraconazole (200 mg b.i.d.) but he suffered from a bout of massive hemoptysis barely 2 weeks after the start of medication and could not be resuscitated. An autopsy was not permitted by his relatives.
Case 3.
A 44-year-old female schoolteacher from Bihar, India, a nonsmoker, presented to hospital in July 2011. She reported persistent productive cough, on-and-off hemoptysis for 8 years, progressive breathlessness for 4 years, a loss of weight and appetite for 2 months, and right-sided chest pain and high-grade fever for 15 days. She also had a history of Raynaud's phenomenon and had received ATT many times in the past 7 years. On examination, the patient was found to have pallor and her blood investigations showed hypochromic microcytic anemia with neutrophilia and hypoproteinaemia. A radiograph of her chest showed cavitating consolidation in the right middle and lower lobes and infiltrates in the left upper and lower lobes along with pleural effusion on the right side. A CECT of thorax done 2 days later revealed pleura-based cavitating lesions in the same distribution as seen on the X ray. Her sputum was negative for aerobic pathogens and AFB for Mycobacterium tuberculosis. This was followed by an ultrasound of the chest, showing loculated pleural fluid on the right side, which upon thoracentesis was found to be purulent. This fluid was of an exudative nature, with low glucose content (7 mg/dl), and cultures of pleural fluid were negative for aerobic, acid-fast, or fungal organisms. The adenosine deaminase (ADA) level of pleural fluid was 154 U/liter (against an upper limit of normal of 24 U/liter); thus, a diagnosis of tuberculous empyema was made. Her blood culture was negative. However, repeated sputum cultures grew Nocardia species. The patient underwent a computed tomography (CT)-guided fine-needle aspiration of the pulmonary lesions, which on histologic examination showed Nocardia, liquefactive necrosis, and an ill-formed granulomata. Thus, a diagnosis of Nocardia pneumonia was made and the patient was started on i.v. therapy of imipenem (500 mg t.i.d.) along with cotrimoxazole (960 mg t.i.d.). She was maintained on cotrimoxazole for the next month but her symptoms persisted. Although her radiograph showed resolution of the consolidation, the pleural effusion increased in amount.
The patient was investigated for other coexisting etiologic agents, and three consecutive sputum specimens were sent for mycological investigation. A KOH wet mount of sputum specimens showed a presence of hyaline, septate, and branched hyphae. Multiple colonies of a white mold were isolated in cultures of sputum samples on SGA at 28°C and 37°C, which were later identified as C. lacerata (VPCI 1603/11; CBS 133712). By this time, a confirmed growth of M. tuberculosis from her sputum sample was received. Also, a repeat aspiration of the pleural fluid was suggestive of tubercular effusion; thus, the patient's diagnosis was updated to Nocardia pneumonia with pulmonary tuberculosis and tubercular pleural effusion and she was started on isoniazid, rifampin, pyrazinamide, and ethambutol in undivided daily doses of 300 mg, 450 mg, 1,250 mg, and 800 mg, respectively, with daily intramuscular injections of 750 mg streptomycin in addition to the cotrimoxazole. A 3-month follow-up of the patient revealed no growth of Nocardia and M. tuberculosis in culture, whereupon streptomycin and pyrazinamide were discontinued. However, fungal culture of the sputum sample still yielded growth of C. lacerata. Finally, ATT was stopped in June 2012 after an ultrasound demonstrated an absence of pleural fluid and a repeat sputum examination was negative for AFB. Currently, the patient is receiving cotrimoxazole only and continues to be on an improving trend. With regard to the isolation of C. lacerata, since the patient showed improvement in her symptoms even without antifungal therapy, it was concluded that this was a case of an asymptomatic colonization of the respiratory tract by this basidiomycete mold and no further therapeutic intervention was undertaken.
Case 4.
