Abstract
Context
Recently, researchers have shown that phototherapy administered to skeletal muscle immediately before resistance exercise can enhance contractile function, prevent exercise-induced cell damage, and improve postexercise recovery of strength and function.
Objective
To critically evaluate original research addressing the ability of phototherapeutic devices, such as lasers and light-emitting diodes (LEDs), to enhance skeletal muscle contractile function, reduce exercise-induced muscle fatigue, and facilitate postexercise recovery.
Data Sources
We searched the electronic databases PubMed, SPORTDiscus, Web of Science, Scopus, and Rehabilitation & Physical Medicine without date limitations for the following key words: laser therapy, phototherapy, fatigue, exercise, circulation, microcirculation, and photobiomodulation.
Study Selection
Eligible studies had to be original research published in English as full papers, involve human participants, and receive a minimum score of 7 out of 10 on the Physiotherapy Evidence Database (PEDro) scale.
Data Extraction
Data of interest included elapsed time to fatigue, total number of repetitions to fatigue, total work performed, maximal voluntary isometric contraction (strength), electromyographic activity, and postexercise biomarker levels. We recorded the PEDro scores, beam characteristics, and treatment variables and calculated the therapeutic outcomes and effect sizes for the data sets.
Data Synthesis
In total, 12 randomized controlled trials met the inclusion criteria. However, we excluded data from 2 studies, leaving 32 data sets from 10 studies. Twenty-four of the 32 data sets contained differences between active phototherapy and sham (placebo-control) treatment conditions for the various outcome measures. Exposing skeletal muscle to single-diode and multidiode laser or multidiode LED therapy was shown to positively affect physical performance by delaying the onset of fatigue, reducing the fatigue response, improving postexercise recovery, and protecting cells from exercise-induced damage.
Conclusions
Phototherapy administered before resistance exercise consistently has been found to provide ergogenic and prophylactic benefits to skeletal muscle.
Key Words: photobiomodulation, laser therapy, skeletal muscle fatigue
Key Points
-
•
Phototherapy administered before resistance exercise may enhance contractile function, reduce exercise-induced muscle damage, and facilitate postexercise recovery.
-
•
The effectiveness of phototherapy is dose dependent, so selecting appropriate treatment variables, such as wavelength and output power, is important.
-
•
In attempting to reproduce clinical outcomes, clinicians and researchers should use evidence-based decision making when selecting treatment variables in phototherapy.
-
•
Given the increased beam reflection and attenuation at the skin interface, a larger treatment dose may be necessary when using light-emitting diodes (LEDs) instead of a semiconductor laser.
Phototherapy involves the therapeutic use of light to treat various pathologic conditions and musculoskeletal injuries. Research addressing the ability of light therapy to modulate physiologic processes associated with injury and healing has yielded promising results. Such modulatory processes associated with phototherapy often are called photobiomodulation (PBM), which involves the use of light to induce biochemical changes in tissue in a stimulatory or inhibitory manner.1
The use of light as a clinical modality has increased greatly over the past decade. The beneficial outcomes of phototherapy for the treatment of acute and chronic musculoskeletal disorders include pain control,2,3 enhanced blood circulation,4 and improved tissue repair.5 Although evidence is available on how light is absorbed by tissue and cells, the biochemical translation to alter clinical outcomes in humans remains poorly understood. The biological effects of phototherapy are mediated by the absorption of photons (light particles) by endogenous chromophores and the subsequent transduction of light energy into chemical energy inside the plasma membrane or cytosolic organelle.6 Membrane-bound chromophores act as photosensitizers that induce changes in membrane permeability and transport mechanisms that give rise to intracellular changes in pH, ion concentrations, and membrane excitability.7,8 Photons that penetrate the cell membrane often will enter mitochondria, where they readily are absorbed by cytochrome enzymes (eg, cytochrome c oxidase), generating physiologic responses conducive to the production of reactive oxygen species and increased rates of adenosine 5′-triphosphate (ATP) and protein synthesis.1,9 The reactive oxygen species concentrations below cytotoxic levels have been shown to create biostimulatory effects for the cell.10
Recently, researchers have begun to explore the ergogenic effects of phototherapy in delaying the onset or resisting the effects of muscle fatigue and exhaustion. Acutely, fatigue impairs muscular strength and motor control and reduces a muscle's capacity to perform work over a designated period.11 The decrease in muscle function associated with fatigue is believed to be a result of metabolic alterations, such as substrate depletion (lack of ATP and glycogen), oxidative stress, tissue hypoxia, and blood acidification.11 Researchers also have indicated that specific doses of phototherapy reduce blood lactate and inflammatory biomarker levels after strenuous upper and lower extremity exercise.12,13 Based on these findings, one may infer that phototherapy also provides a prophylactic effect to tissue by limiting exercise-induced cellular damage. Limiting inflammation and cell damage during exercise also can improve recovery of muscle strength and function postexercise. Therefore, the purpose of our systematic review was to determine the ability of phototherapeutic devices, such as lasers and light-emitting diodes (LEDs), to enhance muscle contractile function, reduce exercise-induced muscle fatigue, and facilitate postexercise recovery.
METHODS
Data Sources
We searched for articles in the electronic databases of PubMed, SPORTDiscus, Web of Science, Scopus, and Rehabilitation & Physical Medicine without date limitations for the following key words: laser therapy, phototherapy, fatigue, exercise, circulation, microcirculation, and photobiomodulation. The articles had to be original research involving human participants and written in English. Citations from related articles also were retrieved and reviewed to identify additional articles for possible inclusion.
Selection Criteria
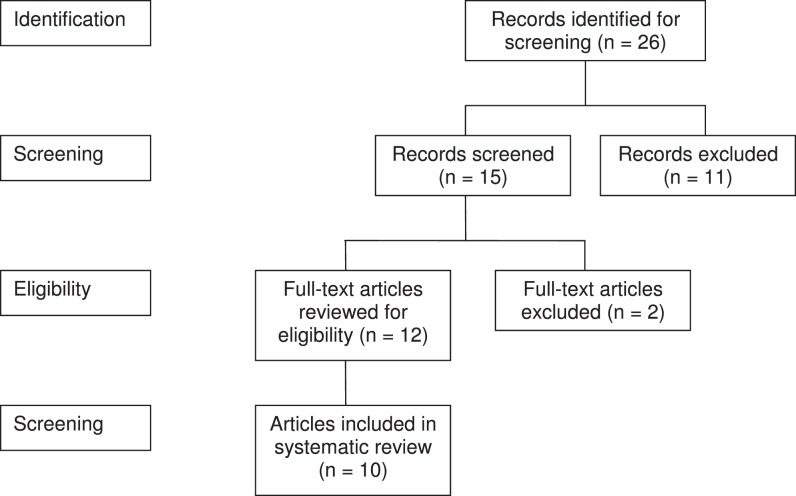
We identified research articles in which investigators evaluated the effect of phototherapy on modulating physiologic functions that result in reducing muscle damage, delaying the onset of muscle fatigue, and improving postexercise recovery during resistance exercise (Figure). Articles in which comparisons were made between phototherapy and a control condition or an alternative therapy were selected for this review. Articles were included in the screening process if the investigators studied humans, evaluated physical function changes from baseline, and assessed changes in muscle function between the control condition and phototherapy. Articles were excluded if they were reviews, meta-analyses, or animal studies.
