Abstract
Systemic low-grade chronic inflammation has been intensively investigated in obese subjects. Recently, various immune cell types, such as macrophages, granulocytes, helper T cells, cytotoxic T cells, and B cells, have been implicated in the pathogenesis of adipose tissue inflammation. However, the roles of invariant natural killer T cells (iNKT cells) and the regulation of iNKT cell activity in adipose tissue are not thoroughly understood. Here, we demonstrated that iNKT cells were decreased in number in the adipose tissue of obese subjects. Interestingly, CD1d, a molecule involved in lipid antigen presentation to iNKT cells, was highly expressed in adipocytes, and CD1d-expressing adipocytes stimulated iNKT cell activity through physical interaction. iNKT cell population and CD1d expression were reduced in the adipose tissue of obese mice and humans compared to those of lean subjects. Moreover, iNKT cell-deficient Jα18 knockout mice became more obese and exhibited increased adipose tissue inflammation at the early stage of obesity. These data suggest that adipocytes regulate iNKT cell activity via CD1d and that the interaction between adipocytes and iNKT cells may modulate adipose tissue inflammation in obesity.
INTRODUCTION
Obesity is a key risk factor of metabolic syndromes, such as hypertension, hyperlipidemia, atherosclerosis, and type 2 diabetes. Given that the adipose tissue of obese animals exhibits low-grade chronic inflammation, which is closely associated with metabolic abnormalities (1–3), recent studies have focused on immune responses in adipose tissue. For instance, accumulating evidences indicate that in the adipose tissue of lean animals, anti-inflammatory immune cells such as M2-type macrophages and regulatory T cells play dominant roles in repressing inflammation and help to maintain insulin sensitivity by enhancing Th2-type cytokine (interleukin 4 [IL-4], IL-10, IL-13) secretion (4–7). On the other hand, the numbers of proinflammatory immune cells, such as M1-type macrophages, Th1 cells, and CD8 T cells, are increased in obese adipose tissue and accelerate adipose tissue inflammation. These proinflammatory immune cells aggravate insulin sensitivity through Th1-type cytokine secretion and other, yet unknown, activities (8–11). Even though various immune cells have been implicated in adipose tissue inflammation and metabolic diseases, the direct regulatory mechanism governing immune responses in adipose tissue has not been clearly elucidated yet.
Natural killer T (NKT) cells are well known as an immune cell population bridging innate and adaptive immune responses (12). There are 3 types of NKT cells, including invariant NKT (iNKT; type I), noninvariant NKT (type II), and NKT-like cells. Invariant NKT (type I) and noninvariant NKT (type II) cells are CD1d dependent, while NKT-like cells are CD1d independent (13). Invariant NKT (type I) cells have a semi-invariant T cell receptor α chain, Vα14Jα18 in mouse and Vα24Jα18 in human (14, 15). iNKT cells are capable of rapid response and secretion of various chemokines and cytokines, including Th1- and Th2-type cytokines (16). Particularly, iNKT cells specifically recognize a variety of lipid antigens loaded on CD1d molecules and do not recognize peptide antigens on major histocompatibility complex (MHC) molecules. For example, phosphatidylethanolamine, phosphatidylcholine, phosphatidylinositol, and isoglobotrihexosylceramide (iGb3) have previously been reported to be lipid antigens of CD1d (17, 18). In particular, α-galactosylceramide (α-GC) is the most potent CD1d-binding lipid antigen for iNKT cell activation (19). It is an MHC class I-like glycoprotein and has a lipid-binding hydrophobic groove (20). CD1d is expressed mainly on professional antigen-presenting cells (APCs), such as dendritic cells, macrophages, B cells, and hepatocytes (21).
Adipocyte constitutes one of the major cell types responsible for the regulation of dynamic lipid metabolisms in response to various energy states. Notably, their lipid metabolism and consequent lipid metabolites are significantly altered in obesity. There is compelling evidence to suggest that altered lipid metabolism and lipid metabolites play critical roles in the regulation of insulin sensitivity in obese and diabetic animals (22–29). These recent findings led us to hypothesize that lipid metabolites produced by adipocytes might be presented by CD1d molecules on the plasma membrane of adipocytes; the recognition of lipid-CD1d complexes in adipose tissue would subsequently modulate iNKT cell activity. Therefore, we investigated whether adipocytes bearing CD1d molecules act as antigen-presenting cells to regulate iNKT cell activities in adipose tissue. In this study, we have revealed the dynamics of the iNKT cell population in the adipose tissue of obese subjects and the role of adipocyte CD1d molecules in iNKT cell activation as well as in adipose tissue inflammation.
MATERIALS AND METHODS
Animals and treatment.
C57BL6/J mice were obtained from Central Lab Animal Inc. (Seoul, South Korea) and were housed in colony cages in 12-h light/12-h dark cycles. After a minimum of 1 week for stabilization, 8-week-old mice were fed a normal chow diet (NCD) and then were administered a 60% high-fat diet (HFD) for the indicated time periods (Research Diets Inc., New Brunswick, NJ). Then, on the day of sacrifice, all of the HFD-fed mice were compared to age-matched NCD-fed mice. db/db mice and ob/ob mice were purchased from Central Lab Animal Inc. and sacrificed at 12 weeks of age. CD1d knockout (KO) mice were generously provided by S. H. Park and Jα18 KO mice by D. S. Lee. Heterozygous mice were bred to generate KO mice and wild-type (WT) littermates. WT and KO Jα18 mice were maintained on NCD until 8 weeks of age before changing to a 60% HFD for 4 weeks. For the oral glucose tolerance test, mice were fasted for 6 h and basal blood samples were taken, followed by oral glucose administration (3 g/kg of body weight). Blood samples were drawn at 15, 30, 45, 60, 90, and 120 min after administration. All experiments with mice were approved by the Institute of Laboratory Animal Resources in Seoul National University.
3T3-L1 adipocyte differentiation and drug treatment.
Preadipocytes were grown to confluence in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% bovine calf serum. Two days postconfluence (day 0), the 3T3-L1 cells were stimulated with DMEM containing 10% fetal bovine serum, dexamethasone (1 μM), methylisobutylxanthine (520 μM), and insulin (167 nM) for 48 h. The culture medium was changed with DMEM containing 10% fetal bovine serum and insulin (167 nM) for 48 h (day 2) and then maintained with DMEM containing 10% fetal bovine serum. Rosiglitazone or all-trans retinoic acid were treated at differentiation day 7.
Quantitative reverse transcription-PCR (qRT-PCR).
cDNA was synthesized using reverse transcriptase with deoxynucleoside triphosphate (dNTP) and random hexamer (Fermentas). These cDNAs were used as templates with specific primers in the presence of dNTPs and TaqDNA polymerase. Real-time PCR amplification reactions were brought to a final volume of 20 μl and contained 20 ng of cDNA, 0.25 μM primers, and SYBR green (Bio-Rad). The MyiQ real-time PCR detection system (Bio-Rad) was used for PCR amplification in 96-well plates. The relative amounts of each mRNA were calculated by using the comparative threshold cycle (CT) method. Cyclophilin mRNA was used as the invariant control. The primer sequence information was added to Table S1 in the supplemental material.
Adipose tissue fractionation.
Fractionation of adipose tissue was performed as previously described (30, 31). Epididymal adipose tissues were weighed and then chopped and incubated in collagenase buffer for 30 min at 37°C with shaking. After centrifugation, adipocytes in the supernatant were collected for RNA extraction or primary cell culture. The pelleted stromal vascular cell (SVC) fraction was used for flow cytometry or RNA extraction.
Flow cytometry analysis.
Splenocytes were prepared by mincing spleen tissues and then lysing red blood cells (RBCs). Adipose tissue SVC pellets were incubated with RBC lysis buffer prior to centrifuging (2,000 rpm, 5 min) and resuspended in phosphate-buffered saline (PBS). SVCs were incubated with CD16/32 (eBioscience, San Diego, CA), as blocking antibody, for 10 min at 4°C prior to staining with fluorescence-labeled primary antibodies for 30 min at 4°C. Phycoerythrin (PE)-conjugated α-GC-loaded CD1d dimer (BD Bioscience, Franklin Lakes, NJ) was prepared as previously described (32). The PE-conjugated immunoglobulin G (IgG) monoclonal antibody (MAb), CD1d dimer, CD3ε MAb, T cell receptor beta (TCR-β) MAb, and CD4 MAb were purchased from BD Biosciences and CD8 MAb from eBioscience. The activation markers (CD25 and CD44 MAbs) were purchased from eBioscience. For macrophage analysis, we stained SVCs with CD11b (BD Bioscience), F4/80 (eBioscience), and CD11c (eBioscience) MAbs for 20 min at 4°C. Cells were gently washed with and resuspended in PBS. SVCs were analyzed using the fluorescence-activated cell sorting (FACS) CantoII instrument (BD Bioscience). For the detection of iNKT cell apoptosis, we used the annexin V staining kit purchased from BD Bioscience.
