Abstract
The neurodegenerative disorder ataxia with oculomotor apraxia 2 (AOA-2) is caused by defects in senataxin, a putative RNA/DNA helicase thought to be involved in the termination of transcription at RNA polymerase pause sites. RNA/DNA hybrids (R loops) that arise during transcription pausing lead to genome instability unless they are resolved efficiently. We found that senataxin forms distinct nuclear foci in S/G2-phase human cells and that the number of these foci increases in response to impaired DNA replication or DNA damage. Senataxin colocalizes with 53BP1, a key DNA damage response protein, and with other factors involved in DNA repair. Inhibition of transcription using α-amanitin, or the dissolution of R loops by transient expression of RNase H1, leads to the loss of senataxin foci. These results indicate that senataxin localizes to sites of collision between components of the replisome and the transcription apparatus and that it is targeted to R loops, where it plays an important role at the interface of transcription and the DNA damage response.
INTRODUCTION
Coordination of efficient DNA replication and transcription is critical for the maintenance of genome integrity and chromosome transmission at mitosis. The transcription of long genes is a particular challenge, as it can often take longer than one cell cycle, and there is evidence that the transcription and replication machineries often collide at certain loci (1, 2). Collisions within long genes, such as those that occur at common fragile sites (CFS), can result in DNA breakage, genome instability, and cancer (1, 3).
Studies carried out in yeast and mammalian cells have shown that DNA double-strand breaks (DSBs) and genome instability arise through replication stress (such as interference between replication and transcription) and often involves the formation of transcription-linked RNA/DNA hybrids (2, 4–6). In Saccharomyces cerevisiae, a protein known as Sen1, a superfamily I RNA/DNA helicase (7), restricts the formation of RNA/DNA hybrids (R loops) that form during transcription (8). Sen1 interacts directly with RNA polymerase II (9), and sen1 mutants exhibit pleiotropic defects in RNA processing and transcription termination (10–13). These defects are thought to be associated with Sen1's role in limiting the accumulation of transcription-directed R-loop structures that would otherwise lead to DSB formation, homologous recombinational repair, and the potential for genome instability (8).
The human SETX gene encodes an ortholog of Sen1 and is known to be defective in the progressive neurological disorder ataxia with oculomotor apraxia 2 (AOA2) and in juvenile amyotrophic lateral sclerosis type 4 (ALS4) (14, 15). Individuals with mutations in SETX exhibit motor neuron degeneration, together with progressive muscle weakness and atrophy. Unfortunately, at this time, little is known about the product of SETX, senataxin, except that it is a 300-kDa protein that has putative RNA/DNA helicase activity and interacts with RNA polymerase II (Pol II) (16, 17). Like yeast sen1 mutants, human cell lines defective for SETX exhibit defects in transcription termination, and recent evidence supports a role in the resolution of R-loop structures that arise at transcription pause sites (17, 18). In addition, SETX cell lines are sensitive to agents that cause DNA damage, in particular those that introduce oxidative stress and single-strand breaks (19). These properties led us to further explore potential links between replication stress, transcription, and the DNA damage response and to determine whether senataxin plays a role in the processing of R loops that might otherwise lead to genome instability. We found that senataxin forms nuclear foci in response to agents that cause replication blockage and in particular that it colocalizes with the DNA damage response marker 53BP1 to sites of collision between components of the replisome and the transcription apparatus. We suggest that senataxin plays an important cellular role at the interface of transcription and the DNA damage response and that the resolution of R-loop structures is a key event in the maintenance of genome stability.
MATERIALS AND METHODS
Yeast methods.
Standard protocols were used for genetic manipulation of S. cerevisiae. A PCR-based strategy was used for the C-terminal 13-Myc or hemagglutinin (HA) tagging of yeast proteins at their endogenous loci in haploid derivatives of BY4741. The yeast tandem affinity purification (TAP) fusion library (45) was purchased from Open Biosystems.
