Abstract
DJ-1 is an oncogene and the causative gene for familial Parkinson's disease. Although the oxidative status of DJ-1 at cysteine 106 (C106) is thought to affect all of the activities of DJ-1 and excess oxidation leads to the onset of various diseases, the precise molecular mechanisms underlying the effects of oxidation of DJ-1 on protein-protein interactions of DJ-1 remain unclear. In this study, we found that DJ-1 bound to the DNA-binding region of p53 in a manner dependent on the oxidation of C106. Of the p53 target genes, the expression level and promoter activity of the DUSP1 gene, but not those of the p21 gene, were increased in H2O2-treated DJ-1−/− cells and were decreased in wild-type DJ-1- but not C106S DJ-1-transfected H1299 cells through sequestration of p53 from the DUSP1 promoter by DJ-1. DUSP1 downregulated by oxidized DJ-1 activated extracellular signal-regulated kinase (ERK) and decreased apoptosis. The DUSP1 and p21 promoters harbor nonconsensus and consensus p53 recognition sequences, respectively, which have low affinity and high affinity for p53. However, DJ-1 inhibited p21 promoter activity exhibited by p53 mutants harboring low DNA-binding affinity but not by wild-type p53. These results indicate that DJ-1 inhibits the expression of p53 target genes and depend on p53 DNA-binding affinity and oxidation of DJ-1 C106.
INTRODUCTION
We identified DJ-1 (also known as Park7) as a novel oncogene that induces anchorage-independent growth of fibroblasts cooperatively with activated ras (1), and we later found it to be the causative gene for a familial form of Parkinson's disease (2). DJ-1 has 3 cysteines located at amino acids 46, 53, and 106 (C46, C53, and C106, respectively). Of the 3 cysteines, C106 is first oxidized as the SOH, SO2H, and SO3H forms, and excessive oxidation then causes oxidation of C46 and C53 (3, 4). The C106S mutant of DJ-1, which is a substitution mutant of DJ-1 at amino acid 106 from cysteine to serine, possesses little or no protective activity against neuronal cell death induced by oxidative stress (4–8), and abnormally oxidized forms of DJ-1 have been observed in patients with sporadic forms of Parkinson's disease (9). From these points, it seems that C106 is the most important cysteine for maintaining the function of DJ-1. Although the oxidative status of DJ-1 affects the activities of DJ-1 toward cells and disease, the precise molecular mechanisms remain unclear.
DJ-1 binds to various factors, including transcriptional factors such as androgen receptor (10, 11), p53 (12, 13), polypyrimidine tract-binding protein-associated splicing factor (PSF) (14), and Keap1, an inhibitor for nuclear factor erythroid 2-related factor 2 (Nrf2) (15). However, it is not known how DJ-1 chooses its suitable binding protein(s) during the course of oxidative stress.
p53 is a tumor suppressor protein that activates transcriptional programs under various types of cellular stress, including oxidative stress. It is not clear, however, how p53 determines a point leading to cell cycle arrest and apoptosis. Recent reports have suggested that the activation of specific promoters by p53 is achieved through its interactions with heterologous transcription factors such as Hzf, human cellular apoptosis susceptibility (hCAS)/CSE1L, and ankyrin repeat-, SH3 domain-, and proline-rich region-containing protein (ASPP) family proteins (16–18). DJ-1 directly binds to p53 to restore p53 transcriptional activity by inhibiting sumoylation of p53 through the interaction of DJ-1 with Topors/p53BP3, a SUMO-1 ligase for p53 (19). Sumoylation of DJ-1 itself is necessary for DJ-1 to localize from the cytoplasm to the nucleus (20), and DJ-1 is a negative regulator for sumoylation (21). Moreover, DJ-1 decreases Bax expression through repression of p53 transcriptional activity by an unknown mechanism (12). Although DJ-1 regulates p53 transcriptional activity through interaction with a SUMO-1 ligase of p53 and regulates its location, it is still unclear whether the oxidative status of DJ-1 affects p53 activity. We hypothesized that the oxidative status of DJ-1 contributes to its binding activity to various proteins to regulate their functions.
In this study, we found that DJ-1 bound to the DNA-binding region of p53 in a manner dependent on the oxidative status of DJ-1 and that the oxidation of C106 was essential for DJ-1 binding to p53, resulting in alteration of the DNA-binding affinity of p53. Furthermore, DJ-1 repressed the transcriptional activity of p53 in a manner dependent on the p53 DNA-binding affinity.
MATERIALS AND METHODS
Cell cultures and mice.
HEK293T, A549, H1299, and mouse embryonic fibroblast cells were cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum. DJ-1 heterozygous knockout mice were kindly provided by J. Shen (22), and DJ-1 homozygous knockout mice (DJ-1−/−) and wild-type (WT) mice with the same background (DJ-1+/+) were obtained. Newborn mice with genotypes of DJ-1−/− and DJ-1+/+ were cut 1 day after birth with scissors, digested with trypsin, and seeded on a 10-cm dish in DMEM with 10% calf serum. Cells obtained were fibroblasts and used as mouse DJ-1−/− and DJ-1+/+ cells. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Committee for Animal Research at Hokkaido University (permit number 08-0468).
RT-PCR and real-time PCR.
The nucleotide sequences of the primers used for reverse transcriptase (RT)-PCR were 5′-TCCTCCCTGGAGAAGAGCTA-3′ (β-actin sense), 5′-CCAGACAGCACTGTGTTGGC-3′ (β-actin antisense [as]), 5′-CCGTGGACAGTGAGCAGTTG-3′ (mouse p21 sense), 5′-GAAGACCAATCTGCGCTTGG-3′ (mouse p21 as2), 5′-GAACGCGCCAGTGAACCCAA-3′ (mouse NOXA sense), 5′-CTTTGTCTCCAATCCTCCGG-3′ (mouse NOXA as), 5′-TCCTCAGCCCTCCCTGTCAC-3′ (mouse PUMA sense), 5′-CCATTTCTGGGGCTCCAGGA-3′ (mouse PUMA as), 5′-CAGCTCCTGGTTCAACGAGG-3′ (mouse DUSP1 sense 2), and 5′-GCAGCTTGGAGAGGTGGTGAT-3′ (mouse DUSP1 as). The nucleotide sequences of the primers used for real-time PCR were 5′-CCTAGGCACCAGGGTGTGAT-3′ (mACTB 192-211F), 5′-GCTCGAAGTCTAGAGCAACA-3′ (mACTB 734-753R), 5′-CCCTAAGGCCAACCGTGAAA-3′ (mACTB F-real-time), 5′-ACGACCAGAGGCATACAGGGA-3′ (mACTB R-real-time), 5′-CCTGGTTCAACGAGGCTATTG-3′ (mDUSP1 F-real-time), 5′-CCAGCTTTACCCGGTTAGTCC-3′ (mDUSP1 R-real-time), 5′-GTATCACGCTTCCCGCAAGG-3′ (human DUSP1 sense 3), 5′-CAAACACCCTTCCTCCAGCATTC-3′ (human DUSP1 as5), 5′-CGGCTGAGGAGTGGCTGG-3′ (human actin sense 3), and 5′-CCAGCCGAGACACGGCAT-3′ (human actin as4). After mouse primary or H1299 cells had been treated with 300 μM H2O2 for 0.25 to 4 h or 0.5 h, total RNAs were prepared and subjected to semiquantitative RT-PCR and quantitative RT-PCR (real-time PCR) analyses. After reactions, PCR products were extracted, separated on 2% agarose gels, and stained with ethidium bromide. PCR conditions for RT-PCR were 1 min at 94°C, 30 s at 94°C, 30 s at 58°C, and 22 cycles of 1 min at 72°C for β-actin, 1 min at 94°C, 30 s at 94°C, 30 s at 58°C, and 25 cycles of 1 min at 72°C for p21, 1 min at 94°C, 30 s at 94°C, 30 s at 58°C, and 28 cycles of 1 min at 72°C for NOXA, 1 min at 94°C, 30 s at 94°C, 30 s at 58°C, and 34 cycles of 1 min at 72°C for PUMA, and 1 min at 94°C, 30 s at 94°C, 30 s at 58°C, and 28 cycles of 1 min at 72°C for DUSP1. PCR conditions for real-time PCR were 10 s at 95°C, 5 s at 95°C, and 44 cycles of 20 s at 60°C for β-actin and 10 s at 95°C, 5 s at 95°C, and 44 cycles of 20 s at 60°C for DUSP1.
Luciferase assays.
pGL4.10-hDUSP1 and pGL3-hp21 were digested with KpnI and HindIII, and each resultant fragment was inserted into KpnI and HindIII sites of pGL4.12[luc2CP] (Promega, Madison, WI). H1299 cells in 6-well dishes were transfected with pGL4.12-hDUSP1 or pGL4.12-hp21 together with pcDNA3-FLAG-p53 and pEF–DJ-1–hemagglutinin (HA) or pEF–DJ-1 C106S-HA by Lipofectamine 2000 (Invitrogen, Carlsbad, CA) per the manufacturer's protocol. pActin-β-galactosidase was also cotransfected with plasmids. At 24 h after transfection, cells were treated with or without 300 μM H2O2 or with 10 μg/ml cycloheximide for 30 min. Whole-cell extracts were then prepared by the addition of a Triton X-100-containing solution from the Pica gene kit (Wako Pure Chemicals, Osaka, Japan) to cells. About a one-fifth volume of the extract was used for the β-galactosidase assay to normalize transfection efficiencies as described previously (23), and the luciferase activity due to the reporter plasmid was determined using a luminometer (Luminocounter Lumat LB 9507, EG&G Berthold, Bad Wildbad, Germany). Proteins in aliquots of the cell extract were analyzed by Western blotting with an anti-FLAG antibody (M2) (Sigma, St. Louis, MO) and an anti-HA antibody (1:2,000) (MBL, Nagoya, Japan) and visualized as described in “Western blotting and antibodies.” The same experiments were repeated at least three times.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays using cultured A549 cells were performed according to the protocol of the ChIP assay kit (Millipore, Billerica, MA). Briefly, after proteins had been cross-linked with DNA, cell pellets were resuspended in an SDS-lysis buffer and sonicated on ice using a sonicator (UR-20P; Tomy, Tokyo, Japan) 4 times for 15 s each time. Genomic DNA was sheared to 300 to 1,200 bp in length. The chromatin solution was preincubated with salmon sperm DNA and protein A-agarose and incubated with species-matched IgG or with specific antibodies overnight at 4°C. Immunoprecipitated DNA fragments were then used as templates for PCR with ExTaq (TaKaRa Bio, Kyoto, Japan) and reacted for 60 s at 94°C, 60 s at 94°C, 30 s at 58°C, and 35 cycles of 30 s at 72°C. The nucleotide sequences of the oligonucleotides used for the PCR primers were 5′-AAGAGCAGGCCGGACAGC-3′ (hDUSP1 sense), 5′-GAGCGCGTTTATATGCGGC-3′ (hDUSP1 as), 5′-CCCAATCCCTCTCCCACTAG-3′ (hDUSP1 sense 3), 5′-CAGAGCCGCTAAAATGGGCA-3′ (hDUSP1 as3), 5′-TGCTGCTCCACCGCACTC-3′ (hp21 sense), 5′-GAAAACAGGCAGCCCAAGGAC-3′ (hp21 as), 5′-CTATCAGCTGCCTCGGGG-3′ (hp21 sense 3), and 5′-GGCGCCCCAAGTTCCTAAC (hp21 as3). PCR products were separated on a 2% agarose gel and stained with ethidium bromide. Reverse images of black and white staining are shown in Fig. 9, 10, and 15.