Whole-mount immunohistochemistry.
Whole-mount immunohistochemistry was performed as previously described (33). Epididymal adipose tissues were removed and fixed with 1% paraformaldehyde for 1 h and washed. The samples were then blocked with 1% bovine serum albumin (BSA), incubated for 1 h, and then stained with primary antibody (perilipin, 1:1,000; CD1d, 1:500) overnight at 4°C. After being washed for 1 h, the samples were incubated with fluorescence-labeled secondary antibody for 4 h at room temperature and washed. Following staining with 4′,6-diamidino-2-phenylindole (DAPI) containing Vectashield solution (Vector Lab Inc.) samples were observed using a Zeiss LSM510NLO confocal microscope.
iNKT cell isolation from adipose tissue.
To isolate iNKT cells from adipose tissue, we pooled epididymal adipose tissues from NCD-fed mice (n = 25) and mice fed an HFD for 2 weeks (n = 15). SVCs were stained by anti-CD16/32 antibody (eBioscience) followed by APC-conjugated TCR-β and PE-conjugated α-GC-loaded CD1d dimer staining for 30 min at 4°C. After PBS washing, we sorted iNKT cells (TCR-β and α-GC-loaded CD1d dimer-double-positive cells) and non-iNKT T cells (TCR-β-positive α-GC-loaded CD1d dimer-negative cells) by FACSAria.
Coculture experiment.
Primary adipocytes were prepared by the fractionation of mouse epididymal adipose tissue or by 3T3-L1 cell line differentiation. DN32.D3 hybridoma cells (provided by S. H. Park) were used as CD1d-restricted NKT cells. In case of α-GC treatment experiment, we pretreated α-GC (100 ng/ml) to adipocytes and washed with PBS and then cocultured with DN32.D3 cells. Adipocytes and DN32.D3 cells were cocultured with or without a Transwell membrane (0.4-μm pore size). The IL-2 cytokine concentration was measured by an IL-2 enzyme-linked immunosorbent assay (ELISA) kit (eBioscience). We transfected negative-control small interfering RNA (siRNA) and CD1d siRNA into differentiated 3T3-L1 adipocytes by electroporation. To assess the attachment of 3T3-L1 and DN32.D3 cells, we prestained DN32.D3 with CellTracker Red CMPTX (Invitrogen, Carlsbad, CA) and then spread it onto the adipocytes. After 24 h of incubation, unattached cells were washed out by vigorous PBS washing and mounted with DAPI containing Vectashield solution (Vector Lab Inc.).
Human samples.
Human adipose tissue samples from 24 obese and overweight nondiabetic patients were provided by the Obesity Research Center at King Saud University and were analyzed for the expression of CD1d and Vα24 mRNA by qRT-PCR. This work is under a program to study adipocyte biology and adipokines. The whole project was approved by the College of Medicine Ethics Committee, King Saud University.
Statistics.
The results are shown as means ± standard errors of the means (SEM). All statistical analysis was performed by the Student t test or analysis of variance (ANOVA) in Excel (Microsoft); P values of <0.05 were considered significant.
RESULTS
iNKT cells are abundant in adipose tissue.
Previously, the NKT cell population in adipose tissue has been characterized either by the presence of NK1.1+ CD3+ cells or by Vα14Jα18 mRNA expression in adipose tissue (34, 35). However, the abundance of the iNKT cell population, especially that of type I NKT cells expressing Vα14Jα18 TCR chains, has not been thoroughly investigated in the adipose tissue of lean or obese mice. To examine the iNKT cell population in adipose tissue, we quantitatively analyzed the proportion of anti-TCR-β- and α-GC-loaded CD1d dimer-double-positive cells by flow cytometry (Fig. 1A). In epididymal adipose tissue, iNKT cells occupied about 3 to 4% of the total lymphocyte population, which was relatively higher than the ratio of iNKT cells in other immune organs, such as the spleen and thymus. When the percentage of iNKT cells was normalized with either total lymphocytes or total T cells in adipose tissue, spleen, and thymus, we noted that the relative percentage of iNKT cells in adipose tissue was significantly higher than the one in the spleen and thymus (Fig. 1B and C). Additionally, when we analyzed the ratio of iNKT cells in several fat depots, including inguinal, epididymal, and mesenteric adipose tissue, epididymal fat tissue exhibited the highest frequency of iNKT cells (Fig. 1D), implying that adipose tissue is relatively enriched with iNKT cells.
Fig 1.
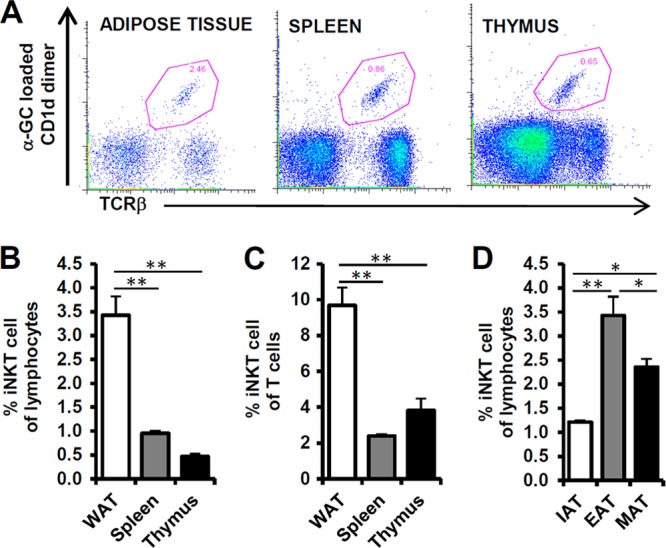
iNKT cells are present in adipose tissue. Eight-week-old C56BL6/J male mice were used for preparation of adipose tissue, spleen, and thymus; n = 5. (A) iNKT cells were detected by staining of α-GC-loaded CD1d dimer and TCR-β. The iNKT cell population is represented as a dot blot graph. (B, C) Percentages of iNKT cells among lymphocytes and T cells in epididymal adipose tissue (EAT), spleen, and thymus. The lymphocyte population was gated by forward scatter (FSC) and side scatter (SSC). (D) Percentages of iNKT cells among lymphocytes in fat depots (inguinal [IAT], epididymal [EAT], and mesenteric [MAT] adipose tissue). *, P < 0.05; **, P < 0.01.
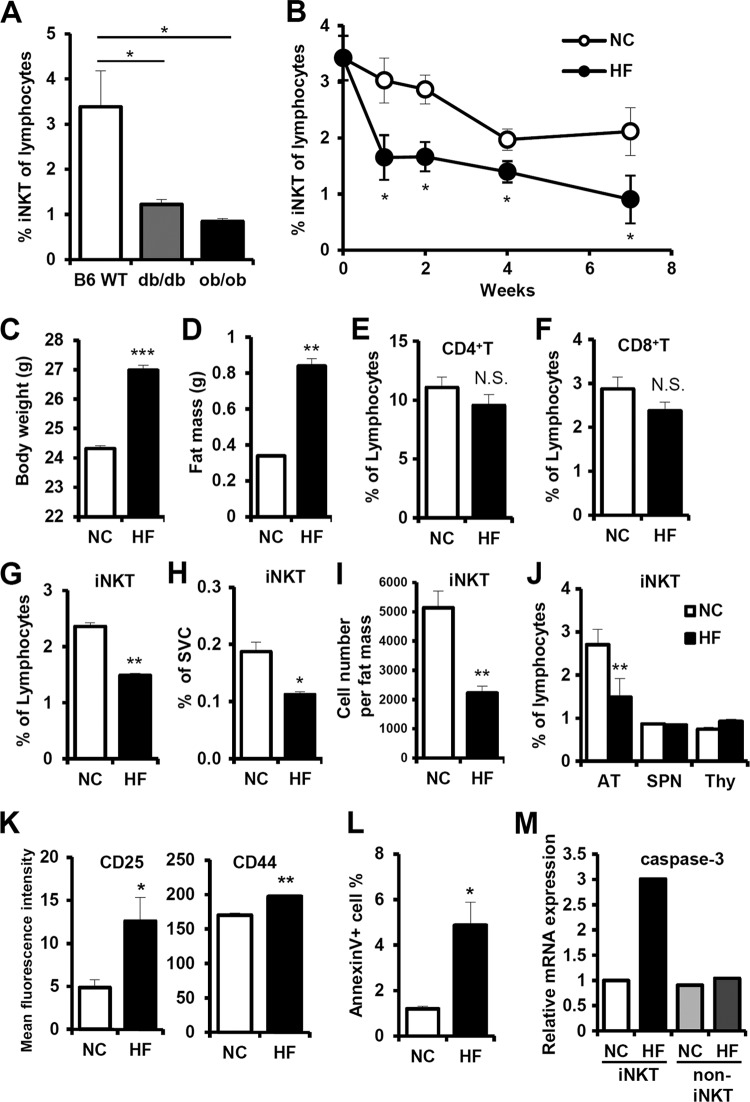
iNKT cell numbers are decreased in the adipose tissue of obese mice.