Figure.
Criteria for selection of articles for review.
Physiotherapy Evidence Database Scale (PEDro)
The 12 articles that were deemed eligible for inclusion in the review were rated and scored using the Physiotherapy Evidence Database (PEDro) Scale14 (Table 1). The PEDro scale was developed as an instrument of evaluation for the Physiotherapy Evidence Database by the Centre for Evidence-Based Physiotherapy. The database provides access to literature on clinical trials and systematic reviews within the field of physiotherapy. The PEDro scale was developed to help users identify studies with the highest methodologic quality. The scale grades research reports based on study design, “believability,” and “interpretability” of the research conducted. It considers all aspects of research, including blinding of research participants, examiners and assessors; group allocation; comparability of groups at baseline; between-groups statistical comparability; and adequacy of follow-up measures. The scale consists of an 11-item checklist that yields a numeric score, with a maximum of 10 points if all criteria are met. No points are awarded for the first criterion. A lower score on the PEDro scale indicates a lack of methodologic techniques, such as randomization, blinding, and other controls that maintain internal or external validity.
Table 1.
Physiotherapy Evidence Database (PEDro) Scale14a
| Criteria |
Yes/No |
Points |
| 1. Eligibility criteria were specified. | Yes | 0 |
| 2. Subjects were randomly allocated in groups. | 1 | |
| 3. Allocation was concealed. | Yes | 1 |
| 4. The groups were similar at baseline regarding the most important prognostic indicators. | Yes | 1 |
| 5. There was blinding of all subjects. | Yes | 1 |
| 6. There was blinding of all therapists who administered therapy. | Yes | 1 |
| 7. There was blinding of all assessors who measured at least one key outcome. | Yes | 1 |
| 8. Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups. | Yes | 1 |
| 9. All subjects from whom outcome measures were available received the treatment or control condition as allocated or, when this was not the case, data for at least one key outcome were analyzed by “intention to treat.” | Yes | 1 |
| 10. The results of between-groups statistical comparisons were reported for at least one key outcome measure. | Yes | 1 |
| 11. The study provides both point measures and measures of variability for at least one key outcome. | Yes | 1 |
| Total | 10 |
Adapted from the Centre for Evidence-Based Physiotherapy (PEDro) scale (http://www.pedro.org.au).
Data Extraction
We used the PEDro scale due to its tested reliability. Data of interest were elapsed time to fatigue, total number of repetitions to fatigue, total work performed, maximal voluntary isometric contraction (strength), electromyographic activity, and postexercise biomarker levels (blood lactate, creatine kinase [CK], C-reactive protein [CRP], lactate dehydrogenase). Creatine kinase and CRP are measured commonly and are used as indicators of exercise-induced muscle damage. Two authors (P.A.B. and K.A.L.) rated all studies. They independently evaluated the 12 randomized controlled trials that met the criteria of evaluation. They scored each of the 12 articles individually based on the PEDro scale criteria and then met to review the final scores for all articles. Individual PEDro scores for all articles yielded an interrater reliability score of κ = 0.94. After the authors met and reviewed the discrepancies, full agreement was achieved with κ = 1.00.
Data Synthesis
Effect sizes for outcome measures were calculated as the difference between the mean score for the active phototherapy treatment and the mean score for the sham treatment (placebo control). The mean difference then was divided by the average of the standard deviation for the 2 measures, giving us the effect size (Cohen d).15 Effect sizes were interpreted as small (0.2), medium (0.5), or large (0.8).15
RESULTS
The PEDro scores ranged from 5 to 10 out of a maximum 10 points. We included only those papers with a PEDro score of 7 or greater. Ten of the 12 research articles met our level of acceptance for this review and contained a total of 32 data sets. Data sets are summarized in Tables 2 through 5. The effect sizes with corresponding P values for all data sets with differences between groups are shown in Table 5. Of the 32 data sets, 24 contained differences between active phototherapy and sham (placebo-control) treatment conditions for the various outcome measures.
Table 2.
Physiotherapy Evidence Database (PEDro) Scores, Beam Characteristics, and Treatment Variables
| Authors |
PEDro Scorea |
Light Source |
Wavelength, nm |
Power Output, mW |
Power Density, W/cm2 |
Energy Density, J/cm2 |
Treatment Points, n |
Energy, Joules per Point |
Treatment Time, s |
Cumulative Dose, J |
| Leal Junior et al16 (2008) | 9 | Single-diode laser | 655 | 50 | 5 | 500 | 4 | 5 | 400 | 20 |
| Leal Junior et al17 (2009) | 10 | Single-diode laser | 830 | 100 | 35.7 | 1785 | 4 | 5 | 200 | 20 |
| Leal Junior et al18 (2009) | 10 | Cluster-diode LED | 660 (34 diodes) | 10 | 0.05 | 1.5 | 1 | 41.7 | 30 | 41.7 |
| 850 (35 diodes) | 30 | 0.15 | 4.5 | |||||||
| Leal Junior et al19 (2009) | 10 | Single-diode laser | 810 | 200 | 5.5 | 165 | 2 | 41.7 | 60 | 12 |
| Cluster-diode LED | 660 (34 diodes) | 10 | 0.05 | 1.5 | 2 | 41.7 | 60 | 41.7 | ||
| 850 (35 diodes) | 30 | 0.15 | 4.5 | 41.7 | ||||||
| Leal Junior et al20 (2009) | 9 | Single-diode laser | 830 | 100 | 35.7 | 1071–1429 | 10 | 3–4 | 300–400 | 30–40 |
| Leal Junior et al21 (2010) | 10 | Multidiode laser | 810 (5 diodes) | 200 | 5.5 | 165 | 2 | 30 | 60 | 60 |
| Leal Junior et al22 (2011) | 10 | Cluster-diode LED | 660 (34 diodes) | 10 | 0.05 | 1.5 | 10 | 41.7 | 300 | 417 |
| 850 (35 diodes) | 30 | 0.15 | 4.5 | |||||||
| Baroni et al23 (2010) | 7 | Multidiode laser | 810 (5 diodes) | 200 | 5.5 | 165 | 6 | 30 | 180 | 180 |
| Baroni et al24 (2010) | 7 | Cluster-diode LED | 660 (34 diodes) | 10 | 0.05 | 1.5 | 3 | 41.7 | 90 | 125.1 |
| 850 (35 diodes) | 30 | 0.15 | 4.5 | |||||||
| Kelencz et al25 (2010) | 7 | Single-diode LED | 640 | 0.116 | 0.222 | 2 | 8 | 1.044 | 72 | 8.4 |
| 0.116 | 0.222 | 4 | 8 | 2.088 | 144 | 16.7 | ||||
| 0.116 | 0.222 | 6 | 8 | 3.132 | 216 | 25.1 |
The PEDro scale scores range from 1 to 10.
Table 5.
