Abstract
Genomic deletions induced by imprecise excision of transposons have been used to disrupt gene functions in Drosophila. To determine the excision properties of Tol2, a popular transposon in zebrafish, we took advantage of two transgenic zebrafish lines Et(gata2a:EGFP)pku684 and Et(gata2a:EGFP)pku760, and mobilized the transposon by injecting transposase mRNA into homozygous transgenic embryos. Footprint analysis showed that the Tol2 transposons were excised in either a precise or an imprecise manner. Furthermore, we identified 1093-bp and 1253-bp genomic deletions in Et(gata2a:EGFP)pku684 founder embryos flanking the 5′ end of the original Tol2 insertion site, and a 1340-bp deletion in the Et(gata2a:EGFP)pku760 founder embryos flanking the 3′ end of the insertion site. The mosaic Et(gata2a:EGFP)pku684 embryos were raised to adulthood and screened for germline transmission of Tol2 excision in their F1 progeny. On average, ∼42% of the F1 embryos displayed loss or altered EGFP patterns, demonstrating that this transposon could be efficiently excised from the zebrafish genome in the germline. Furthermore, from 59 founders, we identified one that transmitted the 1093-bp genomic deletion to its offspring. These results suggest that imprecise Tol2 transposon excision can be used as an alternative strategy to achieve gene targeting in zebrafish.
INTRODUCTION
In Drosophila, genomic deletions induced by transposon excision of P or Minos element are one of the most commonly used methods to disrupt genes of interest (1,2). However, no similar approach has been documented in other organisms. In zebrafish (Danio rerio), several transposons, including the Tol1, Tol2, Sleeping Beauty and Ac/Ds elements, have been used for a variety of purposes (3–6). As the Tol2 transposon is highly efficient, it is widely used in zebrafish, and many transgenic fish lines have been generated through several large-scale genetic screens based on it (7–16).
The Tol2 transposon is a member of the hAT transposon family, whereas P element belongs to the P family. Although they are not closely related, the Tol2 transposon and P element are similar in certain properties, such as transposition through the mode of cut-and-paste, generation of 8-bp DNA duplications at the original insertion sites and leaving footprints after excision (17). Footprints are generated by the error-prone non-homologous end-joining repair of DNA double-strand breaks, which are induced during transposition (18). The excision of the Tol2 transposon is reported to be either precise or imprecise in medaka (Oryzias latipes) and zebrafish (19,20). Small nucleotide deletions and insertions (indels) have been detected after imprecise Tol2 excision; however, relatively large genomic deletions (>1 kb) similar to that induced by P element have not been reported (19–22).
Here, we investigated the excision efficiency and the footprints of the Tol2 transposon using two transgenic fish lines, Et(gata2a:EGFP)pku684 and Et(gata2a:EGFP)pku760, in which the Tol2 transposon containing an enhancer trap cassette with an EGFP reporter gene was inserted at 140-bp upstream of the lmo2 (LIM domain only 2) gene and in the first intron of the nav3 (neuron navigator 3) gene, respectively. We identified 1093-bp and 1253-bp genomic deletions in the Et(gata2a:EGFP)pku684 founder embryos and a 1340-bp deletion in the Et(gata2a:EGFP)pku760 founder embryos adjacent to the Tol2 insertion sites. Furthermore, we identified the 1093-bp genomic deletion in the progeny from one out of 59 Et(gata2a:EGFP)pku684 founder fish, indicating that genomic deletions induced by Tol2 excision is heritable through germline transmission. Our results showed that Tol2 transposon excision may be a feasible and efficient new approach for mutagenesis in zebrafish.
MATERIALS AND METHODS
Zebrafish lines
All the zebrafish used in this study were maintained at 28.5°C in the fish facility of Peking University. The Tol2 transposon insertion sites of the transgenic fish lines Et(gata2a:EGFP)pku684 and Et(gata2a:EGFP)pku760 were mapped using linker-mediated polymerase chain reaction (PCR) as previously described and confirmed by PCR genotyping (23).
Whole-mount in situ hybridization
A 1358-bp fragment of the lmo2 gene was amplified from cDNAs of 24 hours post fertilization (hpf) embryos by PCR (5′-ATAGGACTGAATGCGTGGTGACA-3′ and 5′-AAGATGGGATTGAAGACTGCTGAA-3′). The PCR product was ligated into the pBluescript vector. Antisense RNA probe was prepared by in vitro transcription using T7 RNA polymerase (Promega) and labeled with digoxigenin-UTP RNA labeling mix (Roche). The whole-mount in situ hybridization procedure was carried out as described previously (24,25).
