Abstract
Few studies have correlated the results of interferon (gamma interferon) release assays (IGRAs) with known markers of tuberculosis (TB) treatment response. We report the results of serial QuantiFERON-TB gold in-tube assay (QFT) testing on 149 patients with active tuberculosis and correlate the results with smear and culture conversion. We show that QFT results do not offer much value for treatment monitoring of TB disease.
TEXT
The QuantiFERON-TB gold in-tube assay (QFT) (Cellestis/Qiagen, Australia) is increasingly being utilized in the diagnosis of latent tuberculosis (TB) infection (LTBI). However, the use of interferon (gamma interferon [IFN-γ]) release assays (IGRAs) for other indications is debated. Can IGRAs be used to monitor the response to treatment of active TB? Some reports have suggested that IFN-γ responses decline with decreasing antigenic load during treatment (1–3). Most of these studies have included small numbers of patients in countries where TB is endemic, and few have tried to correlate changes in IFN-γ responses with established, albeit imperfect, markers of treatment response in pulmonary TB (i.e., smear and culture conversion to negativity) (2, 4).
We evaluated 172 patients with active TB at different health centers in Montreal, Canada. The patients received treatment according to the recommendations of the Canadian Tuberculosis Standards (5). Directly observed therapy (DOT) was left to the discretion of the treating physician. QFT was done at the time of diagnosis, after 2 months, and at the end of treatment (between 6 to 9 months for most patients). The study was approved by the institutional review board of McGill University. We performed all QFT testing per the manufacturer's instructions in one laboratory. We evaluated QFT reversion and conversion rates using the manufacturers' definition (i.e., change from negative to positive and vice versa with a cutoff IFN-γ of ≥0.35 IU/ml). We assessed whether QFT reversion or a decline in quantitative IFN-γ results after 2 months of treatment was associated with smear or culture conversion to negativity after 2 months of treatment.
We performed all analyses with Stata, version 12 (Stata Corp., TX). We excluded 2 participants with indeterminate results and 21 participants without QFT results pretreatment from further analysis. Because the distribution of IFN-γ values in the individual groups was not normal, we used nonparametric tests (Kruskal-Wallis test) to assess the difference between median IFN-γ levels between different groups of patients (based on smear and culture status). Differences in the quantitative IFN-γ results at baseline, after 2 months of treatment, and at the end of treatment were compared using a Wilcoxon signed rank test to account for paired data. Logistic regression was used to assess the association of QFT results as a qualitative variable (positive/negative) with culture or smear results after 2 months of treatment after adjustment for other covariates.
The mean age of the patients was 37 years (range, 3 to 82 years), and 59% of patients were male. Most patients (93%) were foreign-born and came from countries with a high prevalence of TB. The age at immigration on average was 22 years. Almost 15% of all cases had some form of immunosuppression (i.e., HIV, therapy with steroids, malignancy, or diabetes). Of the active TB cases, 128 (86%) were pulmonary, and 20 (14%) were extrapulmonary (predominantly lymph node disease). Pulmonary disease was culture confirmed in all but 4 cases and smear positive in 48 (38%) of the cases. Two cases of the 149 patients were multidrug-resistant TB. Adherence to treatment was good except for one patient, who was recorded to have only 75% adherence.