A middle-aged male government employee had been under evaluation since July 2012 for nonresolving cavitating pneumonia at a tertiary care tuberculosis and pulmonary diseases institute in New Delhi, India. The patient's sputum had been repeatedly negative for acid-fast bacilli and aerobic pathogens. He had undergone many bronchoscopies, but no causal organism could be isolated from bronchial secretions. Finally, the BAL specimen and three consecutive sputum samples were investigated to exclude the presence of a fungal pathogen. Surprisingly, a white, cottony mold was cultured on SGA at 28°C and 37°C after 9 days of incubation of the patient's BAL specimen and sputum, which was later confirmed to be C. lacerata (VPCI 2549/11; CBS 133713). He was treated with itraconazole (200 mg t.i.d.), and a follow-up after 4 weeks showed reduction in cough and other symptomatic improvement. Further follow-up of the patient and response to treatment was not known, as the patient could not be tracked. This case might be regarded as a fungal pneumonia since the etiological diagnosis was elusive even after repeated bronchoscopies; the only potential pathogen that could be isolated was the above-stated mold.
MATERIALS AND METHODS
Mycological investigations.
Three consecutive freshly expectorated sputum and BAL specimens of the above-mentioned clinical cases were processed for direct microscopy and culture. The material was cultured on Sabouraud's glucose agar plates supplemented with gentamicin (25 μg/ml). One set of inoculated plates was incubated at 28°C and a second set at 37°C for up to 4 weeks. Colonies of white mold growing on SGA were purified and subcultured on potato dextrose agar (PDA) plates and incubated at 28°C for 4 weeks.
Induction of sporulation on decayed wood.
A thick mycelial suspension of all C. lacerata isolates was prepared in 0.9% saline and inoculated on autoclaved bark pieces of Syzigium cumini (Indian blackberry, Jamun tree, or Jambu), 3 to 4 cm by 0.5 to 1 cm, and stems of tomato (Solanum lycopersicum), 3 to 4 cm long, and placed on PDA medium in 90-mm culture plates and in 150-ml conical flasks. The inoculated petri dishes and flasks were incubated at 28°C under light for 4 weeks.
Molecular identification.
The identification of isolates was done by sequencing of the ITS rDNA and D1/D2 regions of the large ribosomal subunit (LSU) as described previously (4, 19). Briefly, genomic DNA was twice extracted with 700 μl Tris saturated phenol chloroform isoamyl (25:24:1), followed by a chloroform isoamyl (24:1) extraction and ethanol precipitation. The DNA pellet was dried and resuspended in 75 μl of sterile nuclease-free water and treated with 6 μl (10 mg/ml) of RNase (Sigma-Aldrich, Co., St. Louis, MO) for 1 h at 37°C. DNA was amplified using the ITS-1 (5′-TCCGTAGGTGAACCTTGCGG-3′) and ITS-4 (5′-TCCTCCGCTTATTGATATGC-3′) primers, which amplify the ITS region of the ribosomal subunit, and the NL-1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL-4 (5′-GGTCCGTGTTTCAAGACGG-3′) primers, which amplify the ∼600-bp D1/D2 LSU region (20, 21). Amplified DNA was sequenced in both strands on an ABI 3130XL genetic analyzer (Applied Biosystems, Foster City, CA) using the BigDye terminator kit (v3.1, RR-100; Applied Biosystems, Foster City, CA). Sequences were aligned using the Sequencing Analysis 5.3.1 software (Applied Biosystems, Foster City, CA). GenBank basic local alignment search tool (BLAST) searches (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi) were performed for species identification, which was defined as ≥99% homology with the GenBank database reference sequences. Query coverage of ≥95% was considered significant.
AFST.