TAP-Sen1 was purified essentially as described previously (46). Cell pellets (from 30 liters of culture) were lysed using a French press in TAP lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride [PMSF], and protease inhibitors). The extracts were treated with 150 U/ml Benzonase for 2 h at 4°C and centrifuged at 17,000 rpm for 20 min at 4°C. The supernatant was collected, and NP-40 was added to a final concentration of 0.1%. Sen1 was affinity purified from the supernatant using IgG Sepharose 6 fast-flow beads for 2 h at 4°C. The mixture was transferred into a Bio-Rad column and the beads were washed three times with 25 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10% glycerol, 0.1% NP-40, 1 mM PMSF, and protease inhibitors. The column was then washed with 30 ml of TEV cleavage buffer (TCB; 10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.5 mM EDTA, 0.1% NP-40, 10% glycerol, and 1 mM dithiothreitol [DTT]). TCB buffer (3 ml) containing 500 units of recombinant TEV protease was then applied to the column, which was incubated for 2 h at 16°C on a rocking platform. Released proteins were collected by gravity flow, and the column was washed with 0.5 ml of TCB. The eluates were pooled, and CaCl2 was added to a final concentration of 5 mM. Calmodulin binding buffer (CBB; 6 ml; 10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM magnesium acetate [MgOAc], 1 mM imidazole, 2 mM CaCl2, 0.1% NP-40, 10% glycerol, 10 mM β-mercaptoethanol) was added, and the solution was transferred to a Bio-Rad column containing calmodulin Sepharose 4B beads. Following 90 min incubation at 4°C on a rotary wheel, the column was washed three times with CBB buffer and eluted in 10 fractions taken at 5-min intervals using 200 μl of 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM MgOAc, 2 mM EGTA, and 10 mM β-mercaptoethanol. The protein sample was analyzed by gel electrophoresis (NuPAGE, 4 to 12% bis-Tris; NuPAGE, 3 to 8% Tris-acetate; Invitrogen) followed by mass spectrometry.
Affinity purification of human senataxin.
A bacterial artificial chromosome (BAC) containing C-terminally FLAP-tagged senataxin (containing Flag and green fluorescent protein [GFP] tags) was constructed and transfected into HeLa cells (47). Colonies were selected for stable integration using G418 (400 μg/ml) and endogenous levels of protein expression. The cells (20 liters) were harvested and incubated with CSK buffer (10 mM PIPES [pH 6.8], 0.1% Triton X-100, 100 mM KCl, 300 mM sucrose, 1 mM EDTA, 5 mM NaF, 1 mM dithiothreitol, and 20 mM β-glycerophosphate) for 15 min at 4°C, followed by low-speed centrifugation for 10 min to separate the cytoplasmic (supernatant) and nuclear (pellet) fractions. The pellet was resuspended in immunoprecipitation (IP) buffer (50 mM potassium phosphate [pH 7.5], 0.1% Triton X-100, 150 mM KCl, 5 mM NaF, 1 mM dithiothreitol, 20 mM β-glycerophosphate, and 10% glycerol), and the nuclei were broken using a homogenizer (20 strokes with pestle A). The resulting mixture was incubated for 10 min at 4°C and centrifuged at 35,000 rpm in a Beckman 45 Ti rotor for 1 h to separate the nucleoplasm (supernatant) from chromatin (pellet) fractions. The chromatin fraction was resuspended in 150 mM HEPES (pH 7.9), 0.2% NP-40, 1.5 mM MgCl2, 150 mM KCl, 1 mM dithiothreitol, 5 mM NaF, and 10% glycerol and homogenized by 30 strokes with pestle B. The suspension was then treated with 50 units/ml Benzonase for 2 h at 4°C, and centrifuged again at 35,000 rpm for 1 h. The supernatant was taken as the chromatin fraction. All three fractions were then incubated with M2-Flag agarose beads (Sigma) for 2 h at 4°C, which were washed extensively with IP buffer and eluted with 3× Flag peptide. The eluate was then incubated with GFP-Trap beads (ChromoTek) for 1 h at 4°C. The beads were washed extensively and bound proteins analyzed by gel electrophoresis (NuPAGE, 4 to 12% bis-Tris; NuPAGE, 3 to 8% Tris-acetate; Invitrogen). The gels were either stained for SYPRO Ruby or processed for Western blotting using polyvinylidene difluoride (PVDF) membranes (Immobilon, Millipore).
Mass spectrometry.
For mass spectrometric analysis, gel lanes were cut into small slices and peptides were generated by in situ tryptic digestion of protein/gel bands. Sen1 interaction partners were identified by mass spectrometry using SYNAPT HDMS, and the search was performed against a concatenated nonredundant protein database (UniProt 13.6) using the Mascot search engine (Matrix Science, United Kingdom). Senataxin partners were identified in a similar manner but using an LTQ Orbitrap XL mass spectrometer, and in this case the search was carried out against UniProt 15.5.
Senataxin constructs and protein analyses.