Image acquisition and processing
The in situ hybridization results were captured using a Zeiss Stemi 2000-C dissecting microscope equipped with a color digital CCD camera (AxioCam MRc5, Zeiss). Fluorescent images of Et(gata2a:EGFP)pku684 were taken under a Zeiss Axioimager Z1 fluorescence microscope equipped with a monochrome CCD camera (AxioCam MRm, Zeiss) and Zeiss filter set 10. Pseudo-color was added using the supplied AxioVision software (Zeiss). Fluorescent images of Et(gata2a:EGFP)pku760 were taken under a Zeiss Axioimager A1 fluorescence microscope equipped with the color digital CCD camera.
Injection of mRNA encoding Tol2 transposase, footprint analysis and screening for large chromosomal deletions
The mRNA encoding Tol2 transposase was synthesized using pCS-TP plasmid by in vitro transcription using an SP6 mMESSAGE mMACHINE kit (Ambion) (7). One-cell stage homozygous Et(gata2a:EGFP)pku684 or Et(gata2a:EGFP)pku760 embryos were injected with 50-pg transposase mRNA to induce Tol2 transposition. The Et(gata2a:EGFP)pku684 founders were raised to adulthood and outcrossed with homozygous transgenic fish for footprint analysis and with wild-type fish for screening of chromosomal deletions. To examine the footprints of Et(gata2a:EGFP)pku684, the original Tol2 insertion site in founder embryos or individual F1 embryos was amplified by PCR (5′-TTATGTCATTTACTTTTATTGTTG-3′ and 5′-GTTTCTGCTCTTTTCCGACTT-3′) from genomic DNA and analysed by sequencing. To evaluate large deletions of Et(gata2a:EGFP)pku684 in founder embryos, genomic DNA was extracted from groups of three to five 3 days post fertilization (dpf) embryos, and potential deletions were determined by sequencing after PCR amplification and electrophoresis (5′-TCAGGCAGAGATGAGCATCAG-3′ and 5′-ACGAGCTCAAACACGGAGTC-3′ for 5′ detection; 5′-TTATGTCATTTACTTTTATTGTTG-3′ and 5′-GCCCCATTCTCAGATTATTAC-3′ for 3′ detection). To screen for heritable genomic deletions in Et(gata2a:EGFP)pku684, F1 embryos negative for EGFP or expressing EGFP in the non-intermediate cell mass (ICM) region were picked out under a microscope. Twenty selected F1 embryos from the same founder were pooled, and genomic DNA was extracted at 5 dpf. Genomic deletions in these embryos were isolated by electrophoresis after PCR and determined by sequencing (5′-GTTTCTGCTCTTTTCCGACTT-3′ and 5′-ACGATGGAAGTGAATGGTT-3′ for 5′ detection; 5′-TTATGTCATTTACTTTTATTGTTG-3′ and 5′-TTCAGAAAGAAGCGGTCTC-3′ for 3′ detection). Footprints and large deletions in Et(gata2a:EGFP)pku760 founders were examined similarly as for Et(gata2a:EGFP)pku684 (5′-TTCTCAAGAGCCCTTGCTTG-3′ and 5′-AAGGACGCAGCAGGGAAG-3′ for footprint detection, 5′-TTCTCAAGAGCCCTTGCTTG-3′ and 5′-TGTGCTTTTGAGGGCAGTAG-3′ for 5′ deletion detection, 5′-GCGTGTTGTTTGGAGCCT-3′ and 5′-CCCGCATGATGTTTGTATG-3′ for 3′ deletion detection).
Plasmid-based excision assay
Transient excision of the Tol2 transposable element from plasmid DNA pTol2-GT2MP after injection into zebrafish embryos was examined as described previously (20,23). Fifty picograms of the circular plasmid and 50-pg of capped transposase mRNA were co-injected into fertilized eggs at the one-cell stage. Each individual embryo was lysed at the bud stage followed by DNA extraction. Fragments of 560 bp were amplified from the DNA preparation by PCR (5′-CATCAGCCTCCCCGGTCCAT-3′ and 5′-GGCACGACAGGTTTCCCGAC-3′). The PCR products were gel purified and cloned into pMD18-T simple vector (Takara) for sequencing.
Southern blot
A 546-bp DNA fragment was amplified by PCR (5′-ACGATGGAAGTGAATGGTT-3′ and 5′-GATCTGTAAGTGCATAGAAGTC-3′) followed by ligation to pBluescript vector and used as the template for probe synthesis. The digoxigenin-labeled probe was synthesized using a PCR DIG Probe Synthesis Kit (Roche). Genomic DNA was extracted from adult fin clips, and 20 μg was digested with PstI and SacI overnight. The digested DNA was separated on 0.8% agarose gel and transferred to nylon membrane (Amersham Hybond-N+). Hybridization was carried out following the manufacturer’s instructions (Roche). The signal was detected using NBT and BCIP (Promega).