Antifungal susceptibility testing (AFST) of the 4 isolates was performed by the CLSI broth microdilution method (22). The antifungals tested were amphotericin B (Sigma, St. Louis, MO), fluconazole (Pfizer, Groton, CT), itraconazole (Lee Pharma, Hyderabad, India), voriconazole (Pfizer), posaconazole (Schering-Plough [now Astellas], Kenilworth, NJ), isavuconazole (Basilea Pharmaceutica, Basel, Switzerland), flucytosine (Sigma), caspofungin (Merck, Whitehouse Station, NJ), micafungin (Astellas, Toyama, Japan), and anidulafungin (Pfizer). For the broth microdilution test, RPMI 1640 medium with glutamine without bicarbonate (Sigma) buffered to pH 7 with 0.165 mol/liter 3-N-morpholinepropanesulfonic acid (Sigma) was used. Isolates were grown on PDA for 5 days and incubated at 28°C and then 37°C for the next 5 days for sporulation. Final inoculum was adjusted to a density of 1.0 × 104 to 5.0 × 104 hyphal fragments/spores per ml by adjusting an optical density of 0.13 to 0.18 at 530 nm using a spectrophotometer. Drug-free and mold-free controls were included, and microtiter plates were incubated at 35°C for 72 to 96 h. CLSI recommended quality control strains Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019, and reference strains Aspergillus fumigatus ATCC 204305 and Aspergillus flavus ATCC 204304 were included. The MIC endpoints were read visually, which, for azoles and amphotericin B, were defined as the lowest concentration at which there was 100% inhibition of growth compared with the drug-free control wells. For echinocandins, minimal effective concentrations (MEC) were defined as the lowest concentration of drug that led to the growth of small, rounded, and compact hyphal forms.
Nucleotide sequence accession numbers.
Newly determined nucleotide sequences have been deposited in GenBank under accession numbers JX984623 to JX984630.
RESULTS
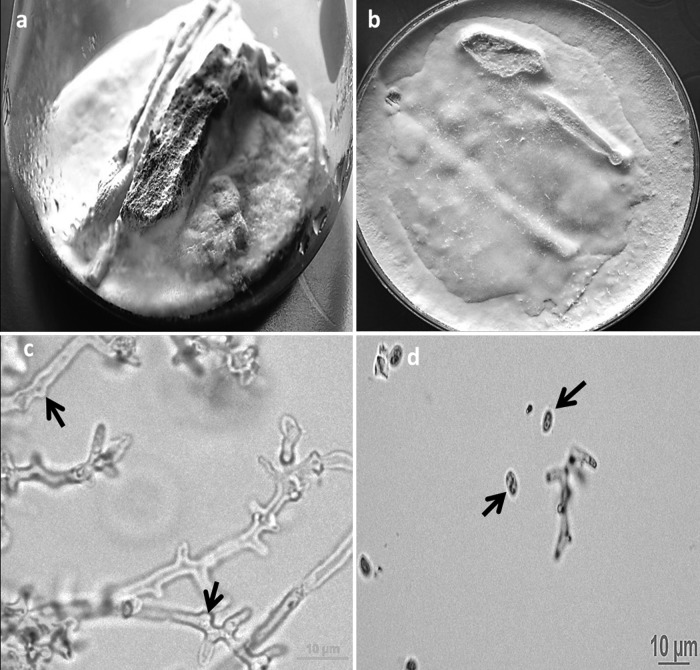
Multiple, white, cottony colonies of a mold grew after 7 to 9 days on SGA plates inoculated with sputum and BAL specimens and incubated at 28°C and 37°C. Microscopically, slide cultures on PDA after 4 weeks of incubation at 28°C revealed hyaline, septate hyphae. No clamp connections or hyphal pegs were seen up to 4 weeks of incubation. All of the isolates showed dense white, cottony growth on wood pieces of S. cumini (Fig. 1a) and twigs of S. lycopersicum (Fig. 1b). The C. lacerata isolate obtained from case 2 (VPCI 1921/11; CBS 133711) showed sporulation on bark pieces of S. cumini, and lactophenol cotton blue mount revealed spicules along hyphae (Fig. 1c) and ellipsoid basidiospores of 3.5 to 4.5 μm by 2.4 to 2.9 μm. (Fig. 1d).
Fig 1.
White-rotting mold growth of Ceriporia lacerata (VPCI 1921/11; CBS 133711) on a Syzigium cumini bark piece (size, 3 to 4 cm by 0.5 to 1 cm) (a) and tomato (Solanum lycopersicum) twigs (3 to 4 cm long) (b), inoculated on potato dextrose agar (PDA) incubated at 28°C under light for 4 weeks. (c) Lactophenol cotton blue mount of the mold (C. lacerata [VPCI 1921/11; CBS 133711]) growing on wooden bark of S. cumini (inoculated on PDA) showing spicules (arrow) along hyphae after 3 weeks of incubation at a magnification of ×400. (d) Lactophenol cotton blue mount of the same mold growing on wooden bark inoculated on PDA showing ellipsoid basidiospores of 3.5 to 4.5 μm by 2.4 to 2.9 μm as seen after 4 weeks at a magnification of ×400.