Full-length senataxin was PCR amplified from a human cDNA library prepared from HeLa cells. Full-length senataxin and four fragments of the protein were N-terminally tagged with GFP by cloning into pDEST53 for expression in human cells. The fragments were GFP-senataxin1–667, GFP-senataxin1–2146, GFP-senataxin624–2146, and GFP-senataxin2099–2677. The GFP-tagged RNase H1 expression vector GFP-M27-H1 is described elsewhere (48, 49). HeLa cells were grown on coverslips in a 10-cm plate and transfected for 24 h with 6 μg DNA using Lipofectamine 2000 (Invitrogen). The cells were harvested and lysed in LAP buffer (47), and insoluble materials were removed by high-speed centrifugation. The cleared lysates were normalized for total protein content and subjected to NuPAGE gel electrophoresis or processed for immunoprecipitation.
Drugs.
The following drugs were used: DNA-dependent protein kinase catalytic subunit (DNA-PKcs) inhibitor NU7441 (Tocris Biosciences), the ATM inhibitor KU55933 (Tocris Biosciences), caffeine (Sigma), aphidicolin (Sigma), α-amanitin (Sigma), diospyrin D1 (a gift of Banasri Hazra), phleomycin (InvivoGen), cisplatin (Sigma), mitomycin C (Sigma), and hydroxyurea (Sigma). Unless indicated otherwise, cells were treated for 24 h with the indicated drug, followed by fixation and processing as described below.
Immunostaining.
Cells were grown overnight on glass coverslips and treated with or without drugs for 24 h. They were washed once with warm phosphate-buffered saline (PBS) and fixed either with 4% paraformaldehyde (20 min at room temperature) or with ice-cold methanol for 1 h. The cells were washed three times with PBS containing 0.1% Tween 20 (PBST) and permeabilized by treatment with PBS containing 0.5% Triton X-100 for 5 min at room temperature. The samples were washed twice with PBST and blocked with 4% bovine serum albumen in PBST for 10 min at room temperature (RT). The primary antibodies were diluted in the same buffer and incubated 2 h at RT. After three 5-min washes in PBST, the samples were incubated with secondary antibodies for 1 h. Coverslips were washed three times for 5 min each time in PBST and mounted using ProLong Gold antifade reagent (Invitrogen) containing DAPI (4′,6′-diamidino-2-phenylindole). Images were acquired on a Zeiss Axio Imager M1 microscope using a Plan Apochromat 63×/1.4 oil objective lens (Zeiss) equipped with an ORCA_ER camera (Hamamatsu) and controlled by Volocity 4.3.2 software (Improvision). Images are displayed projections of deconvolved z planes (generated by Volocity's iterative restoration function). The numbers of foci/cell were quantified from 3 independent experiments.
Live-cell imaging.
Frames were acquired using a 40× EC Plan-Neofluor 40×/1.30 oil DICII objective lens on a Zeiss Axio Observer Z1 microscope controlled by Simple PCI Imaging software (Hamamatsu) and equipped with a full-enclosure environmental chamber heated to 37°C (Digital Pixel Imaging) and an Orca 03GO1 camera (Hamamatsu). Frames were recorded as 3 z planes (5 μm apart) every 5 min.
Antibodies.
Rabbit polyclonal senataxin antibody OY7 was raised against a peptide corresponding to amino acids 1173 to 1193 (PVRPSSSVRNEGQSDTNKRD), and senataxin antibody OY11 was raised against a mixture of four peptides: TPGGASTIDFLKRYASNTPSGEF (residues 8 to 31), CKIQEFHVDGKE (residues 884 to 895), PVRPSSSVRNEGQSDTNKRD (residues 1173 to 1193), and RRNSRWDKRTLEQEDSSSKKRKLL (residues 2654 to 2677). Both antibodies were purified using an AminoLink Plus immobilization kit (Thermo Scientific). OY7 was used for all Western blots and immunofluorescence studies, whereas OY11 was used for immunoprecipitation.
Antibodies used for Western blotting were RNAPII (RPB1-CTD ab5408; Abcam), KAP1 (ab3831; Abcam), CHD4 (ab72418; Abcam), MYC (ab1326; Abcam), GFP (11814460001; Roche), HA (12013819001; Sigma), tubulin (T5168; Sigma), and DNA-PKcs (ab32566; Abcam).
Antibodies used for immunofluorescence studies were: GFP Booster_Atto488 (1:100; Gba488; ChromoTek), cyclin A (a gift from Tim Hunt, Cancer Research UK), 53BP1 (1:300; 612523; BD Bioscience), SMC2 (ab10412; Abcam), UBF (sc-13125; Insight Biotechnology), fibrillarin (ab4566; Abcam), γH2AX (05-636; Millipore), MDC1 (a gift from Ross Chapman, London Research Institute), FANCD2 (NB100-182; Novus), XPA (sc-28353; Santa Cruz), ATRIP (ab19351-100; Abcam), and BRCA1 (sc-6954; Santa Cruz).