Effect Sizes With P Values for Data Sets With Differences Between Groups
| Authors |
No. of Participants and Age (Mean ± SD, y) |
Time to Fatigue |
Repetitions to Fatigue |
Strength Loss, Work, or EMG |
Blood Lactate |
Creatine Kinase |
C-Reactive Protein |
Lactate Dehydrogenase |
Strength Recovery |
| Leal Junior et al16 (2008) | 12 male professional volleyball players (22 ± 3.0) | Cohen d = 2.32, P = .0001 | SD not provided | Not applicable | Not different | Not applicable | Not applicable | Not applicable | Not measured |
| Leal Junior et al17 (2009) | 10 healthy male professional volleyball players (22.3 ± 6.1) | Cohen d = 0.63, P = .04 | Not different | Not applicable | Not different | Not applicable | Not applicable | Not applicable | Not measured |
| Leal Junior et al18 (2009) | 10 healthy male professional volleyball players (23.6 ± 5.6) | Cohen d = 0.50, P = .02 | Cohen d = 0.39, P = .04 | Not applicable | Cohen d = 0.92, P = .04 | Cohen d = 1.12, P = .04 | Cohen d = 0.80, P = .03 | Not applicable | Not measured |
| Leal Junior et al19 (2009) | 8 male volleyball players (18.5 ± 0.93) | Not applicable | Not applicable | Not different | Not applicable | Cohen d = 1.62, P < .01 | Not applicable | Not applicable | Not measured |
| Leal Junior et al20 (2009) | 9 male professional volleyball players (20.7 ± 2.96) and 11 male soccer players (16.2 ± 0.75) | Not applicable | Not applicable | Not different | Cohen d = 0.99; P < .01 | Cohen d = 1.77, P = .01 | Not applicable | Not applicable | Not measured |
| Leal Junior et al21 (2010) | 9 male volleyball players (18.6 ± 1.0) | Cohen d = 1.01, P = .04 | Cohen d = 0.75, P = .03 | Not applicable | Cohen d = 1.67, P < .01 | Cohen d = 1.01, P = .02 | Cohen d = 1.52, P = .047 | Not applicable | Not measured |
| Leal Junior et al22 (2011) | 6 futsal players (20.7 ± 2.96) | Not applicable | Not applicable | Not different | Cohen d = 1.94, P = .004 | Cohen d = 2.07, P = .006 | Not different | Not applicable | Not measured |
| Baroni et al23 (2010) | 36 healthy men (24.8 ± 4.4) | Not applicable | Not applicable | Cohen d = 0.90, P = .01 | Not applicable | 24 ha: Cohen d = 0.89, P = .0248 ha: Cohen d = 1.50, P = .001 | Not applicable | 24 h: not different 48 h: Cohen d = 0.89, P = .02 | 24 h: Cohen d = 1.03, P = .00448 h: Cohen d = 1.16, P = .001 |
| Baroni et al24 (2010) | 17 physically active men (26.3 ± 4.3) | Not applicable | Not applicable | Cohen d = 0.23, P = .03 | Not applicable | Not applicable | Not applicable | Not applicable | Not measured |
| Kelencz et al25 (2010) | 30 men and women | Right: Cohen d = 0.28, P < .05 | Not applicable | Cohen d = 0.65, P < .05 | Not applicable | Not applicable | Not applicable | Not applicable | Not measured |
| Left: Cohen d = 0.29, P < .05 |
Abbreviation: EMG, electromyography.
24 h refers to 24 hours postexercise; 48 h refers to 48 hours postexercise.
DISCUSSION
The important factors related to phototherapy that we discuss include (1) the type of light source used and its treatment variables (wavelength and dose), (2) when the light treatment was applied (before or after exercise), (3) the exercise protocol used, (4) the muscle groups targeted for exercise and testing, and (5) the outcomes of each study.
Beam Characteristics and Tissue Interaction
The phototherapy devices used in the 10 studies were semiconductor or solid-state diode lasers or LEDs. Semiconductor or solid-state laser diodes contain a lasing medium to stimulate photon emission. They emit photons in a more collimated and coherent manner than do LED sources. Another important characteristic of photon emission is the diameter of the beam or spot size. Smaller beam diameters and spot sizes produce greater irradiance of energy density at the point of skin contact. In the studies we included, the laser diodes that were used delivered light using spot sizes16,17,19–21,23 ranging from 0.003 to 0.01 cm2, whereas LEDs used spot sizes18,19,22,24 ranging from 0.2 to 0.5 cm2 and delivered light onto the skin in a much greater divergent pattern. Data on the beam characteristics appear in Table 2.
Pigmentation is known to affect light transmission through skin. Light penetration through darker-pigmented skin is reduced due to the absorption of photons by melanin.26 If the rate of photoabsorption by the skin exceeds the capacity of the tissue to dissipate the light energy, the energy will be converted to heat and may cause thermal discomfort. Practical guidelines recommend that when treating darker-pigmented individuals, the risk of tissue heating can be minimized while maintaining the same treatment dose by reducing the average output power or energy density of the beam and increasing the treatment time. In addition, if more light energy is absorbed by the skin in darker-pigmented individuals, then a larger treatment dose is required to transmit the equivalent amount of light energy to deeper tissues. In clinical settings, the therapist usually chooses the increased treatment dose based on past experience.
Wavelength plays an important role in the ability of light to penetrate soft tissue.27,28 When light therapy is administered directly to the patient's skin, some light is attenuated by the superficial layers. Tunér and Hode7 indicated that from 50% to 90% of energy is absorbed by the skin and subcutaneous tissues and the remaining light energy penetrates into deeper tissue layers (muscle, deep fascia, ligament). Esnouf et al29 used a near-infrared (NIR), 850-nm laser diode with an output power of 100 mW to irradiate a 0.784-mm-thick section of human skin and found that 66% of the initial beam intensity was attenuated by the skin section. Similarly, Kolari and Airaksinen30 exposed dermal tissue to both visible red and NIR lasers and found that a substantial amount of light energy was absorbed within 0.5 mm after penetration. Researchers5,6,28 have indicated that diodes with wavelengths ranging from 820 to 904 nm can transmit light energy from 2 to 4 cm beyond the skin interface and, therefore, are best suited for treating deep soft tissue disorders, such as those involving muscles, ligaments, and tendons. Diodes with wavelengths ranging from 400 to 700 nm can transmit light energy only to the epidermal and dermal tissue layers (<1 cm) and, therefore, are best suited for targeting superficial wounds and skin disorders.31,32
Dose Dependency
Therapeutic dose is reported to have the greatest influence on tissue healing and clinical outcome.28,33,34 Achieving a therapeutic dose without understimulating or overstimulating the target tissues is often the most difficult component of clinical phototherapy practice.33 The Arndt-Schultz principle has been adopted from early toxicology studies of yeast culture to explain the optimal therapeutic dose level of laser. Optimal doses have been established experimentally in cell and tissue cultures. This therapeutic laser dose or level of photostimulation must be attained; if the amount of energy absorbed is insufficient to stimulate the absorbing tissues, no reactions or changes can occur in body tissues.35 Weak stimuli (underdosing) produce no effect or only a minimal effect on cellular function, moderate to strong stimuli positively enhance cellular function, and very strong stimuli (overdosing) suppress or inhibit cellular function.34
The optimal doses that are photostimulating for many human tissues are not known; however, results from animal and cell studies13,36,37 have indicated that the therapeutic effects of light therapy are dose dependent and operate within a therapeutic window when treating musculoskeletal injuries. Therefore, one could surmise that the same dose dependency exists for treating musculoskeletal pathologic conditions in humans. However, problems exist when attempting to translate light therapy studies from animal models to human participants. No known or universally accepted method is available to calculate a comparable treatment dose in humans from those doses used in cell and animal models. In our review, light treatment doses and the type of light source varied among studies, with the trend favoring a higher dose when using an LED device than when using a laser diode. This finding makes sense because the light emanating from LEDs has a wider bandwidth, is not coherent, and is more divergent than the light emanating from laser diodes, resulting in more reflection and less transmission of LED-generated light through the skin. Therefore, a higher dose when using LED therapy may compensate for beam reflection and divergence.