To investigate the correlation between iNKT cells in adipose tissue and obesity, we examined the populations of iNKT cells in the adipose tissue of lean and obese db/db or ob/ob mice. The percentage of iNKT cells among lymphocytes in adipose tissue was significantly lower in obese animal models than in lean mice (Fig. 2A). In addition, we examined the dynamics of the iNKT cell population in fat tissues during HFD consumption. There was an HFD-dependent decrease in iNKT cells in adipose tissue (Fig. 2B). These results are consistent with previously reported data (36). Interestingly, during short-term HFD feeding (∼1 to 2 weeks), the percentage of iNKT cells in fat tissue significantly decreased. Our previous study demonstrated that adipose tissue inflammation and macrophage infiltration were significantly increased by short-term HFD, whereas these phenomena were not observed in other metabolic tissues, such as liver and muscle (33, 37, 38). Therefore, we decided to analyze in detail the population of iNKT cells in short-term (1 week) HFD-fed mice, to unveil changes in adipose tissue in the early stages of obesity. Consistent with previous reports (38), body weight and fat mass increased upon short-term HFD in adult mice (Fig. 2C and D). On the other hand, the percentages of other T cell populations, such as CD4+ T cells and CD8+ T cells, in fat tissue did not change upon short-term HFD (Fig. 2E, F). In adipose tissue, the percentage of iNKT cells calculated based on the total number of lymphocytes or SVCs was significantly diminished. Furthermore, the absolute number of iNKT cells per gram of fat mass was significantly lower in fat tissue (Fig. 2G to I). Surprisingly, the reduction of iNKT cells upon short-term HFD was specific to adipose tissue but not observed in the spleen and thymus, indicating that the decrease in iNKT cells in adipose tissue appears to be independent of any defects in iNKT cell development (Fig. 2J). Furthermore, in HFD-fed mice, the percentage of iNKT cells in circulating lymphocytes in blood was not significantly altered (see Fig. S1 in the supplemental material). We also examined the expression levels of T cell activation/memory markers such as CD25 and CD44 on iNKT cells and observed that these were upregulated in the adipose tissue of short-term HFD-fed mice (Fig. 2K). For further characterization of the decrease in adipose tissue iNKT cell population upon short-term HFD, we carefully examined the degree of apoptosis in adipose tissue iNKT cells. As shown in Fig. 2L, annexin V-positive iNKT cell apoptosis was greatly increased up to about 4-fold following 1 week of HFD. To investigate in more detail about apoptosis of iNKT cells, we analyzed apoptosis-related mRNA and protein expression in iNKT cells in adipose tissue. The levels of caspase 3 mRNA and Fas protein expression on the surface of adipose tissue iNKT cells were elevated in HFD-fed (1 week) mice (Fig. 2M; see also Fig. S2 in the supplemental material), implying that short-term HFD-induced iNKT cell apoptosis is associated with iNKT cell activation during changes in fat tissue in early obesity.
Fig 2.
iNKT cell numbers are lower in adipose tissue of obese mice. (A) Percentage of iNKT cells among lymphocytes in the adipose tissue of C57BL6/J lean mice, db/db mice, and ob/ob mice; n = 5. (B) Eight-week-old C57BL6/J male mice were fed normal chow diet (NCD) or a 60% high-fat diet (HFD) for 1, 2, 4, and 7 weeks. The iNKT cell population was measured at each time point. (C to L) C57BL6/J mice were fed NCD and HFD for 1 week. (C) Body weight (grams); (D) fat mass (grams); (E) percentage of CD4+ T cells among adipose tissue lymphocytes; (F) percentage of CD8+ T cell among adipose tissue lymphocytes; (G) percentage of iNKT cells among adipose tissue lymphocytes; (H) percentage of iNKT cells among adipose tissue SVCs; (I) absolute iNKT cell number per gram of fat mass; (J) percentage of iNKT cells among lymphocytes in adipose tissue, spleen, and thymus; n = 5; (K) expression levels of activation/memory markers (CD25 and CD44) in adipose tissue iNKT cells; (L) percentage of annexin V-positive cells among adipose tissue iNKT cells; (M) the level of caspase-3 mRNA of iNKT cells in adipose tissue. *, P < 0.05; **, P < 0.01.
Adipocytes express high levels of CD1d.
While most T cells recognize peptides loaded on MHC molecules, iNKT cells recognize lipids or glycolipids bound to the MHC-like glycoprotein CD1d. Since iNKT cells are abundant and activated in adipose tissue, we decided to test the expression pattern of CD1d in adipose tissue. Northern blotting and qRT-PCR analyses clearly indicated that CD1d mRNA was highly expressed in adipose tissue (Fig. 3A and B). Among several fat depots, the level of CD1d mRNA was higher in epididymal and mesenteric adipose tissue than that of inguinal adipose tissue (Fig. 3C). Next, we fractionated adipose tissue into adipocytes and SVCs and then compared the levels of CD1d mRNA in these fractions. The expression level of CD1d mRNA was more than 10-fold higher in the adipocyte fraction than in the SVC fraction or in macrophages (Fig. 3D). In addition, the increased level of CD1d mRNA was observed in differentiated 3T3-L1 adipocytes (Fig. 3E). However, neither CD1d knockdown nor its overexpression altered adipogenesis (Fig. 3G to I). In order to determine the subcellular localization of CD1d in adipocytes, we carried out whole-mount immunohistochemistry. As shown in Fig. 3F and in Fig. S3 in the supplemental material, CD1d was abundantly expressed on the plasma membranes of adipocytes. Taken together, these data suggest that mature adipocytes express high levels of CD1d on their cell surfaces, probably to facilitate interaction with iNKT cells and thereby modulate immune responses in adipose tissue.
Fig 3.
CD1d is highly expressed in adipocytes. Tissues were obtained from 8-week-old C57BL6/J male mice. (A) CD1d mRNA expression levels in thymus (THY), spleen (SPN), liver (LIV), epididymal adipose tissue (WAT), kidney (KID), lung (LNG), intestine (INT), and heart (HRT) were detected by Northern blotting. (B) qRT-PCR data of CD1d mRNA levels in each tissue; (C) CD1d mRNA expression level in fat depots; (D) CD1d mRNA expression levels in intraperitoneal macrophages (ip-MACs), SVCs, and adipocytes (AD); (E) mRNA levels of CD1d and PPARγ during 3T3-L1 adipocyte differentiation. (A to E) CT values of CD1d mRNA were around 23, 23, and 26 in adipose tissues, primary adipocytes, and 3T3-L1 adipocytes, respectively. (F) Localization of CD1d in adipocytes, assessed by histological analysis of epididymal adipose tissue. CD1d (red), perilipin (green), and DAPI (blue) are seen. (G) mRNA levels of CD1d, PPARγ, and aP2 in CD1d overexpressing stable cells; (H) mRNA levels of CD1d, PPARγ, and aP2 in CD1d short hairpin RNA (shRNA)-overexpressing stable cells; (I) oil red O staining at day 8 of adipogenesis. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Adipocytes activate iNKT cells through CD1d.
