Abstract
DEAD-box RNA helicases play important roles in remodeling RNA molecules and in facilitating a variety of RNA-protein interactions that are key to many essential cellular processes. In spite of the importance of RNA, our knowledge about RNA helicases is limited. In this study, we investigated the role of the four DEAD-box RNA helicases in the Gram-positive model organism Bacillus subtilis. A strain deleted of all RNA helicases is able to grow at 37°C but not at lower temperatures. The deletion of cshA, cshB, or yfmL in particular leads to cold-sensitive phenotypes. Moreover, these mutant strains exhibit unique defects in ribosome biogenesis, suggesting distinct functions for the individual enzymes in this process. Based on protein accumulation, severity of the cold-sensitive phenotype, and the interaction with components of the RNA degradosome, CshA is the major RNA helicase of B. subtilis. To unravel the functions of CshA in addition to ribosome biogenesis, we conducted microarray analysis and identified the ysbAB and frlBONMD mRNAs as targets that are strongly affected by the deletion of the cshA gene. Our findings suggest that the different helicases make distinct contributions to the physiology of B. subtilis. Ribosome biogenesis and RNA degradation are two of their major tasks in B. subtilis.
INTRODUCTION
RNA molecules participate in a large number of cellular processes. Due to their relative simplicity (they are composed of only four different nucleic acid building blocks) and the molecular crowding found in the cytoplasm of cells, RNAs are prone to form undesired inter- and intramolecular interactions. In order to prevent these interactions, every cell expresses proteins that assist the RNA molecules to function properly. A major class of these proteins are DEAD-box RNA helicases (1).
DEAD-box RNA helicases are ubiquitous enzymes that consist of a highly conserved helicase core comprising two RecA-like domains. Within the two domains, 12 characteristic sequence motifs have been identified. These motifs are involved in ATP and RNA binding, ATP hydrolysis, and the communication between the different sites. Motif II contains the name-giving Asp-Glu-Ala-Asp, or DEAD, amino acid sequence (for reviews, see references 1 and 2). In addition to the conserved helicase core, most DEAD-box proteins contain variable N- or C-terminal extensions. These additional domains often confer specificity for their substrates and interaction partners and are thus involved in the specific functional output and regulation of the DEAD-box protein (3, 4, 5, 6).
As RNA molecules are an integral part of every cell, RNA helicases are involved in a variety of different processes. In eukaryotic cells, RNA helicases are implicated in all processes that involve RNA; among them are transcription, splicing, mRNA export, ribosome biogenesis, translation, and mRNA decay (2). The impact of these enzymes is underlined by the fact that yeasts like Saccharomyces cerevisiae harbor 26 DEAD-box genes, of which 18 are essential (7).
The Gram-negative model organism Escherichia coli encodes five DEAD-box proteins which are mainly involved in ribosome assembly, mRNA decay, and cold adaptation (8). For example, the RNA helicase RhlB is an integral part of the RNA degradosome of E. coli, a protein complex involved in the degradation of mRNA (9). Deletion of the gene affects steady-state levels of hundreds of mRNAs (10). While RhlB participates solely in mRNA decay, other RNA helicases are more versatile. The DEAD-box RNA helicase CsdA is implicated in three different cellular processes: mRNA decay, ribosome biogenesis, and translational initiation. Its involvement in mRNA decay was demonstrated by the fact that overexpression of the protein results in the stabilization of the cspA mRNA, encoding the major cold shock protein A (CspA) (11), and by its binding to the RNA degradosome under cold shock conditions (12). Furthermore, csdA deletion strains are impaired in the proper formation of the large ribosomal subunit (13) and in the translation initiation process of the rpoS mRNA at low temperature (14).
While E. coli encodes five different RNA helicases, the Gram-positive model organism B. subtilis contains four enzymes: CshA, CshB, DeaD, and YfmL (http://www.subtiwiki.uni-goettingen.de/wiki/index.php/DEAD-box_RNA_helicases and reference 15). All four RNA helicases have the conserved catalytic core and possess, with the exception of YfmL, distinctly different C-terminal domains.
CshA is the largest of the four RNA helicases with a specific domain at its C terminus. This protein is suggested to be the functional homolog of RhlB of E. coli with respect to its ability to bind to the RNA degradosome (5, 16). CshA, as well as the RNA helicase CshB, is involved in cold adaptation (17). Both proteins are localized around the nucleoid, a pattern reminiscent of that of ribosomes or cold-shock proteins (CSP). Further fluorescence resonance energy transfer analysis using CshB and the cold shock protein B (CspB) demonstrated interaction of these two proteins, supporting a role of CshB in cold adaptation (17). For the RNA helicase CshA, the RNA unwinding activity of the enzyme was demonstrated in vitro (18). Conflicting results were reported concerning the role of CshA and CshB for viability of B. subtilis. Hunger et al. (17) reported that the deletion of one of the RNA helicases has no effect on growth at low temperature, whereas the loss of both helicases is lethal even at 37°C. In contrast, Ando and Nakamura (18) demonstrated that deletion of cshA resulted in a growth defect at 22°C but not at elevated temperatures. The latter finding is consistent with results obtained with Bacillus cereus: the deletion in the corresponding cshA homolog results in a severe growth defect at lower temperatures in this species as well (19, 20). A further analysis of the CshA and CshB homologs of B. cereus revealed that the RNA helicases are also required for growth at a basic pH and under oxidative conditions (21).
Another DEAD-box RNA helicase of B. subtilis is DeaD/YxiN. This enzyme was extensively investigated with a focus on the molecular mechanisms of its catalytic function, whereas nothing is known about the physiological role of the protein, except that it has the ability to bind the 23S rRNA in vitro (22, 23, 24). The role of the RNA helicase YfmL in B. subtilis is completely unknown.
In this study, we reveal that all DEAD-box RNA helicases are dispensable for the growth of B. subtilis at higher temperatures. In contrast, CshA, CshB, and YfmL have nonredundant functions at low temperature. This is supported by the finding that these three helicases are implicated in ribosome biogenesis. CshA has multiple roles in the physiology of B. subtilis. Cells lacking this RNA helicase exhibit an aberrant morphology at lower temperatures. Furthermore, we show that CshA interacts with components of the predicted RNA degradosome and with ribosomal proteins. Finally, we show that CshA affects the expression of the frlBONMD and ysbAB operons encoding proteins involved in the utilization of fructoselysine and in the modulation of murein hydrolysis, respectively (25, 26).
MATERIALS AND METHODS
Bacterial strains, growth conditions, and transformation.
The B. subtilis strains used in this study are listed in Table 1. All B. subtilis strains are derivatives of the wild-type strain 168. E. coli DH5α (28) was used for cloning experiments.
Table 1.
Bacillus subtilis strains used in this study
| Strain | Genotype | Source or referencea |
|---|---|---|
| 168 | trpC2 | Laboratory collection |
| GP899 | trpC2 Δnfo::aphA3 | 27 |
| GP1010 | trpC2 cshA-FLAG spc | 5 |
| GP1011 | trpC2 cshB-FLAG spc | 5 |
| GP1026 | trpC2 cshA-Strep aphA3 | 5 |
| GP1035 | trpC2 ΔcshA::aphA3 | This study |
| GP1051 | trpC2 ΔcshB::cat | This study |
| GP1052 | trpC2 ΔdeaD::tet | This study |
| GP1053 | trpC2 ΔyfmL::mls | This study |
| GP1063 | trpC2 ΔcshA::aphA3 ΔcshB::cm ΔdeaD::tet ΔyfmL::mls | This study |
| GP1066 | trpC2 yfmL-FLAG spc | This study |
| GP1068 | trpC2 deaD-FLAG spc | This study |
| GP1083 | trpC2 ΔcshA::cat | This study |
| GP1084 | trpC2 ΔcshA::cat lacA::cshA aphA3 | pGP1886→GP1083 |
| GP1086 | trpC2 ΔcshB::cat lacA::cshB aphA3 | pGP1857→GP1051 |
| GP1087 | trpC2 ΔyfmL::mls lacA::yfmL aphA3 | pGP1858→GP1053 |
| GP1095 | trpC2 cshA-Strep aphA3 rplA-FLAG spc | pGP1895→GP1026 |
Arrows indicate construction by transformation.
