Abstract
We report the first case of cerebral abscess due to a novel species of Nocardia in a heart transplant patient and describe the antimicrobial susceptibility of this isolate. As our patient was intolerant to trimethoprim-sulfamethoxazole, we also discuss alternative therapeutic options in brain abscess due to Nocardia sp.
CASE REPORT
A 37-year-old woman underwent heart transplantation in November 2007 in the University Hospital of Lille (France). A rejection occurred in April 2008 (grade IR on International Society for Heart and Lung Transplantation classification), requiring an increase of the corticosteroid therapy. She was maintained on cyclosporine (150 mg twice a day [b.i.d.]), mycophenolate mofetil (500 mg/day), and prednisone (10 mg/day). Trimethoprim-sulfamethoxazole (TMP/SMX) (80/400 mg/day) prophylaxis for Pneumocystis jirovecii was discontinued in March 2008, according to our Heart Transplant Center's protocol. In June 2009, after a pneumonia was treated with ceftriaxone for 2 weeks, the computed-tomography (CT) scan revealed multiple bilateral nodules, one of them cavitated, and bilateral pulmonary infiltrate with hilar adenomegalies. The cultures from the bronchoalveolar lavage (a few days after ceftriaxone discontinuation) were sterile, and the biopsy specimens showed a nonspecific inflammation. In August 2009, she presented with fever and seizures. The leukocyte count was 5,950/mm3, and C-reactive protein was measured at 6 mg/liter. The cerebral CT scan and magnetic resonance imaging (MRI) showed a left frontal brain abscess with perilesional edema. The direct Gram stain of the abscess liquid was negative, but cultures recovered colonies consistent with Nocardia with Gram-positive filaments. The isolate grew on Columbia agar plates as white, rough, irregular, dry, 2-mm-diameter colonies after 48 h of incubation at 37°C in an aerobic atmosphere. Blood cultures and cerebrospinal fluid remained sterile.
The antimicrobial susceptibility of this strain (OFN 09.174) was tested by using a broth microdilution method according to the CLSI standard M24-A2 guidelines (1). The MICs were obtained from Rapid Growing Mycobacteria Plate Format (RAPMYCO) Sensititre plates incubated at 37°C for 72 h, and interpretation was made according to breakpoints established for the Nocardia genus. MIC results for OFN 09.174 indicated that our clinical isolate was susceptible to most of the antibiotics tested and resistant to amoxicillin-clavulanic acid and ciprofloxacin. The results are shown in Table 1.
Table 1.
MICs determinated by broth microdilution method for OFN 09.174 strain
| Drug(s) | MIC (μg/ml) for strain or categorya |
|||
|---|---|---|---|---|
| OFN 09.174 | Susceptible | Intermediate | Resistant | |
| Amikacin | ≤4 | ≤8 | ≥16 | |
| Amoxicillin-clavulanic acid | >16/8 | ≤8/4 | 16/8 | >32/16 |
| Cefotaxime | ≤4 | ≤8 | 16–32 | ≥64 |
| Ceftriaxone | ≤4 | ≤8 | 16–32 | ≥64 |
| Cefepime | ≤4 | ≤8 | 16 | ≥32 |
| Ciprofloxacin | 4 | ≤1 | 2 | ≥4 |
| Clarithromycin | ≤1 | ≤2 | 4 | ≥8 |
| Imipenem | 4 | ≤4 | 8 | ≥16 |
| Linezolid | 1 | ≤8 | ||
| Minocycline | ≤0.5 | ≤1 | 2–4 | ≥8 |
| Moxifloxacin | 1 | ≤1 | 2 | ≥4 |
| Tobramycin | ≤2 | ≤4 | 8 | ≥16 |
| Trimethoprim-sulfamethoxazole | ≤0.5/9.5 | ≤2/38 | ≥4/76 | |
All breakpoints are from reference 1.
Initially treated with TMP/SMX (160/800 mg three times a day [t.i.d.]) and cefotaxime (300 mg/kg of body weight/day), the patient developed a severe neutropenia after 2 weeks. Therapy was changed to cefotaxime and oral ciprofloxacin (750 mg t.i.d.). As the clinical and MRI evolution after 6 weeks was favorable, cefotaxime was replaced with oral minocycline (200 mg b.i.d.), and ciprofloxacin was continued. Minocycline was rapidly discontinued, as the patient developed nausea, diarrhea, and vomiting. A treatment with ceftriaxone (2 g/day) and ciprofloxacin (750 mg b.i.d.) was reintroduced, for 12 months, with a good tolerance and a clinical and radiological improvement.