RESULTS
Identification of the Et(gata2a:EGFP)pku684 and Et(gata2a:EGFP)pku760 fish lines
Using a Tol2 transposon cassette containing an EGFP reporter gene driven by a zebrafish gata2a minimal promoter, we performed a large-scale enhancer trap screen (unpublished data). From this screen, we identified mp684, a fish line also called Et(gata2a:EGFP)pku684, in which the EGFP signal was first detected in the two stripes of lateral mesoderm at 12 hpf. At 24 hpf, the EGFP expression was restricted to the ICM region and the sprouting intersegmental vessels (Supplementary Figure S1a). This indicated that both hematopoietic and endothelial cells were labeled in this line. To identify the trapped gene, we cloned the sequence flanking the Tol2 transposon insertion site by linker-mediated PCR (Supplementary Figure S1c and Supplementary Table S1). Sequencing and Basic Local Alignment Search Tool (BALST) searching results showed that the Tol2 enhancer trap cassette was inserted at 140-bp upstream of the transcription start of lmo2, a gene essential for hematopoiesis and angiogenesis (Supplementary Figure S1d). We further confirmed the insertion site by PCR with two primers across the transposon and one primer located within the transposon (Supplementary Figure S1e). Whole-mount in situ hybridization showed that the EGFP pattern of Et(gata2a:EGFP)pku684 recapitulated endogenous lmo2 expression (Supplementary Figure S1b). In addition, Et(gata2a:EGFP)pku684 showed a fluorescence pattern similar to two previously reported lmo2 transgenic fish lines, which were generated using dsRed and EGFP as reporters under the control of a 2.5-kb lmo2 promoter (26). All these results suggested that the Tol2 transposon trapped the enhancer of the lmo2 gene in Et(gata2a:EGFP)pku684 fish line.
Similarly, we identified another line Et(gata2:EGFP)pku760, also called MP760GFP or mp760a, in which the Tol2 enhancer trap cassette was inserted in the first intron of gene nav3 (Supplementary Figure S2e and Supplementary Table S1) (27). In this line, the EGFP signal was detected in endoderm, central nervous system and spinal cord, which was consistent with the nav3 gene expression pattern (Supplementary Figure S2) (28,29).
Analyses of the efficiency and footprints of Tol2 transposon excision
The Tol2 transposon is mobilized from the genome in the presence of its transposase. We first evaluated the properties of Tol2 transposon excision in Et(gata2:EGFP)pku684 fish line. After injection of Tol2 transposase mRNA into homozygous Et(gata2:EGFP)pku684 embryos, DNA fragments flanking the Tol2 insertion site were amplified by PCR and analysed by sequencing (Figure 1). Among 35 clones that were sequenced, 10 (28.6%) showed exactly the same sequence as the wild-type, indicating precise excision of the Tol2 transposon (i.e. a complete reversal to the wild-type genomic sequence after Tol2 excision). Footprints with various indels were detected in the remaining 25 (71.4%) clones (Figure 2a), suggesting that imprecise excision is dominant during Tol2 transposition. We also examined the Tol2 excision in Et(gata2:EGFP)pku760 fish line using similar method. Among 41 clones that were sequenced, nine (22.0%) showed precise excision, and the remaining 32 (78.0%) clones displayed footprints with different indels (Figure 2b), which is comparable with the situation in Et(gata2:EGFP)pku684.
Figure 1.
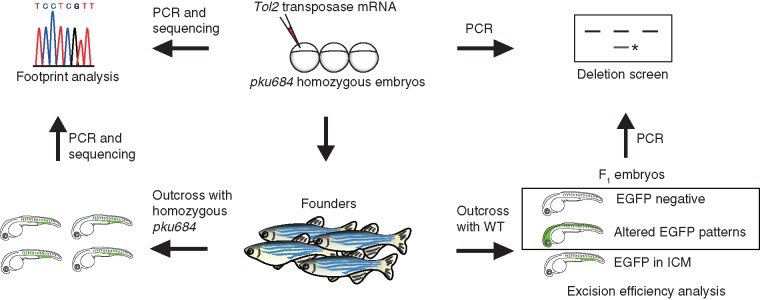
Schematic diagram showing the strategy for Tol2 excision analysis and large genomic deletion screening using Et(gata2a:EGFP)pku684 as an example. The Tol2 transposon was considered to be excised from the original insertion site in embryos with negative or altered EGFP patterns. Asterik, PCR band containing relatively large genomic deletions; WT, wild-type. Et(gata2a:EGFP)pku684 is simplified to pku684.
Figure 2.