ITS sequences of the four C. lacerata isolates (GenBank accession no. JX984623 to JX984626) showed 99% homology (query coverage ranging from 95 to 97%) to eight C. lacerata isolates in GenBank (accession no. HQ331080.1, HQ331032.1, HQ331074.1, HQ331078.1, FJ462746.1, HQ331033.1, JN628149.1, and JN182908.1). Also, LSU sequences of our four isolates (GenBank accession no. JX984627 to JX984630) showed 99% identity (query coverage of 95 to 97%) with three C. lacerata isolates in GenBank (accession no. AB566280.1, JF416691.1, and HM595618.1). The isolates VPCI 1873/11 (CBS 133710), VPCI 1921/11 (CBS 133711), VPCI 1603/11 (CBS 133712), and VPCI 2549/11 (CBS 133713) were deposited at the CBS Fungal Biodiversity Centre, the Netherlands. The isolates had the lowest MICs of posaconazole and isavuconazole (MIC range, 0.06 to 0.125 μg/ml), followed by itraconazole (MIC range, 0.06 to 0.5 μg/ml), voriconazole (MIC range, 0.125 to 0.5 μg/ml), and amphotericin B (MIC range, 0.25 to 1 μg/ml). All three echinocandins showed high MECs of 8 μg/ml. For fluconazole and flucytosine, the MICs ranged from 4 to 32 μg/ml and 8 to 16 μg/ml, respectively.
DISCUSSION
The filamentous basidiomycetes encountered in clinical settings are conventionally classified into Agaricales and Stereales, which in their natural habitat display macroscopically visible fruiting bodies recognizable as mushrooms and shelf fungi, respectively. Most species described from clinical samples are white rotters on dead wood, assimilating polyaromatic ligneous hydrocarbons in the substrate, which leaves an overabundance of white, cellulosic material behind. This ecology is noted for the shelf fungi C. tabacinus (12), I. lacteus (13), I. tropicalis (14, 15), P. undulatus (16), O. corticola (17), P. chrysosporium (7), S. commune, and B. adusta (11), as well as for C. lacerata described in the present communication. Clinical cases of infection by these shelf fungi almost exclusively concern pulmonary colonization, eventually leading to allergic responses but with limited invasion. Fatal systemic infections due to S. commune have been reported (19, 23, 24), but the majority of cases of infection by this fungus are saprobic colonization and allergic sinusoidal or bronchopulmonary disease. Similarly, the recently reported basidiomycete mold Perenniporia sp. has been shown to be involved in the formation of a secondary pulmonary fungal ball but not in invasive disease (4). In contrast, recurrent invasive infections, in addition to pulmonary colonization, are observed with the members of Agaricales, Hormographiella aspergillata and Hormographiella verticillata (5, 6, 8–10, 25–28), and in V. volvacea (18). These mushrooms have a ruderal strategy (29), colonizing compost, dung, self-heated wood chips, and similar substrates. The possibility is not excluded that the differences in ecological background of the clinically relevant basidiomycete species determine their invasive abilities in the human patient.
The four cases presented above, though from geographical regions spaced 50 to 1,500 km from each other, have a number of features in common. All patients resided in rural/suburban areas, with farming being the predominant occupation; two were farmers themselves. Three out of four patients suffered from underlying lung disorders, with the possible exception of case 4, where no clinical details were available. Cases 1 and 3 previously had pulmonary tuberculosis, and case 2 had the background of COPD. With regard to the commonly reported pulmonary ascomycete Aspergillus fumigatus, it is known that fungal colonies may reside in structural lung defects and emphysemas and provoke invasive infection with the onset of immunodeficiency or other debilitating disease (30, 31). A similar notion can be developed with S. commune (19, 32–34). Being abundant in the environment, basidiospores of mushrooms and shelf fungi are easily inhaled and, with their small size, deposited in pulmonary alveoli. Local or systemic impaired function of alveolar macrophages may allow settlement of a fungal thallus. Therefore, the shelf fungi are not to be categorized as primary pathogens. S. commune, for example, is a psychrophilic fungus of which fruiting body formation is stimulated by low winter temperatures. Possibly the shelf fungi, naturally being involved in lignin degradation, have only limited virulence, whereas with the ruderal mushrooms Coprinus cinereus and V. volvacea, a higher degree of invasive potential may be expected. This is evident from a high case fatality (73%) in invasive infections due to C. cinereus reported in 8 of the 11 patients of hematological malignancies described in literature so far (10, 25, 27, 28, 35, 36, 37). Also, a solitary fatal case of V. volvacea in a patient following double umbilical cord blood transplantation has been reported (18).
