Abstract
We have previously demonstrated that the human papillomavirus (HPV) genome replicates effectively in U2OS cells after transfection using electroporation. The transient extrachromosomal replication, stable maintenance, and late amplification of the viral genome could be studied for high- and low-risk mucosal and cutaneous papillomaviruses. Recent findings indicate that the cellular DNA damage response (DDR) is activated during the HPV life cycle and that the viral replication protein E1 might play a role in this process. We used a U2OS cell-based system to study E1-dependent DDR activation and the involvement of these pathways in viral transient replication. We demonstrated that the E1 protein could cause double-strand DNA breaks in the host genome by directly interacting with DNA. This activity leads to the induction of an ATM-dependent signaling cascade and cell cycle arrest in the S and G2 phases. However, the transient replication of HPV genomes in U2OS cells induces the ATR-dependent pathway, as shown by the accumulation of γH2AX, ATR-interacting protein (ATRIP), and topoisomerase IIβ-binding protein 1 (TopBP1) in viral replication centers. Viral oncogenes do not play a role in this activation, which is induced only through DNA replication or by replication proteins E1 and E2. The ATR pathway in viral replication centers is likely activated through DNA replication stress and might play an important role in engaging cellular DNA repair/recombination machinery for effective replication of the viral genome upon active amplification.
INTRODUCTION
Papillomaviruses are species-specific double-stranded DNA (dsDNA) viruses that infect the cutaneous and mucosal epithelia of many vertebrate species (1). Human papillomavirus (HPV) infections are widespread, and this virus is considered a common member of the human epithelial microflora (2). In many cases, infections with papillomaviruses are asymptomatic (3). Nearly 100 different HPV types have been identified (4); infections with low-risk viruses (e.g., HPV type 6b [HPV6b] and HPV11) might induce the formation of benign tumors, such as warts and condylomas, while other types (e.g., HPV16 and HPV18), which are referred to as high-risk types, have been shown to cause anogenital and head and neck cancers (reviewed in reference 5). The viral genomes are maintained in infected cells as extrachromosomal nuclear episomes. The proteins encoded by the E1 and E2 open reading frames (ORFs) load the cellular replication machinery at the origin of the HPV replication (reviewed in reference 6). The E1 protein is an origin recognition factor, which is loaded in a sequence-specific manner by the E2 protein at the replication origin, where it forms the E1 double-hexameric replicative helicase upon oligomerization (7–10). Before the initiation of replication, the oligomeric E1 protein unwinds dsDNA into two single strands, assembles into the double-hexameric, ATP-dependent replicative helicase, and loads the cellular replication complex at replication forks for the initiation of DNA replication (reviewed in reference 11). The E2 protein largely determines the specificity of the E1 protein for initiating replication at specific HPV origins (12).
Many viruses interact with the host cell DNA damage-sensing and -repair machinery, either to protect the viral genome from inactivation or to adjust the cellular milieu for more efficient replication of the viral genome (for a review, see reference 13). For example, the DNA damage response (DDR) is activated and cellular recombination and repair proteins are recruited to the sites of viral DNA replication during the early phase of herpes simplex virus 1 (HSV-1) infection (14–16). Polyomavirus (17) and simian virus 40 (SV40) (18) infections have also been shown to activate the ATM-dependent signaling cascade during the early stages of viral infection. In contrast, Epstein-Barr virus (EBV) activates DDR pathways during the lytic replication phase (19). Although viral replication intermediates or single viral proteins have been shown to induce these pathways in some cases, the specific viral proteins responsible for the activation of DDR are often unknown (13). Some viral proteins, including the SV40 large T antigen (20), three EBV latency proteins (21) and HIV-1 Vpr1 (22), contribute directly to host DNA damage.
It has been demonstrated that HPV might affect the DNA damage response through oncoproteins E6 and E7, which modulate the activity of some key cellular proteins involved in cell cycle control and DNA damage repair (23–25). Changes in the expression levels of these cellular proteins have recently been demonstrated in HPV-positive precancerous lesions and benign hyperplasias (26). The E7 protein-mediated activation of the ATM-dependent signaling cascade is crucial for effective viral replication in the productive phase (27). Our laboratory has previously demonstrated that the coexpression of HPV E1 and E2 proteins in HeLa and SiHa cells strongly induces activation of the DNA damage response (28, 29). We have shown that the expression of the E1 and E2 proteins induces uncoordinated replication at the integrated HPV origin in HPV-positive cell lines through an onion skin-type mechanism, which causes genomic irregularity, triggers the loading of DNA damage response factors at the origin, and activates DDR through an ATM-Chk2-dependent pathway. Interestingly, this study showed that the expression of E1 alone, in the absence of E2 and therefore without the HPV-specific origin of replication, also induces some level of DDR activation. Recently, two groups (30, 31) have demonstrated that the overexpression of the E1 protein activates the DNA damage response in primary keratinocytes and HPV-negative C33A, CV1, and U2OS cell lines.
We have developed a cellular assay system to study mucosal low- and high-risk and cutaneous HPVs in U2OS cells (32). These cells effectively support the replication of various HPV genomes during initial transient amplification and stable replication. Moreover, in confluent U2OS cells, the amplification of the episomal HPV genomes by at least an order of magnitude could be demonstrated, which was reminiscent of vegetative replication (32). We have recently also analyzed the transcriptome of HPV18 in U2OS cells (unpublished data) and detected no difference in the promoter, splice site, or polyadenylation site usage in U2OS cells compared to the keratinocytes (33). This cellular system is therefore ideal to study the relationship between HPV DNA replication and the cellular DDR and to characterize the effects of E1 protein expression, either alone or in combination with other factors, on activation of the DNA damage response.
Our data suggest that overexpression of the E1 protein from expression constructs in U2OS cells could induce double-strand DNA breaks (DSBs) in a concentration-dependent manner and thereby activate the ATM-Chk2 pathway, leading to cell cycle arrest in the S and G2 phases. We also show that DDR is activated, but only locally in the transient-replication centers of the HPV genomes in U2OS cells. As a result of viral genome replication, DDR is activated at much lower levels than those for E1 expression from vectors. We show that viral replication centers contain DNA damage response factors characteristic for replication stress, which is dependent on ATR.
MATERIALS AND METHODS
Cell lines and transfection.
U2OS cell lines with and without the constitutive expression of Epstein-Barr virus (EBV) EBNA1 protein and polyomavirus large T antigen (LT) were grown in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% fetal bovine serum. Electroporation was performed as previously described (9) using a Bio-Rad Gene Pulser XCell II apparatus equipped with a capacitance extender (Bio-Rad Laboratories) at 220 V and a capacitance of 975 μF in all experiments.
Plasmids.
The plasmids pMHE1-18 and pQMNE2-18 for HPV18 E1 and E2 expression and the HPV18 origin-containing plasmid pUC-URR-18 were used; these plasmids have been previously described (29). The pauxoMCS plasmid (Iqosagen, Estonia), which does not encode any gene product in animal cells and has no significant homology with the expression plasmids, was used in the transfections as carrier DNA.
Point mutations were generated in the HPV18 E1 and E2 plasmids using site-directed mutagenesis with sequence-specific primers. The following primers and restriction enzymes were used: E1 K237A, Eco72I, GTACACGTGGTTGCATCACTTTTAAAATTTC; E1 H558A, Esp3I, TTCGTCTCGCTATCAATACTTATTGGATTGCC and ACGTCTCGATAGAAAGGCCAAACCATTAATACAAC; E1 K490A, Esp3I, TTCGTCTCATGATGCTCCTGTATTTGCTGGTCC and ATCGTCTCAATCATATTTTGGAATGAGTTTTATAC; and E2 N336A/C340A, HindIII, CTGTACCGTAAAGCTTTTAAACTGGCTCTGTCACC and GTTTAAAAGCTTTACGGTACAGATTGCG. Frameshift mutations were generated in the HPV18 genome by inserting an XhoI linker at the beginning of the respective ORF. All resulting ORFs encoded proteins shorter than 20 amino acids (aa). The following primers were used to generate mutations in the respective ORFs (the number of unchanged amino acid residues in each protein is indicated in parentheses): E6fs (10 amino acid residues), TAACTCGAGGACCCTACAAGCTACCTG and TAACTCGAGCGCCGTGTTGGATCCTCAAAG; E7fs (7 amino acid residues), TAACTCGAGAACATTGCAAGACATTGTATTGC and TAACTCGAGTTGCCTTAGGTCCATGCATAC; and E1fs (9 amino acid residues), TAACTCGAGAGGGCACGGGTTGTAAC and TAACTCGAGTCCCCGTCTGTACCTTCTG.
Minicircle (34) viral genomes were used in all experiments involving viral genomes. For construction of minicircle viral genomes, a BglII site was introduced into the HPV18 genome after nucleotide 7473 and the minicircle vector pMC.BESPX was cloned into this resulting site. For generation of the HPV18 minicircle upstream regulatory region (mcURR), a BamHI fragment of the viral URR was cloned into a BglII site of the minicircle vector pMC.BESPX. For the production of minicircles, Escherichia coli strain ZYCY10P3S2T was transformed and grown in TB medium until an optical density at 600 nm (OD600) of 4 to 5 was reached. An equal volume of induction mix (0.04 N NaOH and 0.02% l-arabinose in LB broth) was added to induce recombination, and the culture was incubated for an additional 5 h at 32°C. Subsequently, plasmid DNA was extracted from the bacterial cells and gel purified for obtaining only the covalently closed circular DNA (cccDNA) form of the viral genome.
Western blotting and immunoprecipitation.
For experiments involving only 24-hour time points, the cells were washed twice with phosphate-buffered saline (PBS), lysed with 1× SDS sample buffer (50 mM Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 0.002% bromophenol blue [BPB], 100 mM dithiothreitol [DTT]), and boiled at 100°C for 5 min. For the 48-hour experiments (experiments with viral genomes), the cells were lysed with 1× SDS sample buffer without either DTT or BPB. Total protein concentrations were measured using a DC protein assay (Bio-Rad), and the lysates were normalized to contain equal concentrations of total protein. Subsequently, DTT and BPB were added, and the samples were boiled for 5 min. The proteins were separated on 10 to 15% polyacrylamide-SDS gels and transferred to an Immobilon-P membrane (Millipore). The proteins of interest were detected using the antibodies described below. An ImageQuant RT ECL imager (GE Healthcare) was used for Western blot band intensity quantitation. Immunoprecipitations were performed as described previously (28).
IF analysis.