RESULTS
Identification of Sen1/senataxin interaction partners.
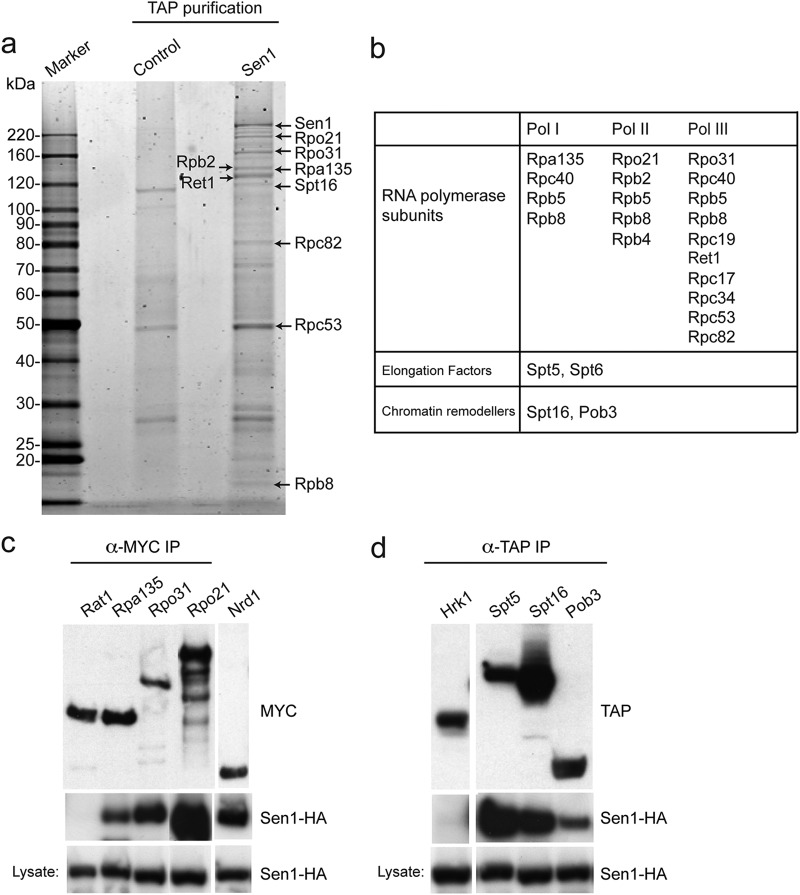
To gain insight into the molecular functions of senataxin, we identified novel interaction partners using affinity purification of human senataxin, as well as its S. cerevisiae ortholog Sen1. Previously, it was shown that SEN1 is an essential gene in yeast and that loss-of-function mutations lead to the accumulation of tRNA and rRNA precursors, mislocalization of core snoRNPs, and the 3′ extension and transcriptional read-through of some snoRNAs, snRNPs, and short protein-encoding mRNAs (9, 10). Consistent with a role in transcription, a combination of global and candidate-specific two-hybrid screens had identified the Rpo21 (Rpb1) subunit of RNA polymerase II (RNAPII) as an interaction partner of Sen1 (9). When affinity purification of Sen1-TAP was carried out, our mass-spectrometric analysis identified Rpo21 together with additional RNAPII subunits such as Rpb2 and Rpb4 (Fig. 1a and b; also, see Table S1 in the supplemental material). However, a number of RNA polymerase I (e.g., Rpa135) and RNA polymerase III (e.g., Rpo31) core subunits, as well as elongation factors and components of the FACT complex, were also identified. The interactions between Sen1 and Rpa135 (RNAPI), Rpo21 (RNAPII), Rpo31 (RNAPIII), Spt5 (transcription elongation), and Spt16 and Pob3 (FACT) were confirmed by pulldown assays using Myc and TAP affinity-tagged proteins (Fig. 1c and d). As negative controls in these experiments we used Rat1 protein (which interacts with the transcription apparatus and, like Sen1, is involved in transcription termination) and Hrk1 (a protein that is expressed at cellular levels similar to those of Sen1). These results indicate that Sen1 is a general transcription factor, consistent with observations showing that sen1 mutants exhibit defects in RNAPI, RNAPII, and RNAPIII transcription (10, 20).
Fig 1.