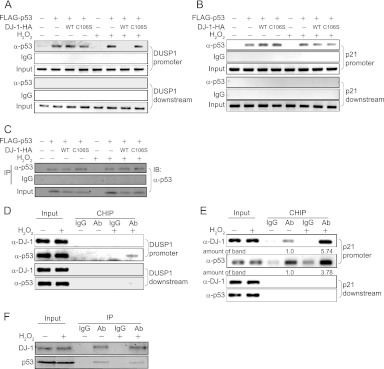
Fig 9.
DJ-1–p53 complex stably binds to the p21 promoter but not to the DUSP1 promoter. H1299 cells were transfected with FLAG-p53 and wild-type or C106S DJ-1–HA. Forty-eight hours after transfection, cells were treated with 300 μM H2O2 for 30 min, and ChIP assays targeting the DUSP1 (A) and p21 (B) genes were carried out. (C) Proteins prepared from transfected H1299 cells were analyzed by Western blotting. A549 cells were treated with 300 μM H2O2 for 30 min, and ChIP assays targeting the DUSP1 (D) and p21 (E) genes were carried out. The intensities of the precipitated DNA bands in lanes 4 and 6 in panel E were quantified, and their relative levels are shown. (F) Proteins prepared from H2O2-treated A549 cells were analyzed by Western blotting. Ab, antibody.
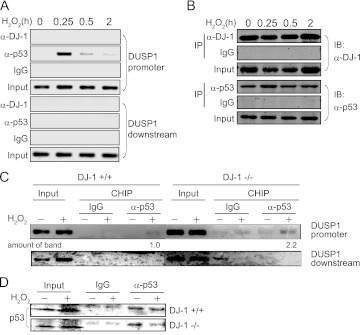
Fig 10.
A time course of endogenous DJ-1–p53 interactions and endogenous p53 ChIP on the DUSP promoter in cells exposed to oxidative stress. (A) A549 cells were treated with 300 μM H2O2 for 0.25 to 2 h, and ChIP assays targeting the DUSP1 gene were carried out. (B) Proteins prepared from H2O2-treated A549 cells were analyzed by Western blotting. (C) DJ-1+/+ and DJ-1−/− mouse cells were treated with 300 μM H2O2 for 0.5 h, and ChIP assays targeting the DUSP1 gene were carried out. (D) Proteins prepared from H2O2-treated mouse DJ-1+/+ and DJ-1−/− cells were analyzed by Western blotting.
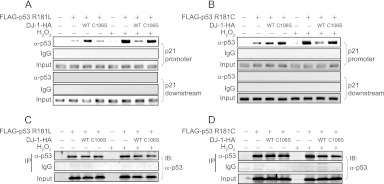
Fig 15.
DJ-1 inhibits the DNA-binding activity of p53 mutants. H1299 cells were transfected with FLAG-p53 R181L (A) or R181C (B) and wild-type or C106S DJ-1–HA. Forty-eight hours after transfection, cells were treated with 300 μM H2O2 for 30 min, and ChIP assays targeting the p21 gene were carried out. Proteins were prepared from H1299 cells transfected with FLAG-p53 R181L (C) or R181C (D) and wild-type or C106S DJ-1–HA and analyzed by Western blotting.
Western blotting and antibodies.
Proteins were extracted from cells with a buffer containing 150 mM NaCl, 5 mM EDTA, 50 mM Tris (pH 7.5), and 0.5% NP-40, loaded on 12% SDS-polyacrylamide gels, and subjected to Western blotting. The antibodies used in this study were anti-HA (1:2,000) (MBL, Nagoya, Japan), anti-FLAG F7425 (1:1,000) (Sigma), anti-T7 (1:1,000) (Novagen, Madison, WI), anti-p53 (DO-1 at 1:1,000) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-p53(serine 6) (1:1,000) (Cell Signaling Technology, Danvers, MA), anti-phospho-p53(serine 9) (1:1,000) (Cell Signaling Technology), anti-phospho-p53(serine 15) (1:1,000) (Cell Signaling Technology), anti-phospho-p53(serine 20) (1:1,000) (Cell Signaling Technology), anti-phospho-p53(serine 37) (1:1,000) (Cell Signaling Technology), anti-phospho-p53(serine 46) (1:1,000) (Cell Signaling Technology), anti-phospho-p53(serine 392) (1:1,000) (Cell Signaling Technology), anti-actin (1:4,000) (Chemicon, Temecula, CA), anti-phospho-ERK1/2 (1:1,000) (Cell Signaling Technology), anti-ERK1/2 (1:1,000) (Santa Cruz Biotechnology), anti-p53 (Pab 240 at 1:1,000) (Santa Cruz Biotechnology), anti-mitogen-activated protein kinase (MAPK) phosphatase 1 (MKP1) (C-19 at 1:500) (Santa Cruz Biotechnology), monoclonal rat anti-DJ-1 (1:1,000), polyclonal anti-DJ-1 (1:4,000), monoclonal mouse anti-DJ-1 (3E8 at 1: 4,000) (MBL), and anti-oxidized DJ-1 (1:1,000). Rabbit anti-DJ-1, rat anti-DJ-1 and anti-oxidized DJ-1 antibodies were established by us as described previously (1, 24). After membranes had been reacted with primary antibodies, they were reacted with Alexa Fluor 680-conjugated anti-mouse IgG antibody (Molecular Probes, Eugene, OR), Alexa Fluor 680-conjugated anti-rabbit IgG antibody (Molecular Probes), IRDye 800-conjugated anti-mouse IgG antibody (Rockland, Philadelphia, PA), or IRDye 800-conjugated anti-rabbit IgG antibody (Rockland) and visualized by using an infrared imaging system (Odyssey; Li-Cor, Lincoln, NE).
Pulldown assays.
35S-labeled p53 was synthesized in vitro using reticulocyte lysate of the TNT coupled transcription-translation system (Promega). Labeled proteins were reacted with glutathione S-transferase (GST), GST–wild-type DJ-1, or GST–C106S DJ-1 expressed in and prepared from Escherichia coli in a G buffer containing 150 mM NaCl, 5 mM EDTA and 50 mM Tris (pH 7.5), 0.05% bovine serum albumin, and 0.1% Nonidet P-40 for 2 h at 4°C, mixed with glutathione Sepharose beads, and centrifuged. After washing pellets with the wash buffer containing 150 mM NaCl, 10 mM Tris-HCl (pH 7.5), and 0.1% Nonidet P-40, Laemmli buffer was added to the pellets. The pellets were then heated at 97°C for 5 min, separated on a 12% polyacrylamide gel containing SDS, and visualized by Coomassie brilliant blue (CBB) staining and fluorography.
HEK293T or H1299 cells were transfected with expression vectors for wild-type DJ-1–HA or C106S DJ-1–HA and One-STrEP-p53 and treated with H2O2. Proteins in the cell extracts were then subjected to pulldown assays with Strep-Tactin Sepharose beads according to the supplier's protocol (IBA, Göttingen, Germany), and coprecipitated DJ-1 was detected by Western blotting with anti-HA and anti-oxidized DJ-1 antibodies.
Coimmunoprecipitation assays.
Proteins were extracted from cultured cells with or without 300 μM H2O2 for 30 min by a procedure described previously (25). Proteins were immunoprecipitated with a rabbit anti-DJ-1 antibody (1:500) (MBL) or normal rabbit IgG, and precipitates were analyzed by Western blotting with anti-p53 (1:1,000) (Santa Cruz Biotechnology) or mouse anti-DJ-1 antibody (3E8 at 1:1,000) (MBL). Proteins on membranes were visualized as described above.
HEK293T cells were transiently transfected with expression vectors for FLAG-p53 and DJ-1–HA by the calcium phosphate method and were lysed by treatment of cells with or without 1 mM H2O2 for 30 min. Proteins were then immunoprecipitated by an anti-FLAG antibody, and precipitates were detected by using an anti-HA antibody or an anti-DJ-1 antibody.
Isoelectric focusing.
Cells were treated with 1 mM H2O2 for 30 min, and cell extracts were prepared according to a procedure described previously (25). Proteins in the extracts were then separated on pH 5 to 8 ranges of an isoelectric focusing gel or on 12.5% polyacrylamide gel containing SDS, transferred onto nitrocellulose membranes, and reacted with an anti-HA antibody.
ELISA.
GST or GST-p53 purified from E. coli was loaded on enzyme-linked immunosorbent assay (ELISA) plates (BD) at 4°C overnight. After the plates had been blocked with 0.25× block A and washed with 0.1% Tween 20-PBS (phosphate-buffered saline), wild-type or C106S DJ-1 was added to the plates and incubated for 1 h at 4°C. The plates were then reacted with an anti-rat DJ-1 antibody (1:1,000) for 1 h at 37°C and reacted with a 2,2-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) solution, and the absorbance of each well was measured using a plate reader (Bio-Rad).
Immunofluorescence.
A549 cells were treated with or without 1 mM H2O2 for 30 min. Cells were then fixed with acetone-methanol and reacted with an anti-p53 or anti-DJ-1 antibody, and immunofluorescence images of proteins were detected by a fluorescein isothiocyanate- or rhodamine-conjugated secondary antibody, respectively. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Cell cycle FACS analyses.