Cells grown on microscope slides were washed twice with PBS, fixed with 4% paraformaldehyde, and permeabilized with 0.5% Triton X-100 in PBS for 15 min at room temperature (RT). The cells were washed twice with PBS and blocked with 5% bovine serum albumin (BSA) in PBS. After blocking, the cells were incubated with primary antibody in antibody-binding solution (3% BSA in PBS) for 1 h at RT and washed 3 times with PBS. Subsequently, the cells were incubated with secondary antibodies in antibody-binding solution for 30 min at RT and washed 3 times with PBS. The cells were placed on glass slides using a mounting medium containing 0.1 mM 4,6-diamidino-2-phenylindole (DAPI) and examined using a confocal microscope. The FV1000-IX81 microscope from Olympus (for Fig. 7 and 8B) and the LSM 710 microscope from Carl Zeiss Microscopy (for Fig. 8A and 9) were used. For ethynyl deoxyuridine (EdU) labeling, the cells were pulse-labeled with 50 μM EdU for 30 min at 48 h posttransfection. The cells were fixed and permeabilized as described above, and the EdU signal was detected using the Click-iT EdU Alexa Fluor 647 imaging kit (Invitrogen) according to the manufacturer's protocol. For immunofluorescence (IF)-fluorescence in situ hybridization (FISH) analysis, the cells were grown on poly-l-lysine-coated slides, fixed, and permeabilized with methanol at −20°C for 15 min. After antibody labeling, the antibodies were cross-linked with 3.7% formaldehyde for 2 min at RT, and the samples were sequentially dehydrated with 70%, 80%, and 96% ethanol at −20°C.
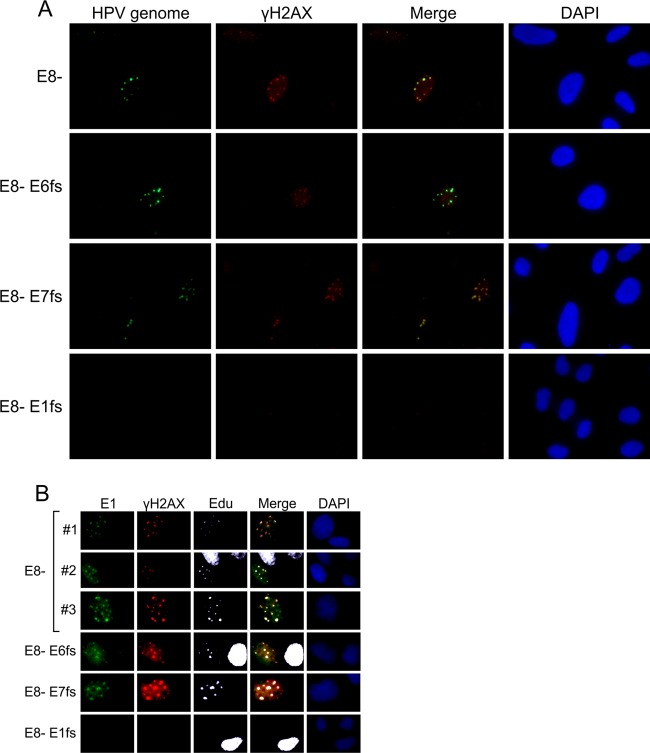
Fig 7.
U2OS cells were transfected with 2 μg of E8− HPV18 minicircle genome or with the double mutants, where not only the E8 repressor but also the expression of the viral early protein E6, E7, or E1 were suppressed by frameshift mutations. (A) Combined immunofluorescence and FISH analysis were performed at 48 h after transfection. The FISH signal of the HPV18 genome is visible in green, and immunostained γH2AX is visible in red. The merged images are presented in the third column, and the DAPI-stained nuclei are shown in the fourth column. (B) After 48 h, the cells were pulse-labeled with EdU and costained with HPV18 E1 antibodies (green), γH2AX antibodies (red), and EdU (white), together with DAPI.
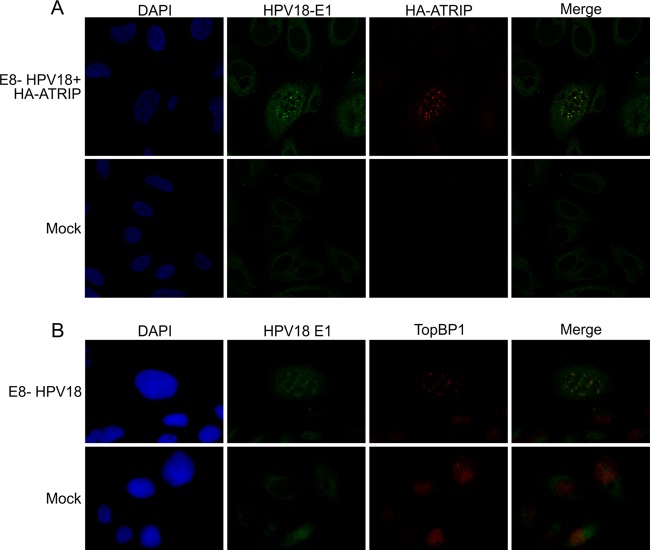
Fig 8.
U2OS cells were transfected with 2 μg of HPV18/E8− genome with (A) or without (B) 50 ng of HA-ATRIP plasmid. Transfection of circular carrier plasmid alone was used as mock control. Costaining of HPV18 E1 with HA-ATRIP (A) or with TopBP1 (B) was performed at 48 h after transfection. The DNA was visualized by DAPI staining.
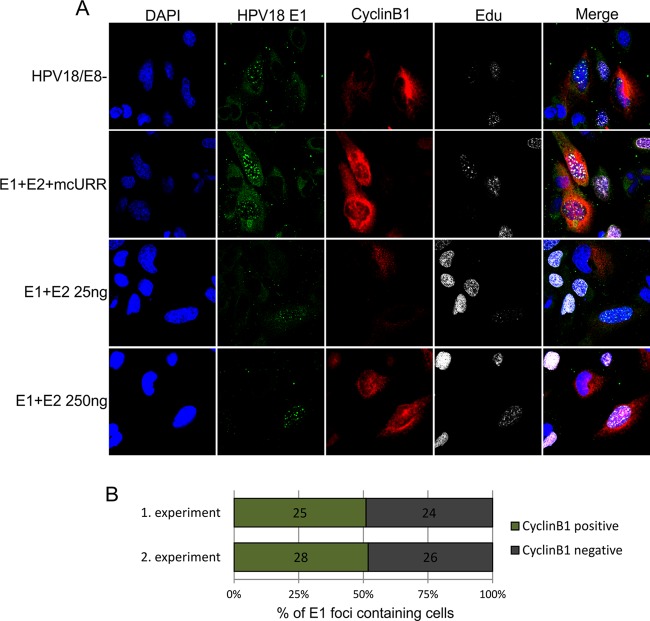
Fig 9.
U2OS cells were transfected with 2 μg of the HPV18/E8− genome, 25 ng of the HPV18 E1 and E2 expression constructs together with 500 ng of mcURR (minicircle URR), and 25 or 250 ng of the E1 and E2 expression constructs without the URR plasmid. (A) At 48 h after transfection, the cells were pulse-labeled with EdU, and costaining of HPV18 E1, cyclin B1, and EdU was performed. The DNA was visualized by DAPI staining. (B) U2OS cells were transfected with the HPV18/E8− genome, and randomly selected E1 focus-containing cells were determined to have or not have cyclin B1 signal in the cytoplasm using confocal microscopy. Results of two independent experiments are shown on the graph, with the numbers representing the amount of cells counted in each subset.
FISH.
After labeling of the target proteins with specific antibodies for IF detection, the samples were incubated in 2× SSC (300 mM NaCl, 30 mM Na-citrate, pH 7.0) for 20 min at 60°C. The samples were then sequentially dehydrated in 70%, 80%, and 96% ethanol at −20°C. RNA was eliminated by incubating the samples in a solution containing RNase and 2× SSC (RNase at 100 μg/ml) for 60 min at 37°C. The samples were washed in 2× SSC and dehydrated as described above. The samples were treated with 0.01 M HCl for 5 min at 37°C, washed with PBS, and subsequently incubated in a 0.95% formaldehyde–PBS solution for 10 min, washed with PBS, and dehydrated as described above. Finally, prior to hybridization, chromosome preparations were denatured at 75°C in 70% formamide–2× SSC for 3 min and immediately dehydrated with an ethanol series as described above. To detect papillomavirus replication centers, the samples were hybridized overnight with an HPV18 genome-specific, biotin-labeled probe at 37°C. The following day, the samples were incubated in a 0.4× SSC solution for 2 min at 69°C and in a 0.1% NP-40–2× SSC solution for 30 s and then dehydrated. The specific signal was detected using a tyramide signal amplification (TSA) kit (Invitrogen) according to the manufacturer's protocol.
Single-cell gel electrophoresis assay (comet assay).
The cells were detached from 60-mm tissue culture plates in PBS–3 mM EDTA (pH 7.5), pelleted for 2 min at 300 × g, and resuspended in PBS to a total cell count of 1.0 × 105 cells in 15 μl. A 75-μl portion of a 0.65% low-melting-point agarose solution in 89 mM Tris-borate–10 mM EDTA (pH 8.3) (TBE) was added to the cells. The suspension was pipetted onto a microscope slide, covered with a coverslip, and incubated on ice for 10 min. The coverslip was then removed, and an additional 75 μl of agarose was added; the suspension was then covered with a coverslip again and incubated on ice for 10 min. The coverslip was removed, and the cells were lysed for 2 h at 4°C in 2 M NaCl–30 mM EDTA–10 mM Tris-HCl (pH 8.3)–1% Triton X-100–10% dimethyl sulfoxide (DMSO), which was added immediately before use. Subsequently, the slides were washed with TBE. Electrophoresis was performed at 2 V/cm for 25 min, and the slides were neutralized in 0.2 M Tris, pH 7.5. Finally, the cells were stained with 1.0 μg/ml DAPI and analyzed on an ArrayScan VTI fluorescence microscope (Thermo Scientific). The tail extent moment was calculated using the Comet V3 protocol.
Cell cycle analysis.
The cells were washed twice with PBS, detached from 60-mm tissue culture plates in PBS–3 mM EDTA (pH 7.5), and collected by centrifugation at 500 × g for 5 min. Subsequently, the cells were resuspended in PBS and permeabilized for 30 min on ice using 80% ice-cold ethanol. The cells were washed with PBS, recovered by centrifugation, resuspended in PBS containing 50 μg/ml propidium iodide and 200 μg/ml RNase, and incubated for 30 min at 37°C. Cell cycle analysis was conducted using a BD LSR II flow cytometer (BD Biosciences). Three independent experiments were performed, and the results were quantitated using FlowJo software. For γH2AX detection, the cells were blocked after fixation with 5% BSA in PBS and incubated with the primary antibody in an antibody-binding solution for 1 h. Subsequently, the cells were washed with PBS and incubated with the secondary antibody in the same antibody-binding solution for 30 min. Finally, the cells were washed with PBS, and cell cycle analysis was performed as previously described.
Transient DNA replication analysis.
Low-molecular-weight DNA was extracted using Hirt lysis (35), digested with an appropriate enzyme for linearization and DpnI, resolved on an 0.8% agarose gel, blotted, and hybridized with an HPV18 genome sequence-specific probe labeled with [α-32P]dCTP using random priming (DecaLabel kit; Fermentas). Specific HPV replication signals were detected using autoradiography exposure of X-ray film (Fuji).
Antibodies.