B. subtilis was grown in LB medium, sporulation (SP) medium (8 g of nutrient broth per liter, 1 mM MgSO4, 13 mM KCl; supplemented after sterilization with 2.5 μM FeSO4, 500 μM CaCl2, and 10 μM MnCl2), or C minimal medium with succinate and glutamate (CSE) (29). The media were supplemented with auxotrophic requirements (at 50 mg/liter). E. coli was grown in LB medium, and transformants were selected on plates containing ampicillin (100 μg/ml). LB plates were prepared by the addition of 17 g Bacto agar/liter to LB.
B. subtilis was transformed with plasmid DNA according to the two-step protocol described previously (30). Transformants were selected on SP plates containing kanamycin (10 μg/ml), chloramphenicol (5 μg/ml), spectinomycin (150 μg/ml), tetracycline (12.5 μg/ml), or erythromycin (3 μg/ml) and lincomycin (25 μl/ml).
DNA manipulation.
Transformation of E. coli and plasmid DNA extraction were performed using standard procedures (28). Restriction enzymes, T4 DNA ligase, and DNA polymerases were used as recommended by the manufacturers. DNA sequences were determined using the dideoxy chain termination method (28). Chromosomal DNA of B. subtilis was isolated as described previously (30). All plasmids and oligonucleotides used in this study are listed in Tables S1 and S2, respectively, in the supplemental material.
Construction of deletion and complementation strains.
Deletion of the genes of cshA, cshB, deaD, and yfmL was achieved by transformation with PCR products constructed using oligonucleotides (see Table S2 in the supplemental material) to amplify DNA fragments flanking the target genes and intervening antibiotic resistance cassettes (31), as described previously (32). The quadruple mutant was generated by successively transforming chromosomal DNA of the RNA helicase mutant strains into strain GP1035.
For complementation analyses, we made use of the integrative vector pGP888, which allows integration into the nonessential lacA gene (33). Briefly, we amplified the cshA, cshB, and yfmL genes using appropriate primer pairs (see Table S2 in the supplemental material). The PCR fragments were digested with XbaI and KpnI and cloned into pGP888, resulting in the formation of plasmids pGP1886 (cshA), pGP1857 (cshB), and pGP1858 (yfmL). Finally, the plasmids were linearized with ScaI and used to transform the appropriate B. subtilis mutant strains.
Analyses of growth and cell morphology of the RNA helicase mutant strains at low temperature.
To investigate the impact of the deletions of the individual RNA helicase genes on growth, the growth of different strains in LB medium at 16 and 37°C was determined.
To investigate the morphology of the RNA helicase mutant strains, the mutants were cultivated as follows. LB medium was inoculated with an overnight culture of the corresponding strains. These cultures were grown at 37°C. At an optical density of 600 nm (OD600) of 1.0, these cultures were used to inoculate new cultures to an OD600 of 0.05. These cultures were grown under vigorous agitation at 20°C. Cells of every mutant strain were analyzed by phase-contrast microscopy at an optical density of 0.8.
Expression analyses.
To monitor the expression patterns of the DEAD-box RNA helicases, we used strains expressing the helicases labeled with a C-terminal triple FLAG tag. The respective strains for CshA and CshB were available (5). For DeaD and YfmL, vector pGP1331 (5) was used to fuse the proteins to a triple FLAG tag. Briefly, the 3′ ends of deaD and yfmL were amplified using primer pairs ML181/ML182 and ML127/ML128 and cloned into pGP1331 using the BamHI and SalI restriction sites. The resulting plasmids were pGP1345 (deaD-3×FLAG) and pGP1346 (yfmL-3×FLAG). Both plasmids integrate by single crossover into the chromosome of B. subtilis, leading to strains GP1068 (deaD::pGP1346) and GP1066 (yfmL::pGP1346). Growth experiments with the B. subtilis strains GP1010, GP1011, GP1068, and GP1066 were performed as described above. Samples for Western blot analysis were taken throughout growth.
Analysis of ribosome profiles.
Relative amounts of the mature 70S ribosomes and of the 30S and 50S ribosomal subunits were investigated by sucrose density gradient centrifugation of cell extracts. For this purpose, B. subtilis cells were grown to an OD600 of 0.4 to 0.6 in 75 ml LB medium at 20°C. Chloramphenicol (100 μg/ml) was added to the culture 5 min prior to harvesting to avoid ribosome runoff. Cells were centrifuged and washed in a minimum of 30 ml magnesium washing buffer (10 mM Tris-HCl, pH 7.5, 60 mM KCl, 10 mM MgCl2). The cell pellets were stored at −20°C or directly resuspended in 1.3 ml lysis buffer (10 mM Tris-HCl, pH 7.5, 60 mM KCl, 2 mM MgCl2, 0.5% Tween 20, 0.5% sodium deoxycholate) containing 10 U/ml DNase I. Cells were lysed using a French press (three cycles each of 1,000 lb/in2). The cell extract was clarified by centrifugation at 20,000 × g for 15 min. Seven A260 units were applied to a precooled linear sucrose gradient of 10 to 40% sucrose. These gradients were prepared by applying 10 and 40% sucrose solutions to the ISCO640 density gradient fractionator. Sucrose solutions were prepared by dissolving the sucrose in a 2× sucrose buffer and subsequently adding water to the desired volume. Loaded gradients were centrifuged for 16 h at 22,000 rpm (82,800 × g) and 4°C using the TH-641 swinging bucket rotor. After centrifugation, the gradients were continuously monitored at 254 nm with the ISCO640 density gradient fractionator.
Northern blotting.
Preparation of total RNA and Northern blot analysis were carried out as described previously (34). Digoxigenin (DIG) RNA probes were obtained by in vitro transcription with T7 RNA polymerase (Roche Diagnostics) using PCR-generated DNA fragments as templates. The primer pairs used to amplify DNA fragments specific for ysbA and frlB are listed in Table S2 in the supplemental material. The reverse primers contained a T7 RNA polymerase recognition sequence. In vitro RNA labeling, hybridization, and signal detection were carried out according to the instructions of the manufacturer (DIG RNA labeling kit and detection chemicals; Roche Diagnostics). The sizes of the transcripts were estimated based on the sizes of the transcripts of the gapA operon (data not shown).
In vivo detection of protein-protein interactions.
To isolate interaction partners of CshA, we used strain GP1026, which harbors a CshA variant with a Strep tag at its C-terminal end that is expressed from the native promoter of cshA (5). The isolation of protein complexes from B. subtilis cells was performed by SPINE technology (35). Briefly, growing cultures of B. subtilis were treated with formaldehyde (0.6% [wt/vol] for 20 min) to facilitate cross-linking of interacting proteins (35). The Strep-tagged proteins and their potential interaction partners were then purified from crude extracts using a Streptactin column (IBA, Göttingen, Germany) and desthiobiotin as the eluent. Twenty-five μl of the elution fraction was subjected to SDS-PAGE. Prior to electrophoresis, the protein samples were boiled for 20 min in Laemmli buffer to reverse the cross-links. Mass spectrometry (MS) to identify protein bands of interest was performed at the Service Unit of the University of Köln (Germany).
To verify the interaction between CshA and the ribosomal protein L1, we fused a triple FLAG tag to the C terminus of L1 using pGP1087 (33). For this purpose the 3′ end of rplA was amplified using the primer pair ML302/ML303 and cloned between the HindIII and KpnI sites of pGP1087. The resulting plasmid was pGP1895 (rplA-3×FLAG). This plasmid was transformed into strain GP1026, giving GP1095. B. subtilis GP1095 was used to purify CshA as described above. To verify that the interaction between CshA and L1 is direct and not RNA mediated, we extensively treated the extract with RNase A (100 μg/ml; 30 min at room temperature). Proteins from the extract and the CshA elution fraction were analyzed for the presence of L1-FLAG.
B2H assay.