Mouse DJ-1+/+ and DJ-1−/− cells were transfected with 50 nM AllStars negative control siRNA (Qiagen), DUSP1 siRNA-1 (Scramble siRNA cocktail that contains 4 sets of siRNA, item no. M-040753-01-0005; Thermo Scientific, San Jose, CA), and DUSP1 siRNA-2 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The nucleotide sequences of DUSP1 siRNA-2 are 5′-GGAUGCAGCUCCUGUAGUATT-3′ (DUSP1-2 sense) and 5′-UACUACAGGAGCUGCAUCCTT-3′ (DUSP1-2 antisense). At 48 h after transfection, the cells were starved for 6 h and then treated with or without H2O2. Cells were harvested at 40 h after the second H2O2 treatment, washed once with PBS, and fixed with 90% ethanol overnight. The cells were then treated with 1 mg/ml RNase for 30 min at 37°C, washed once with PBS, and suspended in 300 μl of PBS containing 50 μg/ml of propidium iodide (Sigma-Aldrich). After leaving for 1 h at room temperature, the cells were subjected to fluorescence-activated cell sorting (FACS) analysis using a FACSCalibur flow cytometer (Becton Dickinson), and the data obtained were analyzed using CellQuest software and ModFit software.
Statistical analyses.
Data are expressed as means ± standard errors (SE). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by unpaired Student's t test. For comparisons of multiple samples, the Tukey-Kramer test was used.
RESULTS
Oxidative stress enhances DJ-1 binding to p53.
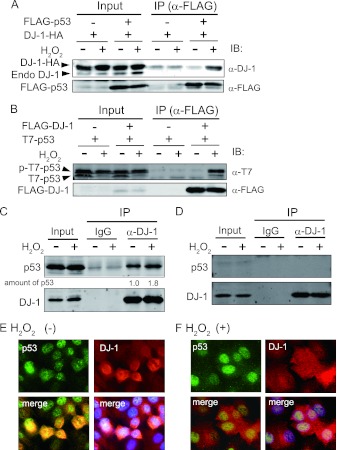
To address the effects of DJ-1 oxidation on interactions of DJ-1 with its target proteins, including p53, FLAG-tagged proteins were cotransfected with DJ-1–HA into HEK293T cells, and the cells were treated with 1 mM H2O2 for 30 min. Coimmunoprecipitation followed by Western blotting analyses showed that p53 and Daxx were strongly bound to DJ-1 in H2O2-treated cells (Fig. 1 and data not shown). Therefore, in this study we focused on the interaction of DJ-1 with p53 under conditions of oxidative stress.
Fig 1.
Oxidative stress enhances DJ-1 binding to p53. (A) HEK293T cells were transfected with FLAG-p53 and DJ-1–HA and treated with 1 mM H2O2 for 30 min at 48 h after transfection. Proteins were analyzed by immunoprecipitation (IP) followed by Western blotting (IB). (B) HEK293T cells were transfected with FLAG–DJ-1 and T7-p53 and analyzed as described for panel A. A549 cells (p53+/+) (C) and H1299 cells (p53−/−) (D) were treated with 300 μM H2O2 for 30 min, and proteins were analyzed by immunoprecipitation followed by Western blotting. The intensities of the precipitated p53 bands in lanes 5 and 6 were quantified, and their relative levels are shown under the blots. A549 cells were treated with (F) or without (E) 300 μM H2O2 for 30 min. Immunofluorescence analyses were carried out using anti-p53 and anti-DJ-1 antibodies as described in Materials and Methods.
The same results as those shown in Fig. 1A were obtained when FLAG–DJ-1 was cotransfected with T7-p53 into HEK293T cells (Fig. 1B). In this case, both phosphorylated and unphosphorylated T7-p53 was bound to DJ-1. Proteins from H2O2-treated A549 cells and H1299 cells in which p53 was not expressed were immunoprecipitated with an anti-DJ-1 antibody, and precipitates were analyzed by Western blotting with an anti-p53 antibody. The results showed that endogenous p53 bound to endogenous DJ-1 under normal culture conditions and that the amount of precipitated p53 was enhanced after treatment of A549 cells, but not H1299 cells, with H2O2 (Fig. 1C and D, respectively), suggesting that the oxidative status of DJ-1 modulates the p53-binding activity of DJ-1.
DJ-1 is localized in the cytoplasm, nuclei, and mitochondria of cells, and its localization is changed by oxidative stress (26). Oxidative stress might therefore alter the localization of p53 and the interaction of p53 with DJ-1. When A549 cells were treated with or without 300 μM H2O2 for 30 min and the localizations of proteins were analyzed by an immunofluorescence technique, DJ-1 and p53 were located in the cytoplasm and nuclei and in the nuclei, respectively, and both proteins were colocalized in the nuclei of cells before and after H2O2 treatment (Fig. 1E and F), indicating that oxidative stress enhances the binding activity of DJ-1 to p53 without affecting the localization of DJ-1.
Cysteine 106 of DJ-1 is essential for binding of DJ-1 to p53 under conditions of oxidative stress.
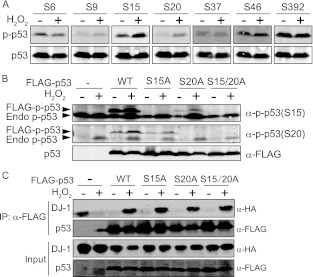
To examine whether translational modification of both p53 and DJ-1 induced by oxidative stress contributes to an H2O2-dependent increase of p53–DJ-1 complex formation, HEK293T cells were exposed to 1 mM H2O2 for 30 min, and phosphorylation of p53 was analyzed by Western blotting with several anti-phosphorylated p53 antibodies. As shown in Fig. 2A, enhanced phosphorylation of p53 at serine residues 15 and 20 but not 6, 9, 37, 46, and 392 was observed. When HEK293T cells were transfected with serine mutants of p53 in which serine was changed to alanine and exposed to 1 mM H2O2 for 30 min, the expected phosphorylation of serines 15 and 20 of FLAG-p53 mutants was not observed with single and double substitutions (Fig. 2B). HEK293T cells were then cotransfected with wild-type and serine mutants of FLAG-p53 and DJ-1–HA and exposed to 1 mM H2O2 for 30 min at 48 h after transfection. Coimmunoprecipitation experiments showed that of the three serine mutants and wild-type p53, no or only slight differences in the binding activity of FLAG-p53 to DJ-1–HA were observed under conditions of oxidative stress (Fig. 2C), indicating that the phosphorylation of p53 does not contribute to H2O2-dependent enhancement of DJ-1–p53 interaction.
Fig 2.
Phosphorylation levels of serines in p53 under conditions of oxidative stress. (A) HEK293T cells were treated with or without 1 mM H2O2 for 30 min, and proteins were analyzed by Western blotting with anti-p53 and respective anti-phospho-p53 (anti-p-p53) antibodies. (B) HEK293T cells transfected with FLAG-tagged wild-type (WT) and FLAG-tagged mutants of p53 were treated with 1 mM H2O2 for 30 min at 48 h after transfection. Proteins were detected by Western blotting. (C) HEK293T cells transfected with DJ-1–HA, FLAG-tagged wild type, and FLAG-tagged mutants of p53 were treated with 1 mM H2O2 for 30 min at 48 h after transfection. Proteins were analyzed by immunoprecipitation followed by Western blotting. A precipitated p53 band in lane 2 is the overflow from a band in lane 3.
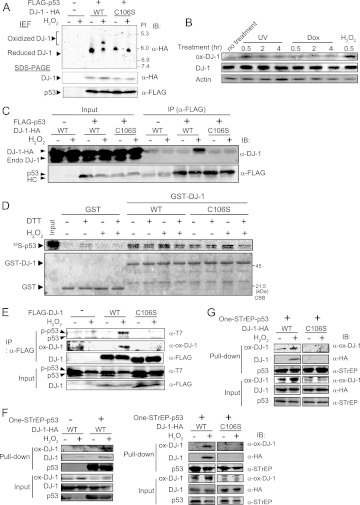
The oxidative status of cysteine 106 (C106) of DJ-1 is critical for all of the functions of DJ-1. HEK293T cells were cotransfected with the wild type or C106S mutant of DJ-1–HA and FLAG-p53 and exposed to H2O2 under the same conditions as those described above. The oxidation levels of wild-type and C106S DJ-1–HA were first examined by using electron-focusing gels. While C106S DJ-1–HA had no shifted band, approximately 50% of wild-type DJ-1–HA was shifted to a more acidic point in H2O2-treated cells (Fig. 3A). Furthermore, when HEK293T cells were treated with H2O2 and with UV or doxorubicin (other stressors activating p53) and proteins were analyzed with anti-DJ-1 and anti-C106-oxidated DJ-1 antibodies, H2O2 exposure clearly enhanced C106 oxidation of DJ-1 (Fig. 3B), suggesting that a shifted band observed in a sample of wild-type DJ-1–HA was derived from C106. The DJ-1–p53 complex was then analyzed by immunoprecipitation using an anti-FLAG antibody followed by Western blotting with an anti-DJ-1 antibody. The results showed that while wild-type DJ-1 was strongly bound to p53 in H2O2-treated cells, the C106S mutant of DJ-1 had lost its p53-binding activity (Fig. 3C). Pulldown experiments were then carried out using GST–wild-type DJ-1, GST–C106S DJ-1, and GST with 35S-labeled p53 in the presence or absence of H2O2 and reducing agent dithiothreitol (DTT). Recombinant DJ-1 purified from E. coli comprises a mixture containing reduced and oxidized forms of C106, and the amounts of the SOH and SO2H forms of C106 are about 10 to 20% of the total forms of C106 (data not shown). Doublet bands of p53 in gels must be unphosphorylated and phosphorylated p53. As in the case of DJ-1 binding to p53 in HEK293T cells, H2O2-treated wild-type DJ-1 was bound to p53 more strongly than was H2O2-nontreated wild-type DJ-1, and the C106S mutant of DJ-1 had lost the enhancement of p53-binding activity (Fig. 3D). The enhancement of binding activity of DJ-1 to p53 was diminished by the addition of DTT, indicating that DJ-1 directly interacts with p53 and that the oxidation of C106 is essential for this interaction.
Fig 3.