The following antibodies were used: topoisomerase IIβ-binding protein 1 (TopBP1) (sc-271043) and cyclin B1 (sc-70898) from Santa Cruz Biotechnology; anti-γH2AX (phospho-S139) (ab22551) from Abcam; anti-α-tubulin B512 from Sigma-Aldrich; Alexa Fluor 488 goat anti-rabbit IgG (A-11008) and Alexa Fluor 568 goat anti-rabbit IgG (A-11004) from Invitrogen; and Chk2 (2662), phospho-Chk2Thr68 (2661), and phospho-Chk2Ser19 (2666) from Cell Signaling Technology. A mouse monoclonal antibody was used for the Chk2 immunoprecipitation experiments (c9233; Sigma-Aldrich). The HPV E1 protein with a hemagglutinin (HA) epitope was detected with a rat monoclonal antibody (3F10) that was conjugated with peroxidase (12013819001; Roche). Secondary antibodies conjugated with peroxidase were purchased from LabAs Ltd. (Estonia). Mouse monoclonal antibody 2E7.1 was raised against bacterially expressed HPV18 E2. The bacterially expressed C-terminal portion of the HPV18 E1 protein (aa 355 to 657) was used to generate a rabbit polyclonal anti-HPV18 E1 antibody. E1-specific antibodies were affinity purified from the rabbit serum.
RESULTS
Overexpression of HPV18 E1 protein triggers the activation of γH2AX in U2OS cells.
We have previously demonstrated that the phosphorylation of histone H2AX is induced and that the ATM-Chk2 pathway is activated upon E1 protein overexpression in HeLa cells (28). The phosphorylation of H2AX is associated with double-strand DNA breaks (DSBs) and is a sensitive marker of these dangerous DNA lesions (for a review, see reference 36). To study the effects of E1 on the HPV-negative cell line, we transfected the expression constructs for HPV18 E1 and E2 proteins at various concentrations into U2OS cells using electroporation. Twenty-four hours later, the cells were lysed, and E1, E2, and phosphorylated histone H2AX (γH2AX) levels were measured using Western blotting with specific antibodies. The results demonstrated that the expression of the HPV18 E1 protein in U2OS cells induced the dose-dependent phosphorylation of H2AX (Fig. 1A, lanes 1 to 4). However, E2 protein expression, at any concentration tested, had no effect on H2AX phosphorylation (Fig. 1A, lanes 5 to 7). The coexpression of E2 and E1 in U2OS cells considerably increased the levels of γH2AX compared with the expression of E1 alone (Fig. 1A, lanes 8 to 10), as also was shown by the quantitation of data from two independent experiments (Fig. 1B). One reason for the observation of elevated levels of γH2AX in the coexpression experiments was the increase in E1 expression level due to the stabilization of E1 by E2 protein-mediated complex formation (Fig. 1A, compare lanes 1 to 3 with lanes 8 to 10). The observation that E1 and E2 can stabilize each other has previously been demonstrated in an HPV16 system, where E1 enhances E2 stability (37). Cotransfection of a HPV18 URR-containing plasmid with E1 and E2 expression vectors resulted in strong replication of the HPV18 origin plasmid (data not shown). However, we reproducibly observed a slight reduction in γH2AX levels compared to those in experiments with the viral replication proteins alone (Fig. 1A and B, lanes 11 to 13). These results indicate that the presence of a specific replication origin might change the functioning of the E1 protein in these cells, which was also previously suggested by another study (30).
Fig 1.
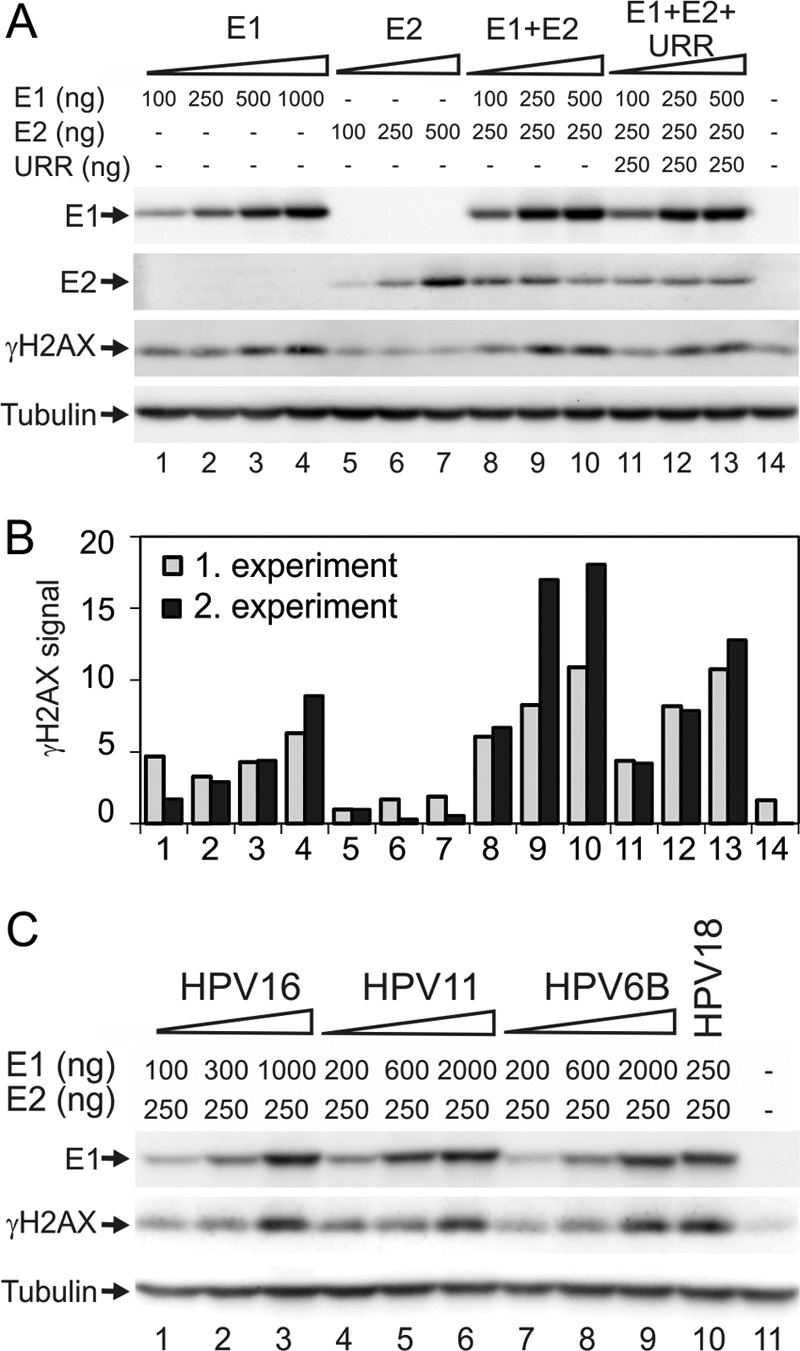
(A) U2OS cells were transfected with HPV18 E1 and E2 expression plasmids and an HPV18 origin-containing plasmid, termed URR, and these are shown as follows: titration of E1 (lanes 1 to 4), titration of E2 (lanes 5 to 7), titration of E1 in the presence of E2 (lanes 8 to 10), and titration of E1 in the presence of E2 and URR (lanes 11 to 13). In every transfection, the amount of plasmid was adjusted to 10 μg with a carrier plasmid (pauxoMCS). For the mock transfection, cells were transfected with 10 μg of carrier plasmid, as shown in lane 14. (B) Two independent experiments were performed as described for panel A. γH2AX signals were quantitated and normalized to the value of 100 ng E2 (lane 4). (C) Expression constructs encoding HPV16 E1 (lanes 1 to 3), HPV11 E1 (lanes 4 to 6), and HPV6B E1 (lanes 7 to 9) were transfected into U2OS cells together with constant amounts of the respective E2 expression constructs. One concentration of HPV18 E1 together with HPV18 E2 was also added (lane 10). The mock transfection of only circular carrier DNA is shown in lane 11. (A and C) Western blot analysis was performed at 24 h after transfection and used to determine the levels of E1, γH2AX, and tubulin. E1 levels were determined using an antibody raised against the HA tag. This tag was engineered onto the N termini of all HPV E1 proteins.
E1 proteins from high- and low-risk HPVs induce the formation of γH2AX.
We transfected E1 protein expression constructs of high-risk (HPV16 and HPV18) and low-risk (HPV11 and HPV6B) types into U2OS cells and measured the E1 concentration-dependent H2AX phosphorylation. All E1 expression constructs were cotransfected into U2OS cells with homologous E2 expression plasmids. Expression of the E1 protein from various HPV types resulted in a concentration-dependent accumulation of γH2AX at similar levels (Fig. 1B). The lack of a difference in the phosphorylation of H2AX between E1 proteins from high- and low-risk types indicates that this finding is a general feature of all HPV E1 proteins and is not specific to high-risk HPVs.
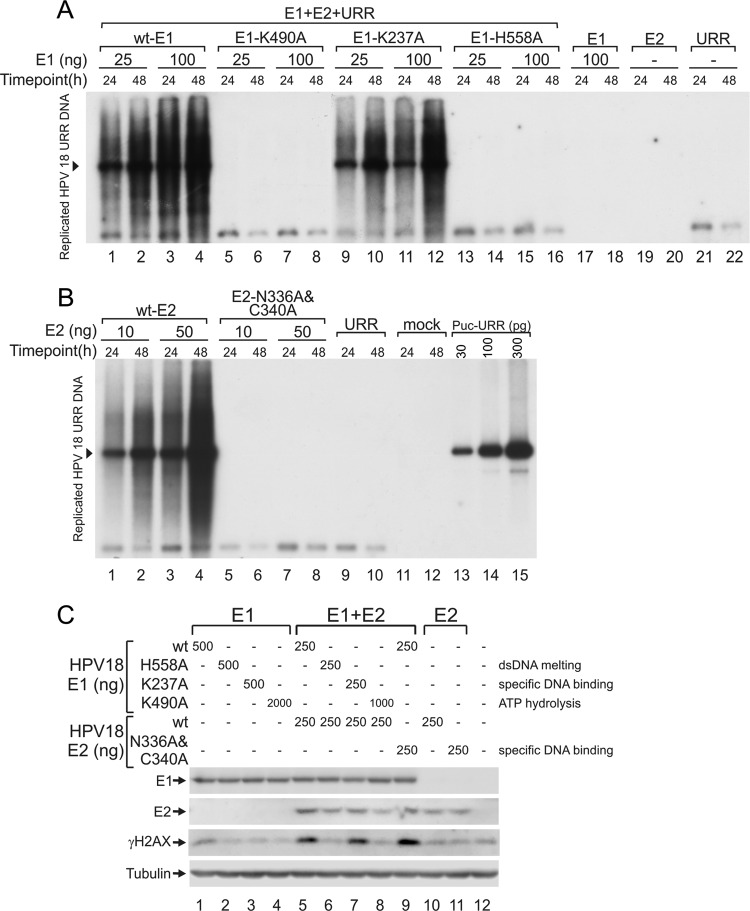
The ATPase and DNA melting activities of E1 are essential for the activation of γH2AX.