Contractile Function, Fatigue Resistance, and Postexercise Recovery
Recently, researchers have shown that phototherapy can provide an ergogenic effect by improving the contractile function of a skeletal muscle.12,13,16–18,21,23,24,38 In several studies, researchers have investigated the effects of laser and LED therapy on skeletal muscle fatigue and muscle damage using both animal12,13 and human populations.16–18,20 Lopes-Martins et al13 and Leal Junior et al12 showed a delay in the fatigue response to repeated, electrically evoked tetanic contractions in the tibialis anterior muscle of rats exposed to laser therapy. They also showed that skeletal muscle exposed to laser displayed lower levels of blood lactate and CK activity after repeated tetanic contractions.12,13 In human studies, skeletal muscle exposed to selected doses of laser16,17,21 or LED18,24 therapy demonstrated enhanced performance by maintaining contractile force output and delaying the onset of fatigue when challenged with resistance exercise. Skeletal muscle exposed to laser or LED therapy also had less cell damage after exercise, indicating that phototherapy provided protection from exercise-induced damage.12,18–23
In a series of studies, Leal Junior et al16–18,21 administered active phototherapy treatments or sham treatments to the biceps brachii muscle of professional volleyball players and instructed them to perform repeated, near-maximal arm-curl exercises until voluntary exhaustion. The phototherapy intervention consisted of having 20 J (5 J per point at 4 points),16,17 42 J (1 point),18 or 60 J (30 J per point at 2 points)21 of light delivered with either a laser diode or multidiode LEDs to the biceps brachii muscle of the dominant upper extremity before exercise. The biceps brachii muscle that was treated with active laser or LED therapy performed more arm-curl repetitions until voluntary exhaustion than did the muscle receiving the sham treatment.16–18,21 Blood lactate levels compared before and after exercise were slightly lower to much lower after exercise when an active light treatment was administered to the biceps.17,18,21 Creatine kinase and CRP levels also were lower after the active light treatment than in the sham (control) condition.18,21 Leal Junior et al16,17 initially assessed the effects of laser therapy on biceps brachii muscle function by comparing 655-nm16 and 830-nm17 single-diode lasers. The cumulative dose level for both studies was 20 J (5 J per point at 4 points). Although both wavelengths produced similar results for muscle performance and postexercise recovery, we found the results interesting because the red (655-nm) lasers emitted light in shorter wavelengths and therefore had poorer light penetration than NIR (830-nm) lasers.32 Researchers28,32 have indicated that less light can be transmitted to muscle tissue using a red 655-nm laser, so we would expect the muscle receiving laser therapy with the 655-nm wavelength to fatigue at a faster rate and incur more exercise-induced cell damage than the muscle receiving the NIR 830-nm laser condition. Because light attenuation and absorption were not measured directly in these studies, we could not determine the actual dose of light absorbed by the muscles that were treated.
In later studies, Leal Junior et al18,21 compared the ergogenic and prophylactic effects of phototherapy using a 5-diode NIR laser (810 nm) and a cluster multidiode (34 red and 35 NIR diodes) LED probe. The cluster LED probe delivered 42 J at 1 point to the biceps, and the multidiode NIR laser delivered 60 J (30 J per point at 2 points). Again, both devices produced similar results for performance enhancement and postexercise recovery. Active LED or laser therapy applied pre-exercise increased the number of repetitions to exhaustion by 12.9% and 14.5%, respectively, and the elapsed time to exhaustion by 11.6% and 8%, respectively.18,21 In addition, the muscle exposed to active light therapy displayed less exercise-induced cell damage than the muscle in the sham treatment condition.
Leal Junior et al19,20 assessed the effects of laser or LED therapy on muscle fatigue, exercise-induced muscle damage, and postexercise recovery after strenuous lower extremity exercise. Participants completed a single bout of resistance exercise for the lower extremity using the Wingate cycle ergometry test. Male volleyball and soccer players were evaluated after receiving either a controlled dose of laser or LED therapy or a sham dose to the rectus femoris muscle before completing the Wingate test. Researchers found no differences in peak or mean power or the amount of muscular work performed between the active-light and sham-control treatment conditions; however, postexercise blood biomarker levels were lower when the athletes received the active dose of light therapy. This finding indicated that light therapy may protect muscles from damage or enhance postexercise recovery mechanisms after strenuous exercise. The doses delivered to the target muscle varied considerably among studies. Laser diode (NIR 810–830 nm) treatment doses consisted of either 12 J (6 J per point at 2 points)19 or 30 to 40 J (3–4 J per point at 10 points)20 per muscle, whereas the multidiode LED dose consisted of 83.4 J (41.7 J per point at 2 points)19 delivered per muscle.
In 2 studies using isokinetic dynamometry, Baroni et al23,24 evaluated muscle fatigue and postexercise recovery of the quadriceps femoris muscle group in response to phototherapy or sham therapy. They investigated the protective effects of laser or LED therapy on exercise-induced muscle damage and postexercise strength loss and recovery using a knee-extensor model. The quadriceps femoris muscle was exposed to an active or sham laser or LED treatment, then the participants performed a challenging resistance exercise regimen for the quadriceps muscle group. The authors then treated the quadriceps muscle group (rectus femoris, vastus medialis, and vastus lateralis) with a multidiode NIR 810-nm laser23 or a cluster (34 at 660 nm or 35 at 850 nm) multidiode LED24 before exercise at cumulative doses of 180 J (30 J per point at 6 points) and 125.1 J (41.7 J per point at 3 points), respectively. In both studies, Baroni et al23,24 showed that active laser or LED therapy before exercise reduced postexercise levels of markers of muscle damage, limited the extent of muscle fatigue, and improved postexercise recovery compared with sham light therapy.