As shown below, four genes (16S rRNA, gyrB, hsp65, and sod) have been used in the genetic analysis with the aim of identifying our isolate at the species level. However, the multigenic analysis was not enough to correctly identify the isolate, so a DNA-DNA hybridization with the most closely phylogenetically related Nocardia species was necessary to conclude the identification process. Strain OFN 09.174 was grown in Bennett medium, and nocardial DNA extraction (2) was performed. For 16S rRNA, the nearly complete gene sequence (1,315-nucleotide [nt] fragment) was determined with primers SQ1 (5′-AGAGTTGATCMTGGCTCAG-3′) and SQ6 (5′-CGGTGTGTACAAGGCCC-3′) (3). For gyrB, a fragment of about 1,230 nt was also amplified by using primers GYRBF1 (5′-ATGGCCTTCCTCAACAAGGG-3′) and GYRBR1 (5′-GTTCCACTGCATCGCGATCT-3′) and sequenced according to a method described by Shen et al. (4). For the hsp65 gene, a fragment of 441 nt was amplified by using TB11 (5′-ACCAACGATGGTGTGTCCAT-3′) and TB12 (5′-CTTGTCGAACCGCATACCCT-3′) primers (2). Finally, for the sod gene, a fragment of 442 nt was amplified by using Z205 (5′-ACGTTCACCACAGCAAGCACCA-3′) and Z212 (5′-TCGGCCCAGTTCACGACGTT-3′) primers (5). PCR products were purified and sequenced on both strands. The resulting sequences were aligned with the corresponding sequences of representative Nocardia species obtained from GenBank by using Clustal X software (6).
The phylogenetic study was performed using MEGA 5 software (7). We obtained evolutionary trees with our strain and the type strains of the most closely phylogenetically related species. These evolutionary trees were inferred with three treeing algorithms: the maximum-likelihood (8), maximum-parsimony (9), and neighbor-joining (10) methods. The robustness of the trees was tested by bootstrap resampling (1,000 replicates each).
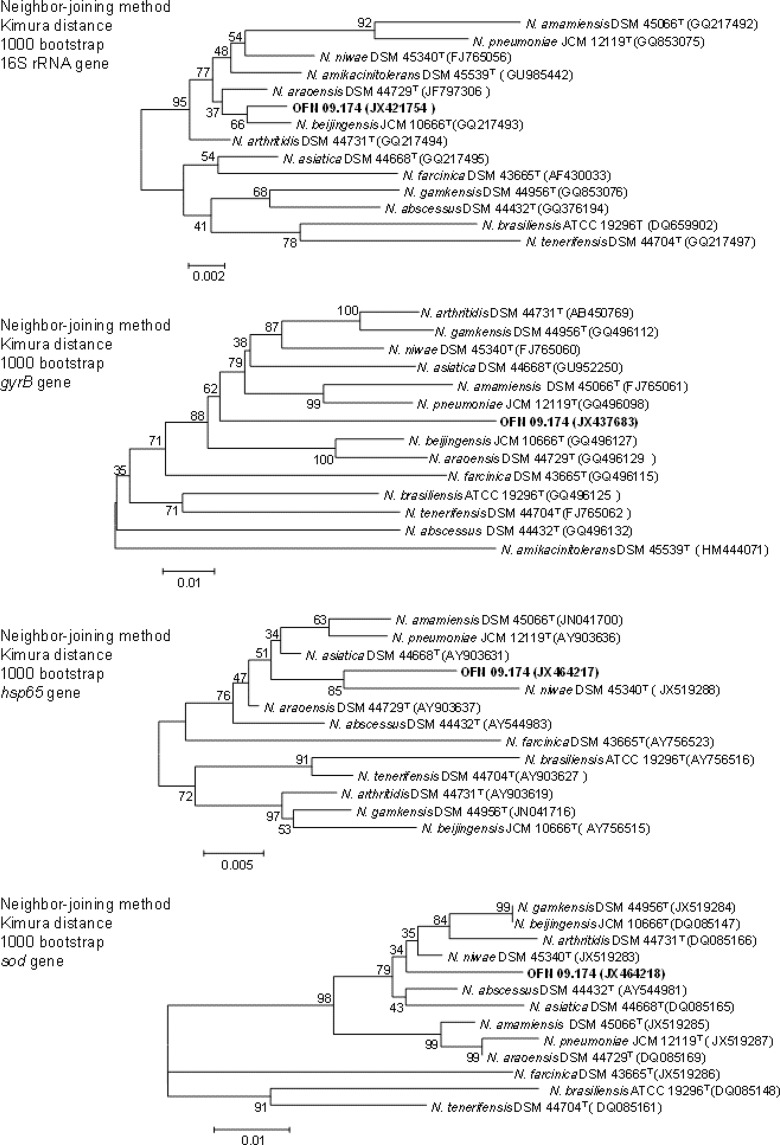
The PCR product for the 16S rRNA gene showed a sequence similarity of 99.5% to N. beijingensis JCM 10666T, 99.4% to N. araoensis DSM 44729T, 99.1% to N. arthritidis DSM 44731T, 99.0% to N. amikacinitolerans DSM 45539T, 98.9% to N. niwae DSM 45340T, and 98.7% to N. asiatica DSM 44668T. As shown in the phylogenetic tree of 16S rRNA (Fig. 1), strain OFN 09.174 forms a clade with the type strains of the Nocardia species listed above.