To identify which activities of the E1 protein are required for the activation of DDR, we inactivated the following HPV18 E1 protein activities by alanine substitution: double-strand DNA melting activity (H558A) (38), sequence-specific DNA-binding activity (K237A) (39), and ATPase activity (K490A) (40). The functionalities of the mutant proteins were first tested in a replication assay (Fig. 2A) by cotransfection of the mutant E1 expression plasmids with the HPV18 E2 expression construct and the replication origin-containing plasmid into U2OS cells. Low-molecular-weight DNA was extracted from the cells at 24 and 48 h posttransfection using Hirt lysis, purified, and subjected to Southern blot analysis. As expected, the dsDNA melting and ATP hydrolysis mutants failed to support HPV DNA replication; however, the sequence-specific DNA-binding mutant supported viral replication at a level comparable to that of wild-type (wt) E1. This finding is consistent with previous studies, which demonstrated that E1 origin-specific binding activity is not important for HPV replication (41, 42). Next, the ability of these mutants to induce the phosphorylation of histone H2AX was assessed. Expression constructs coding for wt or mutant E1 proteins were transfected into U2OS cells alone or together with the E2 expression vector, and H2AX phosphorylation was measured as described above. When E1 was expressed alone, only the wild-type E1 protein caused moderate phosphorylation of the histone (Fig. 2C, lane 1). However, when E1 and E2 were coexpressed, the sequence-specific DNA-binding mutant K237A induced the accumulation of γH2AX to a level similar to that of the wt E1 protein (Fig. 2C, lanes 5 and 7). The remaining two E1 mutants (the dsDNA melting and ATP hydrolysis mutants) did not increase γH2AX levels higher than that observed with the mock transfection. These results suggest that E1 must be capable of hydrolyzing ATP and melting DNA to induce the phosphorylation of H2AX.
Fig 2.
(A and B) Transient DNA replication assay of HPV18. (A) U2OS cells were transfected with 25 or 100 ng of wt or mutant HPV18 E1 expression vectors together with 25 ng of expression vector for HPV18 E2 and 25 ng of HPV18 origin-containing plasmid pUC-URR. Expression vectors for wt E1 or E2 or origin plasmid were transfected separately as negative controls. (B) U2OS cells were transfected with 25 ng of E1 expression construct together with 10 or 50 ng of the E2 construct and 25 ng of the origin plasmid (URR). The transfection of 25 ng of the origin plasmid was used as a negative control. Low-molecular-weight DNA was harvested at 24 and 48 h after transfection using Hirt lysis. The samples were digested with a linearizing enzyme and DpnI to remove the methylated input DNA and analyzed by Southern blotting using an HPV18 origin-specific probe. Amounts of 30, 100, and 300 pg of the linearized pUC-URR plasmid were used as a marker. (C) Expression vectors for wt or mutant E1 proteins were transfected into U2OS cells alone (lanes 1 to 4) or with an expression construct for wt E2 (lanes 5 to 8). A mutant E2 protein was also analyzed by transfecting its expression construct either alone (lane 11) or with a wt E1 construct (lane 9). Transfection of the wt E2 expression construct and mock transfection are shown in lanes 10 and 12, respectively. HA-tagged HPV18 E1, HPV18 E2, γH2AX, and tubulin signals were determined by Western blotting at 24 h after transfection.
Specific DNA-binding activity of the E2 protein is not required for DDR activation.
Thousands of E2 binding sites exist in the human genome (43); therefore, we speculated that E2 might play a role in γH2AX formation by directing E1 onto the chromatin through binding to these sites. To assess this possibility, we mutated the two amino acids in the E2 DNA-binding domain that form direct base-specific DNA contacts (N336A/C340A) (44). In a replication assay, this mutant protein exhibited no ability to support the replication of HPV origin-containing plasmids (Fig. 2B). However, when examined together with E1 protein in an H2AX phosphorylation assay (Fig. 2C), coexpression of the DNA-binding-defective E2 mutant with E1 induced levels of γH2AX accumulation similar to those of the wt E2 protein, indicating that E2-specific DNA-binding activity is not required for the E1 protein-mediated phosphorylation of H2AX; complex formation with E1, stabilization, and compartmentalization of the E1-E2 complex could be more important for this activity.
Overexpression of the E1 protein induces double-strand DNA breaks in the comet assay.
The presence of double-strand DNA breaks in eukaryotic cells induces signal transduction, DNA repair, and cell cycle arrest to avoid further DNA damage. Although the phosphorylation of H2AX is strongly related to double-strand DNA breaks, there are exceptions when this histone is phosphorylated without DNA damage (45). To test whether E1 protein expression indeed induces the formation of DSBs, neutral single-cell gel electrophoresis, commonly known as the “comet assay,” was performed (Fig. 3). The neutral comet assay is used to assess the presence of double-strand DNA breaks by measuring “comet tails,” which are formed by DNA fragments that migrate from the nucleus in the electric field. The length and brightness of these tails have been associated with the extent of DNA damage in a cell (46). Representative images from cells transfected with carrier DNA, E1 expression construct alone, or E1 and E2 expression constructs are shown in Fig. 3A. Three independent experiments were performed, and the average tail extent moments were calculated and normalized to time zero. The results demonstrate that more DNA migrated from the nuclei of E1- and E1/E2-expressing cells than from mock-transfected cells, indicating the presence of an increased amount of double-strand DNA breaks in these cells. The value of a parameter indicative of double-strand DNA breaks increases 2-fold when E1 is expressed compared with the mock transfection, and an even greater increase is observed when E2 is also present. These data clearly demonstrate that overexpression of the E1 protein causes extensive damage to genomic DNA. The E1 mutants were also tested in the comet assay (Fig. 3B). All three tested mutants proved to be negative in this assay. While it was expected in case of the dsDNA melting (H558A) and ATPase (K490A) mutants, because these two were also negative in the H2AX phosphorylation assay, it was somewhat surprising that the specific DNA-binding mutant (K237A) was not able to cause a detectable level of DNA damage. This suggests that E1-K237A causes less DNA damage than wt E1, and while this is still detectable in the H2AX phosphorylation assay, it is not enough to be seen in the less sensitive comet assay.
Fig 3.
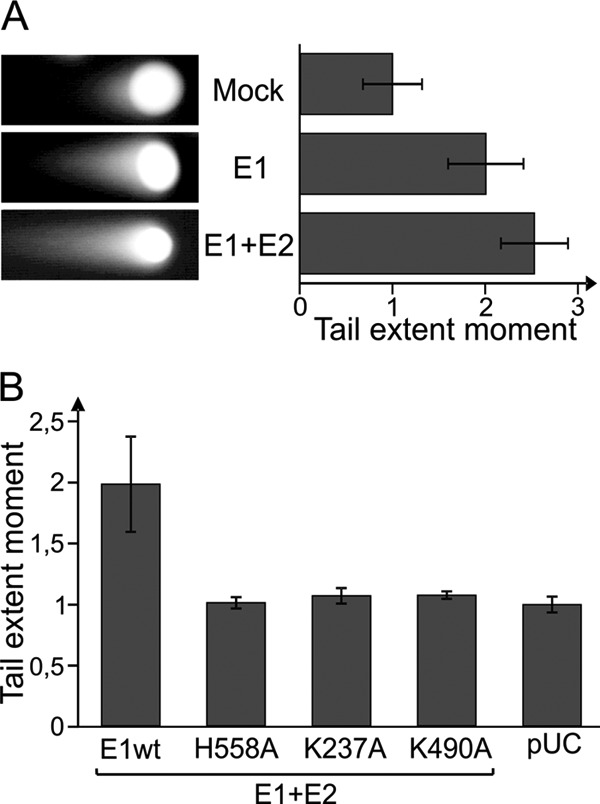
(A) U2OS cells were transfected with 250 ng of HPV18 E1 expression construct alone or with 250 ng of HPV18 E2 vector. Mock transfection with carrier DNA alone is also shown. A neutral comet assay was performed at 24 h after transfection. Representative cells from each sample are shown. Three independent experiments were performed, and results were quantitated using a Thermo Scientific Array scan VTI microscope. The average tail extent moments for each experiment were calculated and normalized to the value for the mock transfection. The mean values of the three average values are shown. Each error bar represents the standard deviation of three averages. (B) wt and mutant HPV18 E1 proteins were expressed together with E2 protein in the U2OS cell line, and a neutral comet assay was performed. The average tail extent moments from two independent experiments were calculated and normalized to the value of a pUC18-transfected sample. Standard deviations are shown.
The ATM-Chk2 pathway is specifically activated in E1-overexpressing cells.
The ATM-Chk2 signaling cascade is responsible for DSB-induced cell cycle arrest. To examine whether this pathway is activated in HPV18 E1-expressing cells, U2OS cells were transfected with expression constructs encoding the wt or mutant E1 proteins, alone or together with the E2 construct. Twenty-four hours later, immunoprecipitation with Chk2 antibody was conducted, and the level of Chk2 activation was evaluated using Western blotting with phosphorylation-sensitive antibodies (Fig. 4). Two phosphorylation sites were analyzed, Thr68 and Ser19. Both of these sites have been shown to be individually sufficient for the activation of Chk2, although Thr68 is considered to be the main phosphorylation site (47). Coexpression of E1 and E2 induced the phosphorylation of Chk2 at both Ser19 and Thr68. Mutant E1 proteins performed in the same way as they did in the H2AX phosphorylation assay, demonstrating that the activation of Chk2 is independent of E1 DNA-specific binding activity but dependent on dsDNA melting activity. Thr68 exhibited higher background phosphorylation in response to transfection; therefore, we were not able to detect clear phosphorylation at this site in cells expressing E1. However, it has previously been demonstrated that Chk2 is phosphorylated specifically at Ser19 in response to DSB (48). Moreover, we did not detect any signal with the pSer19-specific antibody in mock-transfected cells (Fig. 4, lane 8). More importantly, phosphorylation at Ser19 was clearly detectable in wild-type E1- and mutant K237A-expressing cells.
Fig 4.
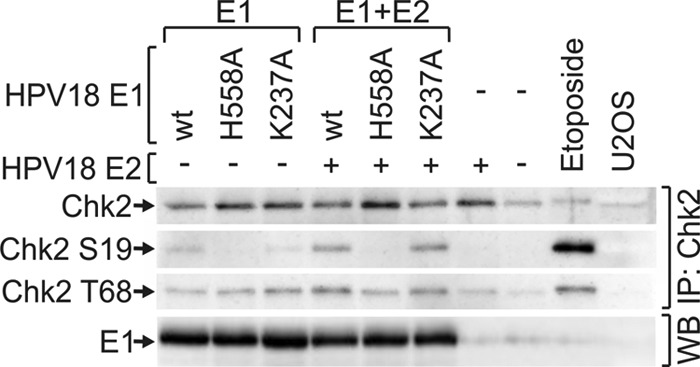
Expression vectors coding for wt or mutant HPV18 E1 proteins were transfected into U2OS cells either alone (lanes 1 to 3) or together with the expression construct for HPV18 E2 protein (lanes 4 to 6). The cells were also transfected with E2 vector alone (lane 7) or with 10 μg of carrier plasmid (lane 8). Nontransfected U2OS cells are shown in lane 10, and U2OS cells treated with 50 μm of etoposide for 1 h before analysis are shown in lane 9. Western blot analysis of Chk2, Chk2 phosphorylated at Ser19, and Chk2 phosphorylated at Thr68 was performed after immunoprecipitation with a Chk2 antibody at 24 h. The HPV18 E1 signals were determined from total cell lysates by Western blotting.