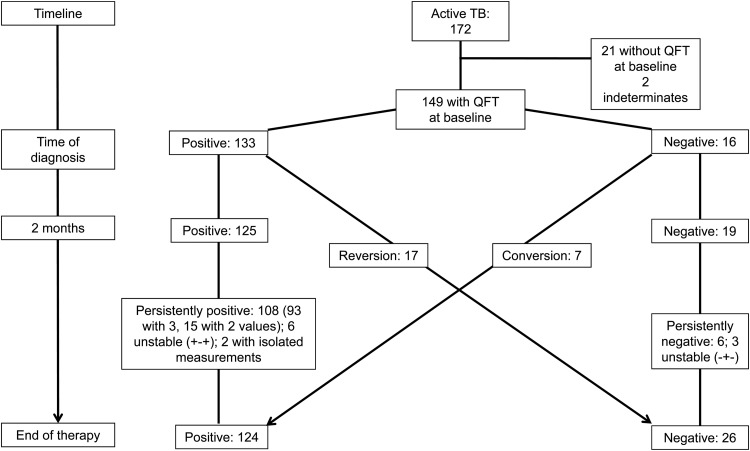
As shown in Fig. 1, at diagnosis (before treatment), 133 of 149 patients (89%; 95% confidence interval [95% CI], 83% to 94%) with valid pretreatment results were QFT positive. After 2 months of treatment, 125 of 144 (87%; 95% CI, 80% to 92%) patients were QFT positive, and at the end of treatment, 108 of 134 (81%; 95% CI, 73% to 87%) were QFT positive. Overall, only 17 out of 133 patients (13%; 95% CI, 8% to 20%) with positive QFT results at diagnosis reverted to negative by the end of treatment (Fig. 1). The median IFN-γ value at the time of diagnosis was significantly higher in the patients who stayed QFT positive (i.e., a stable positive pattern) (9.0 IU/ml; interquartile range [IQR], 4.3 to 9.8) compared to the patients who revert (1.5 IU/ml; IQR, 0.9 to 2.7) (P < 0.001).
Fig 1.
QFT categories for patients with active TB over time. Flow chart of QFT results of patients with active TB and available QFT measurement at the time of diagnosis. The numbers of patients are shown. Conversion and reversion were defined per the manufacturer's cutoff values.
Of the 128 pulmonary TB patients, 35 of 40 (88%) smear-positive patients at diagnosis became smear negative, and 84 of 97 (87%) initially culture-positive patients became culture negative after 2 months of treatment (8 smear-positive and 27 culture-positive patients were unable to produce a sputum sample at diagnosis). There was no evidence that patients who remained smear or culture positive were more likely to have a positive QFT result after 2 months of treatment (Table 1). In addition, there was no difference in the quantitative IFN-γ response after 2 months in patients whose smear and culture results converted to negative and those who did not (Table 1). No other variable (i.e., age, sex, immunocompromised status) was significantly associated with smear or culture conversion after 2 months of treatment. No treatment failures were diagnosed at the end of therapy; therefore, no analysis of correlates with the final treatment outcome was possible.
Table 1.
QFT categories and IFN-γ results stratified by sputum smear and culture results after 2 months of treatmenta
| Test result after 2 mo of treatment | At diagnosis |
After 2 mo of treatment |
||||
|---|---|---|---|---|---|---|
| No. of QFT-positive patients/total no. of patients (%) | Odds ratio (95% CI) for QFT positiveb | Median IFN-γ value (IU/ml) (IQR)c | No. of QFT-positive patients/total no. of patients (%) | Odds ratio (95% CI) for QFT positive | Median IFN-γ value (IU/ml) (IQR) | |
| Smear | ||||||
| Negative | 31/33 (94) | 1.6 (0.1, 40) | 9.5 (2.2, 9.8) | 30/33 (91) | 2.5 (0.2, 30) | 4.9 (1.2, 9.2)d |
| Positive | 5/5 (100) | 4.3 (1.1, 4.7) | 4/5 (80) | 2.6 (0.7, 3.7) | ||
| Culture | ||||||
| Negative | 46/52 (89) | 0.6 (0.03, 13) | 8.2 (1.8, 9.7) | 43/52 (83) | 0.96 (0.1, 9.2) | 3.6 (0.9, 8.5)e |
| Positive | 6/6 (100) | 9.0 (1.0, 9.9) | 5/6 (83) | 9.8 (1.2, 9.9) | ||
Patients were either smear positive or culture positive (and smear negative) at the time of diagnosis. Qualitative QFT and quantitative IFN-γ results are reported stratified by smear or culture result after 2 months of treatment. Abbreviations: QFT, QuantiFERON-TB gold in-tube assay; IFN-γ, gamma interferon; 95% CI, 95% confidence interval; IQR, interquartile range.
Odds ratio of a positive QFT result with respect to a smear/culture status after 2 months of treatment. In order to calculate the odds ratio, 0 in 2-by-2 table was replaced with 0.5.
The median IFN-γ value is shown in international units per milliliter. The interquartile ranges are shown in parentheses.
The median IFN-γ values for smear-negative and smear-positive patients after 2 months of treatment were nonsignificantly different (P = 0.68) by the Kruskal-Wallis test.