Fig 1.
Phylogenetic trees based on the 16S rRNA, gyrB, hsp65, and sod sequences from our isolate and the most closely related Nocardia type strains. The type strains of N. farcinica DSM 43665, N. brasiliensis ATCC 19296, and N. tenerifensis DSM 44704 were used to root the trees. These trees were based on a comparison of stretches of 1,315 nucleotides of the 16S rRNA gene, 1,230 nucleotides of the gyrB gene, 441 nucleotides of the hsp65 gene, and 442 nucleotides of the sod gene. Evolutionary trees were inferred by the neighbor-joining method with bootstrap resampling (1,000 replicates).
For gyrB, the PCR product showed that the isolate had a sequence similarity of 91.7% to N. arthritidis DSM 44731T, 91.6% to N. gamkensis DSM 44956T, 91.5% to N. niwae DSM 45340T, 91.4% to N. asiatica DSM 44668T, 91.2% to N. amamiensis DSM 45066T, 91.2% to N. pneumoniae JCM 12119T, 91.0% to N. beijingensis JCM 10666T, and 86.7% to N. amikacinitolerans DSM 45539T. The phylogenetic tree of Fig. 1 shows that strain OFN 09.174 forms a clade with the type strains of the most closely related Nocardia species.
With the hsp65 gene, we obtained a PCR product sequence with a sequence similarity of 98.4% to N. asiatica DSM 44668T, 98.2% to N. araoensis DSM 44729T, 97.6% to N. niwae DSM 45340T, 97.3% to N. abscessus DSM 44432T, and 97.3% to N. pneumoniae JCM 12119T. No data are available regarding N. amikacinitolerans DSM 45539T. The phylogenetic tree shown in Fig. 1 shows that strain OFN 09.174 forms a clade with these strains.
Finally, using the sod gene, the PCR product showed a sequence similarity of 98.0% to N. niwae DSM 45340T, 97.3% to N. abscessus DSM 44432T, 97.0% to N. beijingensis JCM 10666T, 97.0% to N. gamkensis DSM 44956T, 96.8% to N. arthritidis DSM 44731T, and 96.6% to N. asiatica DSM 44668T. No data are available regarding N. amikacinitolerans DSM 45539T. The phylogenetic tree in Fig. 1 shows that strain OFN 09.174 forms a clade with all the strains listed before.
DNA-DNA hybridization studies were performed using the DNAs of strain OFN 09.174 and the most closely phylogenetically related Nocardia species previously obtained in phylogenetic studies. Two criteria were used to choose these species: the number of times that a species is present in the same clade as our strain and the similarity between those strains. We chose N. niwae and N. asiatica, which appear in the same phylogenetic cluster as strain OFN 09.174 regardless of the gene under study, and N. beijingensis and N. arthritidis, which appear in the same phylogenetic cluster as our strain for three genes. Finally, of the species that appeared in the same cluster of our strain for only two genes, we chose those that presented the higher level of similarity, namely, N. araoensis and N. abscessus. Therefore, N. gamkensis, N. amamiensis, and N. pneumoniae were discarded. Regarding N. amikacinitolerans, even though no data for hsp65 and sod genes of this species are available in databases, the similarity level determined using the gyrB gene was so low that we were also able to discard this species for further studies.