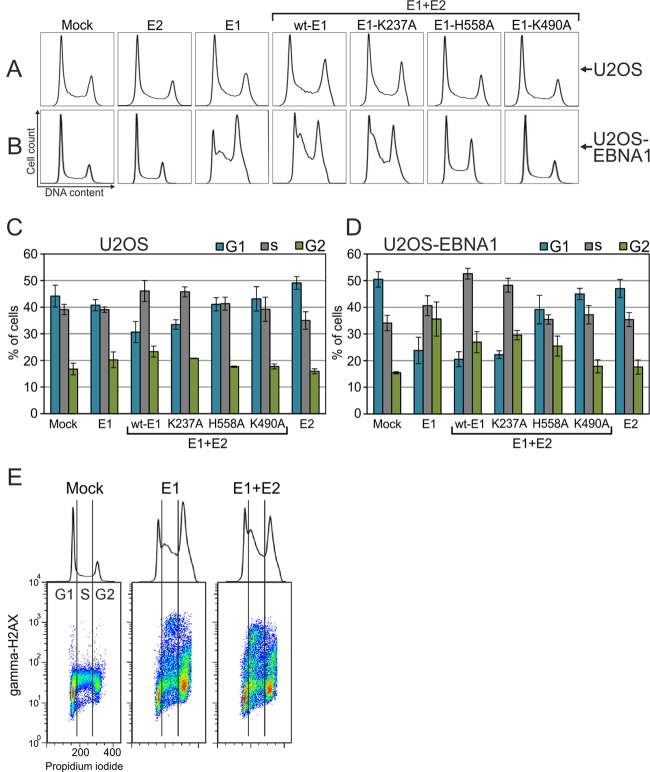
The overexpression of E1 induces cell cycle arrest and γH2AX accumulation in the S and G2 phases.
Activated Chk2 kinase transfers the checkpoint signal to several effector proteins, including p53, Cdc25A, and Cdc25C, which results in the arrest of the cell cycle at the G1/S or G2/M boundary and in the inhibition of ongoing DNA replication (for a review, see reference 49). Because the E1 protein was capable of inducing Chk2 activation in U2OS cells, we examined whether its expression affects the cell cycle. U2OS cells are suitable for such experiments, because we constantly obtain transfection efficiencies of as high as 80 to 90% using electroporation. The wt and mutant E1 proteins were expressed separately in U2OS cells, and a cell cycle analysis was performed at 48 h after transfection. Three independent experiments were performed, and a representative cell cycle profile from every sample is presented in Fig. 5A. The distribution of cells between different phases was quantified, and the average distributions of cells are shown in Fig. 5C. We did not detect considerable changes in the cell cycle of U2OS cells expressing only E1 or E2 protein compared with the mock-transfected cells, but the coexpression of E1 and E2 induced the accumulation of the cells in the S and G2 phases (Fig. 5A and C). In the case of E1 and E2 coexpression, the fraction of G1 cells decreased from 44% to 31% compared with that in the mock-transfected cells. The S and G2 phase fractions both increased equally in the case of E1 and E2 coexpression compared to mock transfection. The DNA-specific binding mutant K237A acted similarly to wt E1, but the cell cycle profiles of cells transfected with the ATPase and dsDNA melting mutants (K490A and H558A, respectively) were indistinguishable from that of the mock-transfected cells.
Fig 5.
U2OS (A and C) or U2OS-EBNA1 (B, D, and E) cells were transfected with expression constructs for HPV18 E2 and wt or mutant HPV18 E1 proteins together with the circular carrier plasmid. Transfection of carrier DNA alone was used as mock control. Cell cycle analysis using propidium iodide for DNA staining was performed at 48 h, and the cell cycle profiles are shown in panels A and B. Quantitated cell cycle distributions were averaged over three experiments and are presented in panels C and D with standard deviations. For panel E, the U2OS-EBNA1 cell line was transfected as follows: 10 μg of circular carrier plasmid, an expression construct coding for HPV18 E1, or cotransfection of expression constructs coding for HPV18 E1 and E2. The cells were stained to determine the γH2AX level and DNA content at 48 h and analyzed using flow cytometry. Dot plots for γH2AX (y axis) and DNA content (x axis) are shown. The respective cell cycle profiles for each sample are shown above the dot plots. The vertical guidelines on the plots represent approximate G1/S and S/G2 boundaries.
In the regular transient-expression system and using conventional expression vectors, the levels of induced protein expression decrease at 48 h after transfection because the expression vectors have no capability for partitioning into daughter cells upon cell division. To extend the expression of E1 and E2 through several cell cycles, we used the U2OS-EBNA1 cell line, which stably expresses EBV nuclear antigen 1, and added the family of repeats into our expression constructs. As a result, equal segregation of the plasmids into the daughter cells was acquired upon cell division, leading to the extended expression of E1 and E2 in these cells (unpublished data). The U2OS-EBNA1 cell line was transfected with expression plasmids encoding wild-type or mutant E1 proteins, and a cell cycle analysis was performed 48 h later (Fig. 5B and D). Expression of the E1 protein severely affected the cell cycle in this expression system. An arrest in early S phase was triggered when E1 was expressed either alone or in combination with E2. Compared with the mock transfection, the percentage of cells in the G1 phase was reduced 2-fold from 50% to less than 25% in those samples. Although the percentage of cells in the G1 phase in E1-positive cells in the presence of E2 was similar to that in the absence of E2, the distributions between S and G2 phases were different in these two samples. Cells expressing both E1 and E2 were arrested earlier in the S phase than cells expressing only E1. When more E1- and E2-expressing cells were in the S phase than in the G2 phase (53% and 27% for S and G2, respectively), the cells expressing E1 alone were divided more equally between the S and G2 cell cycle phases (41% and 36%, respectively). The expression of E2 either alone or in combination with the E1 ATPase mutant K490A or the E1 dsDNA melting mutant H558A did not affect the cell cycle. These data suggest that the overexpression of the E1 protein induces cell cycle arrest due to the induction of double-strand DNA breaks, which triggers cell cycle arrest.
The induction of dsDNA breaks and the activation of the DNA damage response are initiated in the S and G2 phases.
Next, we measured the levels of γH2AX in each cell cycle phase. The levels of γH2AX were determined using a phosphorylation-sensitive antibody and plotted against the DNA content, which was measured by staining with propidium iodide (Fig. 5E). Few γH2AX-positive cells were detected in the mock transfection. In contrast, when E1 was expressed alone or in combination with E2 in the U2OS cells, a large number of cells containing phosphorylated H2AX were detected. Interestingly, the majority of these γH2AX-positive cells were in the S and G2 phases, and few were detected in the G1 phase. Because the plot does not fully describe the transition from G1 to S, some of the positive cells in the G1 phase could actually be in early S phase. The observation that H2AX is phosphorylated in the S and G2 phases of the cell cycle is consistent with data from previous studies showing that E1 is imported into the nucleus during the G1/S transition (50). Taken together, these data suggest that the expression of HPV E1 protein induces cell cycle arrest in the intra-S and G2/M checkpoints, which is caused by DNA damage generated in S phase or in both S and G2 phases.
Induction of the ATM-Chk2 response pathway is not required for HPV DNA replication.
Consistent with published data (30, 31), our results demonstrate that HPV E1 protein can activate the ATM-Chk2 pathway; therefore, we examined whether the activation of DNA repair mechanisms could affect HPV DNA replication. It has been proposed that U2OS cells support the replication of low- and high-risk HPV genomes and HPV origin-containing plasmids when E1 and E2 proteins are transiently expressed (32). Therefore, we examined whether DDR is activated due to viral genome replication in U2OS cells in a manner similar to that by which it was demonstrated for the E1 and E2 expression constructs. In addition to the wt HPV18 genome, we used an HPV18 mutant in which the initiation codon of E8 was inactivated by a point mutation (AUG-ACG). The HPV18/E8− mutant genome does not express the E2 repressor protein E8/E2 and is capable of higher transient-replication levels than the wt genome (51). To evaluate the potential role of viral oncoproteins in DDR activation, frameshift mutations were introduced into the E1, E6, and E7 ORFs to terminate their expression. The wt and mutant genomes were examined in a transient-replication assay (Fig. 6A). As expected, the HPV18/E8− genome (Fig. 6A, lanes 7 to 12) exhibited a much higher level of replication than did the wt genome (Fig. 6A, lanes 1 to 6). While frameshift mutation in E6 (E6fs) (Fig. 6A, lanes 13 and 14) did not change the level of HPV18 transient replication, the frameshift mutation in E7 (Fig. 6A, lanes 15 and 16) induced elevated replication of the viral genome.
Fig 6.
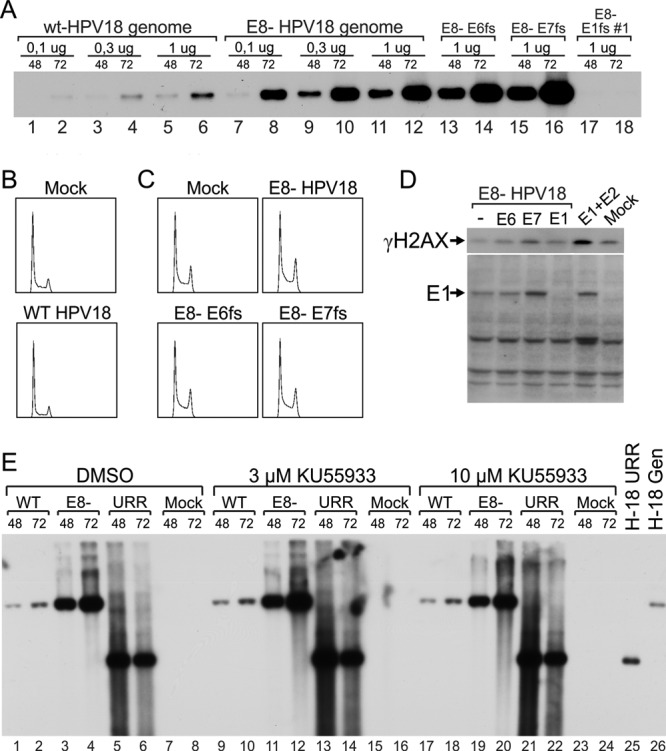
U2OS cells were transfected with 2 μg of HPV18 wild-type or mutant genomes. The following mutants were used: HPV18/E8−, which did not express the E8/E2 repressor, and its double mutants, which also contained frameshifts in the E6, E7, or E1 ORF. Cells transfected with circular carrier DNA alone were used as mock controls. (A) Low-molecular-weight DNA was extracted using Hirt lysis at 48 and 72 h after transfection, digested with a linearizing enzyme and DpnI to remove methylated input DNA, and analyzed by Southern blotting using an HPV-specific probe. (B and C) Cell cycle analysis was performed at 48 h after transfection, and the cell cycle profiles are shown. (D) Western blot analysis was performed at 48 h using anti-γH2AX or polyclonal anti-HPV18 E1 antibodies. Cotransfections of E1 and E2 expression constructs were used as positive controls. (E) In addition to the wt and E8− genomes, U2OS cells were transfected with 100 ng of HPV18 E1 and E2 expression plasmids and complemented with 100 ng of HPV18 origin-containing plasmid (pUC-URR). Immediately after transfection, the ATM small-molecule inhibitor KU559933 was added at two concentrations (3 and 10 μM); DMSO was used as a vehicle control. Total DNA was harvested at 48 and 72 h after transfection. Two micrograms of total DNA was digested with a linearizing enzyme (BglI for the genomes and the mock sample and Bsp1407I for pUC-URR) and DpnI. This digestion was followed by Southern blotting using an HPV18 origin-specific probe. Linearized pUC-URR plasmid and HPV18 genome were used as markers.
