Abstract
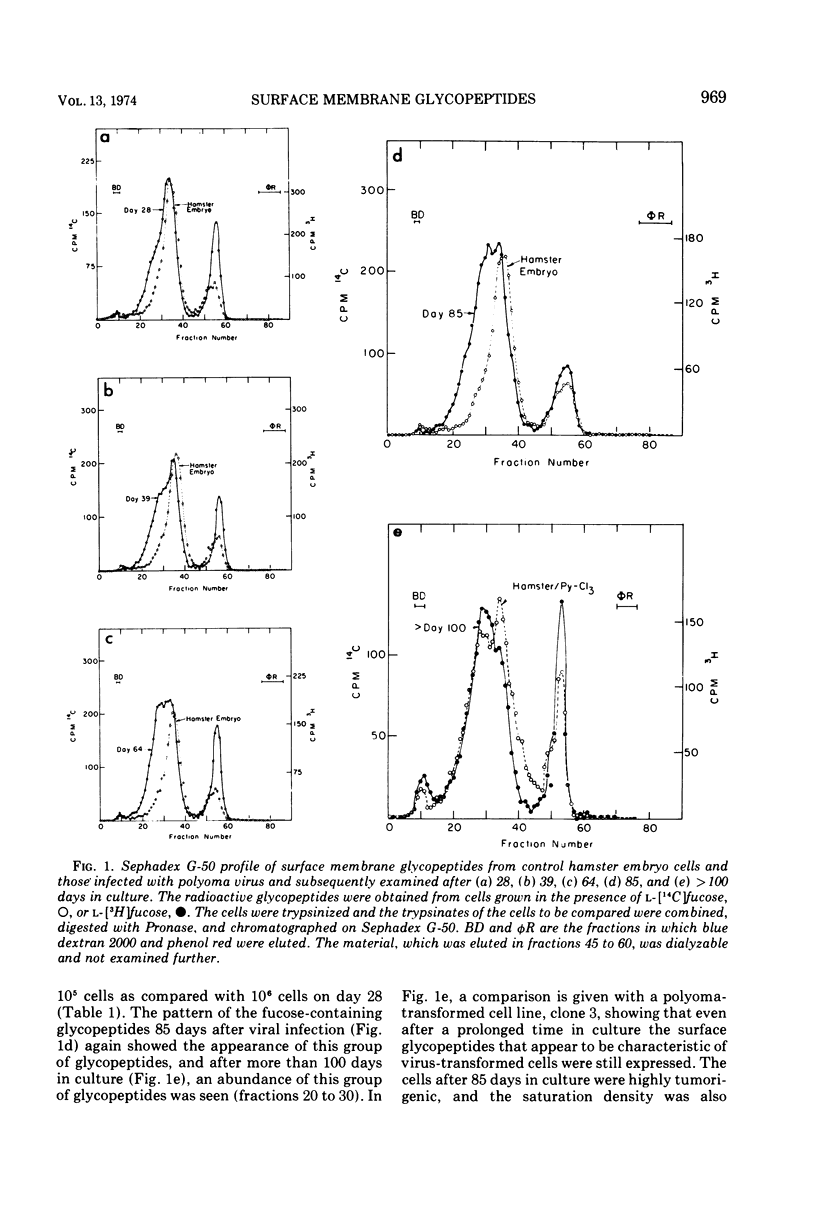
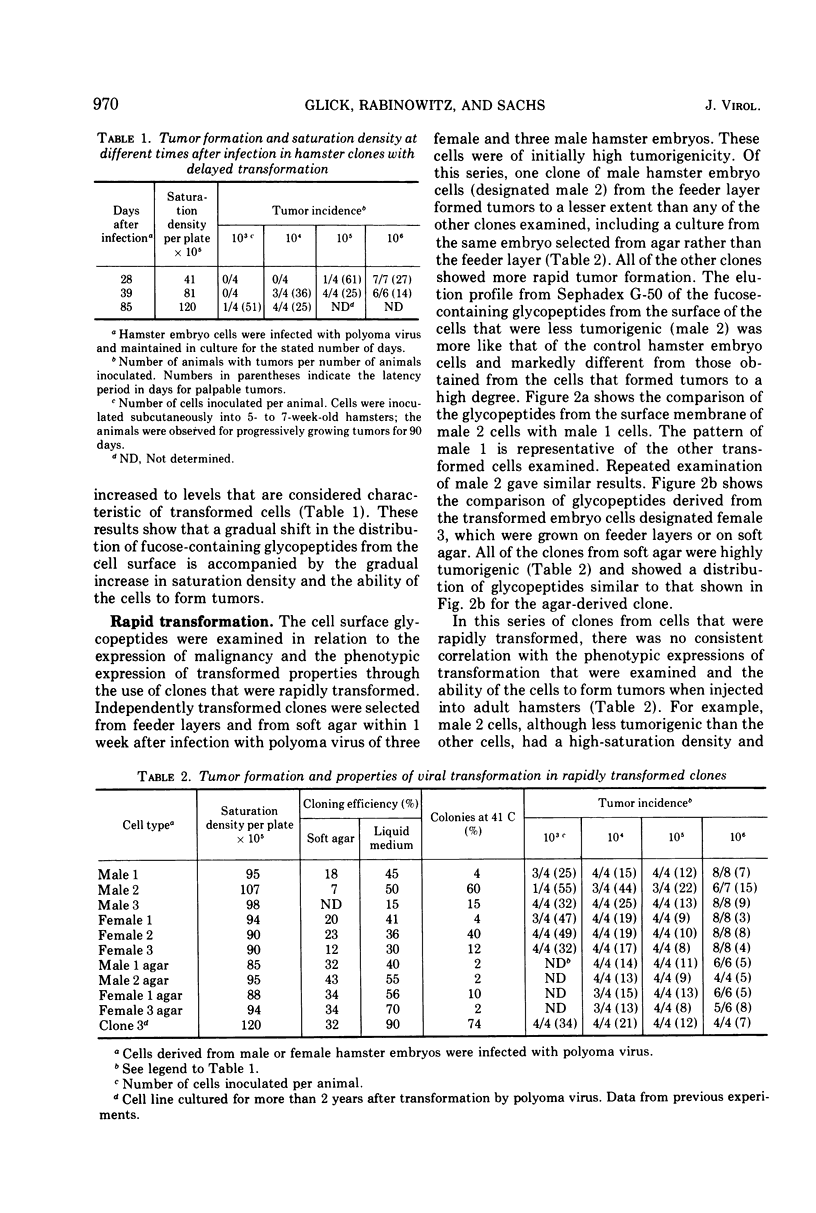
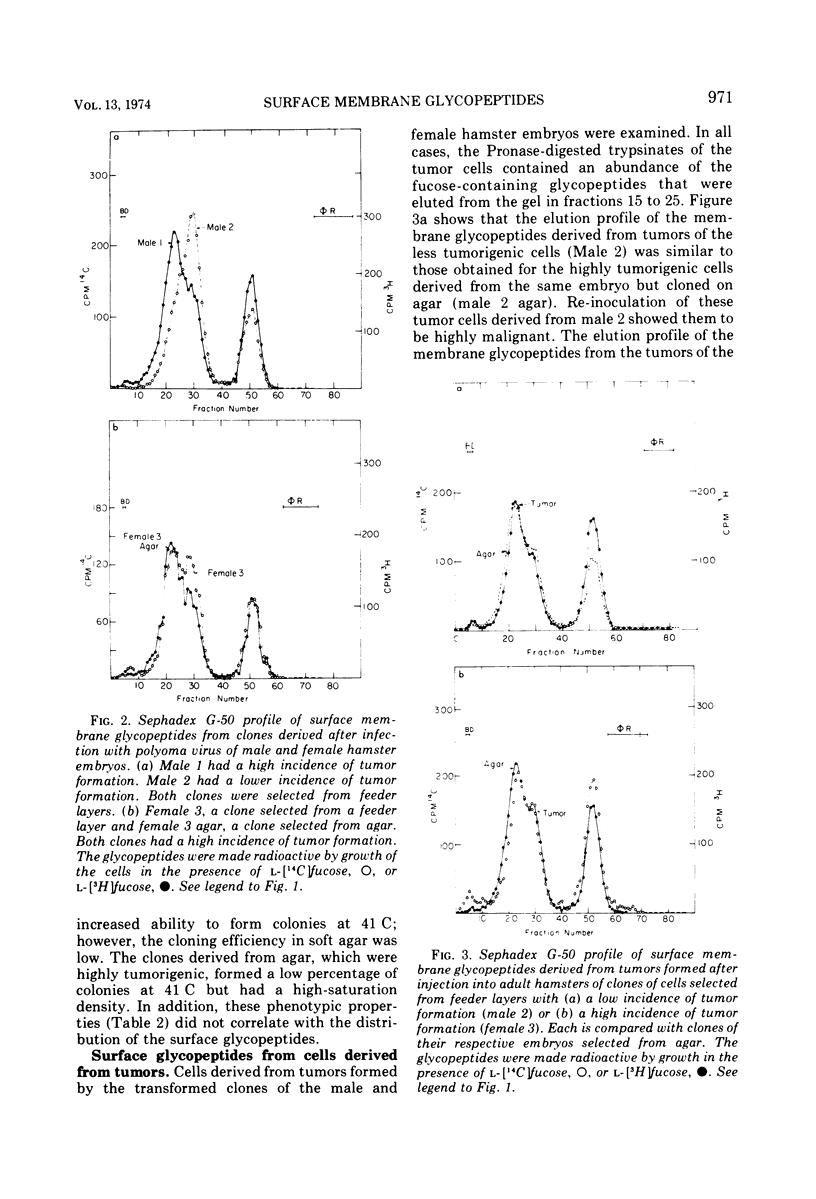
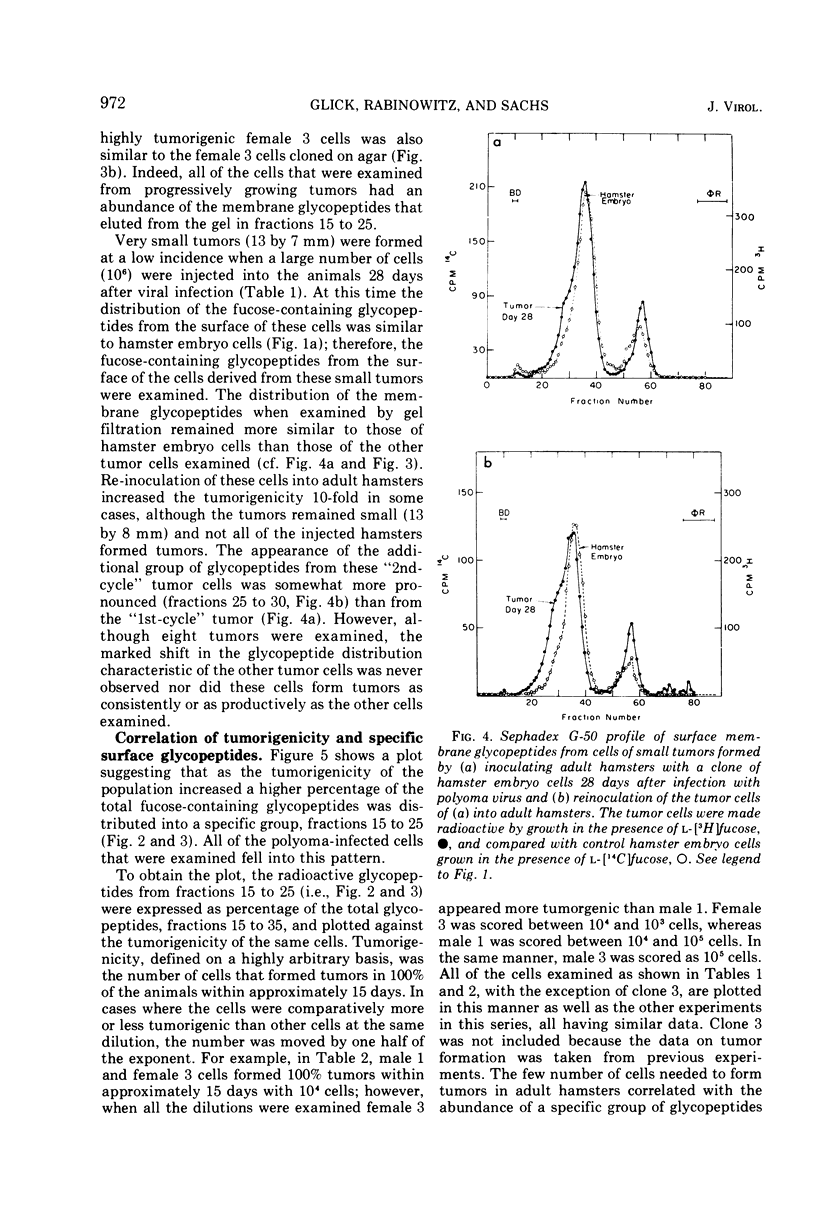
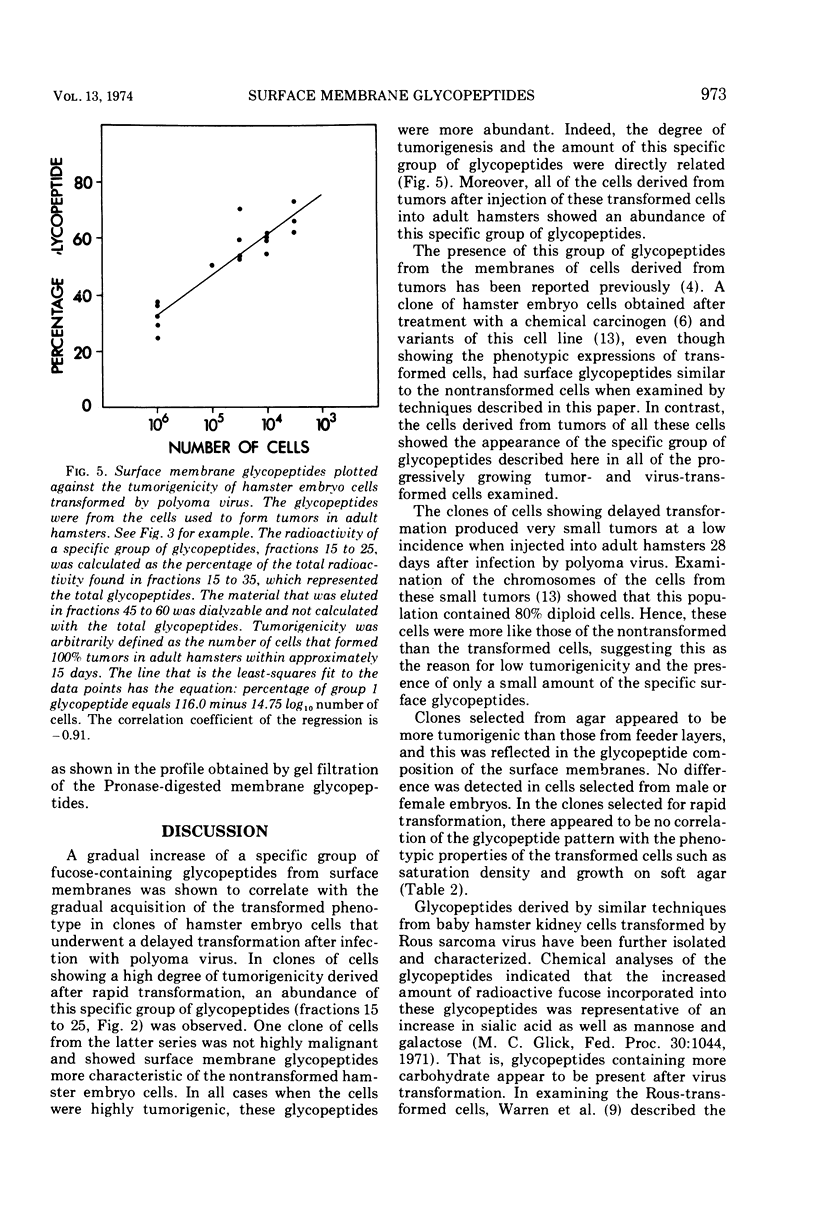
Glycopeptides from the surface of clones of hamster embryo cells were examined at various intervals after infection with polyoma virus. Two types of transformed cells were examined: (i) clones that showed delayed transformation or an initially low tumorigenicity, and (ii) clones that were rapidly transformed showing an initially high tumorigenicity. The glycopeptides were removed from the cell surface by trypsin and, after Pronase digestion, were examined by filtration through Sephadex G-50. With delayed transformation, a specific group of glycopeptides was increasingly evident over an 85-day period