Kelencz et al25 studied the effects of LED (640 nm) therapy on muscle activity and fatigue resistance. The masseter muscle was irradiated under 4 dose conditions (sham and 8.3 J [1.04 J per point at 8 points], 16.7 J [2.09 J per point at 8 points], and 25.1 J [3.13 J per point at 8 points] per muscle). After treatment, the participant bit down on a pressure plate that was equipped with a load cell embedded into the platform. The electromyography (EMG) signals and maximal voluntary isometric contraction levels were recorded before and after treatment and mandibular occlusion. A dose-dependent increase in muscle activity and time before fatigue was observed after treatment. The 8.3-J dose produced greater muscle activity posttreatment, whereas the 16.7-J dose increased the time to fatigue.
In most of the studies in our review, the investigators administered the laser treatments before completing the exercise protocol. In one of these studies, laser therapy was applied after the exercise protocol was completed.22 Leal Junior et al22 compared the effects of cold-water immersion therapy (CWIT), LED therapy, and sham LED therapy on postexercise recovery after high-intensity resistance exercise. Participants completed a single bout of resistance exercise for the lower extremity using the Wingate cycle ergometer test. Multidiode LED therapy was administered to the major muscle groups of the lower extremity (quadriceps, hamstrings, triceps surae) 5 minutes after completing the exercise bout. The cumulative dose for both legs was 417 J (41.7 J per point at 10 points). The LED therapy was more effective than CWIT or sham LED therapy in facilitating muscle recovery postexercise. Blood lactate levels and CK activity were lower after treatment with active light therapy than with CWIT and sham light therapy.
Mechanisms of Actions
Ergogenic Effects
The authors of the reviewed studies postulated that phototherapy could provide an ergogenic effect during exercise by enhancing intramuscular microcirculation,24 decreasing lactic acid production,18,19,21,22 improving mitochondrial function,1,9 and improving the antioxidant capacity16,17,23 of exercising muscle. However, microcirculation and oxidative stress levels were not assessed directly in the studies we reviewed.
Lower levels of blood lactate after strenuous upper and lower extremity exercise for the active laser or LED condition indicate that fewer anaerobic or glycolytic metabolic pathways were used during exercise or that lactic acid was being used as a substrate for oxidative metabolism by the mitochondria.39,40 Well-oxygenated muscle cells are better able to oxidize lactic acid to pyruvate, which then is used by the mitochondria to produce ATP.39 Phototherapy, especially in the NIR spectrum, activates the respiratory chain through the photoacceptor cytochrome c oxidase.9 Respiratory chain activation results in a cascade of biochemical reactions, leading to increased rates of ATP synthesis for sustained muscle function.
Prophylactic Effects
Exercise-induced muscle damage occurs in 2 distinct phases: primary and secondary. Primary muscle damage results from the mechanical stress of exercise, and secondary muscle damage is caused by the cascade of biochemical reactions that occur due to the inflammatory response.41,42 Phototherapy administered before exercise is thought to protect muscle cells from primary and secondary damage, whereas phototherapy administered after injury protects cells from secondary damage only.23 The exact mechanisms by which phototherapy protects muscle from exercise-induced damage are not fully understood; however, the results from in vivo animal studies have revealed important information about how phototherapy can protect muscle from secondary damage after trauma.
Authors of in vivo animal studies have shown that phototherapy administered to injured muscle tissue produces anti-inflammatory and antioxidant effects that protect muscle from secondary damage. Avni et al43 investigated the cytoprotective effect of laser therapy using an ischemia-reperfusion injury (IRI) model in rat gastrocnemius muscle. They showed that laser therapy applied immediately and 1 hour after arterial occlusion blunted the extent of tissue degeneration and improved the antioxidant capacity of injured muscle hours after IRI compared with control muscle.43 Similarly, in a series of experiments, Oron et al44 and Ad and Oron45 investigated the effects of laser therapy on muscle damage and scar tissue formation using an experimentally induced myocardial infarction (MI) model in rats and dogs. Myocardial infarction was induced by occlusion of the left anterior descending coronary artery. In the canine model, laser therapy was applied to the infarcted region of the heart at 15 minutes and again at 3 days after MI. A total dose of 1.08 J/cm2 was delivered at each time using the wavelength of 803 nm with a power density of 6 mW/cm2. Tissue samples were analyzed with computerized morphometry software. The hearts that received laser therapy had a 50% to 70% reduction in infarct size and less scar tissue formation 4 to 6 weeks postinfarction compared with the hearts that did not receive laser therapy. The authors concluded that laser therapy after MI and IRI substantially increased the production of heat shock proteins and preserved mitochondrial integrity, which in turn protected the heart muscle from the damaging effects of ischemia, inflammation, and oxidative stress. Rizzi et al46 investigated the effects of laser therapy on inflammation and repair using male Wistar rats with an induced injury to the gastrocnemius muscle. Laser therapy was administered daily to the injured muscle. Results revealed that laser therapy reduced inflammatory signaling pathways and blocked the damaging effects of reactive oxygen species. Inflammatory and oxidative stress levels were reduced, thereby minimizing secondary muscle damage.
In several of the phototherapy studies in our review, postexercise reductions in the levels of CK, lactate dehydrogenase, and CRP were shown when active laser or LED treatment was administered before exercise.18–23 These reductions indicate that less exercise-induced muscle damage and inflammation occurred during the bout of upper and lower extremity resistance exercise. The enhanced cellular protection during strenuous exercise due to the phototherapy treatment likely had a direct effect on the improved postexercise recovery.22,23
Sample and Effect Sizes
For 9 of the 10 studies that we reviewed, considerable overlap of investigators occurred.16–24 In addition, the studies contained relatively small sample sizes with very similar inclusion and exclusion criteria. Seven of the 9 studies contained sample sizes ranging from 6 to 12 participants and consisted primarily of male professional athletes between the ages of 18 and 36 years who competed in volleyball, soccer, or futsal.16–22 Therefore, an overlap of participant samples may have been present among several of the studies. The small sample sizes and similar participant characteristics among studies severely limit the generalizability of the findings.
The effect sizes were rather robust, ranging from small to large depending on the outcome measure (Table 5). Effect sizes for the muscle performance outcomes (repetitions and time to fatigue, work, strength loss, strength recovery, and EMG) averaged 0.78 (range, 0.28–2.32) and for the outcomes of the biomarkers of muscle damage (CK, lactate dehydrogenase), inflammation (CRP) and recovery (blood lactate) averaged 1.34 (range, 0.80–2.07). The collective strength of the effect sizes lend further support to the effectiveness of phototherapy in providing ergogenic and prophylactic benefits for skeletal muscle during resistance exercise.
Clinical Implications
Skeletal muscle fatigue is a new avenue for research in phototherapy. The traditional use of phototherapy in clinical settings has been directed toward treating injured tissue to control pain and enhance healing. However, new paradigms for clinical practice are expanding the traditional model for phototherapy in clinical situations. We provided an in-depth look at the effects of phototherapy administered pre-exercise on limiting the extent of exercise-induced fatigue and muscle damage and on facilitating postexercise recovery. Our review is novel because we examined phototherapy from a proactive standpoint as an ergogenic aid to therapeutic exercise and prophylaxis to exercise-induced muscle damage.