Primary protein-protein interactions were identified by bacterial two-hybrid (B2H) analysis (36). The B2H system is based on the interaction-mediated reconstruction of Bordetella pertussis adenylate cyclase (CyaA) activity in E. coli. Functional complementation between two fragments (T18 and T25) of CyaA as a consequence of the interaction between bait and prey molecules results in the synthesis of cyclic AMP (cAMP), which is monitored by measuring the β-galactosidase activity of the cAMP-catabolite gene activator protein-dependent promoter of the E. coli lac operon. Plasmids pUT18 and p25N allow the expression of proteins fused to the N terminus of the T18 and T25 fragments of CyaA, respectively, whereas pUT18C allows the expression of proteins fused to the C terminus of the T18 fragment of CyaA. The plasmids constructed for the B2H assay (see Table S1 in the supplemental material) were used for cotransformation of E. coli BTH101, and the protein-protein interactions were then analyzed by plating the cells on LB plates containing 100 μg/ml ampicillin, 50 μg/ml kanamycin, 40 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), and 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The plates were incubated for a maximum of 36 h at 30°C.
Transcriptome analysis.
For transcriptome analysis, cells were grown in CSE-Glu. Samples of wild-type and cshA mutant strains were harvested by centrifugation (10,397 × g, 1 min, 4°C) at mid-exponential phase and 2 h after the transition to the stationary phase. A total of four independent biological replicates were included. The pellets were immediately frozen in liquid nitrogen and stored at −80°C. RNA extraction was performed with the Macaloid/Roche protocol (37). RNA concentration and purity was assessed using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). RNA samples were reverse transcribed into cDNA using the Superscript III reverse transcriptase kit (Invitrogen, Carlsbad, CA) and labeled with Cy3 or Cy5 monoreactive dye (GE Healthcare, Amersham, The Netherlands). Labeled and purified cDNA samples (Nucleospin Extract II; Biokè, Leiden, The Netherlands) were hybridized in Ambion Slidehyb #1 buffer (Ambion Europe, Ltd.) at 48°C for 16 h. The arrays were constructed as described elsewhere (38, 57). Briefly, specific oligonucleotides for all 4,107 open reading frames of B. subtilis 168 were spotted in duplicate onto aldehyde-coated slides (Cell Associates) and further handled using standard protocols for aldehyde slides. Slide spotting, slide treatment after spotting, and slide quality control were done as before (39). After hybridization, slides were washed for 5 min in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) with 0.5% SDS, 2 times for 5 min in 1× SSC with 0.25% SDS, 5 min in 1× SSC 0.1% SDS, dried by centrifugation (2 min, 2,000 rpm), and scanned in a GenePix 4200AL (Axon Instruments, CA). Fluorescent signals were quantified using ArrayPro 4.5 (Media Cybernetics Inc., Silver Spring, MD) and further processed and normalized with MicroPrep (40). CyberT (41) was used to perform statistical analysis. Genes with a Bayes P value of ≤1.0 × 104 were considered significantly affected.
Electron microscopy.
Wild-type and cshA mutant strains were grown as described for the analysis of ribosome profiles and harvested by centrifugation. Concentrated cells were filled into a 200-μm-deep aluminum platelet (Microscopy Services) and frozen using a BalTec HPM 10. Freeze substitution was performed at −90°C in a Leica EM AFS as described previously (42). Ultrathin sectioning and staining of the samples was carried out as described elsewhere (43). Sections were analyzed with a Jeol JEM 1011.
Microarray data accession number.
Microarray data have been deposited in the Gene Expression Omnibus database (accession no. GSE36877).
RESULTS
Consequences of deletions of individual DEAD-box helicase genes and of the complete helicase complement.
To get insight into the physiological role of the DEAD-box RNA helicases in B. subtilis, we constructed a series of strains with deletions of any of the four helicase-encoding genes by long flanking homology PCR. In addition, a strain lacking all four DEAD-box RNA helicases was constructed. One of the main functions of DEAD-box RNA helicases is the binding and remodeling of secondary structures of RNA molecules. The formation of such secondary structures is thermodynamically favored at lower temperatures. Therefore, RNA helicases play an important role for the adaptation to the cold (44). To test whether the B. subtilis DEAD-box RNA helicases are involved in this process, we compared growth of the wild-type and isogenic helicase mutant strains at standard laboratory and reduced temperatures (37 and 16°C, respectively). As shown in Fig. 1, all strains were viable at 37°C. Growth of the cshA mutant was slightly reduced. To exclude the possibility that the mutation caused any polar effect, we constructed a cshA mutant strain harboring an ectopic copy of cshA in the lacA locus (GP1084). This complementation fully restored growth of the cshA mutant. At 37°C, the bacteria are able to grow even in the absence of any DEAD-box RNA helicase, as shown by the growth of the quadruple mutant strain GP1063. Different results were observed at 16°C (Fig. 2). At this temperature, the wild-type strain grew well in LB medium. In contrast, poor growth was visible for the cshA mutant strain GP1083 (Fig. 2A). Ectopic expression of cshA fully restored growth of the strain in the ΔcshA background (Fig. 2B), demonstrating that only the loss of CshA causes the cold sensitivity of the mutant strain. These results indicate that cshA is important for the growth of B. subtilis at low temperatures. For the cshB and yfmL mutant strains, GP1051 and GP1053, respectively, we also observed impaired growth at 16°C (Fig. 2A). While yfmL forms a monocistronic transcription unit, cshB is the first gene of the bicistronic cshB-nfo operon (45). However, the nfo mutant GP899 grew as well as the wild type at low temperature, suggesting that the observed phenotype is indeed caused by the deletion of the DEAD-box RNA helicase CshB. Moreover, a complementation analysis with both mutations revealed the restoration of growth at low temperature (Fig. 2B). Finally, the deletion of the deaD gene did not affect growth at low temperature under our conditions. In contrast to the situation at 37°C, the quadruple mutant was unable to grow at 16°C, suggesting an accumulative effect of the mutations. Taken together, our results indicate that three of the four helicases are individually important for growth at low temperature. These findings imply that the three helicases CshA, CshB, and YfmL play important and nonredundant roles at low temperature.
Fig 1.
DEAD-box RNA helicases are dispensable at 37°C. The B. subtilis wild-type strain 168 and the isogenic ΔcshA, ΔcshB, ΔdeaD, and ΔyfmL mutant strains were grown at 37°C in LB medium. In addition, growth of a quadruple mutant devoid of all four RNA helicases (Δ4) and of a strain with ectopic expression of cshA was recorded.
Fig 2.
cshA, cshB, and yfmL are important for growth at low temperatures. (A) The B. subtilis wild-type strain 168, the isogenic ΔcshA, ΔcshB, ΔdeaD, and ΔyfmL mutant strains, as well as the quadruple mutant devoid of all four RNA helicases (Δ4) were grown at 16°C in LB medium. (B) Complementation analysis of the ΔcshA, ΔcshB, and ΔyfmL mutants.
Furthermore, by phase-contrast microscopy we observed that the cell morphology of the cshB, deaD, and yfmL mutants was indistinguishable from that of the wild-type strain at 20°C. In contrast, the cshA mutant strain had altered cell morphology, with wrinkled and elongated cells at low temperatures (Fig. 3). Further investigation of this effect by electron microscopy revealed that the cells were somewhat smaller in diameter and contained a thickened cell wall (see Fig. S1 in the supplemental material).
Fig 3.
Morphology of the wild-type and helicase mutant cells grown at 20°C in LB medium.
Expression of the DEAD-box RNA helicases in B. subtilis.
To assess the amount of the four DEAD-box RNA helicases in the cell, we fused a triple FLAG to the C terminus of individual genes, grew the resulting strains in rich medium at 20°C, and analyzed the amount of the enzymes during logarithmic growth, at the transition from the exponential to the stationary phase, and in the stationary phase. All strains exhibited identical growth behavior, suggesting that the triple FLAG tag had no influence on the activity of the enzymes.