as the cells showed phenotypic properties of transformation and the ability to form tumors. In the other series, all but one clone of hamster embryo cells showed rapid transformation after infection with polyoma virus. This clone was less tumorigenic and showed little of the specific glycopeptides. In all cases of delayed or rapid transformation examined, the specific group of glycopeptides increased proportionately to the ability of the cells to form tumors. All of the cells derived from progressively growing tumors formed by injection of these transformed hamster cells into adult animals showed an abundance of this group of glycopeptides. These results suggest that specific surface membrane glycopeptides accompany viral transformation and tumorigenesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck C. A., Glick M. C., Warren L. A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1970 Nov 10;9(23):4567–4576. doi: 10.1021/bi00825a016. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. Glycopeptides from the surface of control and virus-transformed cells. Science. 1971 Apr 9;172(3979):169–171. doi: 10.1126/science.172.3979.169. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick M. C., Rabinowitz Z., Sachs L. Surface membrane glycopeptides correlated with tumorigenesis. Biochemistry. 1973 Nov 20;12(24):4864–4869. doi: 10.1021/bi00748a009. [DOI] [PubMed] [Google Scholar]
- Huberman E., Sachs L. Susceptibility of cells transformed by polyoma virus and simian virus 40 to the cytotoxic effect of the carcinogenic hydrocarbon benzo[a]pyrene. J Natl Cancer Inst. 1968 Feb;40(2):329–336. [PubMed] [Google Scholar]