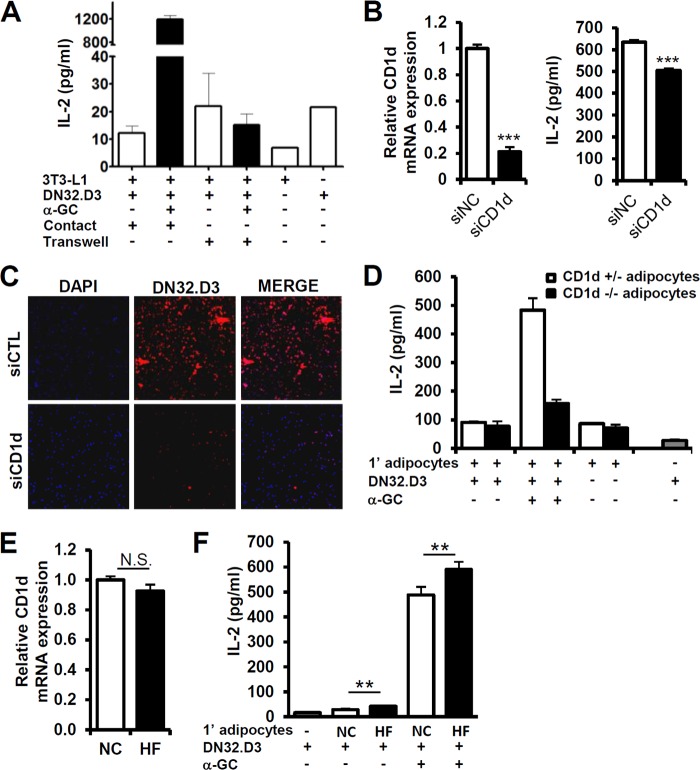
To test whether adipocyte CD1d is able to stimulate iNKT cells in adipose tissue, we carried out coculture experiments with differentiated 3T3-L1 adipocytes and the iNKT cell hybridoma cell line DN32.D3 under various experimental conditions. We designed two different methods of coculture, including the direct physical contact system and the cell-impermeable Transwell system that blocks physical interaction. To monitor iNKT cell activation, α-GC, a potent iNKT cell-activating lipid antigen, was treated to load onto CD1d in adipocytes. Since IL-2 is secreted by activated T cells, we determined the concentration of secreted IL-2 in the culture media of either the contact or Transwell systems. The IL-2 secretion was higher in iNKT cells in the contact system than in iNKT cells in the Transwell system, implying that adipocytes are able to stimulate iNKT cell activation via direct cell-cell contact rather than via indirect cytokine secretion (Fig. 4A). When we examined the ability of dendritic cells and 3T3-L1 adipocytes to activate iNKT cells, both cell types activated iNKT cells upon α-GC pretreatment (see Fig. S4 in the supplemental material).
Fig 4.
Adipocyte CD1d regulates iNKT cell activity. (A) DN32.D3 hybridoma cells were used as iNKT cells. Adipocytes and DN32.D3 cells were directly mixed and cocultured in contact or cultured separated by a Transwell membrane, which is cell impermeable with a 0.4-μm pore size. IL-2 secretion by DN32.D3 cells was induced by coculturing with differentiated 3T3-L1 adipocytes which α-GC (100 ng/ml) pretreated for 4 h. (B, left) CD1d expression in negative control (NC) and CD1d siRNA-transfected adipocytes. (Right) IL-2 secretion by DN32.D3 cells upon coculturing with siRNA-transfected 3T3-L1 adipocytes. Adipocytes were pretreated with α-GC (100 ng/ml) for 4 h and, after PBS washing, cocultured with DN32.D3 cells for 6 h. (C) Immunocytochemical analysis of DN32.D3 cells attached to 3T3-L1 adipocytes. Adipocytes were transfected with control siRNA or CD1d siRNA and cocultured with DN32.D3 cells for 24 h. After being washed with PBS, attached red fluorescence-labeled DN32.D3 cells were monitored. (D) The amount of secreted IL-2 was determined by ELISA analysis. Primary adipocytes were isolated from the adipose tissues of CD1d+/− or CD1d−/− mice and cocultured with DN32.D3 cells for 24 h with or without α-GC (100 ng/ml). (E and F) Primary adipocytes were isolated from mice given NCD or HFD for 1 week. (E) Adipocytes derived from epididymal adipose tissues were used. CD1d mRNA level of adipocytes from NCD-fed mice (NC) or from HFD-fed mice (HF). (F) iNKT cells were activated by treatment with α-GC (100 ng/ml)-pretreated adipocytes. Adipocytes and DN32.D3 cells were cocultured for 36 h. n = 5; *, P < 0.05; **, P < 0.01.
To clarify the role of adipocyte CD1d in iNKT cell activation, we suppressed adipocyte CD1d by using siRNA, followed by direct coculture with iNKT cells. IL-2 secretion was significantly decreased in CD1d-suppressed adipocytes (Fig. 4B). Next, we explored the role of adipocyte CD1d in cell-cell contact with iNKT cells. As shown in Fig. 4C, iNKT cells physically interacted with CD1d-expressing adipocytes, whereas iNKT cell attachment was notably diminished in CD1d-suppressed adipocytes. In addition, the IL-2-producing activity of primary adipocytes isolated from the adipose tissues of CD1d+/− and CD1d−/− mice was determined by coculture with iNKT cells. Primary adipocytes from CD1d+/− mice potentiated IL-2 secretion, whereas CD1d-deficient adipocytes from CD1d−/− mice failed to induce IL-2 secretion in iNKT cells (Fig. 4D). Since we observed that CD25 and CD44 expression on iNKT cells were augmented in adipose tissue after short-term HFD consumption (Fig. 2K), we studied the involvement of adipocytes in iNKT cell activation upon short-term HFD. We isolated primary adipocytes from mice that were given a normal chow diet (NCD) or HFD for 1 week and cocultured them with DN32.D3 cells with or without α-GC pretreatment. Although the levels of adipocyte CD1d mRNA were not significantly different between NCD and HFD groups, primary adipocytes isolated from HFD-fed mice led to a higher degree of iNKT cell activation than those from NCD-fed mice (Fig. 4E and F). These data imply that primary adipocytes from short-term HFD-fed mice appear to be more potent than those from NCD-fed mice in stimulating iNKT cells in adipose tissue. Together, these results propose that adipocytes indeed have the ability to activate iNKT cells via physical interaction with adipocyte CD1d, especially upon short-term HFD.
Adipocyte CD1d expression is regulated by peroxisome proliferator-activated receptor γ.
We next investigated the regulatory mechanism of CD1d expression in adipocytes. Several transcription factors, such as peroxisome proliferator-activated receptor gamma (PPARγ), CCAAT/enhancer-binding protein β (CEBP-β), retinoic acid receptor (RAR), and the Ets family, have been reported to be involved in the regulation of CD1d expression (39–41). PPARγ, the master regulator of adipogenesis, is abundantly expressed in adipocytes. To examine the role of PPARγ in CD1d expression in adipocytes, we determined the expression level of adipocyte CD1d mRNA upon rosiglitazone, a synthetic agonist of PPARγ, and tumor necrosis factor alpha (TNF-α), a functional inhibitor of PPARγ. In adipocytes, CD1d mRNA was increased by rosiglitazone treatment, but TNF-α treatment inhibited CD1d expression (Fig. 5A and B). To confirm whether PPARγ is indeed involved in adipocyte CD1d expression, we investigated the effect of PPARγ knockdown on the expression of CD1d mRNA in adipocytes. Unlike the cells treated with control siRNA, CD1d mRNA expression was not induced by rosiglitazone in cells with PPARγ knockdown (Fig. 5C). Moreover, when we performed the luciferase reporter assay with the mouse CD1d promoter, the CD1d promoter was transactivated by PPARγ (see Fig. S5 in the supplemental material). We then tested whether increased CD1d expression in adipocytes by activated PPARγ affected iNKT cell activity. When iNKT cells were cocultured with adipocytes in the presence of rosiglitazone, IL-2 secretion was slightly but substantially increased (Fig. 5D). Together, these data imply that PPARγ would regulate iNKT cell activity in adipose tissue.
Fig 5.