Subsequently, we evaluated the ability of the viral genomes to induce changes in the cell cycle. Circular wild-type and mutant viral genomes were transfected into U2OS cells using electroporation; cell cycle analysis was then preformed at 48 h posttransfection (Fig. 6B and D). Neither the wt (Fig. 6B) nor the E8 mutant (Fig. 6C) caused detectable changes in the cell cycle. The expression of E6 or E7 in the context of the HPV18/E8− genome also had no effect on the cell cycle, because frameshifts in these ORFs did not affect the assay (Fig. 6C).
The HPV18/E8− genome and its double mutants also containing E6fs or E7fs were examined in an H2AX phosphorylation assay (Fig. 6D). Although E1 is expressed from these genomes at levels comparable to that from the E1 expression construct, as evaluated using E1-specific affinity-purified polyclonal rabbit antibodies in Western blot analysis (Fig. 6D), the formation of γH2AX was much weaker. In fact, the HPV18/E8−, E8− E6fs, and E8− E1fs genomes all failed to induce a level of H2AX phosphorylation higher than that of the background. Only E8− E7fs, which expressed high levels of the E1 protein, induced levels of γH2AX formation that were slightly higher than background levels.
Fradet-Turcotte et al. previously demonstrated that the inhibition of ATM does not affect the replication of the HPV origin-containing plasmids driven by the heterologous expression of E1 and E2 proteins (30). To characterize the role of the ATM-Chk2 pathway in the transient replication of the HPV genome, we used the ATM-specific inhibitor KU55933 (52) at various concentrations. The inhibitor's activity was verified by its ability to inhibit etoposide-induced phosphorylation of Chk2 protein (data not shown). As presented in Fig. 6E, HPV18 wt and E8 mutant genomes and the HPV18 URR reporter replicate efficiently in the presence of any tested concentration of the ATM inhibitor, strongly suggesting that the ATM-Chk2 pathway can be efficiently suppressed without affecting replication of the HPV18 genome or the HPV18 URR plasmid.
Replication of the HPV18 genome occurs in distinct replication centers in the nuclei of U2OS cells.
It is generally accepted that the papillomavirus replication proteins E1 and E2 accumulate into distinct nuclear foci after coexpression from heterologous vectors and that these foci become the sites of viral DNA replication in the presence of the replication origin plasmid (53). These foci have also been shown to contain DDR markers, such as phosphorylated ATM, p53, and γH2AX (31). Therefore, we examined whether similar patterns could be detected during the transient DNA replication of viral genomes. U2OS cells were transfected with the HPV18/E8− genome and HPV mutants containing additional frameshift mutations in the E6, E7, or E1 ORF. FISH analysis of transfected cells indicated that HPV18/E8− genomes indeed accumulate into distinct foci (Fig. 7A). We used an E8 mutant of HPV18 to increase the sensitivity of detection because wt HPV18 genome replication is approximately 10 times lower than that of the E8 mutant (Fig. 6A). More interestingly, immunostaining of cells transfected with HPV18/E8− revealed that viral genome replication centers contain phosphorylated H2AX, in turn suggesting that DDR is activated by ongoing viral DNA replication. HPV18/E8− genomes containing frameshift mutations in viral oncogenes induced a level of γH2AX activation similar to that of the E8− genome, whereas the HPV18/E8− genome containing an E1 frameshift did not establish any visible replication foci or γH2AX centers, demonstrating the need for viral genome replication to recruit DDR to the viral genome.
To further demonstrate that the foci containing HPV genomes are the sites of viral DNA replication, we performed coimmunostaining of the E1 protein and γH2AX and studied EdU incorporation, which indicates the sites of ongoing DNA synthesis (Fig. 7B). For this experiment, we developed affinity-purified rabbit polyclonal antibodies against the HPV18 E1 protein. In the Western blot analysis and the immunofluorescence assays, HPV18 E1 protein expression was clearly detected for the HPV18/E8− genome and its E6/E7 frameshift mutants at 48 h after transfection. We observed that in cells transfected with the HPV18/E8− genome or the E6/E7 frameshift mutants, E1 accumulated into foci, which also invariably contained both γH2AX and EdU. Interestingly, the EdU signal in E1-positive cells was reproducibly much weaker than that in E1-negative S phase cells, suggesting that cellular DNA synthesis is not simultaneous with viral genome replication.
Cellular ATRIP and TopBP1 are localized into viral replication centers.
The localization of the DNA damage response marker γH2AX into viral replication centers indicates that the ATR pathway, which responds to DNA replication stress, might be activated. Many other cellular proteins, such as the ATR-interacting protein (ATRIP), support ATR activation. ATRIP regulates the localization of ATR and is a crucial component of the DNA damage checkpoint pathway (54). Another important protein in the pathway is topoisomerase IIβ-binding protein 1 (TopBP1) (for a review, see reference 55), which is also a binding partner of the HPV E2 protein (56).
Commercially available antibodies against the ATRIP protein are of poor quality. Therefore, the U2OS cells were cotransfected with the HPV18/E8− genome and an expression construct encoding the HA-tagged ATRIP protein (Fig. 8A) to examine whether the ATRIP protein localizes in viral replication centers. At 48 h posttransfection, the cells were costained with the HPV18 E1 antibody for the detection of viral replication centers and with an anti-HA antibody for the detection of the tagged ATRIP protein. E1- and ATRIP-specific foci almost completely overlapped in HPV-positive cells, thereby demonstrating that the tagged-ATRIP protein indeed localizes to the sites of viral DNA replication. For the detection of TopBP1 protein localization, coimmunostaining of HPV18 E1 and TopBP1 was performed at 48 h after transfection of U2OS cells with either carrier DNA or the HPV18/E8− genome. Moreover, TopBP1 always localized to the replication centers of viral E8− genomes as shown by the colocalization of the TopBP1 protein with HPV E1 foci (Fig. 8B). The localization of γH2AX, ATRIP, and TopBP1 to the replication centers of viral genomes indicates that the ATR pathway is activated due to viral DNA replication.
Viral replication centers are present in the G2 phase of the cell cycle.
In previous studies using SiHa and HeLa cells (28) and primary keratinocytes (31), the E1 and E2 proteins formed HPV replication centers at the sites of DNA synthesis; however, no replication of cellular DNA was detected in these cells. The same observation was made in U2OS cells that were actively replicating the HPV genome, where the EdU signal was localized exclusively in the E1 foci (Fig. 7B); this result suggests that cellular DNA replication either is stalled, has been completed, or has not been initiated during HPV genome replication in these centers. At the same time, we cannot exclude possibility that in addition to the DNA synthesis of the HPV genome, some repair synthesis of cellular DNA is also taking place in the E1 foci.
These data suggest that the absence of cellular DNA synthesis during the replication of viral genomes could be caused by temporal separation of these two replication events in the cell cycle. In fact, it has been shown that the vegetative amplification of HPV in raft cultures of primary human keratinocytes occurs during the G2 phase of the cell cycle (57). We examined whether transient replication of the HPV18 genome in U2OS cells could occur outside the S phase. U2OS cells were transfected with the HPV18/E8− genome, pulse-labeled with EdU to detect the sites of ongoing DNA synthesis, and then costained with antibodies against E1 and cyclin B1.
Cyclin B1 regulates cell cycle progression from G2 to M phase. During the G2 phase, cyclin B1 is localized in the cytoplasm, and it moves into the nucleus during mitosis. This protein is expressed at low levels during the G1 and S phases, and its concentration starts to increase in G2 and reaches a peak during early mitosis (58). Levels of cyclin B1 are not sharply increased at the beginning of the G2 phase; therefore, cyclin B1 cannot be used to distinguish between cells in S and early G2 phase. However, this protein does accumulate to clearly detectable levels at the end of G2 and can be used to distinguish these cells from G1/S cells. In our experiments, we were clearly able to detect cells that contained strong cyclin B1 signals in the cytoplasm and had E1 foci in the nucleus (Fig. 9). These E1 foci also always contained EdU signals, suggesting ongoing DNA synthesis. However, not all E1 focus-containing cells had high expression levels of cyclin B1. The exact fraction of E1-positive G2 cells was difficult to assess due to the smooth transition of cyclin B1 levels, but an attempt to quantitate it was made by randomly finding around 50 E1 focus-containing cells in the case of HPV18/E8− genome transfection and determining if they have a strong cyclin B1 signal using confocal microscopy (Fig. 9B). It turned out that approximately half of the E1 and EdU focus-containing cells also exhibited strong cyclin B1 signals in the cytoplasm and were therefore definitely in the G2 phase. The presence of such cells shows that transient replication of the HPV genome can occur in the G2 phase of the cell cycle. This result did not depend on the source of the E1 protein and was the same whether E1 was expressed from the viral genome or from an expression vector, suggesting that no viral factors other than E1 and E2 are required for viral replication to occur in the G2 phase.
DISCUSSION
Our earlier studies as well as those by two other groups have recently addressed the activation of DDR in the context of E1 overexpression from heterologous expression vectors (30, 31). These studies have shown that E1 expression from heterologous expression constructs induces the ATM-dependent DNA damage response, which is accompanied by cell cycle arrest and cell growth suppression. We decided to extend these studies to HPV18 genome replication in order to evaluate how E1 protein activity translates into the viral genome replication context. We used expression of E1 protein from heterologous expression vectors as well as from the viral genome and characterized changes in the DDR status in these cells. Here, we confirm that overexpression of the human papillomavirus type 18 E1 protein induces the dose-dependent activation of the histone H2AX protein in the HPV-negative U2OS cell line through double-strand DNA breaks and the activation of the ATM-Chk2 pathway. E1 dsDNA melting and ATPase activities are essential for the triggering of cellular DNA lesions, whereas sequence-specific DNA binding of the E1 protein is not necessary. Importantly, the inhibition of ATM with a specific inhibitor did not affect the replication of the HPV genome or E1- and E2-dependent HPV18 URR replication in transient assays, suggesting that although the signal transduction pathway is activated, the virus replication machinery does not benefit from this cellular activity. The results of the present study confirm for the first time that the expression of high doses of E1 protein from expression constructs causes double-strand DNA breaks in the host genome, as shown by the “comet assay” (Fig. 3). The comet assay allows the direct visualization of DSBs and unarguably demonstrates the E1-dependent formation of these dangerous DNA lesions; this was previously suggested based on indirect observations, such as the accumulation of γH2AX and the incorporation of nucleotide analogs in the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay. We also provided other evidence in addition to the “comet assay,” such as the phosphorylation of H2AX (Fig. 1) and activation of the ATM pathway (Fig. 4), which supports the hypothesis that DSB formation results from E1 expression.