Analysis of footprints and genomic deletions induced by Tol2 excision in zebrafish founder embryos. (a) Representative footprints detected at the lmo2 locus after Tol2 transposon excision. lFT1: precise excision; lFT2-5: imprecise excision. The arrow indicates the direction of transcription of lmo2 gene. (b) Representative footprints detected at the nav3 locus after Tol2 transposon excision. nFT1: precise excision; nFT2-5: imprecise excision. The arrow indicates the direction of transcription of nav3 gene. (c) Schematic diagram showing the 1093-bp and 1253-bp genomic deletions at the lmo2 locus induced by Tol2 excision. Inverted letters indicate that these elements (miniP and EGFP) were inserted reversely into the endogenous gene. Dashed lines indicate deletions. (d) Schematic diagram showing the 1340-bp genomic deletion at the nav3 locus induced by Tol2 excision. Inverted letters indicate that these elements (Tol2 5′ and Tol2 3′) were inserted reversely into the endogenous gene. Dashed lines indicate deletions. The 8-bp genomic duplication is shown in uppercase. WT, wild-type. Et(gata2a:EGFP)pku684 is simplified to pku684; Et(gata2a:EGFP)pku760 is simplified to pku760.
To analyse the excision efficiency and properties of the Tol2 transposon in the zebrafish germ line, we raised the mosaic Et(gata2a:EGFP)pku684 founder embryos to adulthood and outcrossed the founders with wild-type fish. The excision events were determined according to the EGFP patterns in F1 embryos at 24 hpf (Figure 1). Negative or altered EGFP patterns indicated that the Tol2 transposon had been excised from the original site in these F1 embryos. We analysed 1882 F1 embryos from 18 founders, and the excision efficiency of the Tol2 transposon was 42.0% on average, ranging from 1.4% to 100% (Table 1). To determine the footprints left by Tol2 excision, we crossed 13 mosaic founders from the above with homozygous Et(gata2a:EGFP)pku684 fish. The flanking sequences at the original insertion site of individual F1 embryos were PCR amplified and analysed by sequencing. On average, ∼36.5% of the offspring, ranging from null to 100%, carried exactly the same sequence as the wild-type, resulting from the precise excision of the Tol2 transposon, and 63.5% showed indels compared with the wild-type sequence, indicating that imprecise excision had occurred (Table 2 and Figure 3a). These results indicated that Tol2 transposon could be efficiently excised from the zebrafish genome, and imprecise excision is also dominant in heritable germline cells after remobilization of Tol2 transposon.
Table 1.
Efficiency of Tol2 transposon excision in Et(gata2a:EGFP)pku684 germline cells
| Founder | EGFP patterns of F1 embryosa |
Total F1 embryosa | Tol2 excision efficiency (%)c | ||
|---|---|---|---|---|---|
| ICMb | Negative | Non-ICM | |||
| Male 1 | 45 | 5 | 0 | 50 | 10.0 |
| Male 2 | 127 | 23 | 3 | 153 | 17.0 |
| Male 3 | 0 | 15 | 0 | 15 | 100.0 |
| Male 4 | 91 | 27 | 0 | 118 | 22.9 |
| Male 5 | 51 | 42 | 22 | 115 | 55.7 |
| Male 6 | 79 | 15 | 10 | 104 | 24.0 |
| Male 7 | 12 | 16 | 0 | 28 | 57.1 |
| Male 8 | 18 | 60 | 5 | 83 | 78.3 |
| Male 9 | 0 | 75 | 72 | 147 | 100.0 |
| Male 10 | 155 | 27 | 10 | 192 | 19.3 |
| Male 11 | 24 | 123 | 3 | 150 | 84.0 |
| Male 12 | 5 | 38 | 3 | 46 | 89.1 |
| Male 13 | 127 | 68 | 22 | 217 | 41.5 |
| Male 14 | 69 | 1 | 0 | 70 | 1.4 |
| Female 1 | 69 | 4 | 0 | 73 | 5.5 |
| Female 2 | 141 | 8 | 3 | 152 | 7.2 |
| Female 3 | 71 | 39 | 0 | 110 | 35.5 |
| Female 4 | 54 | 5 | 0 | 59 | 8.5 |
| Average | / | / | / | / | 42.0 |
aThe F1 embryos were obtained from crosses of each corresponding founder with wild-type fish.
bEGFP expression in ICM was considered to indicate no excision.
cEfficiency was calculated as percentage of embryos showing either ‘Negative’ or ‘Non-ICM’ GFP patterns over total embryos.
Table 2.