Two of the patients were administered itraconazole, which showed low in vitro MICs (0.06 to 0.5 μg/ml), but the outcome could not be assessed. The cases presented herein highlight the clinical relevance of this mold in pulmonary diseases. Cases 1, 2, and 4 demonstrate the ability of the mold C. lacerata to produce bronchopneumonia, which was fatal in case 2 and probably in case 1, but the patient could not be followed up and telephonic inquiry revealed that he died. Case 3, however, shows that this mold, similar to other basidiomycetes, can exist in the respiratory tract as a commensal without causing any obvious disease. This is the first time that C. lacerata has been reported from clinical specimens. Environmental strains of the fungus have been isolated from white-rotted trees in Japan and Korea (38, 39). The patients in the first two cases were farmers and likely acquired this infection through exposure to airborne spores at the workplace. It may be emphasized that, though the clinical significance of the isolation of basidiomycetes in healthy patients is probably negligible, a pathological role in debilitated patients cannot be excluded. This communication adds another basidiomycete to the list of fungi associated with humans.
ACKNOWLEDGMENTS
This work was carried out, in part, with financial assistance from the Department of Biotechnology (reference no. BT/39/NE/TBP/2010), Government of India, New Delhi, India.
J.F.M. received grants from Astellas, Merck, Pfizer, Schering-Plough, Gilead, and Janssen Pharmaceuticals. He has been a consultant to Basilea and Merck and received speaker's fees from Merck, Pfizer, Schering-Plough, Gilead, and Janssen Pharmaceuticals. All other authors report no potential conflicts of interest.
The authors alone are responsible for the content and writing of the paper.
Footnotes
Published ahead of print 12 December 2012
REFERENCES
- 1. Balajee SA, Sigler L, Brandt ME. 2007. DNA and the classical way: identification of medically important molds in the 21st century Med. Mycol. 45:475–490 [DOI] [PubMed] [Google Scholar]
- 2. Pounder JI, Simmon KE, Barton CA, Hohmann SL, Brandt ME, Petti CA. 2007. Discovering potential pathogens among fungi identified as nonsporulating molds. J. Clin. Microbiol. 45:568–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. 2010. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J. Clin. Microbiol. 48:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chowdhary A, Agarwal K, Kathuria S, Singh PK, Roy P, Gaur SN, Rodrigues AM, de Hoog GS, Meis JF. 2012. First human case of pulmonary fungal ball due to a Perenniporia species (Basidiomycetes). J. Clin. Microbiol. 50:3786–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gené J, Guillamón JM, Guarro J, Pujol I, Ulfig K. 1996. Molecular characterization, relatedness and fungal susceptibility of the basidiomycetous Hormographiella species and Coprinus cinereus from clinical and environmental sources. Antonie Van Leeuwenhoek 70:49–57 [DOI] [PubMed] [Google Scholar]
- 6. Guarro J, Gené J, de Vroey C, Guého E. 1992. Hormographiella, a new genus of hyphomycetes from clinical sources. Mycotaxon 45:179–190 [Google Scholar]
- 7. Khan ZU, Randhawa HS, Kowshik T, Gaur SN, de Vries GA. 1988. The pathogenic potential of Sporotrichum pruinosum isolated from the human respiratory tract. J. Med. Vet. Mycol. 26:145–151 [PubMed] [Google Scholar]
- 8. Speller DCE, Maclver AG. 1971. Endocarditis caused by a Coprinus species: a fungus of the toadstool group. J. Med. Vet. Mycol. 4:370–374 [DOI] [PubMed] [Google Scholar]