The Western blot analysis revealed that all four DEAD-box RNA helicases were strongly expressed in the exponential phase (Fig. 4, time point A). Since all proteins were detected with the same antibody, the experimental setup used here also allows conclusions about the relative accumulation of the four proteins. Of the four proteins, CshA gave the strongest signal, suggesting that it is the most abundant DEAD-box helicase and accounts for more than 50% of the total RNA helicase pool in growing cells. The expression pattern changed drastically at later time points. While the abundance of CshA remained constant throughout growth even in late stationary phase, the amounts of CshB, DeaD, and YfmL were decreased at later time points. The signals for CshB, DeaD, and YfmL were significantly reduced already in the transient phase (Fig. 4, time point B). In the late stationary phase no signal for CshB and only very weak signals for DeaD and YfmL could be detected (Fig. 4, time point C).
Fig 4.
CshA is the most abundant RNA helicase in B. subtilis. Crude extracts were isolated from the B. subtilis strains GP1010 (cshA-FLAG), GP1011 (cshB-FLAG), GP1068 (deaD-FLAG), and GP1066 (yfmL-FLAG) grown in LB medium at 20°C. Fifteen μg of protein of the crude extract of each culture was loaded onto a 12.5% SDS-polyacrylamide gel. After electrophoresis and blotting onto a polyvinylidene difluoride (PVDF) membrane, the FLAG tag was detected using rabbit polyclonal antibodies. All cultures were harvested in the exponential (A), transition (B), or late exponential phase (C).
This experiment demonstrates (i) that CshA is the major DEAD-box RNA helicase of B. subtilis in terms of cellular levels, and (ii) that the cellular amounts of CshA exhibit a pattern distinct from those of the other RNA helicases. While CshB, DeaD, and YfmL partially disappeared in the stationary phase, the amount of CshA remained constant, giving this protein an even higher impact.
Impact of the RNA helicases on ribosome biogenesis.
The biogenesis of ribosomes is a fundamental process in every organism. Many factors contribute to this elaborate process, including DEAD-box RNA helicases. So far, comprehensive insight into the function of these enzymes is present only for E. coli. In contrast, nothing is known about the contribution of these proteins in Gram-positive bacteria, except for one RNA helicase in Listeria monocytogenes (46). Therefore, we performed sucrose density gradient centrifugation to obtain ribosomal profiles of single and multiple DEAD-box RNA helicase mutants of B. subtilis to assess the contribution of these enzymes to ribosome biogenesis.
At first we compared the abundances of the ribosome and its subunits in the wild type and in a strain lacking all four DEAD-box RNA helicases. The profile of the wild type showed small amounts of 30S and 50S subunits and large amounts of the mature 70S ribosome (Fig. 5A). In cells of B. subtilis GP1063, which is completely devoid of DEAD-box RNA helicases, we observed considerable changes in the biogenesis of the ribosomes (Fig. 5B). First, the intensity of the mature 70S ribosomes was strongly decreased. Furthermore, the abundance of the large 50S subunit was significantly decreased compared to that of the small 30S subunit. In extracts from wild-type cells the intensity of the signal of the 50S subunit was twice as high as that of the 30S subunit; in contrast, the 50S subunit was reduced compared to 30S levels in the quadruple mutant. These findings indicate that one or several DEAD-box RNA helicases are involved in ribosome assembly in B. subtilis.
Fig 5.
Absence of CshA, CshB, and YfmL causes distinct deficiencies in ribosome biogenesis. The B. subtilis wild-type strain 168 (A), the quadruple mutant strain (B), and the individual helicase mutant strains (cshA [C], cshB [D], deaD [E], and yfmL [F]) were grown in LB medium at 20°C. Ribosomal profiles were analyzed as described in Materials and Methods. Peaks of the free 30S and 50S subunits and 70S ribosomes are indicated.
To discern the relative contribution of the four DEAD-box RNA helicases to ribosome biogenesis, we studied the ribosome profiles of the individual mutants. The lack of the DEAD-box RNA helicase CshA resulted in strongly decreased amounts of mature ribosomes. Moreover, the intensity ratio for the 30S and 50S subunits was shifted to the small subunits, as observed in the quadruple mutant (Fig. 5C). These results suggest that the RNA helicase CshA is involved in the assembly of 70S ribosomes in B. subtilis via a reduction of the number of 50S subunits. We next analyzed the profile of extracts obtained from cells deleted of cshB. The lack of this DEAD-box RNA helicase also resulted in an aberrant ribosomal profile, but with a different pattern from that of the cshA mutant. While the 30S/50S ratio of signal intensity remained unchanged with respect to the wild type, we observed a clear reduction of the mature 70S ribosomes (Fig. 5D). This indicates that the DEAD-box RNA helicase CshB is primarily involved in the proper formation of mature 70S ribosome particles. The RNA helicase DeaD was shown to bind the 23S rRNA in B. subtilis (3, 23). To our surprise, the deletion of deaD did not detectably affect the ribosomal profiles (Fig. 5E). This demonstrates that the RNA helicase DeaD is not needed for proper ribosome biogenesis under these conditions. The last single mutant we analyzed lacked the RNA helicase YfmL. Again we observed a pattern that was significantly different from that of the wild type (Fig. 5F). Deletion of yfmL resulted in a 50S signal that was even lower than that for the 30S subunit. In addition, the signal for the mature 70S ribosomes had dropped to the intensity of the 30S subunit. This experiment revealed that the DEAD-box RNA helicase YfmL is involved in ribosome biogenesis, probably by the formation of the proper 50S subunits.
This comprehensive series of experiments about the involvement of the DEAD-box RNA helicases in ribosome formation in B. subtilis suggests that CshA, CshB, and YfmL are involved in the biogenesis of ribosomes. Interestingly, the qualities of these changes are all of a different kind, suggesting that the three DEAD-box RNA helicases have individual and distinct functions in the formation of proper ribosomes.
Scarcity of ribosomes in the cshA mutant.
Three lines of evidence presented here suggest that CshA is the major DEAD-box RNA helicase of B. subtilis. First, the mutant has the most severe cold-sensitive phenotype; second, it is the major helicase based on protein abundance; and third, the cshA mutant exhibits an impaired cell morphology. Therefore, we decided to focus our further analyses on the role of CshA.
Ribosomes are one of the largest and most abundant protein complexes in the cell. Due to their large size, they can be visualized by electron microscopy (47, 48). As shown above, the deletion of cshA leads to a significant defect in the assembly of ribosomes. To investigate this effect in situ, we analyzed a B. subtilis wild-type strain and the isogenic cshA mutant GP1083 by transmission electron microscopy. For this purpose, the two strains were grown at low temperature (20°C). The cells were prepared by high-pressure freezing and freezing substitution to obtain a highly conserved ultrastructure and to reduce the formation of artifacts. Using this technique allowed the visualization of the effect of the loss of CshA on ribosome formation in the cell. As shown in Fig. 6, wild-type cells are densely packed with ribosomes, and these ribosomes are found mostly in the periphery of the cells. In contrast to the wild type and in very good agreement with the results described above, cells devoid of CshA contain a remarkably reduced number of ribosomes. Furthermore, and as stated above, cells deleted for cshA exhibited diminished diameters and thickened cell walls.
Fig 6.
Loss of CshA leads to reduced numbers of ribosomes. The B. subtilis wild-type strain 168 (A) and the isogenic cshA mutant GP1083 (B) were grown in LB medium at 20°C. Cells were harvested at an OD600 of 0.8 and subjected to transmission electron microscopy.
Identification of interaction partners of CshA.
Many RNA helicases are strongly dependent on interaction partners for full catalytic activity (2). Thus, the identification of proteins interacting with RNA helicases is of particular interest. In this study, we performed a pulldown assay with CshA as a bait protein. To avoid secondary effects that might result from CshA overexpression, we used strain GP1026. This strain expresses a CshA variant that contains a Strep tag at its C terminus in its original locus under the control of the native promoter (5). To facilitate the identification of more transient interaction partners, we also fixed the in vivo interactions by adding the cross-linker formaldehyde prior to the harvest of the cells (35). The elution fractions of the CshA pulldown (with and without cross-linker) were analyzed by gel electrophoresis, and potential interaction partners were identified by mass spectrometry.