Footprints after Tol2 transposition in Et(gata2a:EGFP)pku684 germline cells
| Founder | Sequence Readsa | Precise (WT) |
Imprecise (Indels) |
||
|---|---|---|---|---|---|
| Reads | Frequency (%) | Reads | Frequency (%) | ||
| 1 | 28 | 25 | 89.3 | 3 | 10.7 |
| 2 | 39 | 36 | 92.3 | 3 | 7.7 |
| 3 | 37 | 0 | 0.0 | 37 | 100.0 |
| 4 | 7 | 7 | 100.0 | 0 | 0.0 |
| 5 | 6 | 0 | 0.0 | 6 | 100.0 |
| 6 | 22 | 17 | 77.3 | 5 | 22.7 |
| 7 | 1 | 0 | 0.0 | 1 | 100.0 |
| 8 | 10 | 1 | 10.0 | 9 | 90.0 |
| 9 | 19 | 1 | 5.3 | 18 | 94.7 |
| 10 | 11 | 11 | 100.0 | 0 | 0.0 |
| 11 | 7 | 0 | 0.0 | 7 | 100.0 |
| 12 | 24 | 0 | 0.0 | 24 | 100.0 |
| 13 | 14 | 0 | 0.0 | 14 | 100.0 |
| Average | / | / | 36.5 | / | 63.5 |
aThe sequence was obtained from individual F1 embryos from the crosses of each corresponding founder with homozygous Et(gata2a:EGFP)pku684 fish.
Figure 3.
Analysis of footprints and genomic deletions induced by Tol2 excision in the Et(gata2a:EGFP)pku684 germline. (a) Representative footprints detected in F1 embryos. lgFT1: precise excision; lgFT2-5: imprecise excision. The arrow indicates the direction of transcription of lmo2 gene. (b) PCR results to detect large genomic deletion events in F1 embryos. The red arrow denotes the PCR band containing relatively large genomic deletions. (c) Nucleotide sequence alignment showing the 1093-bp genomic deletion in lmo2pku684ld. The transcribed region of the lmo2 gene is underlined. The translation start site (ATG) is set as +1. The 8-bp genomic duplication is shown in uppercase. WT, wild-type. Et(gata2a:EGFP)pku684 is simplified to pku684; lmo2pku684ld is simplified to pku684ld.
The Tol2 transposon usually generates an 8-bp duplication of the flanking genomic target sequence after its integration, as seen in both of the two transgenic fish lines; however, we did not detect any footprints with a remaining duplication of exactly 8-bp after Tol2 remobilization in any of the two lines. Instead, a full reversal to the wild-type genomic sequence (i.e. precise elimination of one copy of the 8-bp duplication) is readily detectable in a relatively high frequency in both fish lines and also in the heritable germline cells of Et(gata2a:EGFP)pku684 fish. These observations are consistent with the previous reports showing that Tol2 excision could be either precise or imprecise (19,20).
To further analyse the properties of Tol2 transposon excision, we performed a plasmid-based excision assay in zebrafish embryos (Supplementary Figure S3a). The pTol2-GT2MP plasmid contains the Tol2 transposon flanked by an 8-bp duplication in the plasmid backbone to mimic the situation after genomic insertion. DNA fragments with expected size (560 bp) were only amplified in the plasmid and transposase co-injected wild-type embryos, suggesting successful excision of the Tol2 element and repair of the plasmid in the presence of the transposase (Supplementary Figure S3b). Among 24 clones that were sequenced, seven (29.2%) were exactly the same as the empty vector, indicating precise excision of the Tol2 transposon. Footprints with various indels were detected in 15 (62.5%) clones. Interestingly, two clones were found to give rise to more than 100-bp deletions flanking the insertion site after Tol2 excision, one being 173 bp at the 5′ end and the other 160 bp at the 3′ end (Supplementary Figure S3c). These results showed that precise excision leading to exact elimination of one copy of the 8-bp duplication flanking the Tol2 integration site can also occur in the plasmids outside the zebrafish genome, and the ratio of the precise excision was also comparable with that of Et(gata2a:EGFP)pku684 and Et(gata2:EGFP)pku760.
Taken together, all the aforementioned results demonstrated that the Tol2 transposon can be efficiently excised from the original insertion site in both precise and imprecise manners with the imprecise excision being dominant. More importantly, both excision types could be efficiently inherited through germline cells.