Yeast Sen1 protein interacts with components of the general transcription machinery. (a) Tandem affinity purification of C-terminally TAP-tagged Sen1 from yeast. Proteins were analyzed by PAGE and stained with SYPRO Ruby, and the bands were excised and identified by mass spectrometry. Control lane, parallel purification from wild-type cells. (b) Partial list of proteins identified by mass spectrometry as possible Sen1 interaction partners following TAP-Sen1 affinity purification. (c and d) Pulldown assays were performed with several of the interaction partners identified by MS. (c) C-terminally HA-tagged Sen1 was pulled down from extracts using C-terminally Myc-tagged Rpa135, Rpo21, Rpo31, or Nrd1. Myc-tagged Rat1, a transcription terminator, was used as a noninteracting control. The Myc-tagged proteins were detected using anti-Myc antibodies. Sen1-HA was detected using anti-HA antibodies. (d) Spt5, Spt16, and Pob3 were C-terminally TAP tagged in cells also expressing Sen1-HA. Tandem affinity purification of the TAP-tagged proteins pulled down Sen1-HA, as detected by Western blotting. Hrk1-TAP, which was expressed at cellular levels similar to those of Sen1, served as a negative control.
Sen1 is known to form a complex with Nrd1 and Nab3, and the Nrd1-Nab3-Sen1 complex plays a role in the termination of small nucleolar RNAs and other short RNAs (21–23). Consistent with these data, we detected interactions of Sen1 with both Nrd1 and Nab3 using pulldown assays and Western blotting (Fig. 1c and data not shown). However, we did not detect Nrd1 or Nab2 in the affinity-purified Sen1-TAP fraction analyzed by mass spectrometry. One possibility is that only a small fraction of Sen1 within the cell is present in a stable complex with Nrd1-Nab3, such that these proteins were below our detection limit. Alternatively, the role that Sen1 plays in transcription termination with Nrd1-Nab3 may be distinct from a more general role in DNA dynamics that takes place independently of Nrd1-Nab3.
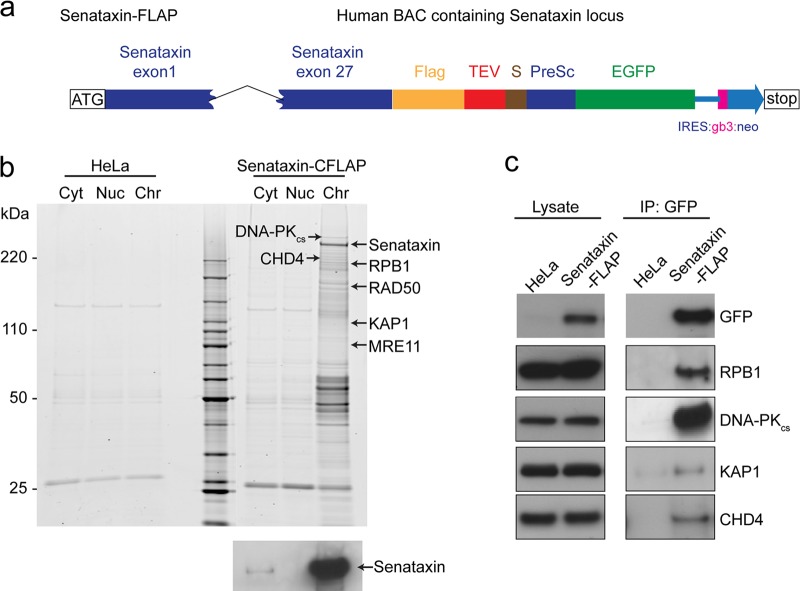
Next, similar experiments were carried out with human senataxin, using stable HeLa cell lines in which the SETX gene was expressed from a bacterial artificial chromosome (BAC). Senataxin was therefore expressed at endogenous levels in human cells, but with C-terminal Flag and GFP tags that enabled affinity purification (Fig. 2a). As a control we used the same cell line (HeLa) but without the BAC. Subcellular fractionation revealed that senataxin was found primarily in the chromatin fraction (Fig. 2b), and mass-spectrometric analysis of this fraction led to the identification of the RNA polymerase II core subunits RPB1, RPB2, and RPB3 and other transcription-associated proteins as senataxin interaction partners (Fig. 2b; also, see Table S2 in the supplemental material). We also identified transcription elongation factors, replication proteins such as RFC2, RFC4, and RFC5, the chromatin remodelers KAP1 and CHD4, and the DNA repair proteins DNA-PKcs, MRE11, and RAD50. Interactions between senataxin and RPB1, DNA-PKcs, KAP1, and CHD4 were confirmed by Western blotting of the Flag and GFP affinity-purified senataxin fraction (Fig. 2c). In contrast to our observations with Sen1, we did not observe RNA polymerase I or III core subunits associated with senataxin, suggesting that the actions of senataxin are restricted to RNA polymerase II transcription.