Phototherapy appears to be a viable treatment modality for skeletal muscle. It is safe, easy to administer, and noninvasive and has no known side effects and few reported contraindications. Study outcomes consistently demonstrated ergogenic and prophylactic benefits to skeletal muscle after a treatment dose of phototherapy. Positive outcomes occurred when phototherapy was administered pre-exercise12,16–21,23–25 and postexercise.22 Investigators could conclude that exposing skeletal muscle to single-diode and multidiode laser or multidiode LED therapy positively affects physical performance by delaying the onset of fatigue,16–18,21 reducing the fatigue response,23–25 improving postexercise recovery,21–23 and protecting cells from exercise-induced damage.18–23 The results we discussed may directly affect how phototherapeutic modalities are prescribed by therapists and used in clinical practice settings.
With respect to light source, laser diodes typically emit light in a very narrow bandwidth compared with LED.47 A more narrow emission intensifies the beam, creating a greater energy density or fluence level (J/cm2). Laser diodes emit light at higher energy densities than LEDs, and this may affect the rate of absorption, depth of penetration, and transmission patterns of light into deeper tissues, regardless of the wavelength.28,47–49 Light-emitting diodes are less expensive to manufacture than semiconductor or solid-state diodes and have been shown to be safe and effective treatment modalities.18,19,24
Being able to quickly and effectively treat an entire muscle or muscle group is an important factor when using laser therapy as an ergogenic aid. Multidiode lasers and LEDs may have an advantage over single-diode lasers for treating large muscles because they can cover a larger surface area over the muscle.18,19 Single-diode laser treatments deliver tight beams of light to small areas on the skin, and consequently the applicator has to be moved constantly to cover multiple points on a muscle or muscle group. Because multidiode LED applicators have greater beam divergence than diode lasers, they do not have to be moved as often during treatment while still covering a large surface area of the muscle per application point. The number of treatment points per muscle or muscle group varied among studies. The biceps brachii was treated on 1 to 2 points with multidiode laser/LEDs and 4 points with a single-diode laser. For the lower extremity, the number of treatment points for the quadriceps femoris ranged from 2 to 6 for multidiode lasers/LEDs and included 5 points for single-diode lasers.
Phototherapy administered immediately before resistance exercise extended the elapsed time to fatigue and the total number of repetitions to fatigue.16–18,21 In addition, phototherapy reduced the fatigue response of skeletal muscle during a bout of resistance exercise,23,24 and it enhanced postexercise recovery and reduced exercise-induced damage when applied before and after strenuous resistance exercise.18,21–23 The effective dose for the biceps brachii ranged from 20 to 60 J, whereas larger muscles in the lower extremity displayed ergogenic and prophylactic effects when higher treatment doses ranging from 125 to 180 J were administered. On average, the treatment doses were higher in the LED therapy studies than in the single-diode and multidiode laser studies to achieve a similar therapeutic outcome.49 Therefore, clinicians may need to adjust the cumulative treatment dose when using LED devices to compensate for the higher beam divergence and reduced energy density.
Individuals recovering from musculoskeletal impairment often experience changes in physical performance, such as early-onset muscle fatigue with associated pain and soreness due to disuse atrophy, degenerative changes (eg, osteoarthritis), and neuromuscular deficits. In clinical situations where progressive resistance exercise is indicated for structural and functional recovery (eg, with postoperative or postinjury rehabilitation), exercise-induced muscle fatigue and pain can be limiting factors.50 This is especially true for muscle groups that maintain balance and locomotor function, such as the quadriceps femoris.51
During a normal rehabilitation session, an injured athlete usually undergoes a combination of therapeutic modality treatments coupled with therapeutic exercise. Therapeutic modalities typically are administered during a treatment session to control pain and other symptoms that may limit the intensity and volume of therapeutic exercise. The aims of therapeutic exercise are for the athlete to regain range of motion, strength, and function so he or she can return safely to participation. Enhanced muscle performance and fatigue resistance would benefit athletes during rehabilitation and recovery from injury when muscle atrophy and weakness can impair muscle function. If phototherapy is found to be beneficial in the rehabilitation and postexercise recovery process, it may improve clinical outcomes and the overall quality of health care for injured athletes.
CONCLUSIONS
Phototherapy administered immediately before a bout of resistance exercise consistently was shown to provide an ergogenic effect to skeletal muscle by improving physical performance (extending the elapsed time and total number of repetitions to fatigue, reducing the deficit in maximal voluntary isometric contraction pre-exercise to postexercise) and improving the clearance of blood lactate immediately after exercise. It also consistently was shown to provide a protective effect for skeletal muscle by reducing postexercise plasma levels of CK and CRP. The information gained from this novel use of laser therapy can open a therapeutic window into the treatment of musculoskeletal conditions in which muscle fatigue and fatigue-related impairment are barriers to treating musculoskeletal injuries.
Table 3.
Effectiveness of Laser Therapy on Selected Outcome Measures Used to Characterize Ergogenic Effects of Laser
| Authors |
Journal |
Type of Fatigue Protocol |
Muscles Exercised |
Time to Fatigue |
Repetitions to Fatigue |
Strength Loss or Work Performed |
Electromyographic Activity |
| Leal Junior et al16 (2008) | Photomed Laser Surg | Isotonic arm curl | Biceps brachii | Effective | Effective | Not applicable | Not applicable |
| Leal Junior et al17 (2009) | Lasers Med Sci | Isokinetic arm curl | Biceps brachii | Ineffective | Effective | Not applicable | Not applicable |
| Leal Junior et al18 (2009) | Lasers Surg Med | Isokinetic arm curl | Biceps brachii | Effective | Effective | Not applicable | Not applicable |
| Leal Junior et al19 (2009) | Photomed Laser Surg | Wingate cycle ergometer | Quadriceps femoris (bilateral) | Not applicable | Not applicable | Ineffective | Not applicable |
| Leal Junior et al20 (2009) | Lasers Med Sci | Wingate cycle ergometer | Quadriceps femoris (bilateral) | Not applicable | Not applicable | Ineffective | Not applicable |
| Leal Junior et al21 (2010) | J Orthop Sports Phys Ther | Isokinetic arm curl | Biceps brachii | Effective | Effective | Not applicable | Not applicable |
| Leal Junior et al22 (2011) | Lasers Med Sci | Wingate cycle ergometer | Quadriceps femoris (bilateral) | Not applicable | Not applicable | Ineffective | Not applicable |
| Baroni et al23 (2010) | Eur J Appl Physiol | Isokinetic (eccentric) leg extension | Quadriceps femoris | Not applicable | Not applicable | Effective | Not applicable |
| Baroni et al24 (2010) | Photomed Laser Surg | Isokinetic (concentric) leg flexion-extension | Quadriceps femoris | Not applicable | Not applicable | Effective | Not applicable |
| Kelencz et al25 (2010) | Photomed Laser Surg | Isometric jaw clench | Masseter | Effective (2.09 J only) | Not applicable | Not applicable | Effective (1.04 J only) |
Table 4.