The results of the flow cytometric analysis indicate that after E1 overexpression, U2OS cells are arrested in the S and G2 phases of the cell cycle. A majority of the γH2AX-positive cells appeared to be in the S and G2 phases; therefore, it is reasonable to conclude that cell cycle arrest is triggered as a result of E1-induced DSB formation (Fig. 5). It has previously been shown that E1 expression can cause S-phase arrest (30); however, in our assay system, the G2 phase is also arrested. The difference between these two results may come from the different E1 proteins used in these two studies. We used HA-tagged E1 protein, while the previous study used a yellow fluorescent protein (YFP)-E1 fusion protein, and some properties (for example, the half-life) of these two E1 variants may be different, which might cause the discrepancy seen in the results.
The mechanism underlying the induction of DNA breaks by E1 has not been clearly demonstrated. The present study shows that direct interactions between E1 and DNA are essential for inducing DNA breaks, because mutant proteins that were incapable of dsDNA melting did not activate DDR (Fig. 2). The E1 protein contains a highly conserved beta-hairpin in its helicase domain, and a mutation of the histidine on the tip of this structure results in retention of the ability to bind DNA, oligomerize, and hydrolyze ATP but loss of dsDNA melting ability (38). We show that such a mutant (HPV18 E1 H558A) does not induce the DNA damage response. This finding, together with the observation that DDR is activated in the replication centers of viral genomes, indicates that E1 might damage the host genome by initiating nonspecific DNA melting. Another possibility would be that E1 overexpression and its interactions with host proteins might disrupt normal cellular replication, which could result in the activation of DDR. The E1 protein interacts with several cellular replication proteins, such as polymerase α and RPA (reviewed in reference 59). Because the H558A mutant should maintain wt interactions with cellular proteins but is not able to induce the accumulation of γH2AX, our data suggest that for the induction of DDR, E1 does not depend on its interactions with cellular proteins but rather interacts directly with the host DNA. E1 may induce DDR by initiating DNA replication from nonspecific DNA, which leads to abortive elongation of the replication fork and generation of DNA damage.
We developed an affinity-purified polyclonal antibody against HPV18 E1 to examine the effect of the E1-induced DNA damage response in the context of replicating viral genomes and expression vectors. In the case of E1 expression from heterologous expression vectors, we observed two patterns of E1 compartmentalization in the nuclei of the cells. First, we detected a uniform distribution of the E1 signal in the nucleus with exclusion from the nucleolus of the cells, and second, we detected concentration of E1 exclusively into the foci. The cells with the uniform distribution of E1 in the nucleus were prevalent at high concentrations, while foci were seen predominantly at low levels of E1 (data not shown). Although E1 expression from the wt HPV18 genome was difficult to detect in a reproducible fashion, this antibody was effectively used to detect the E1 protein in a variety of assays using viral genomes that did not express the E8/E2 repressor protein (Fig. 6D). To our knowledge, this is the first time that the E1 protein has been detected when expressed from the viral genome. We demonstrated, as previously shown by mRNA analysis, that E1 expression indeed increases when the E8/E2 repressor is turned off. We also observed that a frameshift mutation in the E7 ORF in the HPV18/E8− genome increases E1 expression, which is consistent with a previous observation of E1 expression from polycistronic mRNA (60).
In the case of viral genome replication, the activation of DDR was considerably weaker than E1 expression from the heterologous expression construct. When comparable levels of E1 protein were produced from the E8/E2 mutant genome and the E1 expression construct, H2AX phosphorylation was observed in Western blotting experiments, but not in the case of viral genome expression (Fig. 6D). This suggests that (as shown in Fig. 1 and previously demonstrated by another study [30]) addition of the viral replication origin decreases the E1-dependent DDR activation in these cells. It is also possible that E2 protein is expressed at such a ratio to E1 from viral genomes that it inhibits E1 DNA-damaging activity. The ability of E2 to decrease E1-specific DDR activation has also been previously suggested (30). The resulting imbalance in the E1-to-E2 ratio might also be the reason why we were able to detect slight γH2AX formation only in case of the E7fs mutation in the Western blot experiment (Fig. 6D). The E7fs mutation increased E1 expression but should leave E2 expression unchanged.
The facts that H2AX is phosphorylated only in viral replication centers (Fig. 7) and that no cell cycle arrest or large-scale DDR activation occurs (Fig. 6) indicate that no considerable damage to cellular DNA is generated in cells containing replicating viral genomes. H2AX is phosphorylated only on viral genomes, which considerably limits the overall formation of γH2AX compared with DSB formation in the host genome. If a single DSB is formed in the host genome, H2AX phosphorylation spreads along the chromatin to millions of base pairs (61), which corresponds to the amount of DNA in hundreds of viral genomes. These data suggest that the compartmentalization of E1 into replication centers at low E1 levels lowers the nonspecific activity of E1 for DNA damage.
It was recently shown that by activating the ATM pathway, HPV recruits cellular DNA repair and recombination factors into its replication centers during the stable and vegetative phases of its life cycle (62). Our observations that γH2AX, ATRIP, and TopBP1 are mobilized into HPV replication centers indicate that during the transient-replication phase, HPV replication also activates the ATR pathway (Fig. 8). ATR kinase phosphorylates H2AX independently of ATM in response to replication stress (63). In most cases, ATR is activated by stretches of single-stranded DNA (ssDNA) bound to RPA, which forms due to replication fork stalling (for a review, see reference 64). For this reason, replication fork movement might be blocked during the replication of viral genomes, thereby causing the activation of ATR.
The activation of DDR could also be caused by the viral oncoproteins E6 and E7, which interfere with many cellular pathways that are involved in checkpoint control and DNA repair. However, neither of these proteins is necessary for the activation of DDR in viral replication centers because mutant genomes, which do not express E6 or E7, both recruit γH2AX in these centers (Fig. 7). These results indicate that the viral DNA replication process is responsible for DDR activation.
It is unclear how HPV benefits from inducing the DNA damage response. Activation of the ATM pathway is necessary for the effective late amplification of the HPV genome, while it can be inhibited without consequences during the stable maintenance phase (27). Here, we show that ATM inhibition does not decrease the transient-replication levels of viral genomes (Fig. 6E). A similar observation has previously been described for the E1 and E2 expression construct-based replication system (30), which did not involve viral oncogenes that might change the cellular environment by interfering with DDR. Regardless, both of these experiments provide similar results and demonstrate that ATM signaling is not directly involved in HPV replication. Because ATR does not have a specific inhibitor, it is difficult to determine whether ATR-driven DDR plays a role in HPV transient replication. The virus could induce checkpoint activation to arrest the cell cycle in the S or G2 phase, where the virus would have more time for the replication of its genome. Indeed, it has been shown that checkpoint activation does not interfere with viral replication (65). HPV might therefore use DDR activation to stop the cell cycle and thereby make viral replication more efficient. However, we were not able to demonstrate the importance of this mechanism for viral genome replication because no cell cycle arrest was detectable in cells transiently replicating viral genomes (Fig. 6).
It is generally known that HPV genomes are prone to oligomerize upon replication. It is possible that the formation of oligomers facilitates maintenance of the viral genome during latent infection. HPV might promote oligomerization by activating DDR in viral replication centers. The most common type of oligomer involves head-to-tail formation, which indicates that these oligomers have been formed as the result of homologous recombination. It was recently shown that proteins participating in this pathway are localized to viral replication centers (62).
Finally, we provide data that HPV transient replication can occur during the G2 phase of the cell cycle (Fig. 9), as described previously for viral vegetative replication (57). This appears to be the reason why DNA synthesis in cells that replicate viral genomes or origin plasmids is localized only in viral replication centers and no cellular DNA replication occurs at the same time (Fig. 7) (31). The ability of HPV to undergo replication in the G2 phase is not regulated by any proteins other than E1 and E2, as the URR plasmid-based replication system produced results in this experiment similar to those produced in experiments with the full-length viral genome.
ACKNOWLEDGMENTS
We thank David Cortez for providing the HA-tagged ATRIP expression construct, Fernando Rodriguez for comments on the manuscript, Reet Kurg for helpful discussions, Marit Orav for generating the minicircle HPV18 genome, and Liisi Võsa for constructing the HPV18/E8− genome.
This work was supported in part by the target financial projects SF0180175A and SF0180175B, by the European Regional Development Fund through the Center of Excellence in Chemical Biology (3.2.0101.08-0017), and by grants 7192, 7670, 9385, and 9467 from the Estonian Science Foundation.