Fig 2.
Human senataxin interacts with components of the RNA polymerase II transcription machinery. (a) Schematic diagram of the BAC carrying C-terminally FLAP-tagged senataxin. In addition to the Flag and EGFP tags, TEV and prescission (PreSc) sites are indicated. (b) Senataxin was affinity purified using the Flag and EGFP tags from different subcellular fractions prepared from HeLa cells (control) or HeLa cells stably expressing senataxin-FLAP. Proteins were analyzed as described for Fig. 1. The blot at the bottom shows that senataxin localizes primarily to the chromatin fraction (Chr), as determined by Western blotting with antisenataxin antibody. Cyt and Nuc, cytoplasmic and nucleoplasmic fractions, respectively. (c) Senataxin was affinity purified from HeLa cells (with or without the senataxin BAC) using the Flag and GFP tags. The association of senataxin with RNA Pol II (RPB1), DNA-PKcs, KAP1, and CHD4 was confirmed by Western blotting.
Senataxin forms nuclear foci in S/G2- and G1-phase cells.
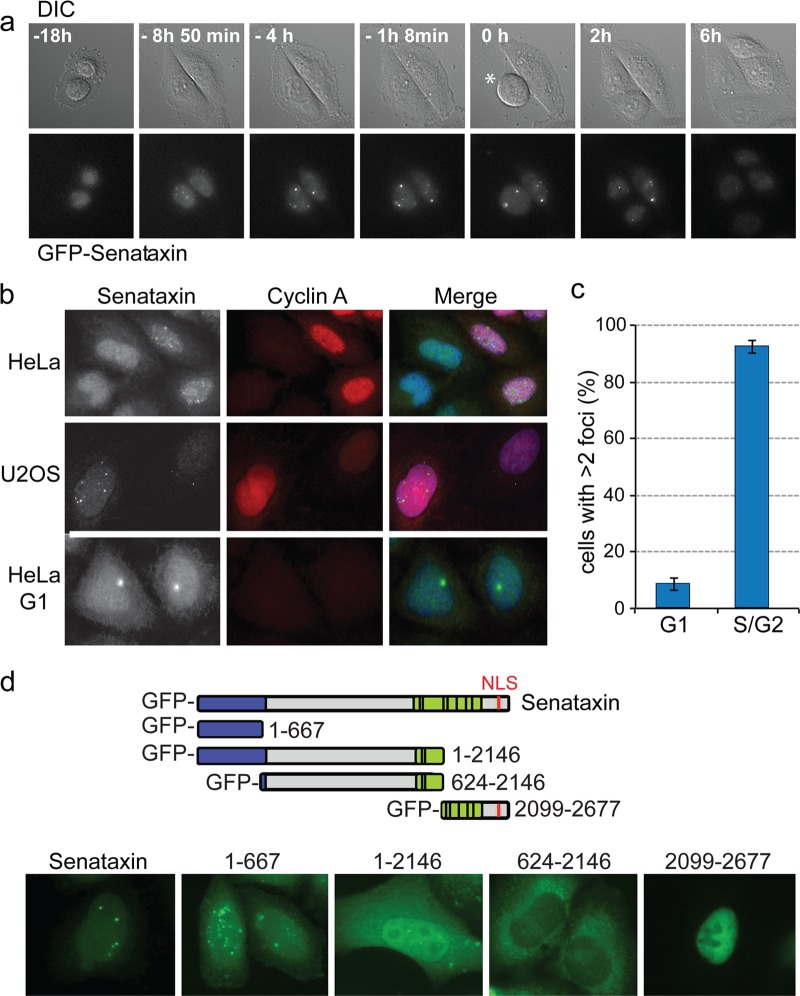
The observation that senataxin was found primarily in the chromatin fraction is important in light of apparently contradictory reports suggesting that senataxin localizes to the nucleus (24) or to the nucleolus (16). We therefore performed live-cell imaging of the senataxin BAC cell lines and visualized senataxin-GFP using automated wide-field microscopy. These studies revealed that the protein forms a small number of nuclear foci approximately 4 h prior to mitosis (Fig. 3a), which in HeLa cells corresponds to the end of S phase and the beginning of G2. At mitotic division, however, some cells had two large visible foci, and these segregated equally into the daughter cells (see Movie S1 in the supplemental material).