The median IFN-γ values for culture-negative and culture-positive patients after 2 months of treatment were nonsignificantly different (P = 0.16) by the Kruskal-Wallis test.
There was a significant difference in the IFN-γ value at the time of diagnosis compared to the measurement after 2 months of treatment and at the end of treatment (median IFN-γ at diagnosis, 6.94 IU/ml [IQR, 1.45 to 9.72 IU/ml] compared to 3.7 IU/ml [IQR, 0.9 to 9.43 IU/ml] after 2 months of treatment and 2.19 IU/ml [IQR, 0.52 to 8.19 IU/ml] at the end of therapy; P < 0.001). However, the absolute change in the IFN-γ value over the first 2 months was not different between the patients who remained smear or culture positive and those who converted to a negative result. There was also no difference in the IFN-γ values measured at different time points for patients who had drug-resistant or drug-sensitive TB or for immunocompromised and immunocompetent patients (data not shown).
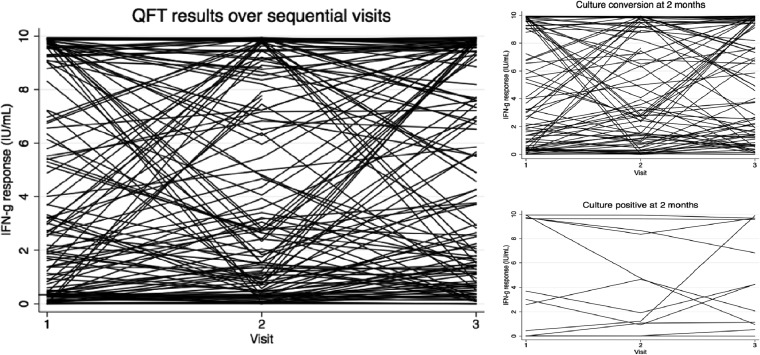
Overall, we observed a large variability of sequential IFN-γ measurements within individual persons independent of whether the culture results for the patients converted, as graphically shown in Fig. 2. The IFN-γ response declined on the whole, but substantial fluctuations were present, and a majority of patients remained QFT positive by the end of treatment.
Fig 2.
IFN-γ response over time in patients treated for active TB (n = 149). Sequential IFN-γ (IFN-g) results are shown for patients with active TB who completed treatment. Visit 1 included only those patients with a QFT result at the time of diagnosis, while visit 2 was follow-up after 2 months of treatment and visit 3 was the end of therapy. All values greater than 10 were rescaled to 10, and negative values were rescaled to 0.
Sputum smear and culture results after 2 months of treatment are currently the only tools for monitoring TB treatment response. Early studies suggested that IGRAs may have some promise as an alternative for TB monitoring, because IFN-γ responses are associated with mycobacterial antigenic load, which declines with treatment (6, 7). However, to date, only one study was able to relate the trajectory of the IGRA response to a treatment outcome that is relevant to the patient (i.e., smear conversion), while three others have not been able to confirm this association (1, 2, 4).
Our study showed that a large proportion of patients remained QFT positive even at the completion of treatment. This proportion is higher than those in prior studies, which could be due to differences in the test procedure, timing of measurement, or underlying genetic differences in the patients (1–3). Furthermore, changes in categorical QFT results or IFN-γ levels in our study were not associated with smear and culture conversion to negative results, reinforcing the lack of utility for treatment monitoring. A nonsignificantly higher IFN-γ response in patients, whose smear results converted (to negative result), could be postulated to be due to an improvement in immune response.
We also found substantial within-subject variability in sequential measurements. This within-subject variability has been reported in serial testing of health care workers (8); however, the driving forces are not well characterized (9, 10). The variation occurred despite all patients getting adequate therapy and having nearly 100% adherence. Exogenous factors that contribute to within-subject variability might include incubation time, contamination with other antigens, different test operators, or other analytical inconsistencies. Endogenous factors might include antigen burden, cumulative TB exposure, time interval from exposure, clearance of infection (spontaneous or due to therapy), recent tuberculin skin testing (TST), concomitant infections, medications, or other unknown factors (9, 11, 12).