- Huberman E., Salzberg S., Sachs L. The in vitro induction of an increase in cell multiplication and cellular life span by the water-soluble carcinogen dimethylnitrosamine. Proc Natl Acad Sci U S A. 1968 Jan;59(1):77–82. doi: 10.1073/pnas.59.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz Z., Sachs L. The formation of variants with a reversion of properties of transformed cells. VI. Stability of the reverted state. Int J Cancer. 1972 Mar 15;9(2):334–343. doi: 10.1002/ijc.2910090211. [DOI] [PubMed] [Google Scholar]
- Sakiyama H., Burge B. W. Comparative studies of the carbohydrate-containing components of 3T3 and simian virus 40 transformed 3T3 mouse fibroblasts. Biochemistry. 1972 Apr 11;11(8):1366–1377. doi: 10.1021/bi00758a007. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E., SACHS L. A plaque assay for the polyoma virus. Virology. 1959 Jul;8(3):397–400. doi: 10.1016/0042-6822(59)90044-3. [DOI] [PubMed] [Google Scholar]
- Warren L., Fuhrer J. P., Buck C. A. Surface glycoproteins of normal and transformed cells: a difference determined by sialic acid and a growth-dependent sialyl transferase. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1838–1842. doi: 10.1073/pnas.69.7.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Meezan E., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry. 1969 Jun;8(6):2509–2517. doi: 10.1021/bi00834a038. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Rabinowitz Z., Sachs L. Identification of the chromosomes that control malignancy. Nat New Biol. 1973 Jun 20;243(129):247–250. doi: 10.1038/newbio243247a0. [DOI] [PubMed] [Google Scholar]



