Abstract
Rasamsonia argillacea (formerly known as Geosmithia argillacea) is a fungus recently recognized as a pathogen of immunocompromised patients. Here we report the first case of Rasamsonia infection in an immunocompetent host, presenting as a pulmonary and aortic graft infection. Its morphological similarity to nonpathogenic Penicillium species delayed the diagnosis and initiation of appropriate treatment.
CASE REPORT
A 56-year-old man presented to our clinic in 2009 for evaluation of progressive, chronic necrotizing pulmonary aspergillosis. In 1998, he had been diagnosed with an aneurysm of the proximal descending thoracic aorta, believed to be secondary to a traumatic aortic injury suffered during a motor vehicle accident in 1979. An endovascular stent graft was placed for treatment of his aortic aneurysm, and he was followed with serial imaging. On routine follow-up imaging of his aneurysm in 2005, he was found to have a cavitary lesion in the left upper lobe of his lung. A bronchoscopy was performed, and cultures revealed Aspergillus fumigatus. He was treated with itraconazole following this diagnosis, and subsequent chest computed tomography (CT) later that year revealed radiographic improvement. In January 2009, repeat CT showed an increase in the size of the cystic cavities in the left lung, and new lesions were visualized in the left lower lobe. He was referred to our clinic for evaluation.
At the time of his visit, the patient described a mild cough and scant hemoptysis several times per week. He denied any constitutional symptoms or pleuritic chest pain. Given the concern for a poorly controlled Aspergillus infection, a decision was made to change to voriconazole, but due to financial constraints, itraconazole was continued. The patient continued to suffer from hemoptysis. The close proximity of the pulmonary infection to the aortic aneurysm was concerning for future direct spread of infection, so the decision of surgical therapy was made. In August 2010, the patient underwent a left lower lobectomy with resection of the pulmonary cavity. The infection was found to abut the aortic aneurysm, but there was no evidence of invasion. Pathological examination of the resected lung lesion revealed pleural and subpleural fibrosis with septate fungal hyphae thought to be consistent with an Aspergillus species (Fig. 1). The intraoperative fungal cultures were reported as Penicillium. It was concluded that the cultures at the time of surgery were negative for Aspergillus due to the concurrent itraconazole therapy and that the Penicillium species was a colonizer, not a pathogen. Shortly after surgery, the patient was switched to voriconazole at 200 mg by mouth twice daily.
Fig 1.
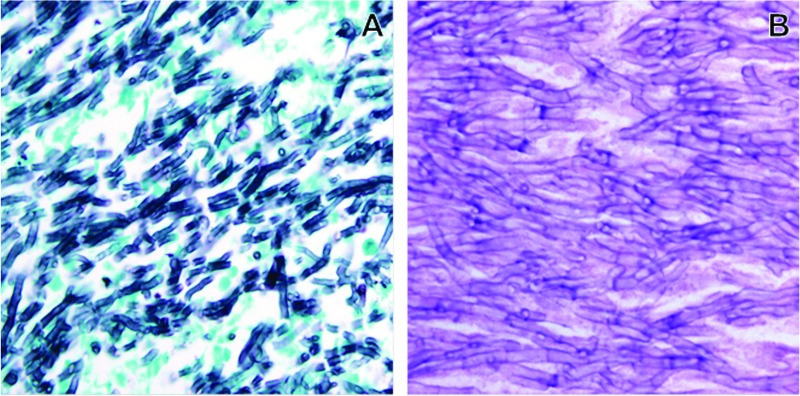
Grocott's methenamine silver (A) or hematoxylin-and-eosin (B) staining of sections from a bronchiectatic cystic structure, showing a mass of interwoven septate hyphae. The hyphae are thin (3 to 5 μm) with parallel walls.