Overall, QFT results and IFN-γ patterns do not seem to offer much value for monitoring of TB treatment, and substantial within-subject variability during serial testing further complicates the use and interpretation of repeated IGRA testing.
ACKNOWLEDGMENTS
C. M. Denkinger is a recipient of a Richard Tomlinson Postdoctoral Award from McGill University. M. Pai is a recipient of salary award from Fonds de Recherche du Québec–Santé, and funding support from EDCTP (TB NEAT grant), CIHR (MOP-81362), and Grand Challenges Canada.
We have no relevant affiliations or financial involvement relating to the subject matter or materials discussed. The funding agencies had no involvement in this publication.
Footnotes
Published ahead of print 21 November 2012
REFERENCES
- 1. Chee CB, KhinMar KW, Gan SH, Barkham TM, Koh CK, Shen L, Wang YT. 2010. Tuberculosis treatment effect on T-cell interferon-gamma responses to Mycobacterium tuberculosis-specific antigens. Eur. Respir. J. 36:355–361 [DOI] [PubMed] [Google Scholar]
- 2. Katiyar SK, Sampath A, Bihari S, Mamtani M, Kulkarni H. 2008. Use of the QuantiFERON-TB Gold In-Tube test to monitor treatment efficacy in active pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 12:1146–1152 [PubMed] [Google Scholar]
- 3. Kobashi Y, Mouri K, Yagi S, Obase Y, Miyashita N, Oka M. 2009. Transitional changes in T-cell responses to Mycobacterium tuberculosis-specific antigens during treatment. J. Infect. 58:197–204 [DOI] [PubMed] [Google Scholar]
- 4. Theron G, Peter J, Lenders L, van Zyl-Smit R, Meldau R, Govender U, Dheda K. 2012. Correlation of Mycobacterium tuberculosis specific and non-specific quantitative Th1 T-cell responses with bacillary load in a high burden setting. PLoS One 7:e37436 doi:10.1371/journal.pone.0037436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Public Health Agency of Canada 2007. Canadian tuberculosis standards, 6th ed Tuberculosis Prevention and Control, Public Health Agency of Canada, Ottawa, Canada: http://www.phac-aspc.gc.ca/tbpc-latb/pubs/tbstand07-eng.php [Google Scholar]
- 6. Lalvani A. 2004. Counting antigen-specific T cells: a new approach for monitoring response to tuberculosis treatment? Clin. Infect. Dis. 38:757–759 [DOI] [PubMed] [Google Scholar]
- 7. Millington KA, Innes JA, Hackforth S, Hinks TS, Deeks JJ, Dosanjh DP, Guyot-Revol V, Gunatheesan R, Klenerman P, Lalvani A. 2007. Dynamic relationship between interferon-gamma and interleukin-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J. Immunol. 178:5217–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ringshausen FC, Nienhaus A, Torres Costa J, Knoop H, Schlosser S, Schultze-Werninghaus G, Rohde G. 2011. Within-subject variability of Mycobacterium tuberculosis-specific gamma-interferon responses in German health care workers. Clin. Vaccine Immunol. 18:1176–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Zyl-Smit RN, Zwerling A, Dheda K, Pai M. 2009. Within-subject variability of interferon-gamma assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One 4:e8517 doi:10.1371/journal.pone.0008517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Veerapathran A, Joshi R, Goswami K, Dogra S, Moodie EE, Reddy MV, Kalantri S, Schwartzman K, Behr MA, Menzies D, Pai M. 2008. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 3:e1850 doi:10.1371/journal.pone.0001850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Detjen AK, Loebenberg L, Grewal HM, Stanley K, Gutschmidt A, Kruger C, Du Plessis N, Kidd M, Beyers N, Walzl G, Hesseling AC. 2009. Short-term reproducibility of a commercial interferon-gamma release assay. Clin. Vaccine Immunol. 16:1170–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dheda K, Pooran A, Pai M, Miller RF, Lesley K, Booth HL, Scott GM, Akbar AN, Zumla A, Rook GA. 2007. Interpretation of Mycobacterium tuberculosis antigen-specific interferon-gamma release assays (T-SPOT.TB) and factors that may modulate test results. J. Infect. 55:169–173 [DOI] [PubMed] [Google Scholar]