Effectiveness of Laser Therapy on Selected Outcome Measures Used to Characterize Prophylactic and Recovery Effects of Laser
| Authors |
Journal |
Type of Fatigue Protocol |
Muscles Exercised |
Postexercise Biomarker Levels |
Strength Recoverya |
Pain and Sorenessb |
|||
| Immediately |
24 h |
48 h |
24 h |
48 h |
|||||
| Leal Junior et al16 (2008) | Photomed Laser Surg | Isotonic arm curl | Biceps brachii | Ineffective | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Leal Junior et al17 (2009) | Lasers Med Sci | Isokinetic arm curl | Biceps brachii | Ineffective | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Leal Junior et al18 (2009) | Lasers Surg Med | Isokinetic arm curl | Biceps brachii | Effective—blood lactate, creatine kinase, and C-reactive protein | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Leal Junior et al19 (2009) | Photomed Laser Surg | Wingate cycle ergometer | Quadriceps femoris (bilateral) | Effective—creatine kinase | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Leal Junior et al20 (2009) | Lasers Med Sci | Wingate cycle ergometer | Quadriceps femoris (bilateral) | Effective—blood lactate and creatine kinase | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Leal Junior et al21 (2010) | J Orthop Sports Phys Ther | Isokinetic arm curl | Biceps brachii | Effective—blood lactate, creatine kinase, and C-reactive protein | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Leal Junior et al22 (2011) | Lasers Med Sci | Wingate cycle ergometer | Quadriceps femoris (bilateral) | Effective—blood lactate and creatine kinase | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Baroni et al23 (2010) | Eur J Appl Physiol | Isokinetic (eccentric) leg extension | Quadriceps femoris | Not applicable | Effective— creatine kinase | Effective— creatine kinase and lactate dehydrogenase | Effective | Effective | Ineffective |
| Baroni et al24 (2010) | Photomed Laser Surg | Isokinetic (concentric) leg flexion-extension | Quadriceps femoris | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Kelencz et al25 (2010) | Photomed Laser Surg | Isometric jaw clench | Masseter | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
Measured with maximal voluntary isometric contraction.
Measured with a visual analog scale.
REFERENCES
- 1.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49(1):1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira DM, Zangaro RA, Villaverde AB et al. Analgesic effect of He-Ne (632.8 nm) low-level laser therapy on acute inflammatory pain. Photomed Laser Surg. 2005;23(2):177–181. doi: 10.1089/pho.2005.23.177. [DOI] [PubMed] [Google Scholar]
- 3.Laakso EL, Cabot PJ. Nociceptive scores and endorphin-containing cells reduced by low-level laser therapy (LLLT) in inflamed paws of Wistar rat. Photomed Laser Surg. 2005;23(1):32–35. doi: 10.1089/pho.2005.23.32. [DOI] [PubMed] [Google Scholar]
- 4.Ihsan FR. Low-level laser therapy accelerates collateral circulation and enhances microcirculation. Photomed Laser Surg. 2005;23(3):289–294. doi: 10.1089/pho.2005.23.289. [DOI] [PubMed] [Google Scholar]
- 5.Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD. The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg. 2004;22(4):323–329. doi: 10.1089/pho.2004.22.323. [DOI] [PubMed] [Google Scholar]
- 6.Reddy GK. Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Laser Med Surg. 2004;22(2):141–150. doi: 10.1089/104454704774076208. [DOI] [PubMed] [Google Scholar]
- 7.Tunér J, Hode L. Laser Therapy: Clinical Practice and Scientific Background. Stockholm, Sweden: Prima Books;; 2002. [Google Scholar]
- 8.Klebanov GI, Kreinina MV, Poltanov EA, Khristoforova TV, Vladimirov YA. Mechanism of therapeutic effect of low-intensity infrared laser radiation. Bull Exp Biol Med. 2001;131(3):239–241. doi: 10.1023/a:1017643230376. [DOI] [PubMed] [Google Scholar]
- 9.Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R. Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol B. 2009;95(2):89–92. doi: 10.1016/j.jphotobiol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Lubart R, Eichler M, Lavi R, Friedman H, Shainberg A. Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg. 2005;23(1):3–9. doi: 10.1089/pho.2005.23.3. [DOI] [PubMed] [Google Scholar]
- 11.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88(1):287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 12.Leal Junior EC, Lopes-Martins RA, de Almeida P, Ramos L, Iversen VV, Bjordal JM. Effect of low-level laser therapy (GaAs 904 nm) in skeletal muscle fatigue and biochemical markers of muscle damage in rats. Eur J Appl Physiol. 2010;108(6):1083–1088. doi: 10.1007/s00421-009-1321-1. [DOI] [PubMed] [Google Scholar]
- 13.Lopes-Martins RA, Marcos RL, Leonardo PS et al. Effect of low-level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol. 2006;101(1):283–288. doi: 10.1152/japplphysiol.01318.2005. [DOI] [PubMed] [Google Scholar]
- 14.Centre for Evidence-Based Physiotherapy. PEDro scale. 2012. http://www.pedro.org.au/english/downloads/pedro-scale/. Accessed August 13.
- 15.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 16.Leal Junior EC, Lopes-Martins RA, Dalan F et al. Effect of 655-nm low-level laser therapy on exercise-induced skeletal muscle fatigue in humans. Photomed Laser Surg. 2008;26(5):419–424. doi: 10.1089/pho.2007.2160. [DOI] [PubMed] [Google Scholar]
- 17.Leal Junior EC, Lopes-Martins RA, Vanin AA et al. Effect of 830 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in humans. Lasers Med Sci. 2009;24(3):425–431. doi: 10.1007/s10103-008-0592-9. [DOI] [PubMed] [Google Scholar]
- 18.Leal Junior EC, Lopes-Martins RA, Rossi RP et al. Effect of cluster multi-diode light emitting diode therapy (LEDT) on exercise-induced skeletal muscle fatigue and skeletal muscle recovery in humans. Lasers Surg Med. 2009;41(8):572–577. doi: 10.1002/lsm.20810. [DOI] [PubMed] [Google Scholar]
- 19.Leal Junior EC, Lopes-Martins RA, Baroni BM et al. Comparison between single-diode low-level laser therapy (LLLT) and LED multi-diode (cluster) therapy (LEDT) applications before high-intensity exercise. Photomed Laser Surg. 2009;27(4):617–623. doi: 10.1089/pho.2008.2350. [DOI] [PubMed] [Google Scholar]
- 20.Leal Junior EC, Lopes-Martins RA, Baroni BM et al. Effect of 830 nm low-level laser therapy applied before high-intensity exercises on skeletal muscle recovery in athletes. Lasers Med Sci. 2009;24(6):857–863. doi: 10.1007/s10103-008-0633-4. [DOI] [PubMed] [Google Scholar]