Following surgery, the patient continued to complain of cough and hemoptysis. A CT scan in September 2011 revealed communication between the superior lingual bronchus and new left-sided loculated hydropneumothorax, as well as fluid within the aneurysm sac near the aortic graft (Fig. 2). These findings were very suspicious for progressive infection now involving the aortic graft, and the patient was taken to the operating room for a thoracic aortic bypass with a graft extending from the ascending aorta to the distal thoracic aorta. Intraoperative samples were sterile, but a subsequent bronchoalveolar lavage (BAL) fluid culture was positive for an isolate again identified as a Penicillium species. Given the recurrence of the Penicillium species without another identified organism and progression of disease on voriconazole, this fungal isolate was forwarded to the Fungus Testing Laboratory at the University of Texas Health Science Center in San Antonio, where it was reidentified as Rasamsonia argillacea. Figure 3 compares the microscopic features of the R. argillacea recovered from the bronchoalveolar lavage sample with those of two morphologically similar genera, Penicillium and Paecilomyces. Antifungal susceptibility testing performed according to the previously published Clinical and Laboratory Standards document M38-A2 for filamentous fungi (1) revealed MICs as follows: amphotericin B, 1 μg/ml; voriconazole, >16 μg/ml; itraconazole, 0.5 μg/ml; posaconazole, 0.5 μg/ml; caspofungin, 0.5 μg/ml; micafungin, ≤0.015 μg/ml. Based on these results, the patient's voriconazole was discontinued, and he was started on posaconazole, 400 mg by mouth twice daily, plus micafungin, 100 mg intravenously once daily. In January 2012, he underwent resection of the remaining infected aortic bypass graft, and intraoperative samples again grew R. argillacea. The patient was continued on posaconazole plus micafungin for an additional 6 weeks after the graft resection, and he was then transitioned to posaconazole monotherapy. Given the association between chronic granulomatous diseases and R. argillacea (2, 3), the neutrophilic oxidative index was determined, which was within normal limits. Testing for HIV was also negative. The patient's postoperative course was complicated by a bronchopleural fistula, but he subsequently was discharged and has improved clinically 9 months after his last surgery. A posaconazole trough was 0.76 μg/ml, and a repeat CT scan of the chest revealed no residual infection of the graft site, but the exam did reveal persistent, slowly resolving hydropneumothorax.
Fig 2.
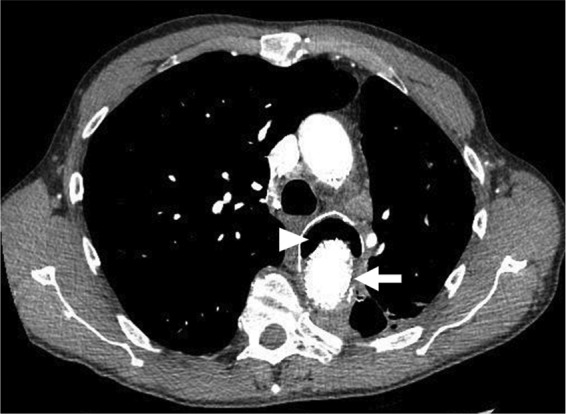
Selected axial image from a contrast-enhanced study of the thorax, with arterial phase timing at the proximal descending thoracic aorta. The arrow identifies an endovascular stent graft for treatment of an aortic aneurysm. The arrowhead indicates gas within the residual aneurysm sac.
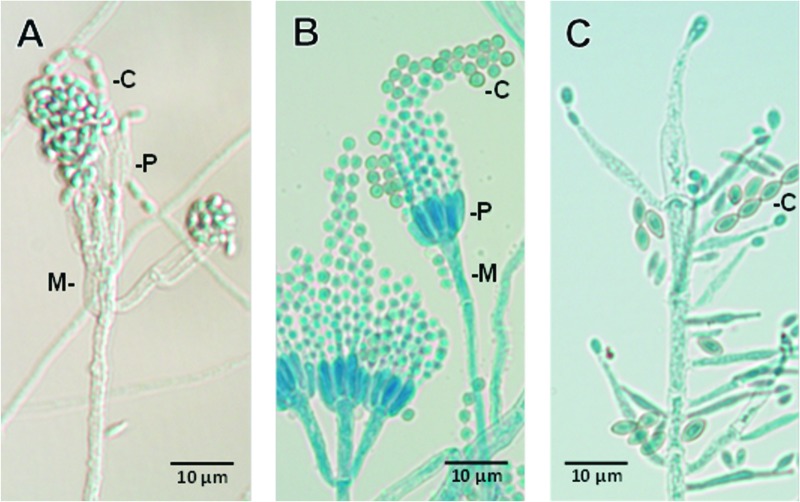
Fig 3.
Photomicrographs show differences in the microscopic morphology of Rasamsonia argillacea (A) from those of a Penicillium species (B) or Paecilomyces variotii (C). All have phialidic conidiogenous cells (P), many of which are supported by metulae (M, cells directly beneath the phialide); however, those in R. argillacea are noticeably roughened. Conidial shape (C) is also distinctive for various species in each of the genera, as shown in the examples above, usually being globose (round) to oval or ellipsoidal in Penicillium or Paecilomyces, respectively, and rectangular to cuneiform (wedge shaped) in R. argillacea. Slide cultures from which photomicrographs were taken were mounted in lactophenol cotton blue.
