Abstract
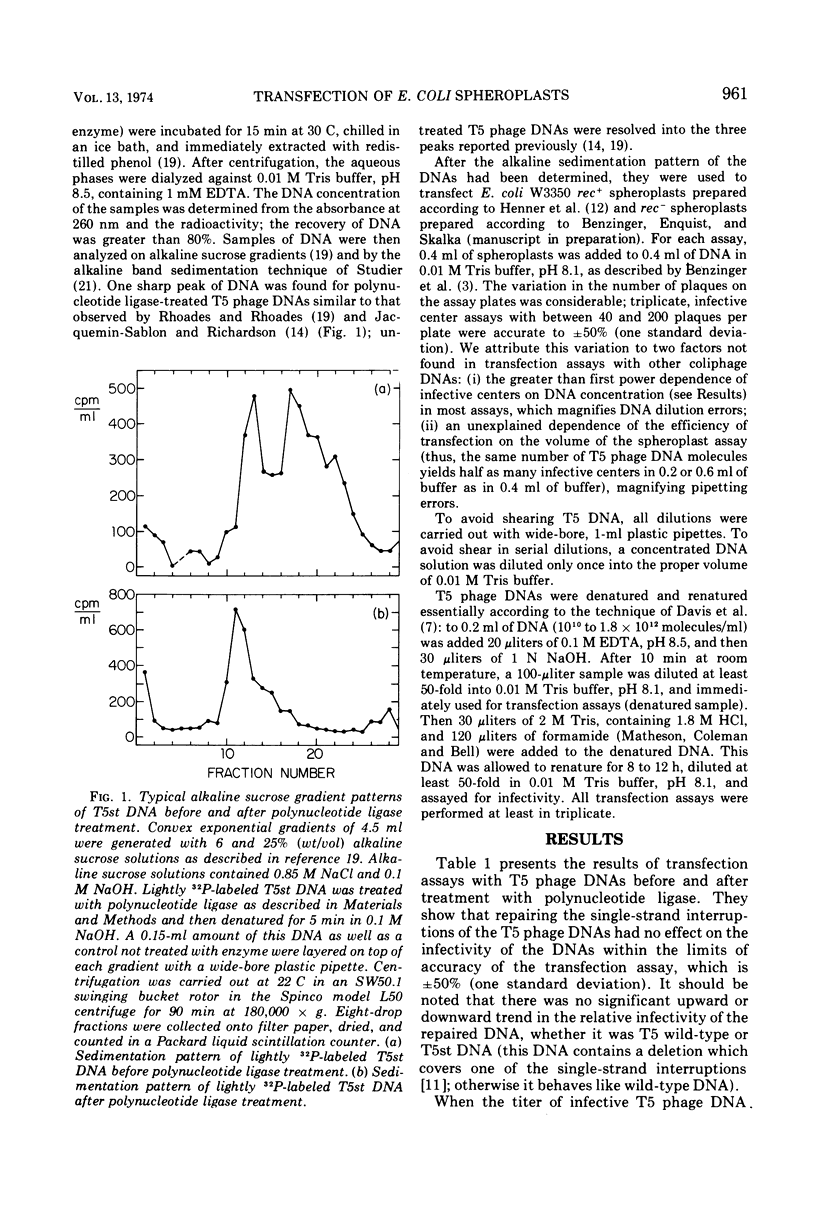
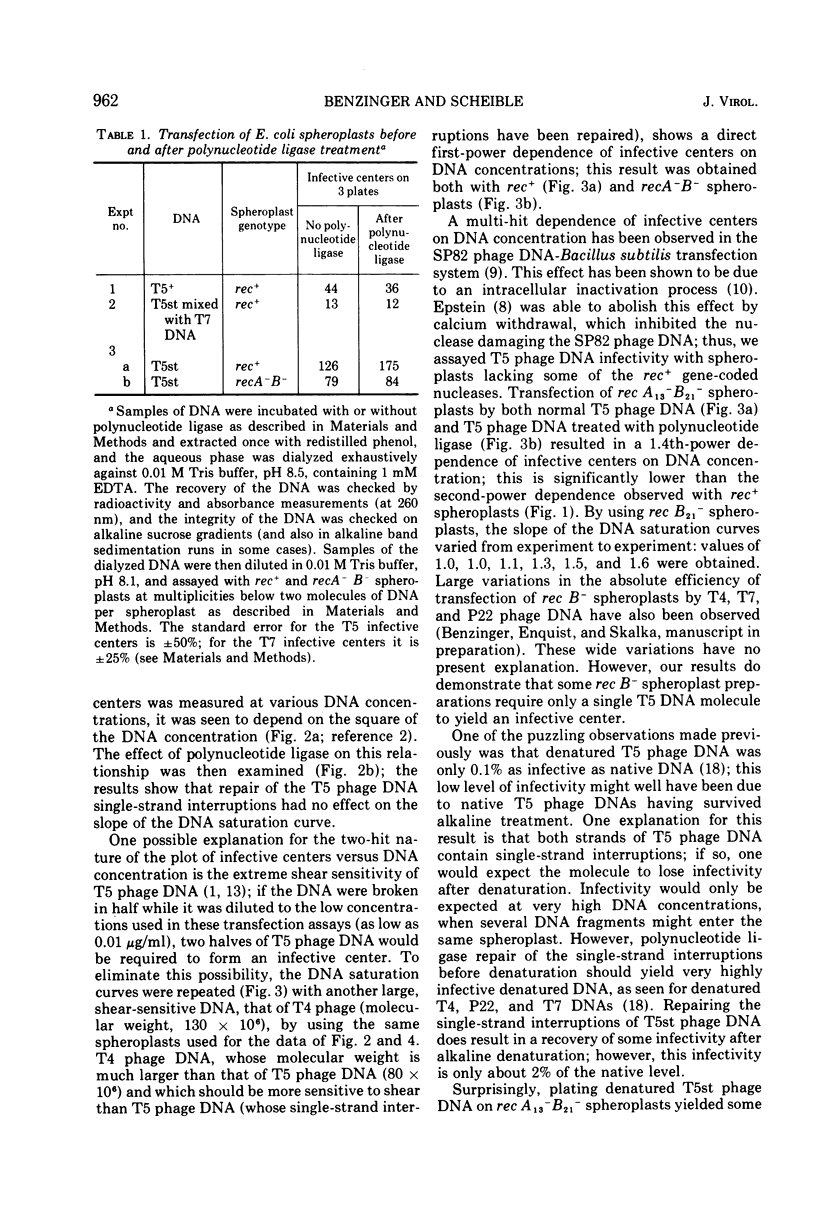
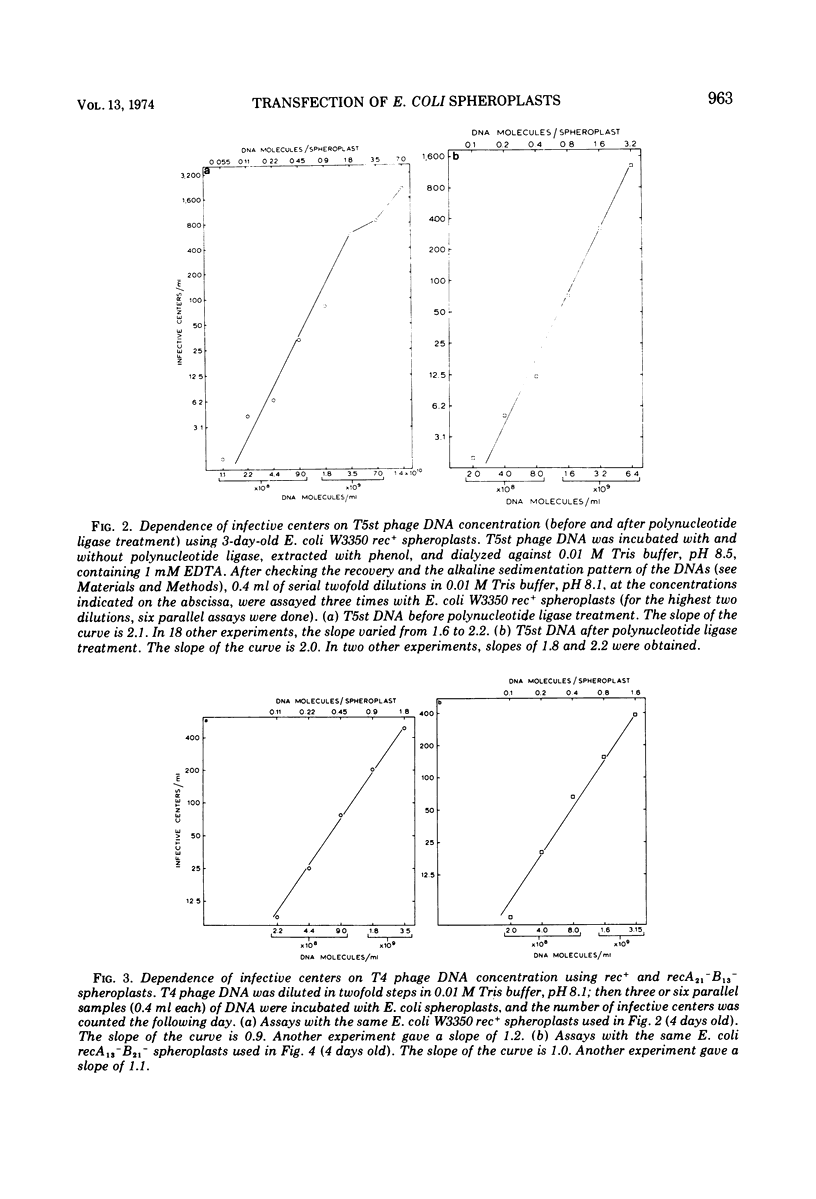
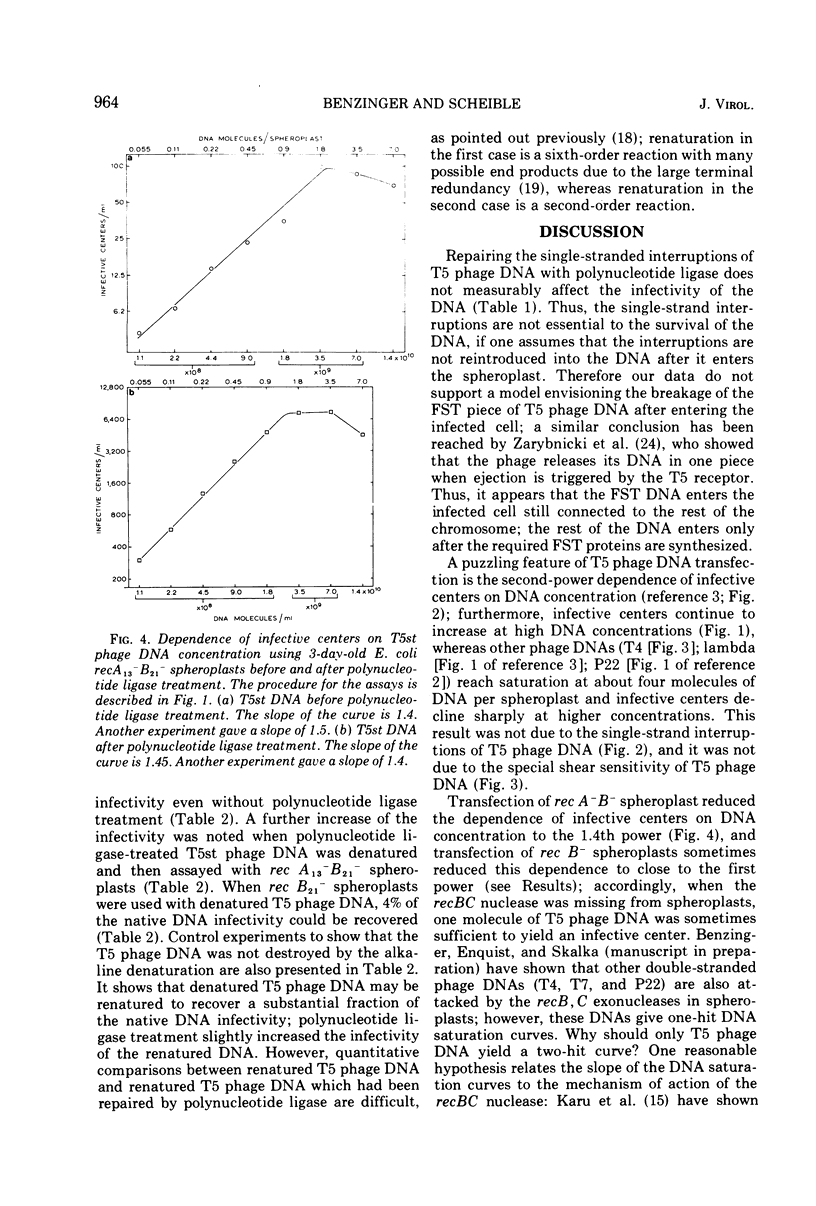
Transfection of Escherichia coli spheroplasts by native T5 phage DNA was not affected by treatment with polynucleotide ligase. Denatured T5 phage DNA infectivity, only 0.1% of the native DNA level, was increased slightly by polynucleotide ligase treatment. Renatured T5 phage DNA infectivity was also increased slightly by polynucleotide ligase treatment. To form an infective center with rec+ spheroplasts, 1.6 to 2.1 native T5 phage DNA molecules were required; however, 1.4 T5 phage DNA molecules were required to form an infective center with recA−B− spheroplasts, and one molecule was sometimes sufficient for rec B− spheroplasts. Polynucleotide ligase treatment of T5 phage DNA had no effect on these parameters. Thus, the single-strand interruptions of T5 phage DNA are probably not essential to the survival of the parental T5 phage DNA, and T5 phage DNA, especially the denatured form, is highly sensitive to some nucleases in E. coli spheroplasts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzinger R., Kleber I. Transfection of Escherichia coli and Salmonella typhimurium spheroplasts: host-controlled restriction of infective bacteriophage P22 deoxyribonucleic acid. J Virol. 1971 Aug;8(2):197–202. doi: 10.1128/jvi.8.2.197-202.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode V. C. Single-strand scissions induced in circular and linear lambda DNA by the presence of dithiothreitol and other reducing agents. J Mol Biol. 1967 May 28;26(1):125–129. doi: 10.1016/0022-2836(67)90266-5. [DOI] [PubMed] [Google Scholar]
- Bujard H., Hendrickson H. E. Structure and function of the genome of coliphage T5. 1. The physical structure of the chromosome of T5 + . Eur J Biochem. 1973 Mar 15;33(3):517–528. doi: 10.1111/j.1432-1033.1973.tb02711.x. [DOI] [PubMed] [Google Scholar]
- Bujard H. Location of single-strand interruptions in the DNA of bacteriophage T5. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1167–1174. doi: 10.1073/pnas.62.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. T. Source of the nonlinear dependence of bacteriophage SP82 transfection on deoxyribonucleic acid concentration. J Virol. 1971 Jun;7(6):749–752. doi: 10.1128/jvi.7.6.749-752.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., GOLDBERG E., BURGI E., INGRAHAM L. Local denaturation of DNA by shearing forces and by heat. J Mol Biol. 1963 Mar;6:230–243. doi: 10.1016/s0022-2836(63)80072-8. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. I. Analysis of the single-stranded DNA fragments by agarose gel electrophoresis. J Mol Biol. 1972 Feb 14;63(3):383–395. doi: 10.1016/0022-2836(72)90435-4. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Kleber I., Benzinger R. Transfection of Escherichia coli spheroplasts. 3. Facilitation of transfection and stabilization of spheroplasts by different basic polymers. J Virol. 1973 Oct;12(4):741–747. doi: 10.1128/jvi.12.4.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin-Sablon A., Richardson C. C. Analysis of the interruptions in bacteriophage T5 DNA. J Mol Biol. 1970 Feb 14;47(3):477–493. doi: 10.1016/0022-2836(70)90316-5. [DOI] [PubMed] [Google Scholar]