- 21.Leal Junior EC, Lopes-Martins RA, Frigo L et al. Effects of low-level laser therapy (LLLT) in the development of exercise-induced skeletal muscle fatigue and changes in biochemical markers related to postexercise recovery. J Orthop Sports Phys Ther. 2010;40(8):524–532. doi: 10.2519/jospt.2010.3294. [DOI] [PubMed] [Google Scholar]
- 22.Leal Junior EC, de Godoi V, Mancalossi JL et al. Comparison between cold water immersion therapy (CWIT) and light emitting diode therapy (LEDT) in short-term skeletal muscle recovery after high-intensity exercise in athletes: preliminary results. Lasers Med Sci. 2011;26(4):493–501. doi: 10.1007/s10103-010-0866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baroni BM, Leal Junior EC, De Marchi T, Lopes AL, Salvador M, Vaz MA. Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur J Appl Physiol. 2010;110(4):789–796. doi: 10.1007/s00421-010-1562-z. [DOI] [PubMed] [Google Scholar]
- 24.Baroni BM, Leal Junior EC, Geremia JM, Diefenthaeler F, Vaz MA. Effect of light-emitting diodes therapy (LEDT) on knee extensor muscle fatigue. Photomed Laser Surg. 2010;28(5):653–658. doi: 10.1089/pho.2009.2688. [DOI] [PubMed] [Google Scholar]
- 25.Kelencz CA, Munoz IS, Amorim CF, Nicolau RA. Effect of low-power gallium-aluminum-arsenium noncoherent light (640 nm) on muscle activity: a clinical study. Photomed Laser Surg. 2010;28(5):647–652. doi: 10.1089/pho.2008.2467. [DOI] [PubMed] [Google Scholar]
- 26.Battle EF, Jr, Hobbs LM. Laser therapy on darker ethnic skin. Dermatol Clin. 2003;21(4):713–723. doi: 10.1016/s0733-8635(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 27.Hashmi JT, Huang YY, Sharma SK et al. Effect of pulsing in low-level light therapy. Lasers Surg Med. 2010;42(6):450–466. doi: 10.1002/lsm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enwemeka CS. Intricacies of dose in laser phototherapy for tissue repair and pain relief. Photomed Laser Surg. 2009;27(3):387–393. doi: 10.1089/pho.2009.2503. [DOI] [PubMed] [Google Scholar]
- 29.Esnouf A, Wright PA, Moore JC, Ahmed S. Depth of penetration of an 850nm wavelength low level laser in human skin. Acupunct Electrother Res. 2007;32(1–2):81–86. doi: 10.3727/036012907815844165. [DOI] [PubMed] [Google Scholar]
- 30.Kolari PJ, Airaksinen O. Poor penetration of infra-red and helium neon low power laser light into the dermal tissue. Acupunct Electrother Res. 1993;18(1):17–21. doi: 10.3727/036012993816357566. [DOI] [PubMed] [Google Scholar]
- 31.Beckerman H, de Bie RA, Bouter LM, De Cuyper HJ, Oostendorp RA. The efficacy of laser therapy for musculoskeletal and skin disorders: a criteria-based meta-analysis of randomized clinical trials. Phys Ther. 1992;72(7):483–491. doi: 10.1093/ptj/72.7.483. [DOI] [PubMed] [Google Scholar]
- 32.Enwemeka CS. Attenuation and penetration of visible 632.8nm and invisible infra-red 904nm light in soft tissues. Laser Ther. 2001;13:95–101. [Google Scholar]
- 33.Bjordal JM, Baxter GD. Ineffective dose and lack of laser output testing in laser shoulder and neck studies. Photomed Laser Surg. 2006;24(4):533–534. [PubMed] [Google Scholar]
- 34.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7(4):358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starkey C. Therapeutic Modalities. 3rd ed. Philadelphia, PA: FA Davis Co;; 2004. p. 379. [Google Scholar]
- 36.Aimbire F, Albertini R, Pacheco MT et al. Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed Laser Surg. 2006;24(1):33–37. doi: 10.1089/pho.2006.24.33. [DOI] [PubMed] [Google Scholar]
- 37.Amaral AC, Parizotto NA, Salvini TF. Dose-dependency of low-energy HeNe laser effect in regeneration of skeletal muscle in mice. Lasers Med Sci. 2001;16(1):44–51. doi: 10.1007/pl00011336. [DOI] [PubMed] [Google Scholar]
- 38.Ferraresi C, de Brito Oliveira T, de Oliveira Zafalon L, et al. Effects of low level laser therapy (808 nm) on physical strength training in humans. Lasers Med Sci. 2011;26(3):349–358. doi: 10.1007/s10103-010-0855-0. [DOI] [PubMed] [Google Scholar]
- 39.Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci U S A. 1999;96(3):1129–1134. doi: 10.1073/pnas.96.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tonkonogi M, Sahlin K. Physical exercise and mitochondrial function in human skeletal muscle. Exerc Sport Sci Rev. 2002;30(3):129–137. doi: 10.1097/00003677-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Leadbetter WB. Soft tissue athletic injury. In: Fu FH, Stone DA, editors. Sports Injuries: Mechanisms, Prevention, Treatment. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins;; 2001. pp. 839–888. [Google Scholar]
- 42.Merrick MA. Secondary injury after musculoskeletal trauma: a review and update. J Athl Train. 2002;37(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- 43.Avni D, Levkovitz S, Maltz L, Oron U. Protection of skeletal muscles from ischemic injury: low-level laser therapy increases antioxidant activity. Photomed Laser Surg. 2005;23(3):273–277. doi: 10.1089/pho.2005.23.273. [DOI] [PubMed] [Google Scholar]
- 44.Oron U, Yaakobi T, Oron A et al. Low-energy laser irradiation reduces formation of scar tissue after myocardial infarction in rats and dogs. Circulation. 2001;103(2):296–301. doi: 10.1161/01.cir.103.2.296. [DOI] [PubMed] [Google Scholar]
- 45.Ad N, Oron U. Impact of low level laser irradiation on infarct size in the rat following myocardial infarction. Int J Cardiol. 2001;80(2–3):109–116. doi: 10.1016/s0167-5273(01)00503-4. [DOI] [PubMed] [Google Scholar]
- 46.Rizzi CF, Mauriz JL, Freitas Correa DS et al. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med. 2006;38(7):704–713. doi: 10.1002/lsm.20371. [DOI] [PubMed] [Google Scholar]
- 47.de Morais NC, Barbosa AM, Vale ML et al. Anti-inflammatory effect of low-level laser and light-emitting diode in zymosan-induced arthritis. Photomed Laser Surg. 2010;28(2):227–232. doi: 10.1089/pho.2008.2422. [DOI] [PubMed] [Google Scholar]
- 48.Enwemeka CS. The place of coherence in light induced tissue repair and pain modulation. Photomed Laser Surg. 2006;24(4):457. doi: 10.1089/pho.2006.24.457. [DOI] [PubMed] [Google Scholar]
- 49.Gundersen B. Clinical trial on laser therapy as a pain control modality. Med Wellness Arch. 2005;2(2):1–3. [Google Scholar]
- 50.Allen DG. Fatigue in working muscles. J Appl Physiol. 2009;106(2):358–359. doi: 10.1152/japplphysiol.91599.2008. [DOI] [PubMed] [Google Scholar]
- 51.Dutta C, Hadley EC, Lexell J. Sarcopenia and physical performance in old age: overview. Muscle Nerve Suppl. 1997;5:S5–S9. [PubMed] [Google Scholar]