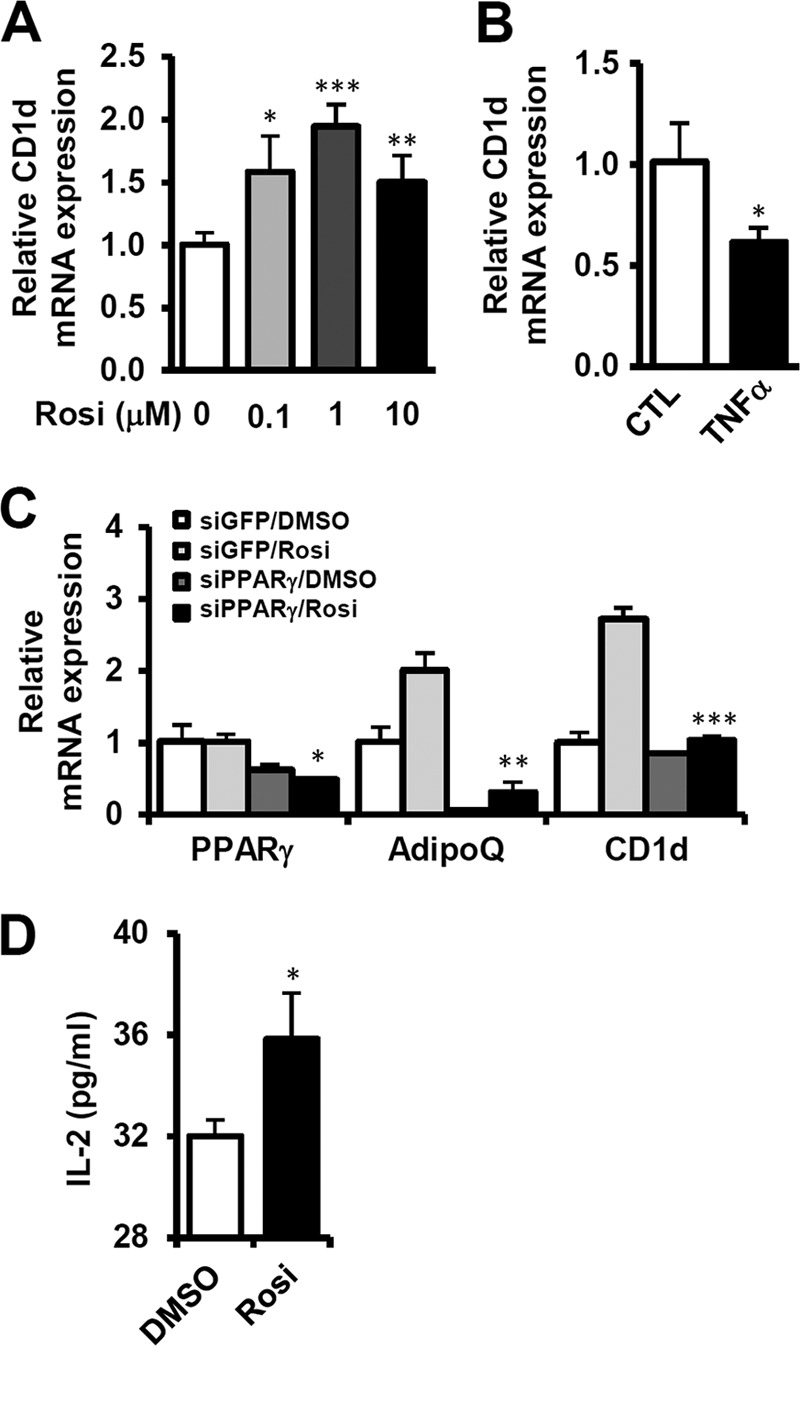
Expression of adipocyte CD1d is stimulated by PPARγ activation. Differentiated 3T3-L1 adipocytes were used as the adipocyte source. (A and B) Level of CD1d mRNA in adipocytes treated with rosiglitazone (0.1, 1, or 10 μM) or TNF-α (10 ng/ml) for 24 h. (C) Effect of PPARγ knockdown on CD1d expression. Differentiated 3T3-L1 adipocytes were transfected with siRNAs (negative control or PPARγ) by electroporation. After 12 h, the cells were treated with rosiglitazone (10 μM). mRNA levels of PPARγ, adiponectin, and CD1d were determined by qRT-PCR. (D) IL-2 secretion by DN32.D3 cells stimulated by adipocytes treated with rosiglitazone (10 μM). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Adipocyte CD1d and PPARγ expression levels are decreased in adipose tissue of obese subjects.
The finding that the iNKT cell population was decreased in the adipose tissue of obese mice prompted us to examine the level of CD1d mRNA in several obese animal models. In concordance with the decrease in iNKT cell population in obesity, CD1d expression in the adipocyte fraction was also significantly reduced in the adipose tissue of db/db, ob/ob, and long-term (8 weeks) HFD-fed obese (diet-induced obesity [DIO]) mice (Fig. 6A). Given that the PPARγ mRNA was reduced in the adipocyte fraction of obese mice, it appears that CD1d expression in adipocytes might be positively correlated with PPARγ (Fig. 6B). Consistent with the mouse models, the mRNA expression levels of Vα24 and CD1d in human visceral adipose tissue were negatively correlated with the body mass index (BMI) (Fig. 7A and B). These results support the idea that the decrease in the iNKT cell population in adipose tissue might be accompanied by reduced CD1d expression in adipocytes in severe obesity.
Fig 6.
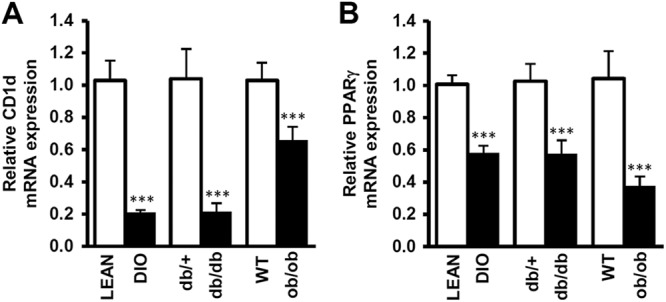
Expression levels of adipocyte CD1d and PPARγ are decreased in adipose tissue in obesity. Primary adipocytes were isolated from the adipose tissue of obese mice. Relative levels of CD1d mRNA (A) and relative levels of PPARγ mRNA (B) in adipocytes between db/+ and db/db mice, WT and ob/ob mice, and lean and DIO mice.
Fig 7.
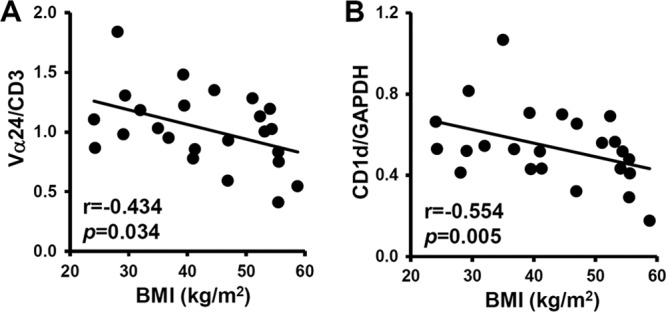
The expression levels of Vα24 and CD1d are reduced in the adipose tissue of obese humans. Vα24 mRNA expression normalized by CD3ε mRNA (A) and CD1d mRNA expression normalized by GAPDH (B), depending on BMI.
Jα18 KO mice are susceptible to DIO.
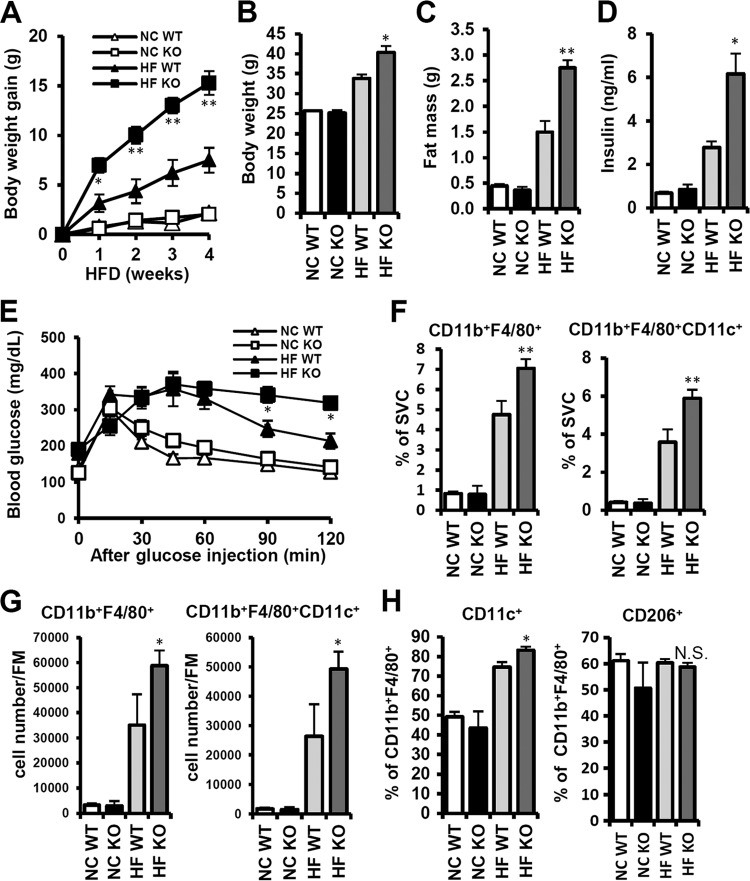
Although the association of iNKT cells with autoimmune diseases and cancers has been reported (42–44), the nature of their pathophysiological roles in various diseases is still controversial. To get insight into the role of iNKT cells in adipose tissue inflammation in obesity, we have compared obesity-associated phenotypes in WT mice with Jα18 KO mice lacking iNKT cells. There were no significant changes in body weight gain between NCD-fed WT and KO mice. In contrast, upon HFD consumption, even for a short duration, the body weight of Jα18 KO mice was predisposed to obesity (Fig. 8A and B). The mass of the epididymal adipose tissue in HFD-fed Jα18 KO mice was significantly higher than in WT mice (Fig. 8C). Since it is well established that obesity is a major risk factor for insulin resistance, we assessed glucose tolerance. As shown in Fig. 8E, Jα18 KO mice revealed a greater degree of glucose intolerance than WT mice upon HFD feeding. Moreover, the plasma insulin level was higher in Jα18 KO mice than in WT mice upon HFD consumption (Fig. 8D). These data indicate that mice deficient in iNKT cells might be susceptible to obesity and glucose intolerance upon HFD.