Strain OFN 09.174 showed a DNA-DNA relatedness of lower than 70% with all the species listed above.
We can conclude that the OFN 09.174 isolate represents a new species because previous results do not allow us to identify our isolate as being an already-described species. We propose to name this new species “Nocardia lillensis” (referring to Lille, where the strain was isolated).
Further studies, including investigation of morphological, physiological, and biochemical characteristics and analysis of cell composition, will be done for completing the characterization of this isolate, which will allow us to publish later the description of the new species to which the N. lillensis OFN 09.174 strain belongs.
Discussion.
The risk factors (heart transplantation, immunosuppressive treatment that includes administration of corticosteroids) as well as the clinical presentation were classic for nocardiosis in our patient. The pneumonia observed 2 months before the diagnosis was possibly already a pulmonary nocardiosis. An initial treatment with ceftriaxone might have been responsible for the negative bronchoalveolar lavage fluid cultures, although the absence of associated pulmonary nocardiosis has been described in up to 34% of patients with nocardial brain abscesses.
The initial empirical treatment with TMP/SMX and cefotaxime was appropriate to the antimicrobial susceptibility of our strain. After TMP/SMX was discontinued due to the development of neutropenia, the patient was treated with ciprofloxacin and cefotaxime. Ciprofloxacin was chosen as a companion drug, despite a MIC at 4 μg/ml (at the cutoff between intermediate susceptibility and resistance), for its tolerance profile and excellent diffusion in the central nervous system (CNS). We expected that continuing high-dosage treatment might be beneficial, although not optimal. We did not consider it reasonable to introduce linezolid, in this context of drug-related acute neutropenia. After 6 weeks, we considered a switch to an oral treatment, for a total duration of 1 year, and decided to introduce minocycline in association with ciprofloxacin.
Linezolid might have been a better option; indeed, there is increasing evidence for its efficacy in disseminated nocardiosis, including cerebral abscesses, with long-lasting treatments (2 to 24 months) (11–16). However, anemia and thrombocytopenia are frequent with prolonged treatments (12, 14, 15), and rare but severe adverse events such as lactic acidosis, neuropathy, and visual impairment have been reported (11–13). In our patient, linezolid was excluded, based on the risk of myelotoxicity.
Minocycline therapy in combination has been proposed for patients showing intolerance to the first-line drugs, after results showing antimicrobial susceptibility (17). Clinical success has been reported with minocycline at 200 mg/day in monotherapy or in association with trimethoprim-sulfamethoxazole for treatment of cutaneous nocardiosis (18, 19). It was also efficient as a maintenance treatment after an initial 3 to 5 weeks of intravenous treatment in pulmonary nocardiosis (20, 21). Regarding nocardial CNS infection, Wren et al. reported a successful treatment with minocycline at 400 mg/day. The isolate was highly susceptible (MIC = 0.049 μg/ml), and drug concentrations obtained in the CNS were 16 to 22 times higher than the MIC (22). Leitersdorf et al. reported an uneventful recovery in 9 out of 10 transplanted patients treated with minocycline (100 to 600 mg/day) for disseminated nocardiosis, including CNS infections (23). Two more cases of successful maintenance treatment with minocycline at 100 mg/day in patients with nocardial cerebral abscesses have also been reported (11, 24). However, a treatment failure has been described in three patients treated with minocycline for pulmonary nocardiosis, with the patients developing brain abscesses under treatment. Susceptibility testing was not available in two cases, but the strain was susceptible in vitro in the third patient (15, 25). The use of minocycline as a second-line oral drug for CNS nocardiosis should be limited to patients with a susceptible isolate (MIC < 0.5 μg/ml) and requires a close follow-up of efficacy. It was not possible to use it in our patient because of gastrointestinal side effects.
This study also showed the difficulties of Nocardia species characterization. Actually, in recent years, the taxonomy of Nocardia has become more complex due to the description of new species that have made some clusters bigger or have even led to the creation of new ones. The use, with identification purposes, of the sequencing of the complete 16S rRNA gene as the sole reference gene has its limitations due to the lack of polymorphism of this gene, especially when the strain under study belongs to a cluster that hosts a high number of different species with a similarity level higher than the “cutoff” determined by the CLSI for this gene.