- 9. Surmont I, van Aelst F, Verbanck J, de Hoog GS. 2002. A pulmonary infection caused by Coprinus cinereus (Hormographiella aspergillata) diagnosed after a neutropenic episode. Med. Mycol. 40:217–219 [DOI] [PubMed] [Google Scholar]
- 10. Verweij PE, van Kasteren M, van de Nes J, de Hoog GS, de Pauw BE, Meis JF. 1997. Fatal pulmonary infection caused by the basidiomycete Hormographiella aspergillata. J. Clin. Microbiol. 35:2675–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. González GM, Sutton DA, Thompson E, Tijerina R, Rinaldi MG. 2001. In vitro activities of approved and investigational antifungal agents against 44 clinical isolates of basidiomycetous fungi. Antimicrob. Agents Chemother. 45:633–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marriott D, Kwong T, Harkness J, Ellis D. 2006. Cyclomyces tabacinus as a cause of deep tissue infection: the first case report. Mycoses 49:147–149 [DOI] [PubMed] [Google Scholar]
- 13. Buzina W, Lass-Flörl C, Kropshofer G, Freund MC, Marth E. 2005. The polypore mushroom Irpex lacteus, a new causative agent of fungal infections. J. Clin. Microbiol. 43:2009–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davis CM, Noroski LM, Dishop MK, Sutton DA, Braverman RM, Paul ME, Rosenblatt HM. 2007. Basidiomycetous fungal Inonotus tropicalis sacral osteomyelitis in X-linked chronic granulomatous disease. Pediatr. Infect. Dis. J. 26:655–656 [DOI] [PubMed] [Google Scholar]
- 15. Sutton DA, Thompson EH, Rinaldi MG, Iwen PC, Nakasone KK, Jung HS, Rosenblatt HM, Paul ME. 2005. Identification and first report of Inonotus (Phellinus) tropicalis as an etiologic agent in a patient with chronic granulomatous disease. J. Clin. Microbiol. 43:982–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williamson D, Pandey S, Taylor S, Rogers K, Storey L, Marshall MR, Holland D. 2011. A case of infection caused by the basidiomycete Phellinus undulatus. J. Med. Microbiol. 60:256–258 [DOI] [PubMed] [Google Scholar]
- 17. Brockus CW, Myers RK, Crandell JM, Sutton DA, Wickes BL, Nakasone KK. 2009. Disseminated Oxyporus corticola infection in a German shepherd dog. Med. Mycol. 47:862–868 [DOI] [PubMed] [Google Scholar]
- 18. Salit RB, Shea YR, Gea-Banacloche J, Fahle GA, Abu-Asab M, Sugui JA, Carpenter AE, Quezado MM, Bishop MR, Kwon-Chung KJ. 2010. Death by edible mushroom: first report of Volvariella volvacea as an etiologic agent of invasive disease in a patient following double umbilical cord blood transplantation. J. Clin. Microbiol. 48:4329–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chowdhary A, Randhawa HS, Gaur SN, Agarwal K, Kathuria S, Roy P, Klaassen CH, Meis JF. 2012. Schizophyllum commune as an emerging fungal pathogen: a review and report of two cases. Mycoses. doi:10.1111/j.1439–0507.2012.02190.x [DOI] [PubMed] [Google Scholar]
- 20. Kurtzman CP, Robnett CJ. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 35:1216–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322 In Innis MA, Gelfand DH, Sninsky JJ, White TJ. (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA [Google Scholar]
- 22. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antimicrobial susceptibility testing of filamentous fungi, 2nd ed, M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 23. Rihs JD, Padhye AA, Good CB. 1996. Brain abscess caused by Schizophyllum commune: an emerging basidiomycete pathogen. J. Clin. Microbiol. 34:1628–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sa HS, Ko KS, Woo KI, Peck KR, Kim YD. 2012. A case of sino-orbital infection caused by Schizophyllum commune. Diagn. Microbiol. Infect. Dis. 73:376–377 [DOI] [PubMed] [Google Scholar]