Fig 8.
Jα18 KO mice are more susceptible to body weight gain and adipose tissue inflammation upon HFD. Eight-week-old WT and Jα18 KO mice were fed NCD or HFD for 4 weeks. (A) Body weight gain was measured every week. (B) Absolute body weight after 4 weeks of HFD; (C) fat mass (grams); (D) plasma insulin levels (ng/ml). (E) Mice were given HFD for 4 weeks and then subjected to the oral glucose tolerance test; n = 4 mice at each time point. *, P < 0.05, HFD WT versus HFD KO. (F) Percentage of CD11b+ F4/80+ and CD11b+ F4/80+CD11c+ cells among adipose tissue SVCs. **, P < 0.01, HFD WT versus HFD KO. (G) Numbers of CD11b+ F4/80+ and CD11b+ F4/80+CD11c+ cells per gram of fat mass. *, P < 0.05, HFD WT versus HFD KO. (H) Percentages of CD11c+ and CD206+ cells among CD11b+ F4/80+ cells in adipose tissue. *, P < 0.05, HFD WT versus HFD KO.
To determine the effect of iNKT cell deficiency on adipose tissue inflammation, we examined macrophage infiltration and inflammatory gene expression in adipose tissue. Upon HFD, the percentages of CD11b+ F4/80+ cells and CD11b+ F4/80+CD11c+ cells among SVCs were significantly elevated in WT mice, and macrophage accumulation in adipose tissue was further pronounced in Jα18 KO mice (Fig. 8F). The number of adipose tissue macrophages per gram of fat mass was also significantly increased in Jα18 KO mice (Fig. 8G). To gain insight into the effect of iNKT cell deficiency on macrophage polarization in adipose tissue, we analyzed the proportion of CD11c+ cells (M1-type macrophages) or CD206+ cells (M2-type macrophages) among CD11b+ F4/80+ cells (total macrophages). The percentage of CD11c+ cells was higher in Jα18 KO mice than in WT mice upon HFD. However, there was no significant change in the proportion of M2-type CD206+ cells (Fig. 8H).
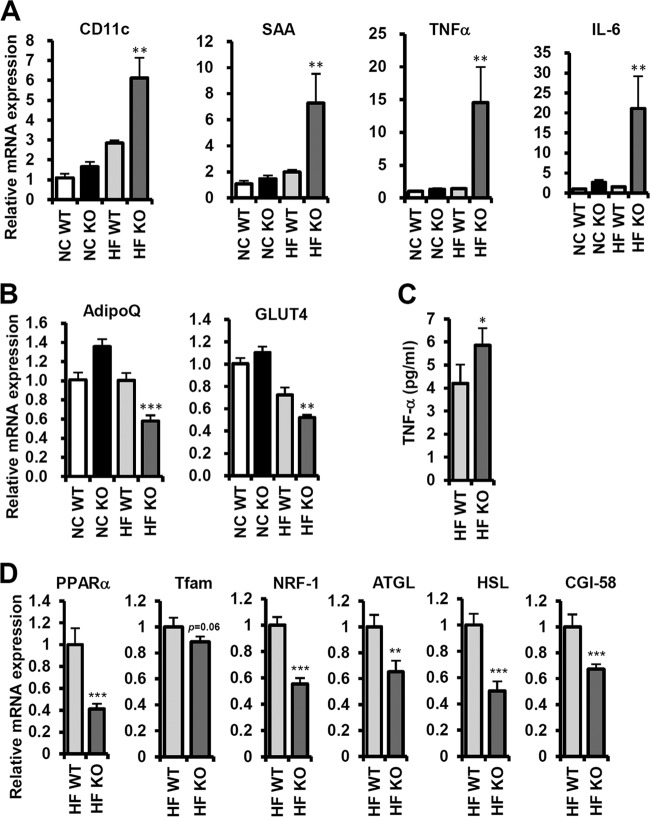
We further examined the expression of genes involved in adipose tissue inflammation and energy metabolism. Consistent with the data from FACS analysis, proinflammatory genes, such as those encoding CD11c, serum amyloid A (SAA), TNF-α, and IL-6, were upregulated in Jα18 KO mice upon HFD (Fig. 9A). On the contrary, adiponectin and GLUT4, whose levels are negatively correlated with insulin resistance in adipocytes, were decreased in Jα18 KO mice upon HFD (Fig. 9B). Furthermore, the serum TNF-α level was elevated in Jα18 KO mice compared to that in WT mice in the HFD-fed group (Fig. 9C). In order to understand the molecular mechanisms by which Jα18 KO mice were prone to obesity upon HFD, we examined gene expression patterns in white adipose tissue (WAT). Compared with WT mice, Jα18 KO mice decreased the level of PPARα, Tfam, NRF-1, ATGL, HSL, and CGI-58 mRNA upon HFD, implying that Jα18 KO mice might be less active for excess fat dissipation than WT mice (Fig . 9D).
Fig 9.
Inflammatory responses and energy metabolism were changed in Jα18 KO mice. (A) Relative levels of CD11c, SAA, TNF-α, and IL-6 transcripts in epididymal adipose tissue by qRT-PCR; (B) relative levels of adiponectin (AdipoQ) and GLUT4 mRNA in epididymal adipose tissue by qRT-PCR; (C) serum TNF-α concentration; (D) mRNA levels of PPARα, Tfam, NRF-1, ATGL, HSL, CGI-58 in epididymal adipose tissue of HFD-fed (4 weeks) WT and Jα18 KO mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001, HFD WT versus HFD KO.
DISCUSSION
In obesity, adipose tissue is inflamed with increased macrophage infiltration and exhibits insulin resistance with dysregulation of glucose and lipid metabolism (9). Recently, we and others have reported that the HFD induces inflammation rapidly and selectively in adipose tissue (33, 37, 38). Notably, on HFD, adipose tissue inflammation initiates within a week, concurrently with insulin resistance (38). These results suggest that, in adipose tissue, there would be a rapid and sensitive process to recognize the change of energy state and induce inflammation. Although various immune cells have been implicated in adipose tissue inflammation (4–11, 45), it has not been completely understood the roles and activation mechanism of acquired immune cells for adipose tissue inflammation in the early stage of obesity. In our previous work, we have reported that the deficiency of B and T lymphocytes in Rag1 KO mice augments adipose tissue inflammation (38). However, the function and regulation of T cells, especially iNKT cells, in adipose tissue inflammation in obesity have remained elusive. As iNKT cells respond to various lipid antigens presented on CD1d molecules in antigen-presenting cells, we hypothesized that adipose tissue iNKT cells might dynamically respond to changes in the lipid metabolism of adipocytes to coordinate whole-body energy homeostasis.
