Abstract
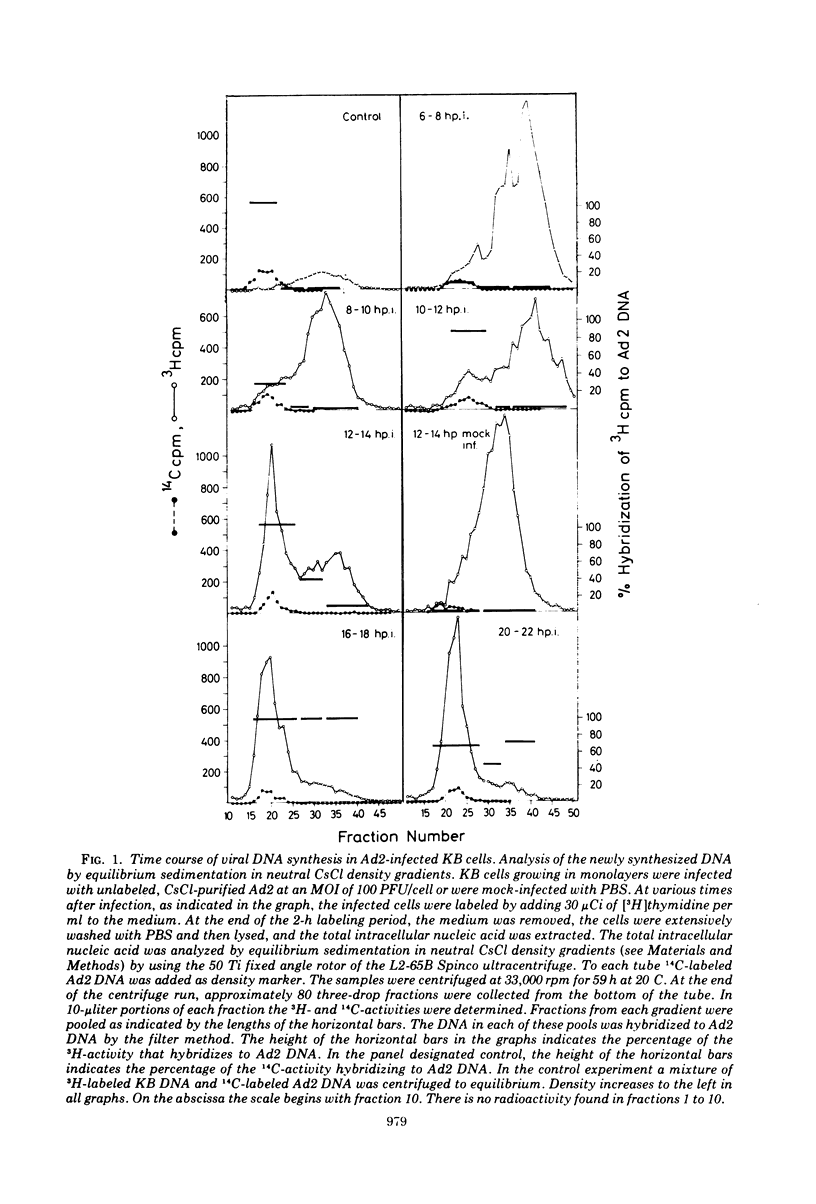
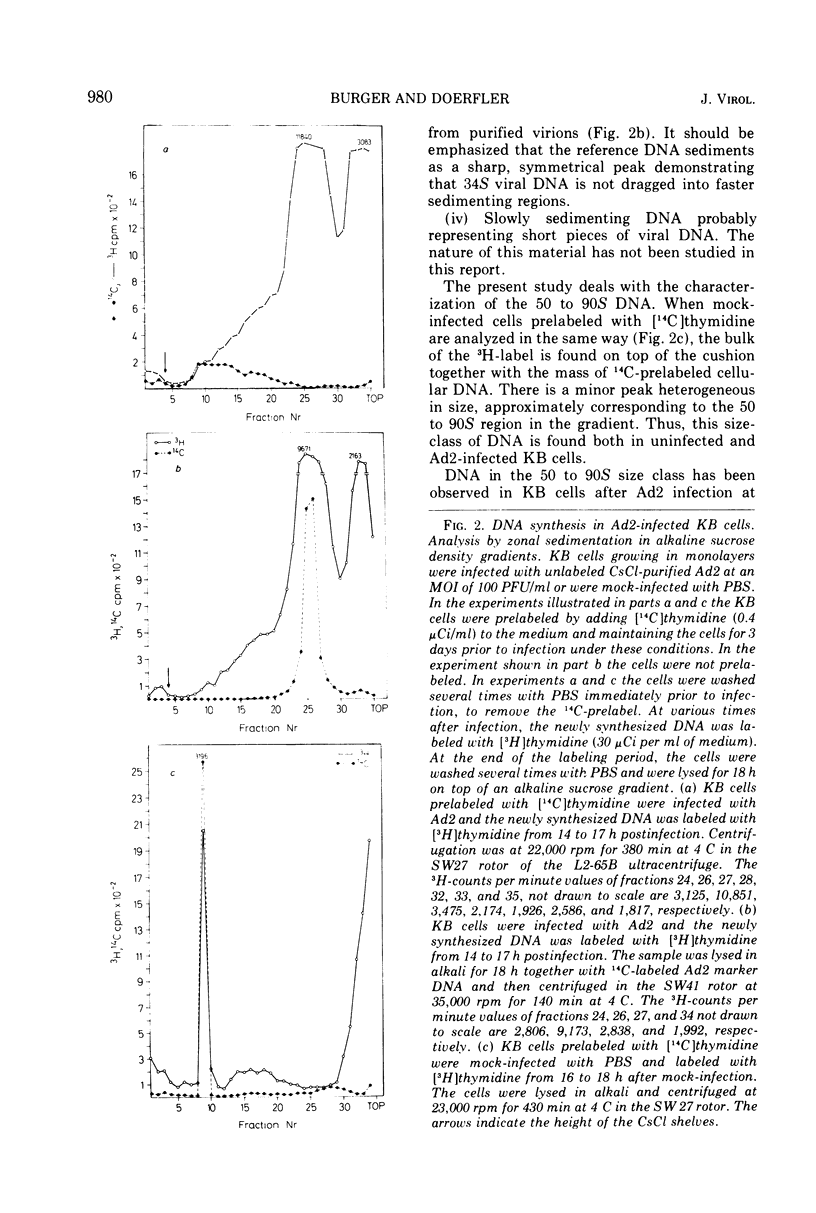
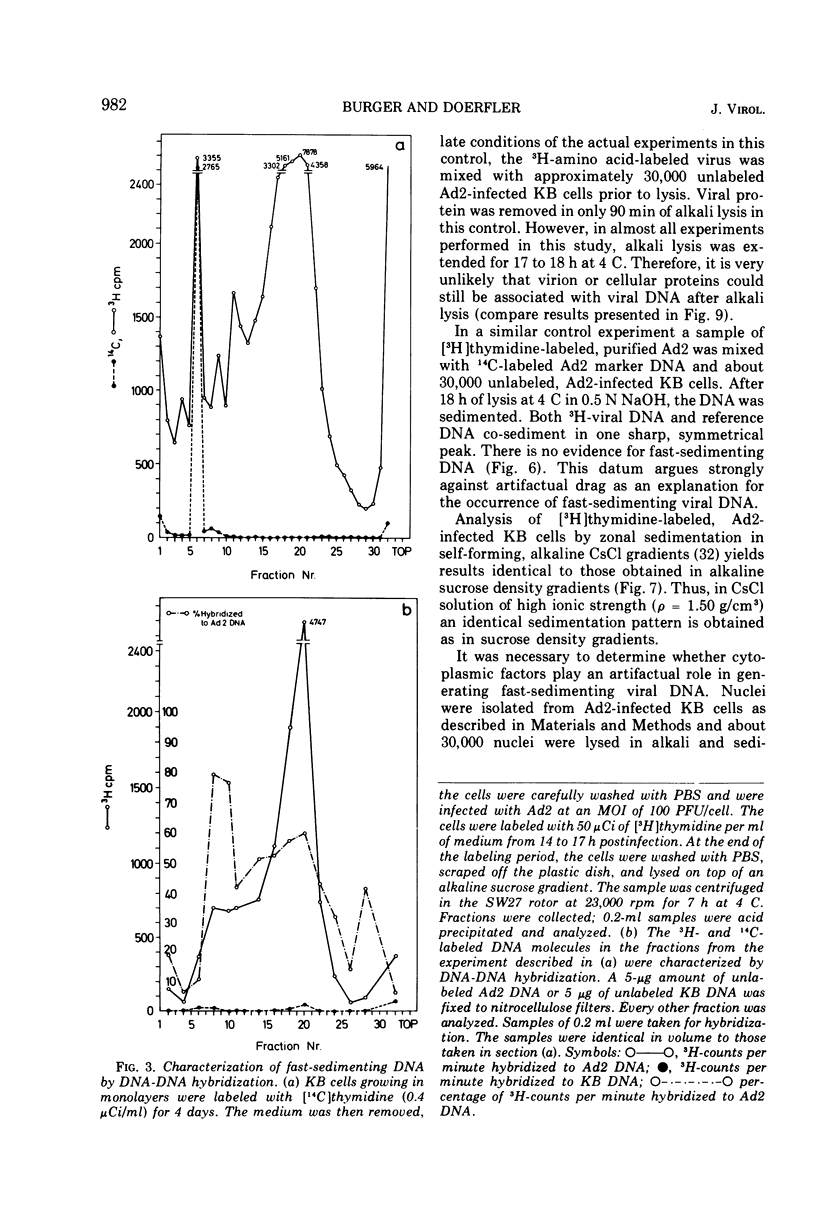
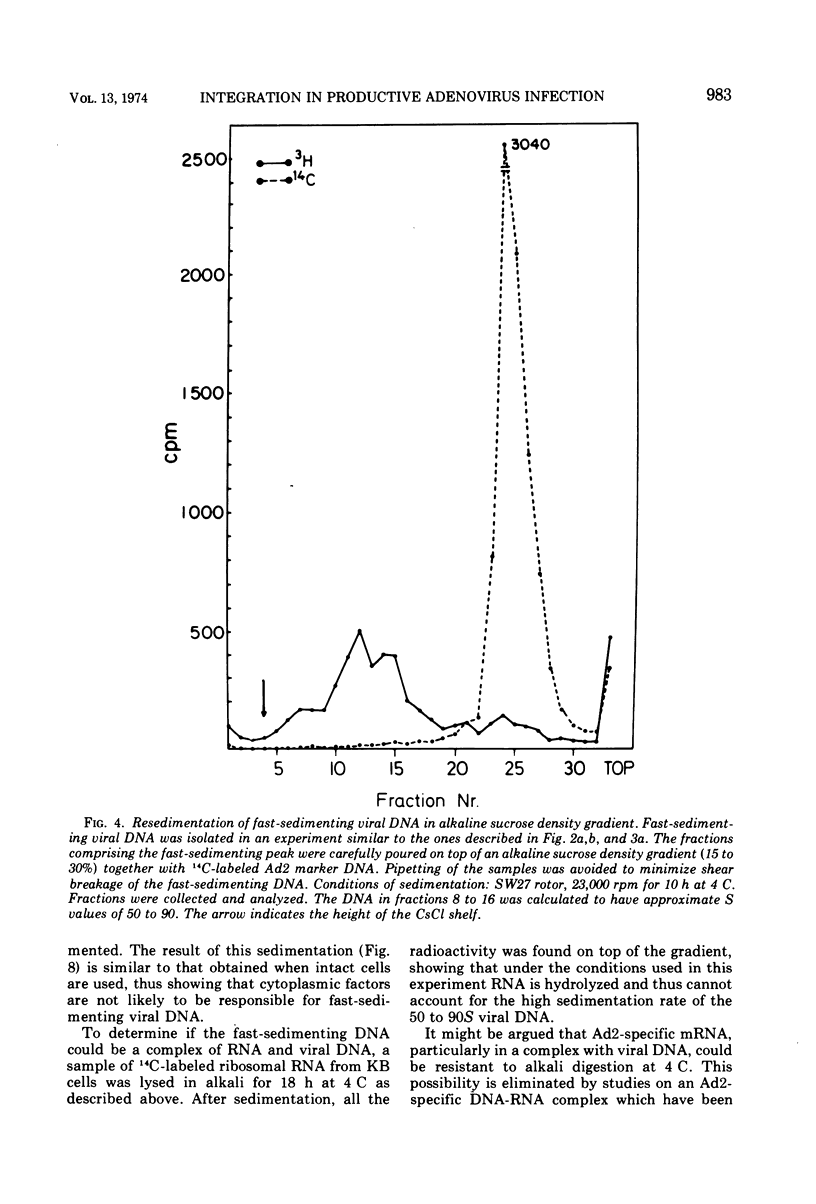
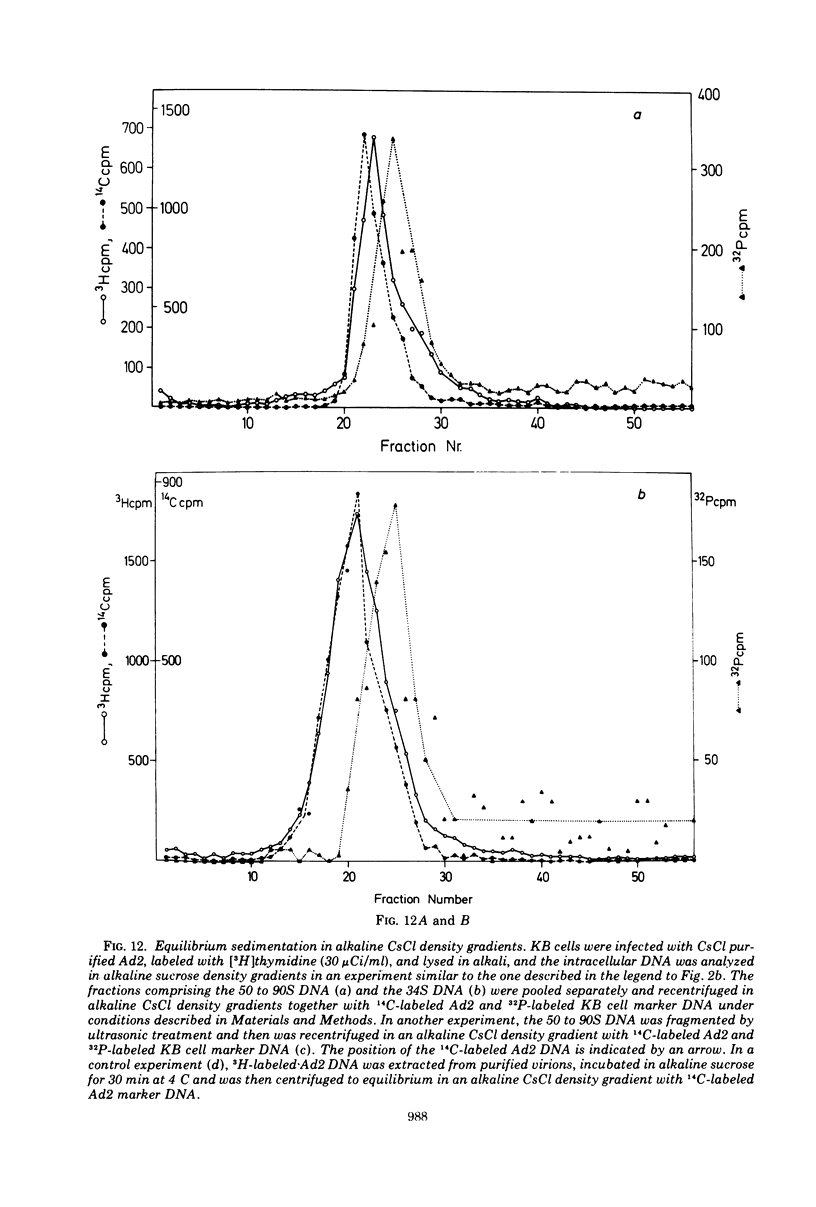
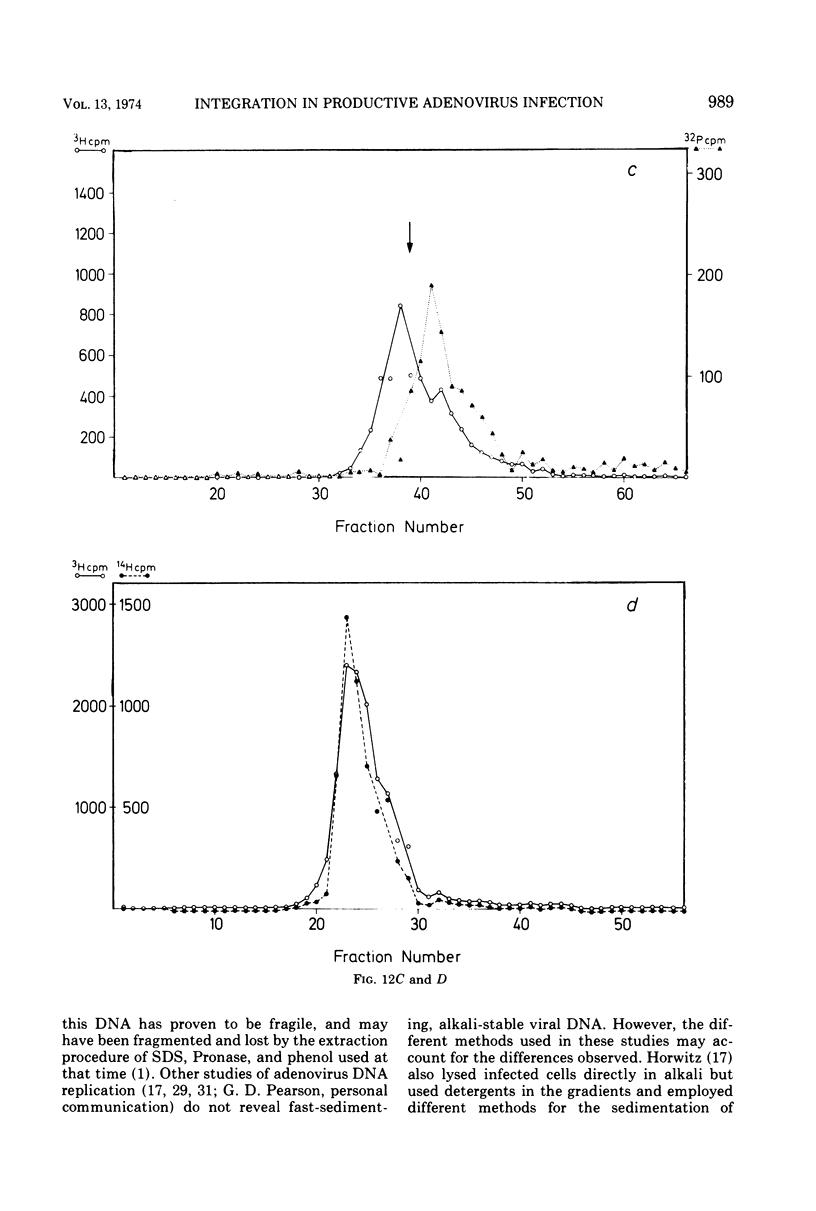
KB cells productively infected with human adenovirus type 2 contain an alkalistable class of viral DNA sedimenting in a broad zone between 50 and 90S as compared to 34S for virion DNA. This type of DNA is characterized as viral by DNA-DNA hybridization. It is extremely sensitive to shear fragmentation. Extensive control experiments demonstrate that the fast-sedimenting viral DNA is not due to artifactual drag of viral DNA mechanically trapped in cellular DNA or to association of viral DNA with protein or RNA. Furthermore, the fast-sedimenting DNA is found after infection with multiplicities between 1 and 1,000 PFU/cell and from 6 to 8 