Cysteine 106 of DJ-1 is essential for binding to p53 under conditions of oxidative stress. (A) HEK293T cells were transfected with wild-type or C106S DJ-1–HA and FLAG-p53. Forty-eight hours after transfection, cells were treated with 1 mM H2O2 for 30 min, and proteins were analyzed by an isoelectric focusing gel (IEF) and subjected to Western blotting with an anti-HA antibody. (B) HEK293T cells were treated with 20 J/m2 UV, 1 μM doxorubicin (Dox), or 1 mM H2O2 for 0.5 to 4 h, and proteins were analyzed by Western blotting. (C) HEK293T cells were transfected with wild-type DJ-1–HA or C106S DJ-1–HA and FLAG-p53 and treated with H2O2 as described for Fig. 2A. Proteins were analyzed by immunoprecipitation followed by Western blotting. HC, heavy chain. (D) GST, GST-wild-type DJ-1, and GST-C106S DJ-1 were incubated with 35S-labeled p53 in the presence or absence of H2O2 and DTT and subjected to pulldown assays. CBB, Coomassie brilliant blue. (E) HEK293T cells were transfected with FLAG-wild-type DJ-1 or FLAG-C106S DJ-1 and T7-p53 and treated with H2O2. Proteins were then analyzed by immunoprecipitation followed by Western blotting. HEK293T cells (F) or H1299 cells (G) were transfected with wild-type or C106S DJ-1–HA and One-STrEP–p53 and treated with H2O2. Proteins were then subjected to pulldown assays using Strep-Tactin Sepharose beads.
To directly examine whether C106-oxidized DJ-1 binds to p53, two experiments were carried out. First, HEK293T cells were cotransfected with FLAG-wild type or the C106S mutant of DJ-1 and T7-p53 and exposed to H2O2. Proteins were subjected to coimmunoprecipitation and Western blotting analyses with an anti-C106–oxidized DJ-1 antibody (Fig. 3E). Second, HEK293T and H1299 cells were transfected with wild-type or C106S DJ-1–HA and One-STrEP-p53 and treated with H2O2. Proteins were pulled down using Strep-Tactin Sepharose, and precipitates were analyzed by Western blotting with the anti-oxidized DJ-1 antibody (Fig. 3F and G, respectively). The results showed that oxidized wild-type DJ-1 but not C106S DJ-1 bound to p53 under conditions of oxidative stress.
DJ-1 downregulates DUSP1 expression under conditions of oxidative stress.
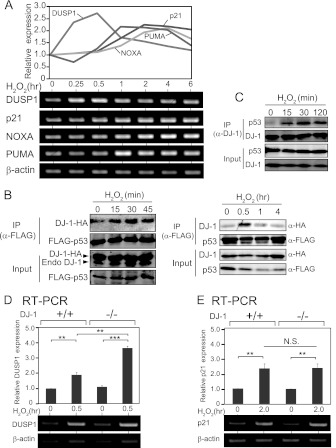
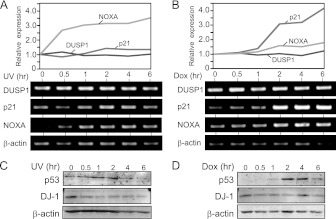
To address the role of increased formation of DJ-1–p53 complex after treatment of cells with H2O2, the expression levels of p53 target genes in mouse primary cells were examined by semiquantitative RT-PCR. As shown in Fig. 4A, the expression level of DUSP1 mRNA was increased at a peak of 30 min after H2O2 treatment, while the transcription levels of other p53 target genes such as p21, NOXA, and PUMA were induced after 1 to 2 h. On the other hand, the other stressors, such as UV exposure and doxorubicin treatment, did not alter the DUSP1 gene expression level, while the expression levels of NOXA and p21 were increased at 0.5 to 6 h after UV exposure and doxorubicin treatment (Fig. 5A to D), suggesting that the DUSP1 gene is a primary target of p53 against oxidative stress.
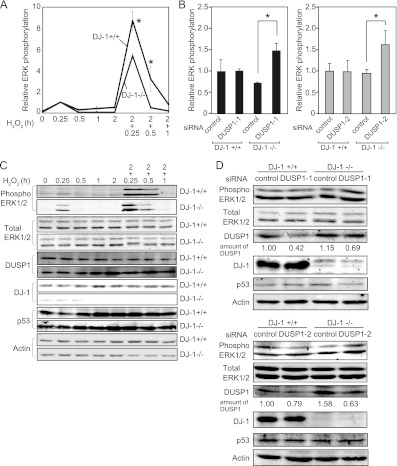
Fig 4.
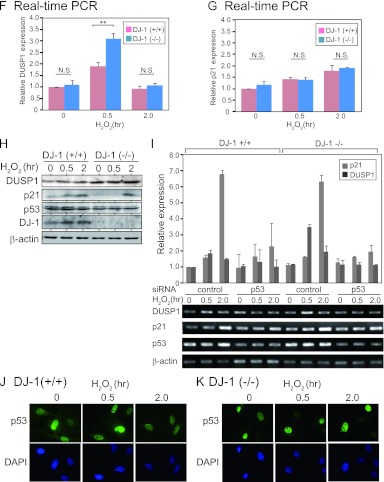
DJ-1 downregulates DUSP1 expression under an oxidative stress condition. (A) Mouse primary cells were treated with H2O2 for 0.25 to 6 h; the expression levels of respective mRNAs were examined by semiquantitative RT-PCR, and their expression levels relative to that of β-actin are shown. (B) HEK293T cells were transfected with FLAG-p53 and DJ-1–HA and treated with 1 mM H2O2 for 15 to 45 min (left panel) or 0.5 to 4 h (right panel) at 48 h after transfection. Proteins were analyzed by immunoprecipitation followed by Western blotting. (C) A549 cells were treated with 300 μM H2O2 for 15 to 120 min. Proteins were analyzed by immunoprecipitation with an anti-DJ-1 antibody followed by Western blotting. (D and E) DJ-1+/+ and DJ-1−/− mouse cells were treated with H2O2 for 0.5 h or 2 h. The expression levels of DUSP1 (D) and p21 mRNA (E) were examined by semiquantitative RT-PCR, and their expression levels relative to that of β-actin are shown. (F and G) DJ-1+/+ and DJ-1−/− mouse cells were treated with H2O2 for 0.5 and 2 h. The expression levels of DUSP1 (F) and p21 mRNA (G) were examined by quantitative RT-PCR. (H) DJ-1+/+ and DJ-1−/− mouse cells were treated with H2O2 for 0.5 and 2 h. The expression levels of DUSP1, p21, p53, DJ-1, and β-actin were analyzed by Western blotting. (I) DJ-1+/+ and DJ-1−/− mouse cells were transfected with control siRNA or p53 siRNA and treated with 300 μM H2O2 for 30 min or 2 h at 72 h after transfection. The expression levels of DUSP1 and p21 mRNAs were examined by semiquantitative RT-PCR. The nucleotide sequences of the p53 siRNA are 5′-CCAGAAGAUAUCCUGCCAUTT-3′ (mp53 sense) and 5′-AUGGCAGGAUAUCUUCUGGTT-3′ (mp53 antisense). (J and K) DJ-1+/+ and DJ-1−/− mouse cells were treated with H2O2 for 0.5 and 2 h. p53 was visualized as described in Materials and Methods. The values shown in panels D through G are means ± SE (n = 3 experiments). **, P < 0.01; ***, P < 0.001. N.S. indicates no significance.
Fig 5.
Expression of DUSP1, p21, and NOXA in UV- and doxorubicin-treated cells. Mouse DJ-1+/+ cells were treated with 20 J/m2 UV irradiation (A and C) or 1 μM doxorubicin (B an D) for 0.5 to 6 h. The expression levels of mRNA and protein of DUSP1, p21, and NOXA were examined by semiquantitative RT-PCR (A and B) and by Western blotting (C and D), respectively.
DUSP1, a mitogen-activated protein kinase phosphatase, regulates the apoptosis signaling pathway through dephosphorylating extracellular signal-regulated kinase (ERK) and is known to be the only p53 target gene that specifically responds to H2O2 treatment (27, 28) (Fig. 4A and 5A and B). Since the amount of p53–DJ-1 complex was also increased at a peak of 30 min after H2O2 treatment in HEK293T cells transfected with DJ-1–HA and FLAG-p53 and in A549 cells (Fig. 4B and C), and p53–DJ-1 complex was not detected at 1 to 4 h after H2O2 treatment (Fig. 4B, right), we focused on the effect of DJ-1 on p53-dependent DUSP1 expression.
The expression levels of DUSP1 and p21 mRNAs were examined using primary cells derived from DJ-1 knockout (DJ-1−/−) and DJ-1+/+ mice. Semiquantitative RT-PCR and real-time PCR analyses showed that the expression level of DUSP1 mRNA in DJ-1+/+ cells was increased at 30 min and then decreased at 2 h after H2O2 treatment and that the level at 30 min was further increased in DJ-1−/− cells (Fig. 4D and F). The expression levels of p21 mRNA were, on the other hand, increased at 2 h, and there were no differences between the expression levels in DJ-1−/− and DJ-1+/+ cells (Fig. 4E and G). The expression levels of DUSP1 protein were increased at 2 h in DJ-1+/+ cells and further increased in DJ-1−/− cells (Fig. 4H). On the other hand, there were few differences in the expression levels of p21 protein at 2 h between DJ-1−/− and DJ-1+/+ cells (Fig. 4H). When DJ-1−/− and DJ-1+/+ cells were transfected with siRNA targeting p53, the expression levels of DUSP1 and p21 were decreased (Fig. 4I), suggesting that the transcriptional activity of p53 was still active even in DJ-1−/− cells. Furthermore, no significant differences in the expression level and subcellular localization of p53 were observed in DJ-1−/− and DJ-1+/+ cells (Fig. 4J and K), indicating that the upregulation of DUSP1 expression in DJ-1−/− cells was not due to a change of p53 localization.
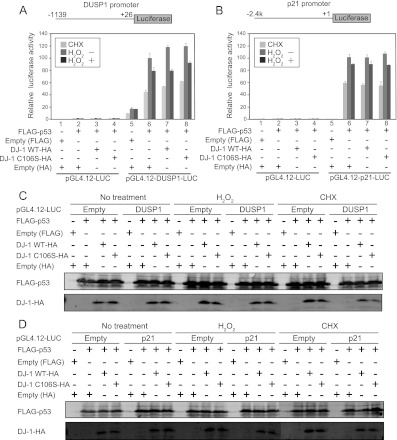
Cysteine 106 of DJ-1 is essential for repression of p53-dependent DUSP1 transcription under conditions of oxidative stress.
p53 binds to the promoter region of the DUSP1 gene (27). Since the luciferase generally used in reporter assays (called the luciferase assay) has a relatively long half-life, it is not suitable for detecting a prompt or immediate change of promoter activity. In the case of a prompt decrease in promoter activity toward a stress response, for instance, the luciferase activity obtained is not parallel to promoter activity due to an accumulation of already synthesized luciferase. To overcome this problem, we set up an experimental condition using pGL4.12-luciferase that harbors a modified version of luciferase with a short half-life of ∼20 min and using cycloheximide (CHX), an inhibitor for translation. Even when pGL4.12-luciferase was used, the short half-life of the modified version of luciferase was still not sufficient to decrease the background caused by its accumulation. Since CHX blocks further translation of luciferase, prompt promoter activity is parallel to promoter activity from which the activity obtained in the presence of CHX is subtracted. Twenty-four hours after cells had been transfected with reporter plasmids, the cells were divided into three sets. One set was a control for measuring the background signal, and the other two were test sets for either an H2O2-treated or nontreated sample. The control set was first treated with CHX for 30 min just before the addition of H2O2 to cells, and then luciferase activity obtained in the control set was subtracted as the background signal from that in the test set. Furthermore, to measure the transcriptional activity of transfected p53 without an effect of endogenous p53, p53-null H1299 cells were used.