Here, we report for the first time the novel role of adipocytes as antigen-presenting cells to stimulate iNKT cells. Interestingly, we found that, in adipose tissue, the ratio of iNKT cells among total lymphocytes was higher than that in the spleen and thymus. Moreover, adipose tissue iNKT cells were selectively and sensitively decreased in obese animals, such as db/db, ob/ob, and DIO mice. The decrease in iNKT cells in adipose tissue became apparent after 1 week of HFD without change in spleen and thymus. To investigate the mechanism by which iNKT cells are modulated in adipose tissue in obese animals or individuals, we examined the expression of CD1d in adipose tissue. Surprisingly, CD1d was highly expressed in differentiated adipocytes, and primary adipocytes from short-term HFD-fed mice potently stimulated iNKT cell activation. Interestingly, however, the mRNA expression levels of CD1d and Vα24 was decreased in obese mouse and human subjects, suggesting that decreased CD1d and Vα24 levels are associated with reduced iNKT cells in adipose tissue and thus increased adipose tissue inflammation. In order to understand the roles of iNKT cells in adipose tissue inflammation, we have carefully investigated the metabolic and inflammatory phenotypes of iNKT cell-deficient Jα18 KO mice. Also, iNKT cell deficiency exacerbated glucose intolerance and adipose tissue inflammation by HFD. These results suggest that iNKT cells can suppress adipose tissue inflammation and insulin resistance in obesity.
CD1d is expressed on the surface of dendritic cells, macrophages, endothelial cells, and hepatocytes (46). It has been reported that the liver is the organ showing the highest CD1d expression (47). However, the expression of CD1d in adipose tissue, especially in adipocytes, has not been studied. Here, we discovered, unexpectedly, that CD1d expression in adipose tissue was as high as in the liver. Furthermore, the major source of CD1d in adipose tissue was the adipocyte fraction, not the SVC fraction. In addition, activation of PPARγ increased CD1d expression in 3T3-L1 adipocytes, while TNF-α decreased CD1d mRNA. Along the same line, it has been reported that CD1d is one of the PPARγ target genes, assessed by chromatin immunoprecipitation with microarray technology (ChIP-chip) analysis (48). Further, it has been shown that RAR regulates CD1d expression in dendritic cells (41). When we tested this in adipocytes, treatment of all-trans retinoic acid, a ligand of RAR, elevated CD1d mRNA in 3T3-L1 adipocytes (see Fig. S6 in the supplemental material).
To date, the underlying regulatory mechanisms directing immune cell activity in adipose tissue inflammation have not been thoroughly understood. In ex vivo coculture studies, we observed that adipocytes are able to directly regulate iNKT cell activity through cell-cell contact, mediated by the CD1d molecule. Moreover, the activation of iNKT cells by adipocytes from mice that were given HFD for 1 week implies that there might be unrevealed dynamic interactions between adipocytes and iNKT cells in adipose tissue to respond to changed nutritional environments.
Adipocytes are active in lipid metabolisms. In this respect, it appears that the species of lipids presented on adipocyte CD1d molecules may determine iNKT cell activity in adipose tissue. Several lipids and lipid derivatives, such as phosphatidylethanolamine, phosphatidylcholine, phosphatidylinositol, iGb3, the ganglioside GD3, globotriaosylceramide (Gb3Cer), and sulfatide, have been reported as CD1d-binding antigens (17). CD1d is also considered a reporting molecule, as it reflects the state of cellular lipid metabolism. One of the distinct characteristics of iNKT cells is their ability to secrete Th1-type cytokines or Th2-type cytokines depending on the species of CD1d-loaded lipid antigens that they encounter, which eventually influence iNKT cell activity (49). In obesity, adipocytes play crucial roles in managing increased lipid metabolism by processing and storing various lipid species in adipose tissue. Thus, it is possible that certain lipid metabolites could be presented on adipocyte CD1d molecules, and the lipid-CD1d complex would be exposed on the adipocyte surface, leading to the modulation of iNKT cell activity in fat tissue. In the case of hepatic inflammation, iNKT cells encounter hepatocytes and become activated upon recognizing specific lipid antigens loaded on hepatocyte CD1d molecules (50). Similarly, it appears that adipose tissue iNKT cells may patrol around adipocytes and respond to various lipid antigens loaded on adipocyte CD1d in fat tissue to coordinate and adapt to nutritional changes.
Several lines of evidences indicate that iNKT cells regulate adipose tissue inflammation. However, there are conflicts among the conclusions from different reports, probably due to analyses of different mouse models in different conditions. Therefore, the role of iNKT cells in adipose tissue inflammation has remained controversial. For example, recently, Ohmura et al. reported that iNKT cells promote adipose tissue inflammation by using beta2-microglobulin knockout mice as an iNKT cell-deficient mouse model (35). However, beta2-microglobulin KO mice lack CD8 T cells as well as NKT cells, and CD8 T cells are well known for their proinflammatory functions. Therefore, their results obtained from this mouse model could be, at least partly, conferred by CD8 T cell deficiency (8). Wu et al. also proposed a proinflammatory role of iNKT cells after long-term HFD. They analyzed the phenotypes of Jα18 KO and CD1d KO mice after more than 10 weeks of HFD feeding and demonstrated decreased adiposity and adipose tissue inflammation in the KO mice (51), which may conflict with our results. However, this study was focused on the function of iNKT cells in the late-stage obesity, whereas we analyzed mainly the function of iNKT cells in the early stage of obesity. Depending on the time point (long- and short-term HFD), it is possible that different lipid antigens could be loaded on CD1d, which will prime iNKT cells either toward anti- or proinflammatory cells producing Th2- and Th1-type cytokines, respectively. The roles of iNKT cells and the types of lipid antigens in relation to the time course of obesity have to be elucidated in further study. On the contrary to these two reports, Satoh et al. observed that there is no difference in body weight, fat mass, and glucose tolerance between Jα18 KO mice and WT mice after 18 weeks of HFD feeding (32). They compared Jα18 KO mice with commercial B6 mice, rather than littermate control WT mice. Recently, it is reported that microbiota in guts can affect energy metabolism by HFD, and it is well recognized that mice touch and sometimes eat feces in the cage (52). Therefore, using commercially purchased WT control mice, but not littermate control mice, may have affected differential commensal microbiota in WT and KO mice. In this study, we used littermate WT mice as a control group and observed that Jα18 KO mice showed repeatedly and consistently increased body weight gain, adiposity, and adipose tissue inflammation compared to WT mice after short-term HFD feeding. In concordance with our study, Ji et al. demonstrated that insulin sensitivity is impaired in CD1d KO mice after 4 days of HFD (53). Moreover, very recently, Lynch's group has reported that HFD-fed Jα18 KO mice are more obese, concomitant with insulin resistance and increased inflammatory responses (54).
To date, most of the studies that are relevant to immune cells in adipose tissue have focused on their roles in prolonged or severe obesity (4, 6–8), which may mask the effects of iNKT cells on adipose tissue inflammation at the early stage of obesity or provoke compensatory responses to resolve a long range of imbalanced energy homeostasis. In contrast, the function of immune cells in the early stages of obesity has not been properly addressed, although there are appreciable changes in adipose tissue inflammation and insulin sensitivity in those phases (33, 37, 38).
Among our observations, several lines of evidence suggest that iNKT cells in adipose tissue might exert anti-inflammatory effects. First, in the adipose tissue of iNKT cell-deficient Jα18 KO mice, the CD11c+ M1 macrophage population was greatly elevated upon HFD. Second, the expression of several proinflammatory genes was stimulated in the fat tissue of HFD-fed Jα18 KO mice. Third, the body weight and fat mass of Jα18 KO mice were higher than those of WT mice upon HFD feeding, resulting from reduced levels of energy metabolism genes in WAT of Jα18 KO mice. Nevertheless, the mass of other peripheral tissues, including the liver, was not significantly changed. Moreover, HFD-fed Jα18 KO mice exhibited glucose intolerance and higher plasma insulin levels. In addition, based on the results of the present study, we postulate that adipocytes act as prominent APCs of iNKT cells by priming adipose iNKT cells toward anti-inflammatory cells via the regulation of lipid antigens loaded onto CD1d molecules during the early phase of obesity. Subsequently, activated iNKT cells would orchestrate the cross talk between innate and adaptive immune cells and constrain the progression of adipose tissue inflammation. On the other hand, in the late stages of obesity, it seems that a dramatic decrease in the number of iNKT cells, concomitant with reduced CD1d expression in adipocytes, may contribute to accumulation of adipose tissue inflammation. Moreover, other immune cells in adipose tissue may act as predominant inflammatory regulators in the late stages of obesity. Therefore, we suggest that iNKT cells in adipose tissue would be involved in maintaining tissue homeostasis and insulin sensitivity, particularly at the early phase of obesity.