h postinfection until very late in infection (24 h). Analysis in dye-buoyant density gradients eliminates the possibility that the fast-sedimenting viral DNA represents supercoiled circular molecules. Upon equilibrium centrifugation in alkaline CsCl density gradients, the fast-sedimenting viral DNA bands in a density stratum intermediate between that of cellular and viral DNA. In contrast, the 34S virion DNA isolated and treated in the same manner as the fast-sedimenting DNA cobands with viral marker DNA. After ultrasonic treatment of the fast-sedimenting viral DNA, it shifts to the density positions of viral DNA and to a lesser extent to that of cellular DNA. The evidence presented here demonstrates that the 50 to 90S viral DNA represents adenovirus DNA covalently integrated into cell DNA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burlingham B. T., Doerfler W. Three size-classes of intracellular adenovirus deoxyribonucleic acid. J Virol. 1971 Jun;7(6):707–719. doi: 10.1128/jvi.7.6.707-719.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. J., Sauer G. Fate of infecting simian virus 40-deoxyribonucleic acid in nonpermissive cells: integration into host deoxyribonucleic acid. J Virol. 1972 Sep;10(3):425–432. doi: 10.1128/jvi.10.3.425-432.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970 Nov;6(5):652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Kleinschmidt A. K. Denaturation pattern of the DNA of adenovirus type 2 as determined by electron microscopy. J Mol Biol. 1970 Jun 28;50(3):579–593. doi: 10.1016/0022-2836(70)90086-0. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Rensing U., Philipson L. Intracellular forms of Adenovirus DNA. II. Isolation in dye-buoyant density gradients of a DNA-RNA complex from KB cells infected with Adenovirus type 2. J Virol. 