It is for this reason that, in these cases, it may be necessary to use the technique of multilocus sequence typing (MLST) with at least four genes (among the 16S rRNA, hsp65, gyrB, sod, rpoB, and secA1 genes). In our study, the results obtained with four genes were not discriminant enough to identify the strain but at least led us to determine a group of the most closely related type species. When the use of several genes is not discriminant enough, analysis of DNA-DNA hybridization between the strain under study and this group of type species must be performed in order to determine exactly the species the strain belongs to or, as happened in this study, to conclude that the strain belongs to a new species.
In conclusion, this report describes a disseminated infection with CNS involvement by a new Nocardia species identified by a molecular method. Despite a wide range of available drugs, the choice of a second-line oral therapy in patients with TMP/SMX intolerance is limited. Linezolid is highly efficient, but its potential as a maintenance treatment is limited, due to frequent and potentially severe side effects. Minocycline might be used when the strain is fully susceptible, with a close follow-up of efficacy analysis, in the absence of other therapeutic options. In severe cases, a long course of intravenous antibiotic administration might be required.
Nucleotide sequence accession numbers.
The nucleotide sequences of the 16S rRNA, gyrB, hsp65, and sod genes from our isolate were deposited in GenBank under the following accession numbers: JX421754 (16S rRNA), JX437683 (gyrB), JX464217 (hsp65), and JX464218 (sod).
ACKNOWLEDGMENTS
C. Flateau, V. Rodríguez-Nava, N. Lemaître, C. Loïez, F. Wallet, E. Bergeron, V. Jurado, and C. Saiz-Jimenez collected data; V. Rodríguez-Nava and C. Flateau wrote the paper; and V. Rodríguez-Nava, F. Wallet, C. Decoene, P. Boiron, K. Faure, and B. Guery critically revised the article.
Footnotes
Published ahead of print 5 December 2012
REFERENCES
- 1. CLSI 2011. Susceptibility testing of Mycobacteria, Nocardia, and other aerobic actinomycetes—2nd ed, vol 31, no. 5. Approved standard M24-A2. National Committee for Clinical Laboratory Standards, Wayne, PA: [PubMed] [Google Scholar]
- 2. Rodríguez-Nava V, Couble A, Devulder G, Flandrois JP, Boiron P, Laurent F. 2006. Use of PCR-restriction enzyme pattern analysis and sequencing database for hsp65 gene-based identification of Nocardia species. J. Clin. Microbiol. 44:536–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodríguez-Nava V, Couble A, Molinard C, Sandoval H, Boiron P, Laurent F. 2004. Nocardia mexicana sp. nov., a new pathogen isolated from human mycetomas. J. Clin. Microbiol. 42:4530–4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen FT, Lu HL, Lin JL, Huang WS, Arun AB, Young CC. 2006. Phylogenetic analysis of members of the metabolically diverse genus Gordonia based on proteins encoding the gyrB gene. Res. Microbiol. 157:367–375 [DOI] [PubMed] [Google Scholar]
- 5. Rodríguez-Nava V, Khan ZU, Potter G, Kroppenstedt RM, Boiron P, Laurent F. 2007. Nocardia coubleae sp. nov., isolated from oil-contaminated Kuwaiti soil. Int. J. Syst. Evol. Microbiol. 57:1482–1486 [DOI] [PubMed] [Google Scholar]
- 6. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Felsenstein J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17:368–376 [DOI] [PubMed] [Google Scholar]
- 9. Fitch WM. 1971. Toward defining the course of evolution: minimum change for a specific tree topology. Syst. Zool. 20:406–416 [Google Scholar]
- 10. Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 11. Lewis KE, Ebden P, Wooster SL, Rees J, Harrison GA. 2003. Multi-system infection with Nocardia farcinica-therapy with linezolid and minocycline. J. Infect. 46:199–202 [DOI] [PubMed] [Google Scholar]