Fig 3.
Immunofluorescence imaging of senataxin foci. (a) Live-cell imaging of HeLa cells transfected with a BAC encoding a FLAP-tagged senataxin. Cells were visualized using differential interference contrast (DIC) and GFP (senataxin) channels. Time zero was defined as the point when the cell indicated with an asterisk entered mitosis. (b) Immunofluorescence imaging of senataxin and cyclin A in fixed HeLa or U2OS cells. Endogenous senataxin was detected using anti-senataxin antibody 1. (c) Quantification of senataxin foci in G1 (cyclin A-negative) and S/G2 (cyclin A-positive) cells (300 cells counted). (d) Identification of the region of senataxin required for S/G2 specific foci. The diagram indicates the five GFP-labeled senataxin constructs expressed in HeLa cells. Senataxin was visualized by GFP immunostaining.
To ensure that the localization pattern observed with the GFP-tagged senataxin, expressed from the BAC, was representative of that of endogenous senataxin, HeLa and U2OS cells were fixed and coimmunostained with antibodies raised against senataxin and cyclin A (used as a marker for S and G2 phases of the cell cycle). An average of 10 to 12 senataxin foci were found in 91% of the cyclin A-positive cells, whereas only a small number of foci (1 or 2) were observed in the cyclin A-negative G1 cells (Fig. 3b and c).
To more precisely correlate the timing of focus formation with the cell cycle, HeLa cells were arrested in S phase using a double thymidine block and fixed at different times after release for immunostaining with a variety of specific markers. Using the rDNA marker protein UBF, we observed that in some cells, senataxin foci were indeed present in the nucleoli at S phase (see Fig. S1a in the supplemental material). However, as the cells progressed from S into G2, approximately 4 h after release, the senataxin foci were distributed throughout the nucleus (see Fig. S1a). Prior to cell division, as revealed by costaining with Smc2, senataxin foci were often found as pairs (see Fig. S1b in the supplemental material). At later stages of the cell cycle, most foci disappeared, although some persisted into mitosis and segregated into the daughter cells to form the morphologically distinct G1 foci described above (Fig. 3a and b; also, see Movie S1 in the supplemental material). One possibility is that these large G1 foci represent unresolved late-S/G2 foci that persist until mitosis and then segregate to the daughter cells. Alternatively, some form of DNA lesion or replication problem might be transmitted to the daughter cells, where they serve as targets for renewed senataxin interaction.
Senataxin protein has two conserved domains: an N-terminal domain, which is required for protein-protein interactions, and a C-terminal domain which contains the RNA/DNA helicase motifs together with a nuclear localization sequence (NLS). Most senataxin mutations associated with AOA2/ALS4 are found within these domains (14, 25–28). To determine which regions of the protein were important for the formation of nuclear foci, full-length GFP-tagged senataxin (2,677 amino acids), GFP-senataxin1–667, GFP-senataxin1–2146, GFP-senataxin624–2146, and GFP-senataxin2099–2677 were transiently expressed in HeLa cells. We found that full-length GFP-senataxin, or fragments containing the N-terminal region of the protein (GFP-senataxin1–667 and GFP-senataxin1–2146), formed nuclear S/G2 foci in a manner similar to that observed with endogenous senataxin or the senataxin-GFP expressed from the BAC (Fig. 3d). In contrast, the C-terminal fragment of senataxin that contained the NLS (GFP-senataxin2099–2677) localized to the nucleus but failed to form foci, whereas the larger fragment lacking the NLS (GFP-senataxin624–2146) was predominantly cytoplasmic. These results indicate that the N-terminal protein-protein interaction domain of senataxin is critical for the formation of nuclear S/G2 nuclear foci, most likely through interactions with other nuclear proteins, and that focus formation can occur in the absence of the C-terminal region containing the helicase domain and the NLS. However, greater numbers of foci were seen with GFP-senataxin1–667 than with full-length senataxin, indicating that the C-terminal region of the protein (containing the RNA/DNA helicase domain) is required for the turnover of these nuclear foci.
Senataxin foci are induced by replication stress.