As the expression of cshA-Strep is controlled by its native promoter and purification of the protein is difficult (49), we obtained only small amounts of the bait protein. Nevertheless, several proteins copurified with CshA (Fig. 7A). MS analysis of the band that corresponds to a protein of around 25 kDa resulted in the identification of the ribosomal proteins L1 and L3, both part of the large subunit of the ribosome (encoded by the rplA and rplC genes, respectively). Furthermore, DegU, the response regulator of the DegSU two-component system, was identified. The strong bands migrating at sizes of 100 and 20 kDa are the pyruvate carboxylase (PycA) and acetyl-coenzyme A carboxylase (AccB), respectively. These two proteins contain biotin and thus intrinsically bind the column resin.
Fig 7.
CshA interacts with the ribosomal proteins L1 and L3. (A) Identification of CshA interaction partners by copurification. Protein complexes were isolated from B. subtilis strain GP1026 encoding CshA-Strep in the absence (−FA) and presence (+FA) of formaldehyde. The strain was grown in minimal medium. Twenty-five μl of the elution fraction of the purification was loaded onto a 12.5% SDS-PAGE gel. Gel bands were visualized by silver staining. Arrows mark the gel bands that were analyzed by mass spectrometry. The asterisks label the bands of PycA and AccB, two biotin-containing proteins that bind to the Streptactin resin. (B) Bacterial two-hybrid analysis to study interactions between CshA and the ribosomal proteins L1, L3, and L4. The ribosomal genes were cloned into the plasmids pUT18 and pUT18C; the cshA gene was cloned into p25N. Plasmids pUT18 and pUT18C allow the expression of the fusion proteins either to the N or C terminus, respectively, of the T18 domain of the B. pertussis adenylate cyclase. Plasmid p25N allows the expression of CshA fused to the N terminus of the T25 domain of the adenylate cyclase. The E. coli transformants were incubated for 48 h at 30°C. The degradation of X-Gal (blue color) indicates the presence of a functional adenylate cyclase owing to the interaction of the two proteins of interest. (C) Verification of the direct interaction between CshA and L1. B. subtilis strain GP1095 expressing CshA fused to a C-terminal Strep tag and the ribosomal protein L1 fused to a triple FLAG tag was grown in LB medium. Extracts of GP1095 were either directly applied to the column (−RNase A) or extensively treated with RNase A (100 μg/ml; 30 min at room temperature) prior to affinity purification of CshA (+RNase A). Twenty-five μl of the first elution fraction of each purification was loaded onto a 12.5% SDS-polyacrylamide gel. After electrophoresis and blotting onto a PVDF membrane, L1-FLAG was detected using commercial antibodies.
To facilitate the purification of more weakly associated proteins, we used formaldehyde as a cross-linker (35). As shown in Fig. 7A, the amount of copurified proteins increased upon the addition of formaldehyde. Several of these bands were analyzed by mass spectrometry. The identification of RNase J1 and enolase was anticipated and is in good agreement with the fact that CshA as well as RNase J1 and enolase are part of the RNA degradosome of B. subtilis (5, 50). Furthermore, we identified the proteins Tig (peptidyl-prolyl isomerase, or Trigger factor, a ribosome-associated protein), GuaA (GMP synthase), Pgk (phosphoglycerate kinase), and PdhC and PdhD (subunits of the pyruvate dehydrogenase) in these bands.
In addition to the known interactions with degradosome components, the pulldown experiment suggests an interaction of CshA with the ribosomal proteins L1 and L3, both part of the large subunit of the ribosome. This observation is in excellent agreement with the implication of CshA in the assembly of the large ribosomal subunit (Fig. 5C). To investigate whether these interactions are direct protein-protein interactions, bacterial two-hybrid analyses were conducted. As shown in Fig. 7B, CshA clearly interacted with L1 and L3 in this heterologous E. coli system. The L4 protein (encoded by rplD) served as a negative control, as the protein was not identified in the pulldown experiment before (Fig. 7A), and also no interaction in the two-hybrid analysis was observed (Fig. 7B).
However, even the heterologous system does not completely rule out the possibility that the interaction between CshA and the ribosomal proteins is mediated by rRNA. To address this issue, we constructed a strain expressing L1 carrying a triple FLAG tag at its C terminus and CshA fused to a Strep tag. This strain was used for copurification of Strep-CshA with its interaction partners. In contrast to the experiment described above (Fig. 7A), the cell extract was extensively treated with RNase A prior to affinity purification of CshA. The resulting eluate was analyzed for the presence of the FLAG-tagged L1 protein. The RNase treatment did not interfere with the copurification of CshA and L1, thus demonstrating that the two proteins interact directly (Fig. 7C). The construction of a corresponding strain for the analysis of FLAG-tagged L3 was impossible, likely due to the inability of tagged L3 to do its job in the ribosome.
Taken together, the unbiased isolation of in vivo interaction partners for CshA, the two-hybrid studies, and the proof of direct interaction provide evidence for the direct and specific interaction of CshA with proteins of the large ribosomal subunit.
Consequences of the deletion of cshA on the transcriptome.
The degradation of mRNAs in E. coli and B. subtilis can be conducted by individual RNases or by multienzyme complexes called RNA degradosomes (see references 9 and 51 for reviews). In B. subtilis, the RNA helicase CshA is part of this complex, suggesting a significant role in mRNA turnover (5). To get further insight into the function of CshA in mRNA decay of B. subtilis, microarray analyses were performed to compare the transcript profiles of the wild-type 168 and the isogenic cshA mutant strain GP1035 in the exponential phase. In order to avoid secondary effects due to very different growth rates at lower temperatures, both strains were cultivated at 37°C.
The microarray analyses revealed that more than 200 mRNAs were changed in abundance due to the deletion of cshA (see NCBI GEO database entry GSE36877). Among these potential targets, we observed an approximately 60-fold increase and a 50-fold decrease in steady-state levels of the ysbA and frlB transcripts, respectively. In addition, we observed increased transcript levels for 118 mRNAs and a decrease of 103 mRNAs upon deletion of the cshA gene. A complete list of the mRNAs affected by the loss of CshA is provided in the GEO database.
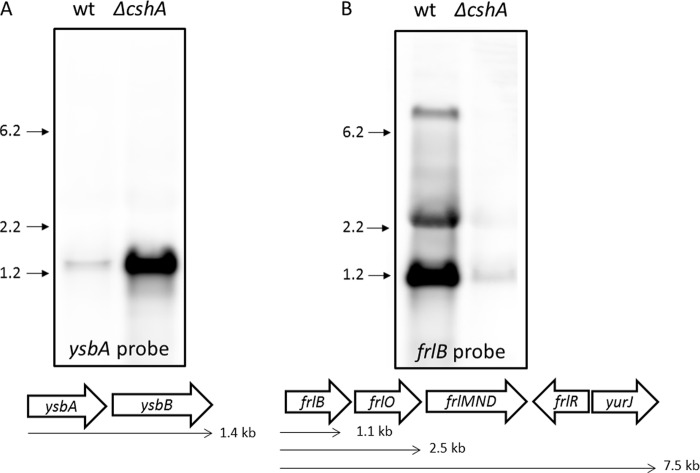
The ysbA and frlB mRNAs were analyzed in more detail by Northern blotting. The most strongly increased target is the mRNA for the antiholin-like protein YsbA, which is 60 times more abundant in the cshA mutant than in the wild-type strain. Using an RNA probe directed against ysbA, a signal of about 1.4 kb was detected by Northern blotting (Fig. 8A). This corresponds to a bicistronic transcript of ysbA with its downstream gene ysbB. This operon organization is also supported by a recent tiling array analysis of the B. subtilis transcriptome (45). The bicistronic ysbAB mRNA strongly accumulated upon deletion of cshA, thus confirming the microarray analysis.
Fig 8.