Footnotes
Published ahead of print 7 November 2012
REFERENCES
- 1. Howley PM, Lowy DR. 2001. Papillomaviruses and their replication, p 2197–2230 In Knipe DM, Howley PM. (ed), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2. Broker TR, Jin G, Croom-Rivers A, Bragg SM, Richardson M, Chow LT, Vermund SH, Alvarez RD, Pappas PG, Squires KE, Hoesley CJ. 2001. Viral latency—the papillomavirus model. Dev. Biol. 106:443–451 [PubMed] [Google Scholar]
- 3. Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. 1998. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 338:423–428 [DOI] [PubMed] [Google Scholar]
- 4. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. 2004. Classification of papillomaviruses. Virology 324:17–27 [DOI] [PubMed] [Google Scholar]
- 5. zur Hausen H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2:342–350 [DOI] [PubMed] [Google Scholar]
- 6. Kadaja M, Silla T, Ustav E, Ustav M. 2009. Papillomavirus DNA replication—from initiation to genomic instability. Virology 384:360–368 [DOI] [PubMed] [Google Scholar]
- 7. Schuck S, Stenlund A. 2005. Assembly of a double hexameric helicase. Mol. Cell 20:377–389 [DOI] [PubMed] [Google Scholar]
- 8. Sedman J, Stenlund A. 1998. The papillomavirus E1 protein forms a DNA-dependent hexameric complex with ATPase and DNA helicase activities. J. Virol. 72:6893–6897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ustav M, Stenlund A. 1991. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 10:449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang L, Mohr I, Fouts E, Lim DA, Nohaile M, Botchan M. 1993. The E1 protein of bovine papilloma virus 1 is an ATP-dependent DNA helicase. Proc. Natl. Acad. Sci. U. S. A. 90:5086–5090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stenlund A. 2003. Initiation of DNA replication: lessons from viral initiator proteins. Nat. Rev. Mol. Cell Biol. 4:777–785 [DOI] [PubMed] [Google Scholar]
- 12. Sedman J, Stenlund A. 1995. Co-operative interaction between the initiator E1 and the transcriptional activator E2 is required for replicator specific DNA replication of bovine papillomavirus in vivo and in vitro. EMBO J. 14:6218–6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weitzman MD, Lilley CE, Chaurushiya MS. 2010. Genomes in conflict: maintaining genome integrity during virus infection. Annu. Rev. Microbiol. 64:61–81 [DOI] [PubMed] [Google Scholar]
- 14. Lilley CE, Carson CT, Muotri AR, Gage FH, Weitzman MD. 2005. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc. Natl. Acad. Sci. U. S. A. 102:5844–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shirata N, Kudoh A, Daikoku T, Tatsumi Y, Fujita M, Kiyono T, Sugaya Y, Isomura H, Ishizaki K, Tsurumi T. 2005. Activation of ataxia telangiectasia-mutated DNA damage checkpoint signal transduction elicited by herpes simplex virus infection. J. Biol. Chem. 280:30336–30341 [DOI] [PubMed] [Google Scholar]
- 16. Wilkinson DE, Weller SK. 2004. Recruitment of cellular recombination and repair proteins to sites of herpes simplex virus type 1 DNA replication is dependent on the composition of viral proteins within prereplicative sites and correlates with the induction of the DNA damage response. J. Virol. 78:4783–4796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dahl J, You J, Benjamin TL. 2005. Induction and utilization of an ATM signaling pathway by polyomavirus. J. Virol. 79:13007–13017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi Y, Dodson GE, Shaikh S, Rundell K, Tibbetts RS. 2005. Ataxia-telangiectasia-mutated (ATM) is a T-antigen kinase that controls SV40 viral replication in vivo. J. Biol. Chem. 280:40195–40200 [DOI] [PubMed] [Google Scholar]
- 19. Kudoh A, Fujita M, Zhang L, Shirata N, Daikoku T, Sugaya Y, Isomura H, Nishiyama Y, Tsurumi T. 2005. Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J. Biol. Chem. 280:8156–8163 [DOI] [PubMed] [Google Scholar]
- 20. Boichuk S, Hu L, Hein J, Gjoerup OV. 2010. Multiple DNA damage signaling and repair pathways deregulated by simian virus 40 large T antigen. J. Virol. 84:8007–8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gruhne B, Sompallae R, Masucci MG. 2009. Three Epstein-Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene 28:3997–4008 [DOI] [PubMed] [Google Scholar]
- 22. Tachiwana H, Shimura M, Nakai-Murakami C, Tokunaga K, Takizawa Y, Sata T, Kurumizaka H, Ishizaka Y. 2006. HIV-1 Vpr induces DNA double-strand breaks. Cancer Res. 66:627–631 [DOI] [PubMed] [Google Scholar]
- 23. Howie HL, Katzenellenbogen RA, Galloway DA. 2009. Papillomavirus E6 proteins. Virology 384:324–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McLaughlin-Drubin ME, Munger K. 2009. The human papillomavirus E7 oncoprotein. Virology 384:335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pim D, Banks L. 2010. Interaction of viral oncoproteins with cellular target molecules: infection with high-risk vs low-risk human papillomaviruses. APMIS 118:471–493 [DOI] [PubMed] [Google Scholar]
- 26. Santegoets LA, van Baars R, Terlou A, Heijmans-Antonissen C, Swagemakers SM, van der Spek PJ, Ewing PC, van Beurden M, van der Meijden WI, Helmerhorst TJ, Blok LJ. 2012. Different DNA damage and cell cycle checkpoint control in low- and high-risk human papillomavirus infections of the vulva. Int. J. Cancer 130:2874–2885 [DOI] [PubMed] [Google Scholar]
- 27. Moody CA, Laimins LA. 2009. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog. 5:e1000605 doi:10.1371/journal.ppat.1000605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kadaja M, Isok-Paas H, Laos T, Ustav E, Ustav M. 2009. Mechanism of genomic instability in cells infected with the high-risk human papillomaviruses. PLoS Pathog. 5:e1000397 doi:10.1371/journal.ppat.1000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kadaja M, Sumerina A, Verst T, Ojarand M, Ustav E, Ustav M. 2007. Genomic instability of the host cell induced by the human papillomavirus replication machinery. EMBO J. 26:2180–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fradet-Turcotte A, Bergeron-Labrecque F, Moody CA, Lehoux M, Laimins LA, Archambault J. 2011. Nuclear accumulation of the papillomavirus E1 helicase blocks S-phase progression and triggers an ATM-dependent DNA damage response. J. Virol. 85:8996–9012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakakibara N, Mitra R, McBride AA. 2011. The papillomavirus E1 helicase activates a cellular DNA damage response in viral replication foci. J. Virol. 85:8981–8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geimanen J, Isok-Paas H, Pipitch R, Salk K, Laos T, Orav M, Reinson T, Ustav M, Jr, Ustav M, Ustav E. 2011. Development of a cellular assay system to study the genome replication of high- and low-risk mucosal and cutaneous human papillomaviruses. J. Virol. 85:3315–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Meyers C, Wang HK, Chow LT, Zheng ZM. 2011. Construction of a full transcription map of human papillomavirus type 18 during productive viral infection. J. Virol. 85:8080–8092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kay MA, He CY, Chen ZY. 2010. A robust system for production of minicircle DNA vectors. Nat. Biotechnol. 28:1287–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirt B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365–369 [DOI] [PubMed] [Google Scholar]
- 36. Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. 2008. GammaH2AX and cancer. Nat. Rev. Cancer 8:957–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. King LE, Dornan ES, Donaldson MM, Morgan IM. 2011. Human papillomavirus 16 E2 stability and transcriptional activation is enhanced by E1 via a direct protein-protein interaction. Virology 414:26–33 [DOI] [PubMed] [Google Scholar]
- 38. Liu X, Schuck S, Stenlund A. 2007. Adjacent residues in the E1 initiator beta-hairpin define different roles of the beta-hairpin in Ori melting, helicase loading, and helicase activity. Mol. Cell 25:825–837 [DOI] [PubMed] [Google Scholar]
- 39. Auster AS, Joshua-Tor L. 2004. The DNA-binding domain of human papillomavirus type 18 E1. Crystal structure, dimerization, and DNA binding. J. Biol. Chem. 279:3733–3742 [DOI] [PubMed] [Google Scholar]
- 40. Liu X, Stenlund A. 2010. Mutations in sensor 1 and Walker B in the bovine papillomavirus E1 initiator protein mimic the nucleotide-bound state. J. Virol. 84:1912–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lu JZ, Sun YN, Rose RC, Bonnez W, McCance DJ. 1993. Two E2 binding sites (E2BS) alone or one E2BS plus an A/T-rich region are minimal requirements for the replication of the human papillomavirus type 11 origin. J. Virol. 67:7131–7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Russell J, Botchan MR. 1995. cis-acting components of human papillomavirus (HPV) DNA replication: linker substitution analysis of the HPV type 11 origin. J. Virol. 69:651–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vosa L, Sudakov A, Remm M, Ustav M, Kurg R. 2012. Identification and analysis of papillomavirus E2 protein binding sites in the human genome. J. Virol. 86:348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matsumoto T, Nakashima N, Takase K, Hirochika H, Mizuno H. 1997. A mutation study of the DNA binding domain of human papillomavirus type11 E2 protein. J. Biochem. 121:138–144 [DOI] [PubMed] [Google Scholar]
- 45. Soutoglou E, Misteli T. 2008. Activation of the cellular DNA damage response in the absence of DNA lesions. Science 320:1507–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh NP, Stephens RE. 1998. X-ray induced DNA double-strand breaks in human sperm. Mutagenesis 13:75–79 [DOI] [PubMed] [Google Scholar]
- 47. Antoni L, Sodha N, Collins I, Garrett MD. 2007. CHK2 kinase: cancer susceptibility and cancer therapy—two sides of the same coin? Nat. Rev. Cancer 7:925–936 [DOI] [PubMed] [Google Scholar]
- 48. Buscemi G, Carlessi L, Zannini L, Lisanti S, Fontanella E, Canevari S, Delia D. 2006. DNA damage-induced cell cycle regulation and function of novel Chk2 phosphoresidues. Mol. Cell. Biol. 26:7832–7845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bartek J, Falck J, Lukas J. 2001. CHK2 kinase—a busy messenger. Nat. Rev. Mol. Cell Biol. 2:877–886 [DOI] [PubMed] [Google Scholar]
- 50. Deng W, Lin BY, Jin G, Wheeler CG, Ma T, Harper JW, Broker TR, Chow LT. 2004. Cyclin/CDK regulates the nucleocytoplasmic localization of the human papillomavirus E1 DNA helicase. J. Virol. 78:13954–13965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kurg R, Uusen P, Vosa L, Ustav M. 2010. Human papillomavirus E2 protein with single activation domain initiates HPV18 genome replication, but is not sufficient for long-term maintenance of virus genome. Virology 408:159–166 [DOI] [PubMed] [Google Scholar]
- 52. Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ, Smith GC. 2004. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 64:9152–9159 [DOI] [PubMed] [Google Scholar]
- 53. Swindle CS, Zou N, Van Tine BA, Shaw GM, Engler JA, Chow LT. 1999. Human papillomavirus DNA replication compartments in a transient DNA replication system. J. Virol. 73:1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cortez D, Guntuku S, Qin J, Elledge SJ. 2001. ATR and ATRIP: partners in checkpoint signaling. Science 294:1713–1716 [DOI] [PubMed] [Google Scholar]
- 55. Sokka M, Parkkinen S, Pospiech H, Syvaoja JE. 2010. Function of TopBP1 in genome stability. Subcell. Biochem. 50:119–141 [DOI] [PubMed] [Google Scholar]
- 56. Boner W, Taylor ER, Tsirimonaki E, Yamane K, Campo MS, Morgan IM. 2002. A Functional interaction between the human papillomavirus 16 transcription/replication factor E2 and the DNA damage response protein TopBP1. J. Biol. Chem. 277:22297–22303 [DOI] [PubMed] [Google Scholar]
- 57. Wang HK, Duffy AA, Broker TR, Chow LT. 2009. Robust production and passaging of infectious HPV in squamous epithelium of primary human keratinocytes. Genes Dev. 23:181–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pines J, Hunter T. 1989. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 58:833–846 [DOI] [PubMed] [Google Scholar]
- 59. Wilson VG, West M, Woytek K, Rangasamy D. 2002. Papillomavirus E1 proteins: form, function, and features. Virus Genes 24:275–290 [DOI] [PubMed] [Google Scholar]
- 60. Remm M, Remm A, Ustav M. 1999. Human papillomavirus type 18 E1 protein is translated from polycistronic mRNA by a discontinuous scanning mechanism. J. Virol. 73:3062–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273:5858–5868 [DOI] [PubMed] [Google Scholar]
- 62. Gillespie KA, Mehta KP, Laimins LA, Moody CA. 2012. Human papillomaviruses recruit cellular DNA repair and homologous recombination factors to viral replication centers. J. Virol. 86:9520–9526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ward IM, Chen J. 2001. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276:47759–47762 [DOI] [PubMed] [Google Scholar]
- 64. Cimprich KA, Cortez D. 2008. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9:616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. King LE, Fisk JC, Dornan ES, Donaldson MM, Melendy T, Morgan IM. 2010. Human papillomavirus E1 and E2 mediated DNA replication is not arrested by DNA damage signalling. Virology 406:95–102 [DOI] [PubMed] [Google Scholar]