In this work, we have newly identified the regulatory mechanism involved in the cross talk between adipocytes and iNKT cells in adipose tissue. We believe that adipose tissue iNKT cells play a pivotal role in sensing and mediating metabolic changes, which would eventually affect fine-tuning between the innate and adaptive immune response to confer systemic energy homeostasis. The exact roles and regulatory mechanisms of iNKT cells in adipose tissue and the identification of obesity-related lipid antigens remain to be investigated.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Korea Science and Engineering Foundation funded by the Korean government (Ministry of Education, Science and Technology) (2012-0001241, 2012000655, 20120006079, and R31-10032). J.Y.H, J.I.K, I.J.H, and J.H.S were supported by BK21 Research Fellowships from the Ministry of Education and Human Resources Development.
We have no conflicts of interest to declare.
We thank S. H. Park for providing CD1d knockout mice and DN32.D3 hybridoma cells and D. S. Lee for providing Jα18 knockout mice.
Footnotes
Published ahead of print 12 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00552-12.
REFERENCES
- 1. Hotamisligil GS, Shargill NS, Spiegelman BM. 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259:87–91 [DOI] [PubMed] [Google Scholar]
- 2. Shoelson SE, Lee J, Goldfine AB. 2006. Inflammation and insulin resistance. J. Clin. Invest. 116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. 2009. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15:930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lumeng CN, Bodzin JL, Saltiel AR. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. 2008. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57:3239–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. 2009. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15:921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. 2009. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15:914–920 [DOI] [PubMed] [Google Scholar]
- 9. Olefsky JM, Glass CK. 2010. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72:219–246 [DOI] [PubMed] [Google Scholar]
- 10. Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. 2008. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. 2008. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ. Res. 103:467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taniguchi M, Seino K, Nakayama T. 2003. The NKT cell system: bridging innate and acquired immunity. Nat. Immunol. 4:1164–1165 [DOI] [PubMed] [Google Scholar]
- 13. Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. 2004. NKT cells: what's in a name? Nat. Rev. Immunol. 4:231–237 [DOI] [PubMed] [Google Scholar]
- 14. Bendelac A, Rivera MN, Park SH, Roark JH. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535–562 [DOI] [PubMed] [Google Scholar]
- 15. Van Kaer L. 2007. NKT cells: T lymphocytes with innate effector functions. Curr. Opin. Immunol. 19:354–364 [DOI] [PubMed] [Google Scholar]
- 16. Godfrey DI, Kronenberg M. 2004. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114:1379–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brutkiewicz RR. 2006. CD1d ligands: the good, the bad, and the ugly. J. Immunol. 177:769–775 [DOI] [PubMed] [Google Scholar]
- 18. Moody DB. 2006. TLR gateways to CD1 function. Nat. Immunol. 7:811–817 [DOI] [PubMed] [Google Scholar]
- 19. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278:1626–1629 [DOI] [PubMed] [Google Scholar]
- 20. Barral DC, Brenner MB. 2007. CD1 antigen presentation: how it works. Nat. Rev. Immunol. 7:929–941 [DOI] [PubMed] [Google Scholar]
- 21. Wu L, Gabriel CL, Parekh VV, Van Kaer L. 2009. Invariant natural killer T cells: innate-like T cells with potent immunomodulatory activities. Tissue Antigens 73:535–545 [DOI] [PubMed] [Google Scholar]
- 22. Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. 2008. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134:933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chait A, Kim F. 2010. Saturated fatty acids and inflammation: who pays the toll? Arterioscler. Thromb. Vasc. Biol. 30:692–693 [DOI] [PubMed] [Google Scholar]
- 24. Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. 2011. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473:528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. 2011. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 121:1858–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. 2007. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5:167–179 [DOI] [PubMed] [Google Scholar]
- 27. Samuel VT, Shulman GI. 2012. Mechanisms for insulin resistance: common threads and missing links. Cell 148:852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Summers SA. 2010. Sphingolipids and insulin resistance: the five Ws. Curr. Opin. Lipidol. 21:128–135 [DOI] [PubMed] [Google Scholar]
- 30. Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, Amidi A, Watkins SM, Nguyen U, Olefsky JM. 2010. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J. Biol. Chem. 285:15333–15345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142:687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, Nakayama T, Taniguchi M, Hirata N, Ishimori N, Tsutsui H, Onoe K, Iwabuchi K. 2012. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS One 7:e30568 doi:10.1371/journal.pone.0030568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, Miller YI. 2009. 12/15-Lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One 4:e7250 doi:10.1371/journal.pone.0007250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Caspar-Bauguil S, Cousin B, Andre M, Nibbelink M, Galinier A, Periquet B, Casteilla L, Penicaud L. 2006. Weight-dependent changes of immune system in adipose tissue: importance of leptin. Exp. Cell Res. 312:2195–2202 [DOI] [PubMed] [Google Scholar]
- 35. Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, Andoh Y, Fujii S, Iwabuchi K, Onoe K, Tsutsui H. 2010. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler. Thromb. Vasc. Biol. 30:193–199 [DOI] [PubMed] [Google Scholar]
- 36. Lynch L, O'Shea D, Winter DC, Geoghegan J, Doherty DG, O'Farrelly C. 2009. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur. J. Immunol. 39:1893–1901 [DOI] [PubMed] [Google Scholar]
- 37. Kleemann R, van Erk M, Verschuren L, van den Hoek AM, Koek M, Wielinga PY, Jie A, Pellis L, Bobeldijk-Pastorova I, Kelder T, Toet K, Wopereis S, Cnubben N, Evelo C, van Ommen B, Kooistra T. 2010. Time-resolved and tissue-specific systems analysis of the pathogenesis of insulin resistance. PLoS One 5:e8817 doi:10.1371/journal.pone.0008817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB. 2011. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60:2474–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geng Y, Laslo P, Barton K, Wang CR. 2005. Transcriptional regulation of CD1D1 by Ets family transcription factors. J. Immunol. 175:1022–1029 [DOI] [PubMed] [Google Scholar]
- 40. Sikder H, Zhao Y, Balato A, Chapoval A, Fishelevich R, Gade P, Singh IS, Kalvakolanu DV, Johnson PF, Gaspari AA. 2009. A central role for transcription factor C/EBP-beta in regulating CD1d gene expression in human keratinocytes. J. Immunol. 183:1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szatmari I, Pap A, Ruhl R, Ma JX, Illarionov PA, Besra GS, Rajnavolgyi E, Dezso B, Nagy L. 2006. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J. Exp. Med. 203:2351–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berzins SP, Smyth MJ, Baxter AG. 2011. Presumed guilty: natural killer T cell defects and human disease. Nat. Rev. Immunol. 11:131–142 [DOI] [PubMed] [Google Scholar]
- 43. Berzofsky JA, Terabe M. 2009. The contrasting roles of NKT cells in tumor immunity. Curr. Mol. Med. 9:667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Exley MA, Lynch L, Varghese B, Nowak M, Alatrakchi N, Balk SP. 2011. Developing understanding of the roles of CD1d-restricted T cell subsets in cancer: reversing tumor-induced defects. Clin. Immunol. 140:184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suganami T, Ogawa Y. 2010. Adipose tissue macrophages: their role in adipose tissue remodeling. J. Leukoc. Biol. 88:33–39 [DOI] [PubMed] [Google Scholar]
- 46. Brigl M, Brenner MB. 2004. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22:817–890 [DOI] [PubMed] [Google Scholar]
- 47. Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. 2005. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 3:e113 doi:10.1371/journal.pbio.0030113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ, Jr, Liu XS, Lazar MA. 2008. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 22:2941–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Florence WC, Bhat RK, Joyce S. 2008. CD1d-restricted glycolipid antigens: presentation principles, recognition logic and functional consequences. Expert Rev. Mol. Med. 10:e20 doi.org/10.1017/S1462399408000732 [DOI] [PubMed] [Google Scholar]
- 50. Swain MG. 2008. Hepatic NKT cells: friend or foe? Clin. Sci. (Lond.) 114:457–466 [DOI] [PubMed] [Google Scholar]
- 51. Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA, Kim S, Mendez-Fernandez YV, Besra GS, Lomenick JP, Williams B, Wasserman DH, Van Kaer L. 2012. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc. Natl. Acad. Sci. U. S. A. 109:E1143–E1152 doi:10.1073/pnas.1200498109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Musso G, Gambino R, Cassader M. 2010. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care 33:2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ji Y, Sun S, Xia S, Yang L, Li X, Qi L. 2012. Short-term high-fat-diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J. Biol. Chem. 287:24378–24386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O'Shea D, O'Farrelly C, Exley MA. 2012. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37:574–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.