Effects of the deletion of the RNA helicase cshA on the ysbA (A) and frlB (B) mRNAs. Total RNA was isolated from the B. subtilis wild-type strain 168 and the isogenic cshA mutant GP1083 grown in minimal medium supplemented with 0.5% glucose. Five μg of total RNA was loaded per lane. A schematic illustration of the operon structure is given below each Northern blot.
The target that was most severely reduced upon cshA deletion was the frlB transcript, encoding a fructoselysine-6-P-glycosidase with a 50-fold decreased abundance. The frlB gene is suggested to be part of a large frlBONMD operon encoding proteins involved in the utilization of fructoselysine (25). The Northern blot analysis revealed three distinct signals (Fig. 8B). One signal, in the range of 1.1 kb, represents the frlB monocistronic transcript. A second signal, in the range of 2.5 kb, probably contains frlB and the downstream gene frlO. The third signal, in the range of 7.5 kb, is consistent in size with a message containing the complete frlBONMD cluster, the downstream genes for the regulator of the operon FrlR, and the ATP-binding subunit of the fructoselysine ABC transporter YurJ. This large operon is also supported by a recent tiling array analysis (45). As already observed in the microarray analysis, all three transcripts were significantly less abundant in the cshA mutant strain than in the wild type. Taken together, we interpret the results to indicate that the loss of the RNA helicase CshA affects gene expression in B. subtilis.
DISCUSSION
Most organisms harbor multiple DEAD-box RNA helicases. It is assumed that these enzymes fulfill different functions. This study on the role of RNA helicases in B. subtilis suggests that three of the four helicases participate in distinct aspects of one common process, ribosome biogenesis. Moreover, the different RNA helicases may play additional roles, as shown for the involvement of CshA in transcription.
The distinct functions of the DEAD-box helicases were already indicated when the growth of the individual mutants was studied. Deletion of any one of the three helicase-encoding genes cshA, cshB, and yfmL results in a cold-sensitive phenotype. Obviously, the helicases are unable to replace each other. Similar results have recently been obtained with the Gram-positive pathogens Listeria monocytogenes and Bacillus cereus. In both organisms the lack of the homologues of B. subtilis CshA, CshB, and YfmL results in a cold-sensitive phenotype (20, 52). In contrast, one previous study found CshA to be nonessential at low temperatures and synthetically lethal with a cshB mutation even at ambient temperature (17). Our results are in disagreement with the latter observation (Fig. 1). The discrepancy might result from the use of different media or different genetic backgrounds.
The reason for cold sensitivity of mutants affected in the DEAD-box RNA helicases has not been clearly identified. However, the increased stability of RNA secondary structures at low temperatures immediately suggests that such structures are not unwound at low temperatures in the absence of the RNA helicases. As a candidate process for causing the cold sensitivity of the cshA, cshB, and yfmL mutants, this study identified ribosome biogenesis, which is distinctly impaired in these three mutant strains. While loss of CshA or YfmL activity differentially affected the assembly of the 50S subunit, loss of CshB primarily affected biogenesis of mature 70S ribosome particles. Therefore, we propose that the compromised growth ability of the mutant strains is caused by the inability of the cells to maintain proper ribosome biogenesis. Defects in ribosome biogenesis were already investigated in E. coli. There, deletion of srmB or csdA leads to a decrease in free 50S subunits and accumulation of 40S particles representing incompletely assembled large subunits (14, 53). Interestingly, even though we also observe a clear reduction of the 50S subunits, no 40S premature particles were detected. As recently suggested, this may reflect some fundamental differences in the assembly of the 50S subunit between E. coli and Gram-positive bacteria (46). Indeed, deletion of the L. monocytogenes homolog of B. subtilis yfmL also leads to aberrant ribosomal profiles without the formation of any premature particle (46).
So far, the DEAD-box RNA helicase DeaD is the only one of the four B. subtilis enzymes that has been linked to ribosomes due to its ability to bind 23S rRNA in vitro (23, 54). Unexpectedly, loss of DeaD activity had no effect on either cold sensitivity or ribosome biogenesis. In agreement with our observations, loss of DeaD did not lead to a cold-sensitive phenotype in L. monocytogenes and B. cereus (20, 52). Furthermore, deletion of dbpA, the deaD homolog of E. coli, does not affect growth at low temperatures or change the ribosome profile (55). In light of all these results, it is conceivable (i) that DeaD has a completely different function that has not been addressed by the experiments in this study, (ii) that the function of DeaD can be replaced by one of the other RNA helicases, or (iii) that DeaD exerts its function under conditions not applied in this study.
Another interesting aspect of this work is the in-depth analysis of CshA. We found that CshA is the most abundant RNA helicase in B. subtilis, and in contrast to the other RNA helicases, CshA is constantly expressed throughout growth. This is in good agreement with a recent transcriptome study that revealed that cshA is, under more than 100 different conditions, one of the most strongly expressed genes of B. subtilis (45). Furthermore, CshA is involved in multiple functions in the cell: the protein participates in ribosome biogenesis, and it interacts with the essential RNases J1 and Y. Due to this interaction, CshA is also implicated in the control of gene expression of certain operons in B. subtilis. This multitasking capability of CshA is not unprecedented: the E. coli DEAD-box protein CsdA is also involved in both ribosome assembly and the control of gene expression (12, 14, 55, 56). Moreover, CsdA is also required for growth at low temperatures, and csdA mutants also exhibit an aberrant morphology (57, 58).
The interaction of CshA with components of the RNA-degrading machinery (5, 51) (Fig. 7) has several implications. First, as observed here for the cshA mutant, lack of the nonessential RNase polynucleotide phosphorylase that is also part of the RNA degradosome results in cold sensitivity (59). Moreover, the association with the RNA degradosome suggests that CshA is also involved in the control of gene expression. A transcriptome analysis revealed that the amounts of the mRNAs of the ysbAB and frl operons are strongly increased and decreased, respectively, as a result of cshA deletion (Fig. 7). Interestingly, the two mRNAs are also targets of the essential endoribonuclease RNase Y (60). However, the amounts of the two mRNAs are increased upon depletion of RNase Y. This suggests differential roles for CshA in the control of RNase Y-mediated RNA degradation in B. subtilis. It is interesting that RNase Y is involved in the control of expression of about 25% of all B. subtilis genes. In contrast, CshA seems to play a rather specific role with an effect on a few transcripts. In fact, the different components of the E. coli RNA degradosome also have a very different impact on global mRNA stability. While RNase E acts at a global scale that is comparable to that of RNase Y, other components, such as the enolase or the RNA helicase RhlB, have significantly fewer targets (10). However, the impact of the E. coli DEAD-box RNA helicase RhlB is much more pronounced than that of CshA in B. subtilis. This difference may result from the presence of a 5′-3′ exoribonuclease activity (RNase J1) in B. subtilis but not in E. coli (61, 62). It is tempting to speculate that the DEAD-box RNA helicases of the RNA degradosome are required to facilitate the access of the RNases to structured RNA. In the absence of a 5′-3′ exoribonuclease, this task may be much more important in the degradosome of E. coli, thus explaining the more pronounced effect of the loss of the degradosome helicase in this organism compared to that in B. subtilis.
One last aspect of this work is the existence and the analysis of a quadruple mutant devoid of all RNA helicases. We found that this strain is viable at 37°C, but its ability to grow and to maintain proper ribosome biogenesis at low temperatures are strongly compromised. Nevertheless, these defects are not a mere addition of the defects of the individual helicase mutants. We propose that the different properties of the quadruple mutant reflect the phenotype of the mutation of that helicase that is most upstream or most strongly involved in a given process. We observed that the cold sensitivity is slightly more pronounced than that in the cshA mutant (data not shown), and the changes in the morphology of the strain are likely caused by the loss of cshA as well. On the other hand, the absence of YfmL might be the limiting factor for ribosome biogenesis in the quadruple mutant, as the profile of both the yfmL mutant and the quadruple mutant are very similar.
Based on the results presented in this study, it seems most likely that the defect in ribosome biogenesis is causative for the impaired growth of the cshA, cshB, and yfmL mutants at low temperatures. Further work will be devoted to the examination of this hypothesis.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Christina Herzberg for excellent technical assistance and to Dominik Tödter for help with some experiments.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (through SFB860) to J.S.