Identification of genomic deletions induced by Tol2 transposon excision
To determine whether genomic deletions larger than the indel mutations (e.g. >1 kb) can be induced by Tol2 excision, we first examined the flanking regions of the Tol2 insertion sites in mosaic Et(gata2:EGFP)pku684 and Et(gata2:EGFP)pku760 founders by PCR. If large genomic deletions occurred, smaller PCR products should be detected by electrophoresis (Figure 1). In Et(gata2:EGFP)pku684 founders, of 34 pools (107 founder embryos in total), we identified relatively large deletions in 10 pools (i.e. a much shorter PCR fragment than expected) 5′ to the original Tol2 insertion site. Sequencing results revealed two kinds of slightly different deletions. A 1093-bp deletion was identified in nine pools, and a 1253-bp deletion was identified in one pool (Figure 2c and Supplementary Figure S4). In Et(gata2:EGFP)pku760 founders, of 20 pools (100 founder embryos in total), we identified a 1340-bp deletion 3′ to the original Tol2 insertion site in one pool (Figure 2d and Supplementary Figure S5). We did not find comparable large deletions either at 3′ of the Tol2 transposon in Et(gata2:EGFP)pku684 or at the 5′ of the Tol2 transposon in Et(gata2:EGFP)pku760 founders embryos.
Next, we went on to determine whether similar large genomic deletions can be induced in germline cells. We crossed the mosaic Et(gata2a:EGFP)pku684 founders with wild-type zebrafish and picked out F1 embryos with either negative or altered EGFP patterns, in which the Tol2 transposon had been mobilized from the original insertion site. Of 59 founders, we identified one that transmitted a genomic deletion 5′ to the original Tol2 insertion site to its EGFP-negative offspring (Figure 3b). Sequencing result showed that the corresponding allele (called lmo2pku684ld) carried a 1093-bp deletion, the same as one of the two large deletions detected in the mosaic founder embryos (Figure 3c). However, we did not find any large deletion at 3′ to the Tol2 insertion site from these founders. To further confirm this deletion, we performed Southern blot using a probe located 5′ upstream of the deletion region (Figure 4a). The fish heterozygous for lmo2pku684ld showed a 4.7-kb wild-type band and a 3.7-kb band owing to the deletion (Figure 4b), as expected. These results showed that Tol2 transposon excision can induce relatively large genomic deletions after imprecise repair in both somatic and germline cells.
Figure 4.
Southern blot analysis of the genomic deletion at the lmo2 locus. (a) Schematic diagrams of the lmo2 locus in wild-type, Et(gata2a:EGFP)pku684 and lmo2pku684ld fish. Inverted letters indicate that these elements (miniP and EGFP) were inserted reversely into the endogenous gene. Blue bars indicate the position of the probe used for Southern blot. P and S indicate PstI and SacI, respectively. (b) Southern blot results of the lmo2 locus. WT, wild-type. Et(gata2a:EGFP)pku684 is simplified to pku684; lmo2pku684ld is simplified to pku684ld.
DISCUSSION
With the development of engineered endonuclease technologies, targeted disruption of genes of interest is now feasible in zebrafish (30–36). However, targeted deletion of relatively large DNA fragments in the zebrafish genome has not been reported, although it is also useful, and sometimes indispensable, for the dissection of gene functions and developmental mechanisms, as well as disease modeling. Here, we provide a strategy to achieve this type of genome modification by deleting the genomic region flanking the Tol2 insertion site through imprecise excision of this transposon. We have detected >1-kb genomic deletions in two independent transgenic fish lines, with one deletion showing inheritable germline transmission. Several large-scale enhancer trap and gene trap screens based on the Tol2 transposon have been carried out in our laboratory and by others (7–16,23). It has been shown that the Tol2 transposon tends to insert into or near genes, and many of its insertion sites have been identified (9,23). The collections of Tol2 insertions within or near a variety of genes comprise unique resources to achieve gene disruption through transposon excision. In addition, here we reported the identification of two enhancer trap lines Et(gata2:EGFP)pku684 and Et(gata2:EGFP)pku760, which faithfully recapitulates the expression pattern of the endogenous genes lmo2 and nav3, respectively. To our knowledge, Et(gata2:EGFP)pku760 is the first transgenic fish available to trace the live and dynamic expression of nav3, which is a valuable tool for studies of the function and regulation of this gene.
Our results and those of others have shown that Tol2 transposon excision is either precise or imprecise (Table 2) (19,20). When P element mobilizes from the chromosomal DNA, the original insertion site can be repaired by using its wild-type homologous chromosome as the template, resulting in precise excision (1). As we injected Tol2 transposase mRNA into homozygous transgenic embryos devoid of wild-type homologous chromosome, the wild-type sequence generated by Tol2 mobilization could not come from its homologous chromosome. This indicates that mobilization of the Tol2 transposon and elimination of the 8-bp duplication is an intrinsic activity/property of Tol2 transposase. We propose that Tol2 transposase may create a complementary sticky end from each of the two 8-bp duplications during transposition at the original insertion site. In this way, the original insertion site could be restored exactly to the wild-type sequence after repair/ligation. The imprecise footprints left after Tol2 excision may result from the modification of the DNA double-strand breaks through the non-homologous end-joining repair pathway. However, the precise mechanism of Tol2 transposition still needs further investigation.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Table 1 and Supplementary Figures 1–5.