1973 Oct;12(4):793–807. doi: 10.1128/jvi.12.4.793-807.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Pettersson U., Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973 Mar;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B., Cassai E., Nahmias A. A DNA fragment of Herpes simplex 2 and its transcription in human cervical cancer tissue. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3784–3789. doi: 10.1073/pnas.69.12.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of permissive monkey kidney cells. J Virol. 1972 Apr;9(4):705–707. doi: 10.1128/jvi.9.4.705-707.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S. Intermediates in the synthesis of type 2 adenovirus deoxyribonucleic acid. J Virol. 1971 Nov;8(5):675–683. doi: 10.1128/jvi.8.5.675-683.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN H. J., CHARGAFF E. METAPHASE CHROMOSOMES AS A SOURCE OF DNA. Biochim Biophys Acta. 1964 Dec 16;91:691–694. doi: 10.1016/0926-6550(64)90032-5. [DOI] [PubMed] [Google Scholar]
- Lett J. T., Caldwell I., Dean C. J., Alexander P. Rejoining of x-ray induced breaks in the DNA of leukaemia cells. Nature. 1967 May 20;214(5090):790–792. doi: 10.1038/214790a0. [DOI] [PubMed] [Google Scholar]
- Lundholm U., Doerfler W. Temperature-sensitive mutants of human adenovirus type 12. Virology. 1971 Sep;45(3):827–829. doi: 10.1016/0042-6822(71)90206-6. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Hanawalt P. C. Isolation of DNA replication complexes from uninfected and adenovirus-infected HeLa cells. J Mol Biol. 1971 Nov 28;62(1):65–80. doi: 10.1016/0022-2836(71)90131-8. [DOI] [PubMed] [Google Scholar]
- Pettersson U. Letter: Some unusual properties of replicating adenovirus type 2 DNA. J Mol Biol. 1973 Dec 25;81(4):521–527. doi: 10.1016/0022-2836(73)90521-4. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C., Ellens D. J., Jansz H. S. Linear intermediates in the replication of adenovirus DNA. Nat New Biol. 1972 Sep 13;239(89):47–49. [PubMed] [Google Scholar]
- Tao M., Doerfler W. Phosphorylation of adenovirus polypeptides. Eur J Biochem. 1972 Jun 9;27(3):448–452. doi: 10.1111/j.1432-1033.1972.tb01859.x. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. D., Kates J. State of adenovirus 2 deoxyribonucleic acid in the nucleus and its mode of transcription: studies with isolated viral deoxyribonucleic acid-protein complexes and isolated nuclei. J Virol. 1972 Apr;9(4):627–635. doi: 10.1128/jvi.9.4.627-635.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eb A. J. Intermediates in type 5 adenovirus DNA replication. Virology. 1973 Jan;51(1):11–23. doi: 10.1016/0042-6822(73)90361-9. [DOI] [PubMed] [Google Scholar]