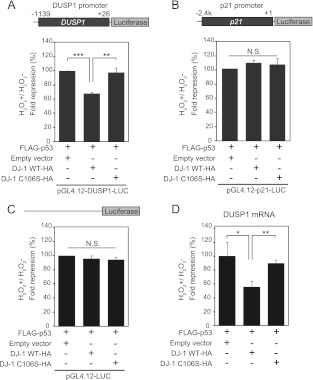
Figure 6A shows histograms of raw data of luciferase activities obtained in the three sets after transfection of the luciferase reporter construct linked to the DUSP1 promoter; p53 strongly activated DUSP1 promoter activity, and the treatment of cells with H2O2 reduced its activation activity, which is consistent with results reported previously (27). When wild-type or C106S DJ-1 was cotransfected with p53, luciferase activities tended to increase compared to those without p53, both in wild-type DJ-1- and C106S DJ-1-transfected cells, but luciferase activity was lower in wild-type DJ-1-transfected cells than in C106S DJ-1-transfected cells in the presence of CHX (Fig. 6A). Similar expression levels of introduced proteins were confirmed by Western blotting (Fig. 6C). To compare accurate changes among these samples, the ratios of luciferase activities in H2O2-treated cells to those in nontreated cells were calculated by subtracting the background signal obtained as the control set (Fig. 7A). The results showed that in H2O2-treated cells, the transcriptional activity of p53 toward the DUSP1 promoter was significantly reduced by wild-type DJ-1 to 60% of that with p53 alone or with C106S DJ-1. No significant changes of promoterless luciferase activity were observed in either the wild-type-DJ-1-transfected or the C106S DJ-1-transfected cells (Fig. 7C). Furthermore, to confirm that DUSP1 promoter activity detected by this assay was parallel with the expression level of DUSP1 mRNA, H1299 cells were transfected with FLAG-p53 and wild-type DJ-1–HA or C106S DJ-1–HA. Twenty-four hours after transfection, cells were treated with H2O2 in the absence of CHX. The expression levels of DUSP1 mRNA examined by quantitative RT-PCR (real-time PCR) were decreased by wild-type DJ-1 but not C106S DJ-1 in H2O2-treated cells (Fig. 7D). These results suggest that DJ-1 downregulates p53-dependent DUSP1 expression in a C106-dependent manner in H2O2-treated cells and that the oxidation of C106 is necessary for this activity. When the same assay using the p21 promoter linked to the modified version of the luciferase gene was carried out, there were no significant changes in promoter activity after transfection of cells with wild-type or C106S DJ-1 (Fig. 6B and D and Fig. 7B), indicating that the H2O2-dependent inhibitory effect of DJ-1 on p53 transactivation activity is specific to the DUSP1 gene.
Fig 6.
Raw data from three sets of luciferase assays. H1299 cells were cotransfected with pGL4.12-DUSP1-luciferase (A) or pGL4.12-p21-luciferase (B) and expression vectors for FLAG-p53 and wild-type or C106S DJ-1–HA. Twenty-four hours after transfection, cells were treated with 300 μM H2O2 or with 10 μg/ml cycloheximide (CHX) for 30 min. Luciferase activities were then calculated. (C and D) Proteins were prepared from H1299 cells transfected with FLAG-p53 and wild-type or C106S DJ-1–HA and analyzed by Western blotting.
Fig 7.
Cysteine 106 of DJ-1 is essential for the repression of p53-dependent DUSP1 transcription under conditions of oxidative stress. H1299 cells were cotransfected with pGL4.12-DUSP1-luciferase (A), pGL4.12-p21-luciferase (B), or pGL4.12-luciferase (C) and FLAG-p53 and wild-type or C106S DJ-1–HA. Cells were treated as described for Fig. 6. The fold repression values of luciferase activities were calculated as described in the text. (D) H1299 cells were transfected with FLAG-p53 and DJ-1–HA and treated with H2O2 for 30 min in the absence of cycloheximide as described for Fig. 6. The expression levels of DUSP1 mRNA were examined by quantitative RT-PCR. Values are means ± SE (n = 3 experiments). **, P < 0.01; ***, P < 0.001. N.S. indicates no significance.
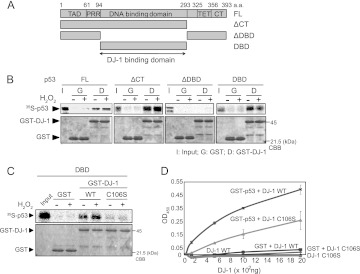
DJ-1 directly binds to the p53 DNA-binding region.
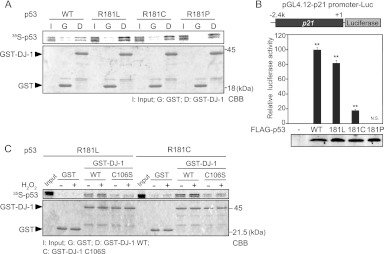
p53 comprises five domains: an N-terminal transactivation domain (TAD) followed by a proline-rich region (PRR), a central DNA-binding domain (DBD), a tetramerization domain (TET), and an extreme C terminus (CT) (see reference 29 for a recent review). To determine the DJ-1-binding region of p53, GST–wild-type DJ-1 and GST were reacted with 35S-labeled full-length p53 and three p53 deletion mutants (Fig. 8A). Pulldown experiments showed that DJ-1 bound to full-length p53, p53-ΔCT, and p53-DBD but not to p53-ΔDBD and that H2O2-treated DJ-1 more strongly bound to full-length p53 and p53-ΔCT than did non-H2O2-treated DJ-1 (Fig. 8B), indicating that DJ-1 binds to p53-DBD (Fig. 8B). Furthermore, pulldown assays (Fig. 8C) and ELISAs (Fig. 8D) showed that DJ-1 bound to p53-DBD in a C106-dependent manner.
Fig 8.
DJ-1 directly binds to the p53 DNA-binding region. (A) Schematic diagram of p53 deletion mutants. FL, full-length; CT, C terminus; DBD, DNA-binding domain. (B) GST and GST–DJ-1 were incubated with 35S-labeled p53 in the presence or absence of H2O2 and then subjected to pulldown assays. (C) GST, GST–DJ-1, and GST-C106S DJ-1 were incubated with 35S-labeled p53-DBD and subjected to pulldown assays. (D) ELISAs were carried out using GST-p53, GST, DJ-1, and C106S DJ-1.
DJ-1–p53 complex stably binds to the p21 promoter but not to the DUSP1 promoter.
A previous study showed that p53 binds to a 10-bp perfect palindromic site in the DUSP1 promoter to activate its transcription, although the p53-binding sequence in the DUSP1 promoter is not a sequence similar to the consensus sequence found in the p21 promoter (27). Chromatin immunoprecipitation (ChIP) assays were carried out using the DUSP1 and p21 genes. First, H1299 cells were cotransfected with FLAG-p53 and wild-type or C106S DJ-1–HA, treated with H2O2 for 30 min, and subjected to ChIP assays with an anti-p53 antibody targeting two regions containing the promoter, and other regions (downstream region: intron 4 and first exon of the DUPS1 and p21 genes, respectively) were amplified by PCR. As shown in Fig. 9A, the anti-p53 antibody precipitated the DUSP1 promoter region but not the downstream region under conditions with no oxidative stress. In H2O2-treated cells, precipitation of the DUSP1 promoter region by the anti-p53 antibody was cancelled by transfection of the wild-type DJ-1–HA but not by that of the C106S DJ-1–HA. Although the p21 promoter region contains the consensus p53-binding sequence, the precipitated p21 promoter region was not cancelled by transfection of the wild-type DJ-1–HA or the C106S DJ-1–HA under conditions with or without oxidative stress (Fig. 9B). The p53 levels precipitated in the reactions were in a range similar to that with Western blotting (Fig. 9C). Furthermore, ChIP assays were carried out using A549 cells. While the anti-p53 antibody precipitated the DUSP1 promoter region but not the downstream region in H2O2-treated cells, an anti-DJ-1 antibody did not precipitate regions even after more than 50 cycles of amplification by PCR (Fig. 9D). Both the anti-p53 and anti-DJ-1 antibodies precipitated the p21 promoter region before and after H2O2 treatment, and the precipitated levels were increased after H2O2 treatment (Fig. 9E). Equal levels of precipitated p53 and DJ-1 were confirmed (Fig. 9F). These results indicate that the binding activity of p53 to the DUSP1 promoter is weaker than that to the p21 promoter and that the p53–DJ-1 complex endogenously binds to the p21 promoter but not to the palindromic site in the DUSP1 promoter. It is thought that DJ-1-free p53 binds to the DUSP1 promoter and that the DJ-1–p53 complex binds to the p21 promoter (Fig. 9D). Time course analyses of ChIP assays were then carried out using A549 cells. p53 bound to the DUSP1 promoter in H2O2-treated cells at a peak of 15 min of exposure to H2O2, and then the level of promoter-bound p53 decreased (Fig. 10A). No binding of DJ-1 to the DUSP1 promoter was observed. The amount of promoter-bound p53 in DJ-1−/− cells was larger than that in DJ-1+/+ cells (Fig. 10C). The precipitated levels of p53 and DJ-1 were similar during the course of H2O2 exposure (Fig. 10B and D). Taken together, these results suggest that p53 binding to the DUSP1 promoter is sequestered by DJ-1 through its binding to p53-DBD in a C106-dependent manner under conditions of oxidative stress, resulting in suppression of the transactivation activity of p53 toward the DUSP1 gene.
DJ-1 increases phosphorylation of ERK and reduces apoptosis under conditions of oxidative stress.