- Karu A. E., MacKay V., Goldmark P. J., Linn S. The recBC deoxyribonuclease of Escherichia coli K-12. Substrate specificity and reaction intermediates. J Biol Chem. 1973 Jul 25;248(14):4874–4884. [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. II. Dissociation of calcium-independent and calcium-dependent processes. Virology. 1960 Apr;10:514–529. doi: 10.1016/0042-6822(60)90133-1. [DOI] [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawhorne L., Kleber I., Mitchell C., Benzinger R. Transfection of Escherichia coli spheroplasts. II. Relative infectivity of native, denatured, and renatured lambda, T7, T5, T4, and P22 bacteriophage DNAs. J Virol. 1973 Oct;12(4):733–740. doi: 10.1128/jvi.12.4.733-740.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades M., Rhoades E. A. Terminal repetition in the DNA of bacteriophage T5. J Mol Biol. 1972 Aug 21;69(2):187–200. doi: 10.1016/0022-2836(72)90224-0. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S. RNA in coliphage T5. Nature. 1973 Mar 30;242(5396):327–329. doi: 10.1038/242327a0. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Zarybnicky V., Zarybnicka A. Infection process of T5 phages. I. Ejection of T5 DNA on isolated T5 receptors. Virology. 1973 Aug;54(2):318–329. doi: 10.1016/0042-6822(73)90146-3. [DOI] [PubMed] [Google Scholar]