Footnotes
Published ahead of print 23 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01475-12.
REFERENCES
- 1.Linder P, Jankowsky E. 2011. From unwinding to clamping—the DEAD box RNA helicase family. Nat. Rev. Mol. Cell Biol. 12:505–516 [DOI] [PubMed] [Google Scholar]
- 2.Rocak S, Linder P. 2004. DEAD-box proteins: the driving forces behind RNA metabolism. Nat. Rev. Mol. Cell Biol. 5:232–241 [DOI] [PubMed] [Google Scholar]
- 3.Karginov FV, Caruthers JM, Hu Y, McKay DB, Uhlenbeck OC. 2005. YxiN is a modular protein combining a DEx(D/H) core and a specific RNA-binding domain. J. Biol. Chem. 280:35499–35505 [DOI] [PubMed] [Google Scholar]
- 4.Klostermeier D, Rudolph MG. 2009. A novel dimerization domain in the C-terminal domain of the Thermus thermophilus DEAD box helicase Hera confers substantial flexibility. Nucleic Acids Res. 37:421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehnik-Habrink M, Pförtner H, Rempeters L, Pietack N, Herzberg C, Stülke J. 2010. The RNA degradosome in Bacillus subtilis: identification of CshA as the major RNA helicase in the multi-protein complex. Mol. Microbiol. 77:958–971 [DOI] [PubMed] [Google Scholar]
- 6.Mohr G, Del Campo M, Mohr S, Yang Q, Jia H, Jankowsky E, Lambowitz AM. 2008. Function of the C-terminal domain of the DEAD-box protein Mss116p analyzed in vivo and in vitro. J. Mol. Biol. 375:1344–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linder P, Gasteiger E, Bairoch A. 2000. A comprehensive web resource on RNA helicases from the baker's yeast Saccharomyces cerevisiae. Yeast 16:507–509 [DOI] [PubMed] [Google Scholar]
- 8.Iost I, Dreyfus M. 2006. DEAD-box RNA helicases in Escherichia coli. Nucleic Acids Res. 34:4189–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpousis AJ. 2007. The RNA degradosome of Escherichia coli: an mRNA-degrading machine assembled on RNase E. Annu. Rev. Microbiol. 61:71–87 [DOI] [PubMed] [Google Scholar]
- 10.Bernstein JA, Lin PH, Cohen SN, Lin-Chao S. 2004. Global analysis of Escherichia coli RNA degradosome function using DNA microarrays. Proc. Natl. Acad. Sci. U. S. A. 101:2758–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandi A, Spurio R, Gualerzi CO, Pon CL. 1999. Massive presence of the Escherichia coli ‘major cold-shock protein’ CspA under non-stress conditions. EMBO J. 18:1653–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prud'homme-Généreux A, Beran RK, Iost I, Ramey CS, Mackie GA, Simons RW. 2004. Physical and functional interactions among RNase E, polynucleotide phosphorylase and the cold-shock protein, CsdA: evidence for a “cold shock degradosome.” Mol. Microbiol. 54:1409–1421 [DOI] [PubMed] [Google Scholar]
- 13.Charollais J, Dreyfus M, Iost I. 2004. CsdA, a cold-shock RNA helicase from Escherichia coli, is involved in the biogenesis of 50S ribosomal subunits. Nucleic Acids Res. 32:2751–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Resch A, Vecerek B, Palavra K, Bläsi U. 2010. Requirement of the CsdA DEAD-box helicase for low temperature riboregulation of rpoS mRNA. RNA Biol. 7:796–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mäder U, Schmeisky AG, Flórez LA, Stülke J. 2012. SubtiWiki—a comprehensive community resource for the model organism Bacillus subtilis. Nucleic Acids Res. 40:D1278–D1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Commichau FM, Rothe FM, Herzberg C, Wagner E, Hellwig D, Lehnik-Habrink M, Hammer E, Völker U, Stülke J. 2009. Novel activities of glycolytic enzymes in Bacillus subtilis: interactions with essential proteins involved in mRNA processing. Mol. Cell. Proteomics 8:1350–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunger K, Beckering CL, Wiegeshoff F, Graumann PL, Marahiel M. 2006. Cold-induced putative DEAD box RNA helicases CshA and CshB are essential for cold adaptation an interact with cold shock protein B in Bacillus subtilis. J. Bacteriol. 188:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ando Y, Nakamura K. 2006. Bacillus subtilis DEAD protein YdbR possesses ATPase, RNA binding, and RNA unwinding activities. Biosci. Biotechnol. Biochem. 70:1606–1615 [DOI] [PubMed] [Google Scholar]
- 19.Broussolle V, Pandiani F, Haddad N, Michaud C, Carlin F, Nguyen T, Brillard CJ. 2010. Insertional mutagenesis reveals genes involved in Bacillus cereus ATCC 14579 growth at low temperature. FEMS Microbiol. Lett. 306:177–183 [DOI] [PubMed] [Google Scholar]
- 20.Pandiani F, Brillard J, Bornard I, Michaud C, Chamot S, Nguyen T, Broussolle CV. 2010. Differential involvement of the five RNA helicases in adaptation of Bacillus cereus ATCC 14579 to low growth temperatures. Appl. Environ. Microbiol. 76:6692–6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandiani F, Chamot S, Brillard J, Charlin F, Nguyen T, Broussolle CV. 2011. Role of the five RNA helicases in adaptive response of Bacillus cereus ATCC 14579 to temperature, pH, and oxidative stress. Appl. Environ. Microbiol. 77:5604–5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karow AR, Klostermeier D. 2009. A conformational change in the helicase core is necessary but not sufficient for RNA unwinding by the DEAD box RNA helicase YxiN. Nucleic Acids Res. 37:4464–4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kossen K, Uhlenbeck OC. 1999. Cloning and biochemical characterization of Bacillus subtilis YxiN, a DEAD protein specifically activated by 23S rRNA: delineation of a novel sub-family of bacterial DEAD proteins. Nucleic Acids Res. 27:3811–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theissen B, Karow AR, Köhler J, Gubaev A, Klostermeier D. 2008. Cooperative binding of ATP and RNA induces a closed conformation in a DEAD box RNA helicase. Proc. Natl. Acad. Sci. U. S. A. 105:548–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deppe VM, Klatte S, Bongaerts J, Maurer KH, O'Connell T, Meinhardt F. 2011. Genetic control of amadori product degradation in Bacillus subtilis via regulation of frlBONMD expression by FrlR. Appl. Environ. Microbiol. 77:2839–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groicher KH, Firek BA, Fujimoto DF, Bayles KW. 2000. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J. Bacteriol. 182:1794–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunka K, Tholen S, Gerwig J, Herzberg C, Stülke J, Commichau FM. 2012. A high-frequency mutation in Bacillus subtilis: requirements for the decryptification of the gudB glutamate dehydrogenase gene. J. Bacteriol. 194:1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 29.Commichau FM, Herzberg C, Tripal P, Valerius O, Stülke J. 2007. A regulatory protein-protein interaction governs glutamate biosynthesis in Bacillus subtilis: the glutamate dehydrogenase RocG moonlights in controlling the transcription factor GltC. Mol. Microbiol. 65:642–654 [DOI] [PubMed] [Google Scholar]
- 30.Kunst F, Rapoport G. 1995. Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J. Bacteriol. 177:2403–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guérout-Fleury AM, Shazand K, Frandsen N, Stragier P. 1995. Antibiotic resistance cassettes for Bacillus subtilis. Gene 167:335–336 [DOI] [PubMed] [Google Scholar]
- 32.Wach A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12:259–265 [DOI] [PubMed] [Google Scholar]