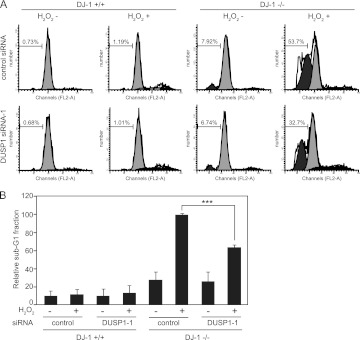
Since DUSP1 dephosphorylates ERK as MAPK phosphatase, we examined whether the dephosphorylation level of ERK is upregulated in DJ-1−/− cells by the increased level of DUSP1. DJ-1−/− and DJ-1+/+ cells were exposed to H2O2 twice, and the phosphorylation level of ERK1/2 was analyzed by Western blotting. Since the culture medium was changed before the second H2O2 treatment, the cells were treated with the same concentrations of H2O2 at each respective stimulation. As shown in Fig. 11A and C, the expression level of DUSP1 was increased 2 h after the first H2O2 treatment in DJ-1−/− cells but not in DJ-1+/+ cells, and the phosphorylation level of ERK1/2 was lower in DJ-1−/− cells than in DJ-1+/+ cells after the second H2O2 treatment. The similar decreased curves of ERK1/2 phosphorylation levels after the second H2O2 treatment were obtained in both DJ-1−/− and DJ-1+/+ cells, indicating that DJ-1 did not prolong the ERK activation. There was few or no differences in the expression levels of p53 and the total ERK1/2 in DJ-1−/− and DJ-1+/+ cells regardless of H2O2 treatment (Fig. 11C). Furthermore, knockdown of DUSP1 using two different siRNAs (siDUSP1-1 and siDUSP1-2) increased ERK phosphorylation in DJ-1−/− cells but not in DJ-1+/+ cells (Fig. 11B and D), indicating a reverse correlation between the expression levels of DUSP1 and phosphorylated ERK in DJ-1−/− cells under conditions of oxidative stress. Since DUSP1 activates the apoptosis pathway in cells after oxidative stress, DJ-1−/− and DJ-1+/+ cells were treated twice with H2O2 and then treated with propidium iodide, and their apoptosis levels were analyzed by using FACS, in which apoptotic cells are observed in the sub-G1 phase. As shown in Fig. 12A and B, H2O2 treatment induced apoptosis in both DJ-1−/− and DJ-1+/+ cells, but the level of apoptosis in DJ-1−/− cells was about 10 times higher than that in DJ-1+/+ cells. Furthermore, knockdown of DUSP1 expression in DJ-1−/− cells reduced apoptosis to 60% of that in control siRNA-treated cells (Fig. 12B). There were no differences between the expression levels of p53 in DJ-1−/− and DJ-1+/+ cells (Fig. 11D). These results suggest that DJ-1 decreases apoptosis in cells under conditions of intermittent oxidative stress through downregulation of DUSP1 expression.
Fig 11.
Phosphorylation levels of ERK in cells under conditions of oxidative stress. DJ-1+/+ and DJ-1−/− mouse cells were starved for 6 h and then treated with or without two pulses of H2O2. Proteins were analyzed by Western blotting (C), and the relative phosphorylation levels of ERK were quantified (A). DJ-1+/+ and DJ-1−/− mouse cells were transfected with control siRNA, DUSP1 siRNA-1, or DUSP1 siRNA-2, starved for 6 h at 48 h after transfection, and then treated with two pulses of H2O2. Proteins were analyzed by Western blotting (B), and the relative phosphorylation levels of ERK were quantified (D). Values are means ± SE (n = 3 experiments). *, P < 0.05.
Fig 12.
DJ-1 regulates cell survival under conditions of oxidative stress. (A) DJ-1+/+ and DJ-1−/− mouse cells were transfected with control siRNA or DUSP1 siRNA-1 and treated as described for Fig. 11. Forty hours after the addition of H2O2, cell cycle profiles were examined by using flow cytometry. (B) The sub-G1 phase of the cell cycle shown in panel A was quantified, and the relative number of cells in the sub-G1 fraction compared to that in DJ-1+/+ cells with control siRNA and H2O2 was calculated. Values are means ± SE (n = 3 experiments). ***, P < 0.001.
DJ-1 inhibits p53 activity in a manner dependent on DNA-binding affinity.
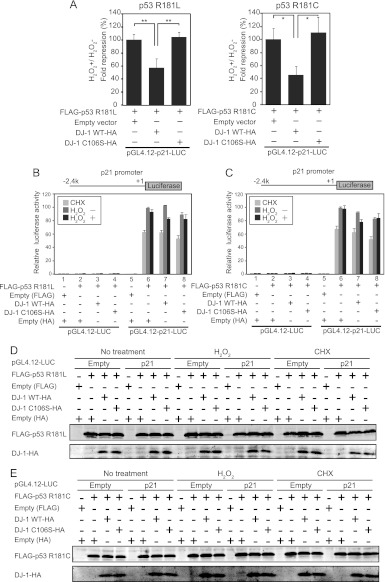
We showed that DJ-1 inhibited the transcriptional activity of p53 targeting the DUSP1 gene but not that of p53 targeting the p21 gene by inhibiting the DNA-binding activity of p53 in the DUSP1 promoter but not in the p21 promoter. Since DUSP1 and p21 promoters contain nonconsensus and consensus p53-binding sequences and since nonconsensus and consensus p53-binding sequences possess low and high binding affinities for p53, respectively, we considered two possible mechanisms by which DJ-1 inhibited p53-binding activity only to the DUSP1 promoter. One possibility is the difference in the p53-binding region between the consensus and nonconsensus sequences, and the other is the difference in binding affinity of p53 for DNA. To address this point, we used substitution mutants of p53 at amino acid 181 from arginine to leucine (R181L), to cysteine (R181C), and to proline (R181P), which are linked to tumor development in families with the hereditary Li-Fraumeni or Li-Fraumeni-like cancer susceptibility syndrome (30). The DNA-binding activities of these p53 mutants to the p21 promoter changed most in the wild type (WT) and decreasingly less in the R181L, R181C, and R181P proteins (31). Pulldown assays using GST–wild-type DJ-1, GST, and 35S-labeled p53 mutants showed that DJ-1 bound to R181L, R181C, R181P, and WT p53 with the same or similar affinities (Fig. 13A). Assays using the luciferase reporter gene conjugated to the p21 promoter showed that the transcriptional activity of p53 mutants toward the p21 promoter was lower than that of WT p53 in a manner dependent on the DNA-binding activity and that the R181P mutant had no transcriptional activity (Fig. 13B). We therefore used R181L and R181C mutants of p53 for further study. Pulldown assays showed that H2O2 treatment enhanced the DJ-1-binding activity of R181L and R181C p53 (Fig. 13C). H1299 cells were then cotransfected with wild-type DJ-1 or C106S DJ-1 together with R181L or R181C p53 and treated with 300 μM H2O2 or with 10 μg/ml cycloheximide for 30 min at 20 h after transfection, and their luciferase activities toward the p21 promoter were examined. Luciferase activities and similar expression levels of introduced proteins in transfected cells are shown in Fig. 14B through E, and the ratios of luciferase activities in cells treated with or without H2O2 were calculated. The results showed that wild-type DJ-1 significantly inhibited the transcriptional activities of R181L p53 and R181C p53 toward the p21 promoter in H2O2-treated cells to 50% and 40%, respectively, of that in cells without DJ-1 or in cells transfected with C106S DJ-1 (Fig. 14A). Wild-type DJ-1 suppressed the p21 promoter activity exhibited by R181C p53 more effectively than that by R181L p53, suggesting that DJ-1 inhibits p53 transcriptional activity in a manner that depends on p53 DNA-binding affinity. Furthermore, H1299 cells were cotransfected with FLAG-R181L p53 or FLAG-R181C p53 together with wild-type DJ-1–HA or C106S DJ-1–HA, treated with or without H2O2, and subjected to ChIP assays targeting the p21 promoter. As shown in Fig. 15A and B, the anti-p53 antibody precipitated the p21 promoter region but not the downstream region under conditions of no oxidative stress, and precipitation of the p21 promoter region was inhibited by the transfection of wild-type DJ-1–HA but not by that of C106S DJ-1–HA under conditions of oxidative stress. The precipitated p53 levels were similar with Western blotting (Fig. 15C and D). These results suggest that DJ-1 inhibits p53 activity in a manner that depends on the DNA-binding affinity.
Fig 13.
Binding activity of p53 mutants to DJ-1. GST and GST–DJ-1 were incubated with 35S-labeled wild-type and mutant p53 in the presence (A) or absence (C) of H2O2 and subjected to pulldown assays. (B) H1299 cells were cotransfected with pGL4.12-p21-luciferase and expression vectors for the wild-type and R181L, R181C, and R181P of FLAG-p53. Twenty-four hours after transfection, luciferase assays were carried out. Values are means ± SE (n = 3 experiments). **, P < 0.01. N.S. indicates no significance.
Fig 14.
DJ-1 inhibits transcriptional activity of p53 to the p21 promoter under conditions of oxidative stress in a manner dependent on DNA affinity. (A) H1299 cells were cotransfected with pGL4.12-p21-luciferase and expression vectors for FLAG-p53 mutants and for wild-type or C106S DJ-1–HA. Twenty-four hours after transfection, their luciferase activities were examined. Values are means ± SE (n = 3 experiments). *, P < 0.05; **, P < 0.01. H1299 cells were cotransfected with pGL4.12-p21-luciferase and an expression vector for wild-type or C106S DJ-1–HA together with a vector for FLAG-p53 R181L (B) or FLAG-p53 R181C (C). Twenty-four hours after transfection, the cells were treated with H2O2 and cycloheximide (CHX) as described for Fig. 6, and their luciferase activities were examined. (D and E) Proteins were prepared from H1299 cells transfected with FLAG-p53 mutants and wild-type or C106S DJ-1–HA and analyzed by Western blotting.
DISCUSSION
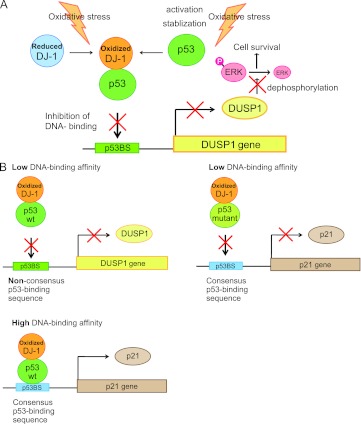
In this study, we showed that oxidized DJ-1 at C106 induced by oxidative stress strongly binds to p53 and that the enhanced interaction of DJ-1 with p53 under conditions of oxidative stress is required to suppress p53-dependent transcriptional activation of the DUSP1 gene by preventing promoter recognition of p53 (Fig. 16A). C106 of DJ-1 is essential for an increase of complex formation with p53 (Fig. 3C). Although several studies, including studies by us, have shown direct interaction of DJ-1 with p53 (12, 13), this is the first evidence that the oxidative status of DJ-1 regulates its interaction.
