TEXT
A fundamental decision made by bacteria is to exist in a free-swimming planktonic state or transition into a sessile biofilm. Bacteria form biofilms for a number of reasons, including anchoring and persistence in a favorable environment or as a defense mechanism against environmental stress, predation, or clearance during infection by the host's immune system. It is important to remember that this transition between motility and biofilm formation is a cyclical process. Motile bacteria that encounter a surface undergo a specific attachment phase, leading to secretion of extracellular matrix material. Upon the biofilm reaching a sufficient size, intraspecies competition for nutrients or space within it activates dispersal processes in which pioneering planktonic cells leave the biofilm community. In the report by Zorraquino et al. in this issue of the Journal of Bacteriology, the authors explore the intersection of biofilm formation and motility in Salmonella enterica serovar Enteritidis by discovering that extracellular polysaccharide (EPS) physically inhibits flagellar rotation (1).
The authors seek to understand how the second messenger molecule cyclic di-GMP (c-di-GMP) negatively regulates motility in S. Enteritidis. c-di-GMP, synthesized by diguanylate cyclase (DGC) enzymes containing a GGDEF domain and degraded by phosphodiesterase (PDE) enzymes containing either an EAL or HD-GYP domain, is the linchpin that controls the transitions between motility and sessility in the majority of bacteria (2). High levels of c-di-GMP promote biofilm formation and repress motility, while low levels of c-di-GMP favor dispersion from a biofilm and increased motility. c-di-GMP is a regulatory maestro, exerting its effects at all levels of phenotypic expression from induction of transcription to direct control of protein activity. For example, in both Vibrio cholerae and Pseudomonas aeruginosa, c-di-GMP inhibits motility at the level of transcription by repressing expression of the flagellar biosynthesis operons (3, 4). Moreover, c-di-GMP directly modulates flagellar activity by binding to the PilZ-containing protein YcgR in multiple bacterial species (5–7). This interaction impacts both the direction and frequency of flagellar rotation and has been described to act as a flagellar brake. Curiously, mutation of ycgR does not completely abolish the ability of c-di-GMP to inhibit motility (5, 7), suggesting that other mechanisms by which c-di-GMP negatively influences motility remain to be identified.
Consistent with S. Enteritidis containing a YcgR-independent mechanism by which c-di-GMP inhibits motility, Zorraquino et al. observed that motility of S. Enteritidis was completely abolished at high levels of c-di-GMP even in the absence of ycgR (1). This inhibition of motility could be explained by reduced expression or assembly of the peritrichous flagella at a high intracellular concentration of c-di-GMP. However, increased c-di-GMP had no impact on the expression of the flagellin genes or the amount or nature of flagella on the surface. Interestingly, all strains that had high c-di-GMP, including the ΔycgR mutant, exhibited no flagellum-based rotation during a classical flagellar tethering assay, hinting that the YcgR-independent mechanism stimulated by c-di-GMP is at the level of flagellar function.
To identify this mechanism, the authors performed a clever screen based on the fact that suppressor mutants insensitive to c-di-GMP in the ΔycgR mutant background simply swim away from the rest of the colony. If only all suppressor mutants were as cooperative! These suppressors mapped to the bcsABZC operon, which is responsible for synthesis of the EPS cellulose. Binding of c-di-GMP to bcsA increases cellulose synthesis, leading to the hypothesis that cellulose itself was responsible for negatively inhibiting motility. This hypothesis was further supported by the observation that overexpression of the endoglucanase BcsZ encoded by S. Enteritidis, which degrades cellulose, increased motility. This experiment argued against the possibility that BcsA directly impacts flagellar function analogously to the EpsE glyosyltransferase of Bacillus subtilis, which functions as a flagellar clutch (8). Furthermore, either deletions in the bcsA pathway or a mutation in bcsA that abolished its interaction with c-di-GMP rendered motility of the ΔycgR mutant completely insensitive to c-di-GMP. However, if either ycgR or the bcsABZC operon was intact, overproduction of c-di-GMP completely abolished motility, showing that these mechanisms function redundantly and both must be inactivated for S. Enteritidis motility to be completely insensitive to c-di-GMP.
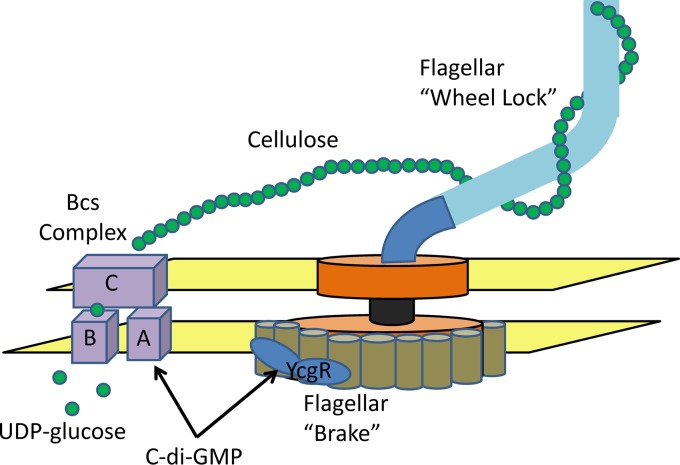
If we consider EpsE to function as a flagellar clutch and YcgR to act as a brake, EPS inhibition of flagellar rotation would be akin to a flagellar wheel lock (Fig. 1). EPS most likely sterically hinders flagellar motion, although it is possible that additional signaling mechanisms may be involved. Because c-di-GMP does not inhibit the expression or production of flagella, S. Enteritidis in a biofilm may be fully flagellated. In a sense, these bacteria are primed for dispersal. If conditions change or biofilm dispersion pathways are initiated, dispersing bacteria can quickly swim away from the biofilm. Whether EPS inhibition of flagella is specific to cellulose and S. Enteritidis or a more widespread phenomenon generally applicable to different bacterial species will be an interesting question of future research.