- 33.Diethmaier C, Pietack N, Gunka K, Wrede C, Lehnik-Habrink M, Herzberg C, Hübner S, Stülke J. 2011. A novel factor controlling bistability in Bacillus subtilis: the YmdB protein affects flagellin expression and biofilm formation. J. Bacteriol. 193:5997–6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludwig H, Meinken C, Matin A, Stülke J. 2002. Insufficient expression of the ilv-leu operon encoding enzymes of branched-chain amino acid biosynthesis limits growth of a Bacillus subtilis ccpA mutant. J. Bacteriol. 184:5174–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herzberg C, Flórez Weidinger LA, Dörrbecker B, Hübner S, Stülke J, Commichau FM. 2007. SPINE: a method for the rapid detection and analysis of protein-protein interactions in vivo. Proteomics 7:4032–4035 [DOI] [PubMed] [Google Scholar]
- 36.Karimova G, Pidoux J, Ullmann A, Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 95:5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Hijum SA, de Jong A, Baerends RJ, Karsens HA, Kramer NE, Larsen R, den Hengst CD, Albers CJ, Kok J, Kuipers OP. 2005. A generally applicable validation scheme for the assessment of factors involved in reproducibility and quality of DNA-microarray data. BMC Genomics 6:77 doi:10.1186/1471-2164-6-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Hijum SA, de Jong A, Buist G, Kok J, Kuipers OP. 2003. UniFrag and GenomePrimer: selection of primers for genome-wide production of unique amplicons. Bioinformatics 19:1580–1582 [DOI] [PubMed] [Google Scholar]
- 39.Kuipers OP, de Jong A, Baerends RJ, van Hijum SA, Zomer AL, Karsens HA, den Hengst CD, Kramer NE, Buist G, Kok J. 2002. Transcriptome analysis and related databases of Lactococcus lactis. Antonie Van Leeuwenhoek 82:113–122 [PubMed] [Google Scholar]
- 40.van Hijum SA, Garcia de la Nava J, Trelles O, Kok J, Kuipers OP. 2003. MicroPreP: a cDNA microarray data pre-processing framework. Appl. Bioinformatics 2:241–244 [PubMed] [Google Scholar]
- 41.Baldi P, Long AD. 2001. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17:509–519 [DOI] [PubMed] [Google Scholar]
- 42.Rostaing P, Real E, Siksou L, Lechaire JP, Boudier T, Boeckers TM, Gertler F, Gundelfinger ED, Triller A, Marty S. 2006. Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur. J. Neurosci. 24:3463–3474 [DOI] [PubMed] [Google Scholar]
- 43.Witte K, Schuh AL, Hegermann J, Sarkeshik A, Mayers JR, Schwarze K, Yates JR, Eimer S, Audhya A. 2011. TFG-1 function in protein secretion and oncogenesis. Nat. Cell Biol. 13:550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phadtare S, Severinov K. 2010. RNA remodeling and gene regulation by cold shock proteins. RNA Biol. 7:788–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicolas P, Mäder U, Dervyn E, Rochat T, Leduc A, Pigeonneau N, Bidnenko E, Marchadier E, Hoebeke M, Aymerich S, Becher D, Bisicchia P, Botella E, Delumeau O, Doherty G, Denham EL, Devine KM, Fogg M, Fromion V, Goelzer A, Hansen A, Härtig E, Harwood CR, Homuth G, Jarmer H, Jules M, Klipp E, Le Chat L, Lecointe F, Lewis P, Liebermeister W, March A, Mars RAT, Nannapaneni P, Noone D, Pohl S, Rinn B, Rügheimer F, Sappa PK, Samson F, Schaffer M, Schwikowski B, Steil L, Stülke J, Wiegert T, Wilkinson AJ, van Dijl JM, Hecker M, Völker U, Bessières P, Noirot P. 2012. The condition-dependent whole-transcriptome reveals high-level regulatory architecture in bacteria. Science 335:1103–1106 [DOI] [PubMed] [Google Scholar]
- 46.Ortiz JO, Forster F, Kurner J, Linaroudis AA, Baumeister W. 2006. Mapping 70S ribosomes in intact cells by cryoelectron tomography and pattern recognition. J. Struct. Biol. 156:334–341 [DOI] [PubMed] [Google Scholar]
- 47.Vendeville A, Lariviere D, Fourmentin E. 2011. An inventory of the bacterial macromolecular components and their spatial organization. FEMS Microbiol. Rev. 35:395–414 [DOI] [PubMed] [Google Scholar]
- 48.Newman J, Hewitt L, Rodrigues C, Solovyova AS, Harwood CR, Lewis RJ. 2012. Dissection of the network of interactions that links RNA processing with glycolysis in the Bacillus subtilis degradosome. J. Mol. Biol. 416:121–136 [DOI] [PubMed] [Google Scholar]
- 49.Lehnik-Habrink M, Newman J, Rothe FM, Solovyova AS, Rodrigues C, Herzberg C, Commichau FM, Lewis RL, Stülke J. 2011. RNase Y in Bacillus subtilis: a natively disordered protein that is the functional equivalent of RNase E from Escherichia coli. J. Bacteriol. 193:5431–5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehnik-Habrink M, Lewis RJ, Mäder U, Stülke J. 2012. RNA degradation in Bacillus subtilis: an interplay of essential endo- and exoribonucleases. Mol. Microbiol. 84:1005–1017 [DOI] [PubMed] [Google Scholar]
- 51.Markkula A, Lindström M, Johansson P, Björkroth J, Korkeala H. 2012. Role of four putative DEAD-box RNA helicase genes in growth of Listeria monocytogenes EGD-e under heat, pH, osmotic, ethanol, and oxidative stresses. Appl. Environ. Microbiol. 78:6875–6882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charollais J, Pflieger D, Vinh J, Dreyfus M, Iost I. 2003. The DEAD-box RNA helicase SrmB is involved in the assembly of 50S ribosomal subunits in Escherichia coli. Mol. Microbiol. 48:1253–1265 [DOI] [PubMed] [Google Scholar]
- 53.Netterling S, Vaitkevicius K, Nord S, Johansson J. 2012. A Listeria monocytogenes RNA-helicase essential for growth and ribosomal maturation at low temperatures, uses its C-terminus for appropriate interaction with the ribosome. J. Bacteriol. 194:4377–4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kossen K, Karginov FV, Uhlenbeck OC. 2002. The carboxy-terminal domain of the DExDH protein YxiN is sufficient to confer specificity for 23S rRNA. J. Mol. Biol. 324:625–636 [DOI] [PubMed] [Google Scholar]
- 55.Peil L, Virumäe K, Remme J. 2008. Ribosome assembly in Escherichia coli strains lacking the RNA helicase DeaD/CsdA or DbpA. FEBS J. 275:3772–3782 [DOI] [PubMed] [Google Scholar]
- 56.Shajani Z, Sykes MT, Williamson JR. 2011. Assembly of bacterial ribosomes. Annu. Rev. Biochem. 80:501–526 [DOI] [PubMed] [Google Scholar]
- 57.Awano N, Xu C, Ke H, Inoue M, Phadtare S. 2007. Complementation analysis of the cold-sensitive phenotype of the Escherichia coli csdA deletion strain. J. Bacteriol. 189:5808–5815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pierce A, Gillette D, Jones PG. 2011. Escherichia coli cold shock protein CsdA effects an increase in septation and the resultant formation of coccobacilli at low temperature. Arch. Microbiol. 193:373–384 [DOI] [PubMed] [Google Scholar]
- 59.Wang W, Bechhofer DH. 1996. Properties of a Bacillus subtilis polynucleotide phosphorylase deletion strain. J. Bacteriol. 178:2375–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lehnik-Habrink M, Schaffer M, Mäder U, Diethmaier C, Herzberg C, Stülke J. 2011. RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol. Microbiol. 81:1459–1473 [DOI] [PubMed] [Google Scholar]
- 61.Condon C. 2010. What is the role of RNase J in mRNA turnover? RNA Biol. 7:316–321 [DOI] [PubMed] [Google Scholar]
- 62.Durand S, Gilet L, Bessières P, Nicolas P, Condon C. 2012. Three essential ribonucleases–RNase Y, J1, and III–control the abundance of a majority of Bacillus subtilis mRNAs. PLoS Genet. 8:e1002520 doi:10.1371/journal.pgen.1002520 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.