Fig 16.
Schematic models of the role of DJ-1 in the inhibition of p53 transactivation. (A) DJ-1 activates cell survival pathways under conditions of oxidative stress through downregulation of DUSP1 expression. (B) DJ-1 suppresses transactivation activity of p53 depending on the p53 DNA-binding affinity.
C106 of DJ-1 is oxidized from forms of SH (reduced), SOH, SO2H, and SO3H, and excess oxidation of C106, probably as an SO3H form, has been found in brains of patients with Parkinson's disease and Alzheimer's disease (9, 32). Which oxidation form of C106 is active is still under debate. Zhou et al. reported that DJ-1 at C106 with SO2H is an active form in terms of chaperone activity toward α-synuclein (33). We, on the other hand, have reported that stimulating the activity of DJ-1 toward tyrosine hydroxylase and l-3,4-dihydroxyphenylalanine (l-DOPA) decarboxylase requires the presence of reduced and SOH forms of C106, which account for more than 50% of the total forms (25). In this study, cells were treated with 1 mM H2O2 for 30 min, and the pI values of DJ-1 spots were shifted from 6.2 to 5.8 (Fig. 3B). Since a spot with a pI of 5.8 contained more than 50% of the SO2H form of C106 to the total forms of C106 (data not shown), it is possible that DJ-1 with SO2H of C106 preferentially binds to p53 to inhibit p53-dependent DUSP1 expression. Since other forms of C106 were also involved in the spot with a pI of 5.8, however, we cannot rule out the possibility that C106 forms other than SO2H work much more effectively toward p53. Establishment of methods to purify DJ-1 possessing respective forms of C106 is necessary for the analysis of DJ-1.
p53 bound to the DUSP1 promoter at 15 min after exposure of cells to H2O2 (Fig. 10A), and the expression level of DUSP1 mRNA then peaked at 30 min (Fig. 4A). The DJ-1-binding level with p53 also peaked at 30 min after H2O2 exposure and then decreased (Fig. 4B) concomitant with decreased binding of p53 to the DUSP1 promoter (Fig. 10A). When the same experiments were carried out using DJ-1−/− cells, the expression level of DUSP1 mRNA was increased at 30 min after H2O2 exposure (Fig. 4D and E). If the p53–DJ-1 interaction is a negative feedback that shuts down DUSP1 after its initial activation, then binding of DJ-1 to p53 must be correlated with repression of DUSP1 expression at time points after the addition of H2O2. As described above, this was not the case. As shown in Fig. 11A and C, DJ-1 increased the phosphorylation level of ERK through downregulation of DUSP1 expression at 15 min after the second exposure to H2O2, but thereafter, there were no differences in the decreasing curves of ERK phosphorylation between DJ-1+/+ and DJ-1−/− cells, suggesting that DJ-1 determines the maximal level of ERK phosphorylation. From these results, it may be that the mechanism of DJ-1 action toward DUSP1 expression involves DJ-1 receiving oxidative stress as an oxidative stress sensor and its C106 is oxidized. Oxidized DJ-1 binds to p53 at the limited time point, resulting in suppression of DUSP1 expression, thereby regulating the activation level of ERK.
p53 is posttranslationally modified by various stresses, and tetramerization of p53 is necessary for transcriptional activity of p53. We did not find any evidence that the phosphorylation of p53 affects its interaction with DJ-1. p53, however, undergoes other posttranslational modifications such as lysine acetylation. The acetylation of p53 enhances the DNA-binding activity of p53 to specific DNA regions (34), and it is therefore possible that acetylation of p53 affects DUSP1 expression. Furthermore, it has been reported that p53 induces phosphorylation of DJ-1 (35). Thus, it is thought that in addition to oxidation, DJ-1 chooses its binding proteins on the basis of various modifications of DJ-1.
A 30-min exposure of cells to H2O2 induced phosphorylation of serines 15 and 20 but not serine 46 of p53, and the p53–DJ-1 complex level was increased at a peak of 30 min after H2O2 treatment (Fig. 2 and 4B). Since severe DNA damage leads to phosphorylation of p53 serine 46 (36), it is thought that weak DNA damage occurred under the conditions of oxidative stress used in this study and that DJ-1 contributes to suppression of p53 under conditions of such weak damage.
The p21 and DUSP1 promoters contain consensus and nonconsensus p53-binding sequences, respectively, which have a high affinity and low affinity for p53 (27, 37). The protein level of Bax, the promoter of which contains the consensus p53-binding sequence, is downregulated by DJ-1 before and after treatment of cells with H2O2 (12, 38), suggesting that the suppressive activity of DJ-1 toward Bax does not depend on the oxidative status of DJ-1. It is of interest that DJ-1 inhibited p53 transcriptional activity toward the DUSP1 promoter but not toward the p21 promoter (Fig. 7) and that these phenomena were obtained through interference of the DNA-binding activity of p53 toward the DUSP1 promoter by DJ-1 (Fig. 9). However, when p53 mutants with low DNA-binding affinity for the p21 promoter were used, DJ-1 inhibited their transcriptional activity even to the p21 promoter (Fig. 14A). Binding activities of DJ-1 to the p53 mutants were the same as that to wild-type p53 (Fig. 13A). It is therefore thought that DJ-1 interferes with p53-binding activity to the DNA region that possesses weak association with p53 and that DJ-1 does not affect p53-binding activity to the DNA region that strongly interacts with p53 under conditions of oxidative stress. In other words, the binding affinity of p53 for the respective DNA-binding region determines whether or not DJ-1 inhibits the DNA-binding and transcriptional activities of p53 (Fig. 16B). The present findings suggest that DJ-1 plays a novel role in suppressing the expression of p53 target genes through preventing promoter recognition of p53 in a DNA-binding affinity-dependent manner. Furthermore, DJ-1 and p53 were colocalized in nuclei before and after H2O2 treatment (Fig. 1E and F), and p53 was localized to nuclei in both DJ-1−/− and DJ-1+/+ cells with or without H2O2 treatment (Fig. 4J and K), suggesting that DJ-1 inhibits binding of p53 to specific DNA regions without affecting the localization of p53.
The exposure of cells to H2O2 quickly induces phosphorylation of p53 and ERK. Activated p53 then upregulates the expression of proapoptotic genes (39, 40). Activation of ERK via the Ras/Raf/MEK pathway, on the other hand, supports cell survival (41, 42). It has been reported that the inhibition of p53 activates ERK after H2O2 treatment of cells, suggesting the existence of cross-talk of the negative signaling pathway between p53 and ERK (43). Oxidized DJ-1 inhibited p53-dependent DUSP1 expression under conditions of oxidative stress (Fig. 4D and F and 7A), and it increased ERK phosphorylation and cell survival (Fig. 11 and 12). Suppression of DUSP1 expression also results in a decrease of oxidative stress-induced cell death in SH-SY5Y cells (44), and activation of ERK regulates tyrosine hydroxylase transcription by activating the orphan nuclear receptor Nurr1 (45, 46). In addition to ERK, DJ-1 inhibits p38, MKK3, and MKK6 activity by inactivating ASK1 under conditions of oxidative stress (47). It is therefore thought that DJ-1 contributes to the activation of survival pathways by suppressing the apoptosis pathway through regulation of the expression of MAPK phosphatase under oxidative conditions.
It has been reported that DJ-1 is overexpressed in many types of cancers, especially in cancer cells with poor prognosis, and that almost half of these cancer cells possess p53 mutations (48–52). In this study, DJ-1 inhibited the transcriptional activities of p53 mutants more effectively than that of wild-type p53 after the cells had been exposed to H2O2 (Fig. 14A). p21 promoter activity exhibited by R181L p53 was decreased to 82% of that exhibited by wild-type p53 and was inhibited by wild-type DJ-1 to 57.2% of that without DJ-1, indicating that the final promoter activity by R181L p53 became 47% of that by wild-type p53. In the case of R181C p53, the final activity was reduced only to 8% of that obtained by wild-type p53 (Fig. 13B and 14A). DJ-1 activates the ERK (Fig. 11A) and Akt pathways by binding to PTEN (53) and has a cooperative transforming activity with H-Ras, which is located upstream of both pathways (1). These results suggest that, in addition to the stimulation of cell proliferation pathways, overexpressed DJ-1 contributes to the poor prognosis in cancer cells by suppressing cell death pathways.
ACKNOWLEDGMENTS
This work was supported by grants-in-aid from the Ministry of Education, Science, Culture and Sports and by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO) in Japan.
We thank Kiyomi Takaya for her technical assistance.