Fig 1.
c-di-GMP inhibits motility of S. Enteritidis by binding to two PilZ-containing proteins, YcgR and BcsA. Binding of c-di-GMP to YcgR functions as a flagellar “brake” by altering the rotation direction and frequency of the flagella, while an interaction of c-di-GMP with BcsA stimulates cellulose synthesis through the Bcs complex. Extracellular cellulose inhibits flagellar rotation, functioning effectively as a flagellar “wheel lock.”
Over the last few decades, many of the molecular details describing initiation of biofilm formation have been deciphered. However, as much as we know about this side of the biofilm coin, the research by Zorraquino et al. reminds us that the flip side, biofilm dispersion, is equally important. Determining what mechanisms S. Enteriditis and bacteria in general use to disperse from a biofilm could be applicable to treating biofilm-based disease. From this research, we can speculate that one key step in dispersion must be degradation of EPS, perhaps by BcsZ, freeing the flagella to rotate. Similarly, dispersin B, discovered in Aggregatibacter actinomycetemcomitans, degrades poly-N-glucosamine in multiple species of bacteria, catalyzing dispersion (9). Do all bacteria encode similar EPS degradation enzymes that are induced to promote dispersion from a biofilm?
It is apparent that bacterial flagella are an important appendage that not only generates motility but also senses the physical properties of the local environment. The peritrichous flagella of Vibrio parahaemolyticus sense surface contact, globally altering gene expression (10). Likewise, breaking of the flagella of V. cholerae during transit of the thick mucus layer blanketing epithelial cells induces a genetic regulatory pathway that increases virulence factor production (11). Contact of a surface in Caulobacter crescentus stimulates synthesis of the polysaccharide holdfast structure, and it is thought that inhibition of flagellar rotation is key to this signaling process (12). Clearly, the physical forces that drive the interaction of flagella with a bacterium's surrounding environment, be it a surface or the matrix of a biofilm, play a key role in the transition of bacteria between the planktonic and biofilm lifestyles, and this article by Zorraquino et al. adds one more sticky piece to this complex puzzle.
ACKNOWLEDGMENTS
C.M.W. is supported by NIH awards (U54-AI-057153 and U19-AI-090872-03) and the NSF-sponsored BEACON Science and Technology Center (Cooperative Agreement no. DBI-0939454).
Footnotes
Published ahead of print 30 November 2012
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1. Zorraquino V, García B, Latasa C, Echeverz M, Toledo-Arana A, Valle J, Lasa I, Solano C. 2013. Coordinated cyclic-di-GMP repression of Salmonella motility through YcgR and cellulose. J. Bacteriol. 195:417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jenal U, Dorman CJ. 2009. Small molecule signaling. Curr. Opin. Microbiol. 12:125–128 [DOI] [PubMed] [Google Scholar]
- 3. Hickman JW, Harwood CS. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol. 69:376–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krasteva PV, Fong JCN, Shikuma NJ, Beyhan S, Navarro MVAS, Yildiz FH, Sondermann H. 2010. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 327:866–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fang X, Gomelsky M. 2010. A post-translational, c-di-GMP-dependent mechanism regulating flagellar motility. Mol. Microbiol. 76:1295–1305 [DOI] [PubMed] [Google Scholar]
- 6. Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, Kaever V, Sourjik V, Roth V, Jenal U. 2010. Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141:107–116 [DOI] [PubMed] [Google Scholar]
- 7. Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM. 2010. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol. Cell 38:128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blair KM, Turner L, Winkelman JT, Berg HC, Kearns DB. 2008. A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science 320:1636–1638 [DOI] [PubMed] [Google Scholar]
- 9. Kaplan JB, Velliyagounder K, Ragunath C, Rohde H, Mack D, Knobloch JKM, Ramasubbu N. 2004. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J. Bacteriol. 186:8213–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gode-Potratz CJ, Kustusch RJ, Breheny PJ, Weiss DS, McCarter LL. 2011. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol. Microbiol. 79:240–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Z, Miyashiro T, Tsou A, Hsiao A, Goulian M, Zhu J. 2008. Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing. Proc. Natl. Acad. Sci. U. S. A. 105:9769–9774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li G, Brown PJ, Tang JX, Xu J, Quardokus EM, Fuqua C, Brun YV. 2012. Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol. Microbiol. 83:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]