- 12. Moylett EH, Pacheco SE, Brown-Elliott BA, Perry TR, Buescher ES, Birmingham MC, Schentag JJ, Gimbel JF, Apodaca A, Schwartz MA, Rakita RM, Wallace RJ., Jr 2003. Clinical experience with linezolid for the treatment of nocardia infection. Clin. Infect. Dis. 36:313–318 [DOI] [PubMed] [Google Scholar]
- 13. Rivero A, Garcia-Lazaro M, Perez-Camacho I, Natera C, del Carmen Almodovar M, Camacho A, Torre-Cisneros J. 2008. Successful long-term treatment with linezolid for disseminated infection with multiresistant Nocardia farcinica. Infection 36:389–391 [DOI] [PubMed] [Google Scholar]
- 14. Shen T, Wu L, Geng L, Wei Z, Zheng S. 2011. Successful treatment of pulmonary Nocardia farcinica infection with linezolid: case report and literature review. Braz. J. Infect. Dis. 15:486–489 [DOI] [PubMed] [Google Scholar]
- 15. Tanioka K, Nagao M, Yamamoto M, Matsumura Y, Tabu H, Matsushima A, Uemura K, Matsumoto R, Ito Y, Takakura S, Takahashi R, Ichiyama S. 2012. Disseminated Nocardia farcinica infection in a patient with myasthenia gravis successfully treated by linezolid: a case report and literature review. J. Infect. Chemother. 18:390–394 [DOI] [PubMed] [Google Scholar]
- 16. Viganò SM, Edefonti A, Ferraresso M, Ranzi ML, Grossi P, Righini A, Rusconi R, Santambrogio L, Ghio L. 2005. Successful medical treatment of multiple brain abscesses due to Nocardia farcinica in a paediatric renal transplant recipient. Pediatr. Nephrol. 20:1186–1188 [DOI] [PubMed] [Google Scholar]
- 17. Minero MV, Marin M, Cercenado E, Rabadan PM, Bouza E, Munoz P. 2009. Nocardiosis at the turn of the century. Medicine (Baltimore) 88:250–261 [DOI] [PubMed] [Google Scholar]
- 18. Akasaka E, Ikoma N, Mabuchi T, Tamiya S, Matuyama T, Ozawa A, Saito E, Wakabayashi T, Yamada C, Aoyama K, Mikami Y. 2011. A novel case of nocardiosis with skin lesion due to Nocardia araoensis. J. Dermatol. 38:702–706 [DOI] [PubMed] [Google Scholar]
- 19. Takahara M, Imafuku S, Matsuda T, Uenotsuchi T, Matsumoto T, Padhye AA, Furue M. 2005. Concurrent double infections of the skin: phaeohyphomycosis and nocardiosis in a patient with idiopathic thrombocytopenic purpura. J. Am. Acad. Dermatol. 53:S277–S280 [DOI] [PubMed] [Google Scholar]
- 20. Ogawa T, Kasahara K, Yonekawa S, Nakagawa C, Maeda K, Konishi M, Mikasa K, Kikuchi K. 2011. Nocardia beijingensis pulmonary infection successfully treated with intravenous beta-lactam antibiotics and oral minocycline. J. Infect. Chemother. 17:706–709 [DOI] [PubMed] [Google Scholar]
- 21. Patel MP, Kute VB, Gumber MR, Shah PR, Patel HV, Dhananjay KL, Jain SH, Trivedi HL, Vanikar AV. 2012. Successful treatment of Nocardia pneumonia with cytomegalovirus retinitis coinfection in a renal transplant recipient. Int. Urol. Nephrol. [Epub ahead of print.] doi:10.1007/s11255-011-0113-9 [DOI] [PubMed] [Google Scholar]
- 22. Wren MV, Savage AM, Alford RH. 1979. Apparent cure of intracranial Nocardia asteroides infection by minocycline. Arch. Intern. Med. 139:249–250 [PubMed] [Google Scholar]
- 23. Leitersdorf I, Silver J, Naparstek E, Raveh D. 1997. Tetracycline derivatives, alternative treatment for nocardiosis in transplanted patients. Clin. Nephrol. 48:48–51 [PubMed] [Google Scholar]
- 24. Kilincer C, Hamamcioglu MK, Simsek O, Hicdonmez T, Aydoslu B, Tansel O, Tiryaki M, Soy M, Tatman-Otkun M, Cobanoglu S. 2006. Nocardial brain abscess: review of clinical management. J. Clin. Neurosci. 13:481–485 [DOI] [PubMed] [Google Scholar]
- 25. Weber L, Yium J, Hawkins S. 2002. Intracranial Nocardia dissemination during minocycline therapy. Transpl. Infect. Dis. 4:108–112 [DOI] [PubMed] [Google Scholar]