- 25. Conen A, Weisser M, Hohler D, Frei R, Stern M. 2011. Hormographiella aspergillata: an emerging mould in acute leukaemia patients? Clin. Microbiol. Infect. 17:273–277 [DOI] [PubMed] [Google Scholar]
- 26. Greer EL, Kowalski TJ, Cole ML, Miller DV, Baddour LM. 2008. Truffle's revenge: a pig-eating fungus. Cardiovasc. Pathol. 17:342–343 [DOI] [PubMed] [Google Scholar]
- 27. Lagrou K, Massonet C, Theunissen K, Meersseman W, Lontie M, Verbeken E, Van Eldere J, Maertens J. 2005. Fatal pulmonary infection in a leukaemic patient caused by Hormographiella aspergillata. J. Med. Microbiol. 54:685–688 [DOI] [PubMed] [Google Scholar]
- 28. Suarez F, Olivier G, Garcia-Hermoso D, Randriamalala E, Ghez D, Bruneau J, Kauffmann-Lacroix C, Bougnoux ME, Lortholary O. 2011. Breakthrough Hormographiella aspergillata infections arising in neutropenic patients treated empirically with caspofungin. J. Clin. Microbiol. 49:461–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Hoog GS, Guarro J, Gene J, Figueras MJ. 2000. Atlas of clinical fungi, 2nd ed Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands, and Universitat Rovira i Virgili, Reus, Spain [Google Scholar]
- 30. Davies D. 1963. Pulmonary aspergillosis. Can. Med. Assoc. J. 89:392–395 [PMC free article] [PubMed] [Google Scholar]
- 31. Smith JA, Kauffman CA. 2012. Pulmonary fungal infections. Respirology 17:913–926 [DOI] [PubMed] [Google Scholar]
- 32. Bulajic N, Cvijanovic V, Vukojevic J, Tomic D, Johnson E. 2006. Schizophyllum commune associated with bronchogenous cyst. Mycoses 49:343–345 [DOI] [PubMed] [Google Scholar]
- 33. Iizasa T, Kamei K, Chiyo M, Suzuki M, Baba M, Toyosaki T, Hiroshima K, Ohwada H, Kanno S, Nishimura K, Fujisawa T. 2001. Colonization with Schizophyllum commune of localized honeycomb lung with mucus. Respiration 68:201–203 [DOI] [PubMed] [Google Scholar]
- 34. Miyazaki Y, Sakashita H, Tanaka T, Kamei K, Nishimura K, Yoshizawa Y. 2000. Mucoid impaction caused by monokaryotic mycelium of Schizophyllum commune in association with bronchiectasis. Intern. Med. 39:160–162 [DOI] [PubMed] [Google Scholar]
- 35. Abuali MM, Posada R, Del Toro G, Roman E, Ramani R, Chaturvedi S, Chaturvedi V, LaBombardi VJ. 2009. Rhizomucor variabilis var. regularior and Hormographiella aspergillata infections in a leukemic bone marrow transplant recipient with refractory neutropenia. J. Clin. Microbiol. 47:4176–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nenoff P, Friedrich T, Schwenke H, Mierzwa M, Horn LC, Haustein UF. 1997. Rare fatal simultaneous mould infection of the lung caused by Aspergillus flavus and the basidiomycete Coprinus sp. in a leukemic patient. J. Med. Vet. Mycol. 35:65–69 [PubMed] [Google Scholar]
- 37. Pang KA, Godet C, Fekkar A, Scholler J, Nivoix Y, Letscher-Bru V, Massias L, Kauffmann-Lacroix C, Elsendoorn A, Uzunov M, Datry A, Herbrecht R. 2012. Breakthrough invasive mould infections in patients treated with caspofungin. J. Infect. 64:424–429 [DOI] [PubMed] [Google Scholar]
- 38. Suhara H, Maekawa N, Kaneko S, Hattorui T, Sakkai K, Kondo R. 2003. A new species of Cereporia lacerata isolated from white-rotted wood. Mycotaxon 86:335–347 [Google Scholar]
- 39. Jang Y, Ha Eun C, Young Woon L, Jin Sung L, Jae-Jin K. 2012. The first report of Ceriporia lacerata (Phanerochaetaceae, Basidiomycota) in Korea. Mycotaxon 119:397–403 [Google Scholar]