Fungal identification.
The BAL fluid and aortic tissue isolates were forwarded to the Fungus Testing Laboratory at the University of Texas Health Science Center in San Antonio for species identification and antifungal susceptibility testing and were accessioned into their culture collection as UTHSC 11-3152 and UTHSC 12-238, respectively. Salient phenotypic features, previously described in detail elsewhere (2–5), included cream to buff to pale brown colonies on potato dextrose agar prepared in-house, growth at elevated temperatures (37, 40, and 45°C), roughened conidiophores and conidiogenous cells (phialides), and smooth, hyaline, cylindrical to cuneiform (wedge-shaped) conidia borne in long columnar chains (Fig. 3A). Based on these features, both isolates were identified morphologically as R. argillacea. Molecular characterization of the BAL fluid and aortic tissue isolates as conducted by PCR amplification and sequencing of the ribosomal internal transcribed spacer (ITS) region and 5′ end of the nuclear large subunit ribosomal DNA (rDNA) at the UTHSCSA Advanced Nucleic Acids Core facility, as previously described (6). Sequences were then used to perform a BLASTn search of the GenBank database at the NCBI (http://www.ncbi.nlm.nih.gov). Outputs were sorted based on percent identity and were considered significant at ≥99% identity and ≥90% query coverage. The top three matches for the UTHSC 12-238 ITS rDNA search were all Rasamsonia argillacea (EU862337.1, 99% identity; EU862335.1, 99% identity; and GU165730.1, 99% identity), and the top three matches for the LSU D1/D2 sequence were also all Rasamsonia argillacea (EU862338.1, 99% identity; EU862336.1, 99% identity; and AB047236.1, 99% identity). The search results with the UTHSC 11-3152 ITS sequence were all Rasamsonia argillacea (GU165733.1, 99% identity; EU862337.1, 99% identity; and GU165730.1, 99% identity), as were the search results for the LSU D1/D2 rDNA sequence (EU862338.1, 100% identity; EU862336.1, 100% identity; and AB047236.1, 100% identity). Based on the phenotypic and genotypic results, both isolates were identified as Rasamsonia argillacea. The case isolates were deposited in the University of Alberta Microfungus Collection under accession numbers UAMH 11662 and UAMH 11663.
Discussion.
Rasamsonia argillacea is a rare fungal pathogen first described by Stolk et al. in 1969 as a thermotolerant fungus under the name Penicillium argillaceum (7). In 1979, Pitt (8) erected a new genus, Geosmithia, to accommodate species whose colonies were tan to buff colored and never green/blue-green like those in most other Penicillium species and whose conidia were rectangular rather than globose or ellipsoidal, as in Penicillium or Paecilomyces, respectively (Fig. 3). Geosmithia was also distinguished by rough-walled metulae (cells supporting the phialides) and phialides. More recently, molecular characterization of thermotolerant members of the family Trichocomaceae, including Geosmithia, using several gene sequences (RPB2, RNA polymerase II gene encoding the second-largest protein submit; TSR1, encoding a putative ribosome biogenesis protein; CCT8, encoding the putative chaperonin complex component TCP-1) have shown that these species form a clade distinct from other genera in the Trichocomaceae family (9). Thus, the genus Rasamsonia was proposed, with the name honoring Robert A. Samson at the CBS-KNAW Fungal Biodiversity Center on the occasion of his 65th birthday, his 40 years of service at the CBS, and for his many contributions to fungal taxonomy. The genus currently contains six species (9).
Rasamsonia argillacea was only recently identified as an etiologic agent in humans and other animals, reported under the name Geosmithia argillacea (2–5, 10, 11). In 2009, a fatal case of disseminated infection in a German shepherd dog was reported (5). Subsequently, eight cases of airway colonization in patients with cystic fibrosis, without evidence of clinical infection, were reported (4, 10). Nine cases of pulmonary infections in patients with chronic granulomatous disease have been reported, two of which involved the chest wall and ribs and one of which disseminated to the brain (2, 3). Four of these patients died from complications of their infections. Finally, fatal G. argillacea infection was recently found in a stem cell transplant recipient receiving immune-suppressive therapy for graft-versus-host disease (11). Other Geosmithia species have not been reported as etiologic agents of human infection.