FUNDING
The 973 program [2012CB945101 to B.Z.]; the National Natural Science Foundation of China [31110103904 and 30730056 to B.Z.]. Funding for open access charge: National Natural Science Foundation of China [31110103904 to B.Z.].
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank K. Kawakami for the Tol2 transposon and transposase plasmids; Y. Shen, Y. Gao and J. Zhang for technical support and lab management; Y. Jia, J. Chen, X. Yang and H. Cui for zebrafish maintenance; and I. C. Bruce for language editing.
REFERENCES
- 1.Adams MD, Sekelsky JJ. From sequence to phenotype: reverse genetics in Drosophila melanogaster. Nat. Rev. Genet. 2002;3:189–198. doi: 10.1038/nrg752. [DOI] [PubMed] [Google Scholar]
- 2.Witsell A, Kane DP, McVey M. Super-sized deletions: improved transposon excision screens using a mus309 mutant background. Fly. 2010;4:137–140. doi: 10.4161/fly.4.2.10918. [DOI] [PubMed] [Google Scholar]
- 3.Davidson AE, Balciunas D, Mohn D, Shaffer J, Hermanson S, Sivasubbu S, Cliff MP, Hackett PB, Ekker SC. Efficient gene delivery and gene expression in zebrafish using the Sleeping Beauty transposon. Dev. Biol. 2003;263:191–202. doi: 10.1016/j.ydbio.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Emelyanov A, Gao Y, Naqvi NI, Parinov S. Trans-kingdom transposition of the maize dissociation element. Genetics. 2006;174:1095–1104. doi: 10.1534/genetics.106.061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koga A, Cheah FS, Hamaguchi S, Yeo GH, Chong SS. Germline transgenesis of zebrafish using the medaka Tol1 transposon system. Dev. Dyn. 2008;237:2466–2474. doi: 10.1002/dvdy.21688. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami K, Shima A, Kawakami N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc. Natl Acad. Sci. USA. 2000;97:11403–11408. doi: 10.1073/pnas.97.21.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell. 2004;7:133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Parinov S, Kondrichin I, Korzh V, Emelyanov A. Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev. Dyn. 2004;231:449–459. doi: 10.1002/dvdy.20157. [DOI] [PubMed] [Google Scholar]
- 9.Kondrychyn I, Garcia-Lecea M, Emelyanov A, Parinov S, Korzh V. Genome-wide analysis of Tol2 transposon reintegration in zebrafish. BMC Genomics. 2009;10:418. doi: 10.1186/1471-2164-10-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark KJ, Balciunas D, Pogoda HM, Ding Y, Westcot SE, Bedell VM, Greenwood TM, Urban MD, Skuster KJ, Petzold AM, et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nat. Methods. 2011;8:506–512. doi: 10.1038/nmeth.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinh LA, Hochgreb T, Graham M, Wu D, Ruf-Zamojski F, Jayasena CS, Saxena A, Hawk R, Gonzalez-Serricchio A, Dixson A, et al. A versatile gene trap to visualize and interrogate the function of the vertebrate proteome. Genes Dev. 2011;25:2306–2320. doi: 10.1101/gad.174037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakami K, Abe G, Asada T, Asakawa K, Fukuda R, Ito A, Lal P, Mouri N, Muto A, Suster ML, et al. zTrap: zebrafish gene trap and enhancer trap database. BMC Dev. Biol. 2010;10:105. doi: 10.1186/1471-213X-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagayoshi S, Hayashi E, Abe G, Osato N, Asakawa K, Urasaki A, Horikawa K, Ikeo K, Takeda H, Kawakami K. Insertional mutagenesis by the Tol2 transposon-mediated enhancer trap approach generated mutations in two developmental genes: tcf7 and synembryn-like. Development. 2008;135:159–169. doi: 10.1242/dev.009050. [DOI] [PubMed] [Google Scholar]
- 14.Urasaki A, Asakawa K, Kawakami K. Efficient transposition of the Tol2 transposable element from a single-copy donor in zebrafish. Proc. Natl Acad. Sci. USA. 2008;105:19827–19832. doi: 10.1073/pnas.0810380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asakawa K, Suster ML, Mizusawa K, Nagayoshi S, Kotani T, Urasaki A, Kishimoto Y, Hibi M, Kawakami K. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl Acad. Sci. USA. 2008;105:1255–1260. doi: 10.1073/pnas.0704963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asakawa K, Kawakami K. The Tol2-mediated Gal4-UAS method for gene and enhancer trapping in zebrafish. Methods. 2009;49:275–281. doi: 10.1016/j.ymeth.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kempken F, Windhofer F. The hAT family: a versatile transposon group common to plants, fungi, animals, and man. Chromosoma. 2001;110:1–9. doi: 10.1007/s004120000118. [DOI] [PubMed] [Google Scholar]