Footnotes
Published ahead of print 12 November 2012
REFERENCES
- 1. Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. 1997. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 231:509–513 [DOI] [PubMed] [Google Scholar]
- 2. Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. 2003. Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science 299:256–259 [DOI] [PubMed] [Google Scholar]
- 3. Kinumi T, Kimata J, Taira T, Ariga H, Niki E. 2004. Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 317:722–728 [DOI] [PubMed] [Google Scholar]
- 4. Takahashi-Niki K, Niki T, Taira T, Iguchi-Ariga SM, Ariga H. 2004. Reduced anti-oxidative stress activities of DJ-1 mutants found in Parkinson's disease patients. Biochem. Biophys. Res. Commun. 320:389–397 [DOI] [PubMed] [Google Scholar]
- 5. Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. 2004. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. U. S. A. 101:9103–9108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, Binari RC, Manoukian AS, Bray MR, Liu FF, Tsao MS, Mak TW. 2005. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell 7:263–273 [DOI] [PubMed] [Google Scholar]
- 7. Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, Floss T, Abeliovich A. 2004. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol. 2:e327 doi:10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. 2004. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 5:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T, Canet-Aviles R, Miller DW, McLendon C, Strand C, Leonard AJ, Abou-Sleiman PM, Healy DG, Ariga H, Wood NW, de Silva R, Revesz T, Hardy JA, Lees AJ. 2004. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson's disease. Brain 127:420–430 [DOI] [PubMed] [Google Scholar]
- 10. Niki T, Takahashi-Niki K, Taira T, Iguchi-Ariga SM, Ariga H. 2003. DJBP: a novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol. Cancer Res. 1:247–261 [PubMed] [Google Scholar]
- 11. Takahashi K, Taira T, Niki T, Seino C, Iguchi-Ariga SM, Ariga H. 2001. DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx alpha to the receptor. J. Biol. Chem. 276:37556–37563 [DOI] [PubMed] [Google Scholar]
- 12. Fan J, Ren H, Jia N, Fei E, Zhou T, Jiang P, Wu M, Wang G. 2008. DJ-1 decreases Bax expression through repressing p53 transcriptional activity. J. Biol. Chem. 283:4022–4030 [DOI] [PubMed] [Google Scholar]
- 13. Shinbo Y, Taira T, Niki T, Iguchi-Ariga SM, Ariga H. 2005. DJ-1 restores p53 transcription activity inhibited by Topors/p53BP3. Int. J. Oncol. 26:641–648 [PubMed] [Google Scholar]
- 14. Xu J, Zhong N, Wang H, Elias JE, Kim CY, Woldman I, Pifl C, Gygi SP, Geula C, Yankner BA. 2005. The Parkinson's disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum. Mol. Genet. 14:1231–1241 [DOI] [PubMed] [Google Scholar]
- 15. Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. 2006. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. U. S.A. 103:15091–15096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Das S, Raj L, Zhao B, Kimura Y, Bernstein A, Aaronson SA, Lee SW. 2007. Hzf determines cell survival upon genotoxic stress by modulating p53 transactivation. Cell 130:624–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samuels-Lev Y, O'Connor DJ, Bergamaschi D, Trigiante G, Hsieh JK, Zhong S, Campargue I, Naumovski L, Crook T, Lu X. 2001. ASPP proteins specifically stimulate the apoptotic function of p53. Mol. Cell 8:781–794 [DOI] [PubMed] [Google Scholar]
- 18. Tanaka T, Ohkubo S, Tatsuno I, Prives C. 2007. hCAS/CSE1L associates with chromatin and regulates expression of select p53 target genes. Cell 130:638–650 [DOI] [PubMed] [Google Scholar]
- 19. Weger S, Hammer E, Engstler M. 2003. The DNA topoisomerase I binding protein topors as a novel cellular target for SUMO-1 modification: characterization of domains necessary for subcellular localization and sumolation. Exp. Cell Res. 290:13–27 [DOI] [PubMed] [Google Scholar]
- 20. Shinbo Y, Niki T, Taira T, Ooe H, Takahashi-Niki K, Maita C, Seino C, Iguchi-Ariga SM, Ariga H. 2006. Proper SUMO-1 conjugation is essential to DJ-1 to exert its full activities. Cell Death Differ. 13:96–108 [DOI] [PubMed] [Google Scholar]
- 21. Fan J, Ren H, Fei E, Jia N, Ying Z, Jiang P, Wu M, Wang G. 2008. Sumoylation is critical for DJ-1 to repress p53 transcriptional activity. FEBS Lett. 582:1151–1156 [DOI] [PubMed] [Google Scholar]
- 22. Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. 2005. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron 45:489–496 [DOI] [PubMed] [Google Scholar]
- 23. Graham FL, van der Eb AJ. 1973. Transformation of rat cells by DNA of human adenovirus 5. Virology 54:536–539 [DOI] [PubMed] [Google Scholar]
- 24. Saito Y, Hamakubo T, Yoshida Y, Ogawa Y, Hara Y, Fujimura H, Imai Y, Iwanari H, Mochizuki Y, Shichiri M, Nishio K, Kinumi T, Noguchi N, Kodama T, Niki E. 2009. Preparation and application of monoclonal antibodies against oxidized DJ-1. Significant elevation of oxidized DJ-1 in erythrocytes of early-stage Parkinson disease patients. Neurosci. Lett. 465:1–5 [DOI] [PubMed] [Google Scholar]
- 25. Ishikawa S, Taira T, Niki T, Takahashi-Niki K, Maita C, Maita H, Ariga H, Iguchi-Ariga SM. 2009. Oxidative status of DJ-1-dependent activation of dopamine synthesis through interaction of tyrosine hydroxylase and 4-dihydroxy-l-phenylalanine (l-DOPA) decarboxylase with DJ-1. J. Biol. Chem. 284:28832–28844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li HM, Niki T, Taira T, Iguchi-Ariga SM, Ariga H. 2005. Association of DJ-1 with chaperones and enhanced association and co-localization with mitochondrial Hsp70 by oxidative stress. Free Radic. Res. 39:1091–1099 [DOI] [PubMed] [Google Scholar]
- 27. Liu YX, Wang J, Guo J, Wu J, Lieberman HB, Yin Y. 2008. DUSP1 is controlled by p53 during the cellular response to oxidative stress. Mol. Cancer Res. 6:624–633 [DOI] [PubMed] [Google Scholar]
- 28. Sun H, Charles CH, Lau LF, Tonks NK. 1993. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75:487–493 [DOI] [PubMed] [Google Scholar]
- 29. Freed-Pastor WA, Prives C. 2012. Mutant p53: one name, many proteins. Genes Dev. 26:1268–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santibanez-Koref MF, Birch JM, Hartley AL, Jones PH, Craft AW, Eden T, Crowther D, Kelsey AM, Harris M. 1991. p53 germline mutations in Li-Fraumeni syndrome. Lancet 338:1490–1491 [DOI] [PubMed] [Google Scholar]
- 31. Schlereth K, Beinoraviciute-Kellner R, Zeitlinger MK, Bretz AC, Sauer M, Charles JP, Vogiatzi F, Leich E, Samans B, Eilers M, Kisker C, Rosenwald A, Stiewe T. 2010. DNA binding cooperativity of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol. Cell 38:356–368 [DOI] [PubMed] [Google Scholar]
- 32. Choi J, Sullards MC, Olzmann JA, Rees HD, Weintraub ST, Bostwick DE, Gearing M, Levey AI, Chin LS, Li L. 2006. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J. Biol. Chem. 281:10816–10824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou W, Zhu M, Wilson MA, Petsko GA, Fink AL. 2006. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J. Mol. Biol. 356:1036–1048 [DOI] [PubMed] [Google Scholar]
- 34. Gu W, Roeder RG. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595–606 [DOI] [PubMed] [Google Scholar]
- 35. Rahman-Roblick R, Hellman U, Becker S, Bader FG, Auer G, Wiman KG, Roblick UJ. 2008. Proteomic identification of p53-dependent protein phosphorylation. Oncogene 27:4854–4859 [DOI] [PubMed] [Google Scholar]
- 36. Oda K, Arakawa H, Tanaka T, Matsuda K, Tanikawa C, Mori T, Nishimori H, Tamai K, Tokino T, Nakamura Y, Taya Y. 2000. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102:849–862 [DOI] [PubMed] [Google Scholar]
- 37. Freeman J, Schmidt S, Scharer E, Iggo R. 1994. Mutation of conserved domain II alters the sequence specificity of DNA binding by the p53 protein. EMBO J. 13:5393–5400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miyashita T, Reed JC. 1995. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293–299 [DOI] [PubMed] [Google Scholar]
- 39. Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, Fornace AJ., Jr 1999. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 18:6845–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shono T, Tofilon PJ, Schaefer TS, Parikh D, Liu TJ, Lang FF. 2002. Apoptosis induced by adenovirus-mediated p53 gene transfer in human glioma correlates with site-specific phosphorylation. Cancer Res. 62:1069–1076 [PubMed] [Google Scholar]
- 41. Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. 1999. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286:1358–1362 [DOI] [PubMed] [Google Scholar]
- 42. Wang JK, Gao G, Goldfarb M. 1994. Fibroblast growth factor receptors have different signaling and mitogenic potentials. Mol. Cell. Biol. 14:181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lange-Carter CA, Pleiman CM, Gardner AM, Blumer KJ, Johnson GL. 1993. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science 260:315–319 [DOI] [PubMed] [Google Scholar]
- 44. Kim GS, Choi YK, Song SS, Kim WK, Han BH. 2005. MKP-1 contributes to oxidative stress-induced apoptosis via inactivation of ERK1/2 in SH-SY5Y cells. Biochem. Biophys. Res. Commun. 338:1732–1738 [DOI] [PubMed] [Google Scholar]
- 45. Jacobsen KX, MacDonald H, Lemonde S, Daigle M, Grimes DA, Bulman DE, Albert PR. 2008. A Nurr1 point mutant, implicated in Parkinson's disease, uncouples ERK1/2-dependent regulation of tyrosine hydroxylase transcription. Neurobiol. Dis. 29:117–122 [DOI] [PubMed] [Google Scholar]
- 46. Lu L, Sun X, Liu Y, Zhao H, Zhao S, Yang H. 2012. DJ-1 upregulates tyrosine hydroxylase gene expression by activating its transcriptional factor Nurr1 via the ERK1/2 pathway. Int. J. Biochem. Cell Biol. 44:65–71 [DOI] [PubMed] [Google Scholar]
- 47. Mo JS, Jung J, Yoon JH, Hong JA, Kim MY, Ann EJ, Seo MS, Choi YH, Park HS. 2010. DJ-1 modulates the p38 mitogen-activated protein kinase pathway through physical interaction with apoptosis signal-regulating kinase 1. J. Cell. Biochem. 110:229–237 [DOI] [PubMed] [Google Scholar]
- 48. Chow SN, Chen RJ, Chen CH, Chang TC, Chen LC, Lee WJ, Shen J, Chow LP. 2010. Analysis of protein profiles in human epithelial ovarian cancer tissues by proteomic technology. Eur. J. Gynaecol. Oncol. 31:55–62 [PubMed] [Google Scholar]
- 49. Le Naour F, Misek DE, Krause MC, Deneux L, Giordano TJ, Scholl S, Hanash SM. 2001. Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin. Cancer Res. 7:3328–3335 [PubMed] [Google Scholar]
- 50. Melle C, Ernst G, Escher N, Hartmann D, Schimmel B, Bleul A, Thieme H, Kaufmann R, Felix K, Friess HM, Settmacher U, Hommann M, Richter KK, Daffner W, Taubig H, Manger T, Claussen U, von Eggeling F. 2007. Protein profiling of microdissected pancreas carcinoma and identification of HSP27 as a potential serum marker. Clin. Chem. 53:629–635 [DOI] [PubMed] [Google Scholar]
- 51. Yuen HF, Chan YP, Law S, Srivastava G, El-Tanani M, Mak TW, Chan KW. 2008. DJ-1 could predict worse prognosis in esophageal squamous cell carcinoma. Cancer Epidemiol. Biomarkers Prev. 17:3593–3602 [DOI] [PubMed] [Google Scholar]
- 52. Zhu XL, Wang ZF, Lei WB, Zhuang HW, Jiang HY, Wen WP. 2010. DJ-1: a novel independent prognostic marker for survival in glottic squamous cell carcinoma. Cancer Sci. 101:1320–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim YC, Kitaura H, Taira T, Iguchi-Ariga SM, Ariga H. 2009. Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int. J. Oncol. 35:1331–1341 [PubMed] [Google Scholar]