Several common themes arise from the Rasamsonia cases (reported as Geosmithia) described above. This pathogen was often misidentified microscopically as a Penicillium or Paecilomyces species. All isolates of this species tested were resistant to voriconazole but were variably resistant to itraconazole, amphotericin B, and posaconazole. Echinocandin resistance was infrequent. Until the present case, R. argillacea had been associated with significant morbidity and mortality only in immunocompromised patients.
Here we report the first case of invasive Rasamsonia argillacea infection in an immunocompetent patient. This patient developed a pulmonary infection in the context of airway compression precipitated by a large posttraumatic aortic aneurysm. The original Aspergillus infection was likely cleared with itraconazole. In the setting of this treatment regimen, the patient developed a secondary Rasamsonia argillacea infection that was characterized by septate hyphae in tissue similar to those seen in aspergillosis (Fig. 1) and a microscopic morphology that was similar to that of Penicillium and Paecilomyces (Fig. 3). Similar to the reported cases, this patient's Rasamsonia isolate was initially misidentified microscopically as a Penicillium species, and in vitro antifungal susceptibility testing suggested resistance to voriconazole. This case highlights that clinical suspicion for a Rasamsonia argillacea infection needs to be raised for patients whose fungal infections worsen and whose cultures are reported as a Penicillium species, regardless of the patient's immune status, especially if these patients are receiving voriconazole.
Nucleotide sequence accession numbers.
The ITS and LSU D1/D2 rDNA sequence data were deposited in GenBank under accession numbers JX514398 to JX514401.
ACKNOWLEDGMENTS
J.E.K. received funding support from a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research.
We acknowledge Dora McCarthy for performing antifungal susceptibility testing on the case isolates.
Footnotes
Published ahead of print 12 December 2012
REFERENCES
- 1. CLSI 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 2nd ed. Approved standard M38-A2. CLSI, Wayne, PA [Google Scholar]
- 2. De Ravin SS, Challipalli M, Anderson V, Shea YR, Marciano B, Hilligoss D, Marquesen M, Decastro R, Liu YC, Sutton DA, Wickes BL, Kammeyer PL, Sigler L, Sullivan K, Kang EM, Malech HL, Holland SM, Zelazny AM. 2011. Geosmithia argillacea: an emerging cause of invasive mycosis in human chronic granulomatous disease. Clin. Infect. Dis. 52:e136–e143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Machouart M, Garcia-Hermoso D, Rivier A, Hassouni N, Catherinot E, Salmon A, Debourgogne A, Coignard H, Lecuit M, Bougnoux ME, Blanche S, Lortholary O. 2011. Emergence of disseminated infections due to Geosmithia argillacea in patients with chronic granulomatous disease receiving long-term azole antifungal prophylaxis. J. Clin. Microbiol. 49:1681–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giraud S, Pihet M, Razafimandimby B, Carrere J, Degand N, Mely L, Favennec L, Dannaoui E, Bouchara JP, Calenda A. 2010. Geosmithia argillacea: an emerging pathogen in patients with cystic fibrosis. J. Clin. Microbiol. 48:2381–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grant DC, Sutton DA, Sandberg CA, Tyler RD, Jr, Thompson EH, Romanelli AM, Wickes BL. 2009. Disseminated Geosmithia argillacea infection in a German shepherd dog. Med. Mycol. 47:221–226 [DOI] [PubMed] [Google Scholar]
- 6. Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. 2010. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J. Clin. Microbiol. 48:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stolk AC, Evans HC, Nilsson T. 1969. Penicillium argillaceum sp. nov., a thermotolerant Penicillium. Trans. Br. Mycol. Soc. 53:307–311 [Google Scholar]
- 8. Pitt JI. 1979. Geosmithia gen. -nov. for Penicillium lavendulum and related species.Can. J. Bot. 57:2021–2030 [Google Scholar]
- 9. Houbraken J, Spierenburg H, Frisvad JC. 2012. Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Antonie Van Leeuwenhoek 101:403–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barton RC, Borman AM, Johnson EM, Houbraken J, Hobson RP, Denton M, Conway SP, Brownlee KG, Peckham D, Lee TW. 2010. Isolation of the fungus Geosmithia argillacea in sputum of people with cystic fibrosis. J. Clin. Microbiol. 48:2615–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valentin T, Neumeister P, Pichler M, Rohn A, Koidl C, Haas D, Heiling B, Asslaber M, Zollner-Schwetz I, Hoenigl M, Salzer HJ, Krause R, Buzina W. 2012. Disseminated Geosmithia argillacea infection in a patient with gastrointestinal GvHD. Bone Marrow Transplant. 47:734–736 [DOI] [PubMed] [Google Scholar]