- 18.Mates L, Izsvak Z, Ivics Z. Technology transfer from worms and flies to vertebrates: transposition-based genome manipulations and their future perspectives. Genome Biol. 2007;8(Suppl. 1):S1. doi: 10.1186/gb-2007-8-s1-s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koga A, Suzuki M, Inagaki H, Bessho Y, Hori H. Transposable element in fish. Nature. 1996;383:30. doi: 10.1038/383030a0. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami K, Koga A, Hori H, Shima A. Excision of the tol2 transposable element of the medaka fish, Oryzias latipes, in zebrafish, Danio rerio. Gene. 1998;225:17–22. doi: 10.1016/s0378-1119(98)00537-x. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami K, Shima A. Identification of the Tol2 transposase of the medaka fish Oryzias latipes that catalyzes excision of a nonautonomous Tol2 element in zebrafish Danio rerio. Gene. 1999;240:239–244. doi: 10.1016/s0378-1119(99)00444-8. [DOI] [PubMed] [Google Scholar]
- 22.Kawakami K, Imanaka K, Itoh M, Taira M. Excision of the Tol2 transposable element of the medaka fish Oryzias latipes in Xenopus laevis and Xenopus tropicalis. Gene. 2004;338:93–98. doi: 10.1016/j.gene.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Xue YL, Xiao A, Wen L, Jia Y, Gao Y, Zhu ZY, Lin S, Zhang B. Generation and characterization of blood vessel specific EGFP transgenic zebrafish via To12 transposon mediated enhancer trap screen. Prog. Biochem. Biophys. 2010;37:720–727. [Google Scholar]
- 24.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 25.Xue YL, Kuok C, Xiao A, Zhu ZY, Lin S, Zhang B. Identification and expression analysis of mical family genes in zebrafish. J. Genet. Genomics. 2010;37:685–693. doi: 10.1016/S1673-8527(09)60086-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhu H, Traver D, Davidson AJ, Dibiase A, Thisse C, Thisse B, Nimer S, Zon LI. Regulation of the lmo2 promoter during hematopoietic and vascular development in zebrafish. Dev. Biol. 2005;281:256–269. doi: 10.1016/j.ydbio.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Z, Song J, Qi F, Xiao A, An X, Liu NA, Zhu Z, Zhang B, Lin S. Exdpf is a key regulator of exocrine pancreas development controlled by retinoic acid and ptf1a in zebrafish. PLoS Biol. 2008;6:e293. doi: 10.1371/journal.pbio.0060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein C, Mikutta J, Krueger J, Scholz K, Brinkmann J, Liu D, Veerkamp J, Siegel D, Abdelilah-Seyfried S, le Noble F. Neuron navigator 3a regulates liver organogenesis during zebrafish embryogenesis. Development. 2011;138:1935–1945. doi: 10.1242/dev.056861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thisse B, Pflumio S, Fürthauer M, Loppin B, Heyer V, Degrave A, Woehl R, Lux A, Steffan T, Charbonnier XQ, et al. Expression of the zebrafish genome during embryogenesis. ZFIN online publication. 2001 [Google Scholar]
- 30.Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, Amora R, Hocking TD, Zhang L, Rebar EJ, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat. Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B. Heritable gene targeting in zebrafish using customized TALENs. Nat. Biotechnol. 2011;29:699–700. doi: 10.1038/nbt.1939. [DOI] [PubMed] [Google Scholar]
- 33.Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, Yeh JR. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat. Biotechnol. 2011;29:697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foley JE, Yeh JR, Maeder ML, Reyon D, Sander JD, Peterson RT, Joung JK. Rapid mutation of endogenous zebrafish genes using zinc finger nucleases made by Oligomerized Pool ENgineering (OPEN) PLoS ONE. 2009;4:e4348. doi: 10.1371/journal.pone.0004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sander JD, Dahlborg EJ, Goodwin MJ, Cade L, Zhang F, Cifuentes D, Curtin SJ, Blackburn JS, Thibodeau-Beganny S, Qi Y, et al. Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA) Nat. Methods. 2011;8:67–69. doi: 10.1038/nmeth.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang P, Zhu Z, Lin S, Zhang B. Reverse genetic approaches in zebrafish. J. Genet. Genomics. 2012;39:421–433. doi: 10.1016/j.jgg.2012.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