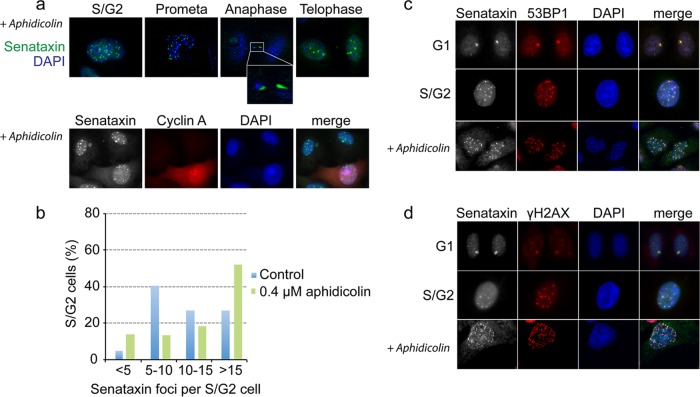
Because the number of senataxin foci was greatest in S/G2 phase, at the time when DNA replication takes place, we determined whether their formation might be linked to replication problems. To do this, cells were treated for 24 h with a low dose of aphidicolin, an agent that selectively impairs replication fork progression through physiological barriers (29). Aphidicolin-induced replication stress resulted in a 2-fold increase in the number of senataxin foci in S/G2-phase cells (Fig. 4a, top, and b). Moreover, aphidicolin treatment led to an increase in the number of unresolved senataxin foci in prometaphase cells, and these foci persisted until mitosis. In some aphidicolin-treated cells, senataxin was coincident with DAPI-stained anaphase bridges (Fig. 4a, anaphase; also, see Fig. S2 in the supplemental material). Aphidicolin treatment also increased the number of large foci present in untreated G1 cells from an average of 1 or 2 in untreated cells to more than 4 foci per cell after treatment (Fig. 4a, bottom; see G1 cells that are not stained with cyclin A). These results indicate that senataxin foci form in response to replication stress.
Fig 4.
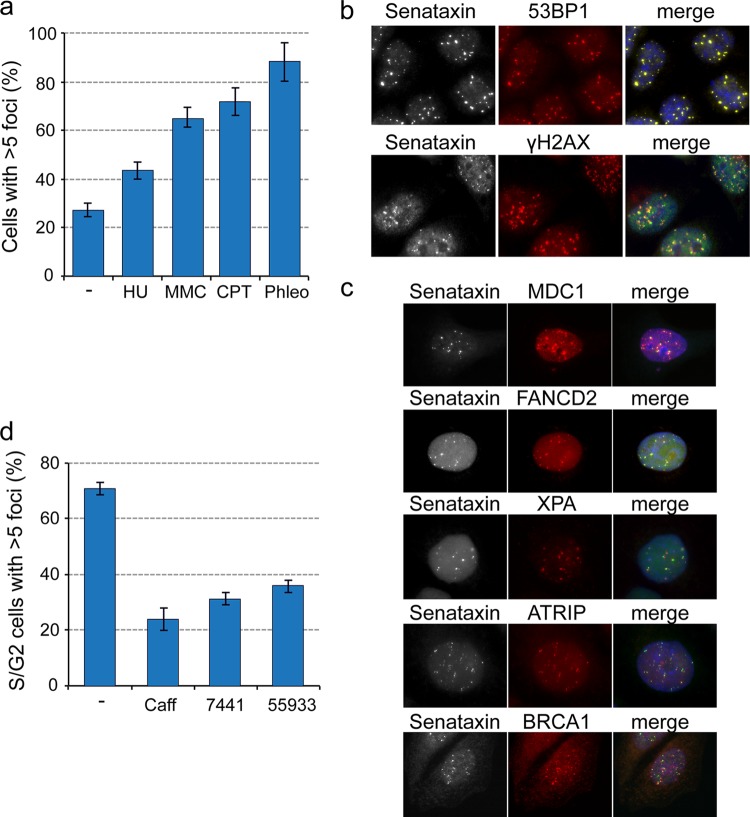
Senataxin foci increase upon replication stress and colocalize with 53BP1 and H2AX. (a) Cells were treated with 0.4 μM aphidicolin for 24 h, fixed, and immunostained with the indicated antibodies. (Top) Cells taken at different mitotic stages. (Bottom) Costaining with cyclin A indicates that replication stress induces senataxin foci. (b) Quantification of senataxin foci in S/G2-phase HeLa cells (n = 120) treated with or without aphidicolin. (c and d) Cells were treated as described for panel a, and senataxin foci were colocalized with 53BP1 and H2AX.
Senataxin colocalizes with the 53BP1 DNA damage response protein.
Previously, it was shown that 53BP1 foci are induced by replication stress and that G1 cells contain large nuclear bodies that contain 53BP1 (30, 31). Since these bodies arise in parallel in both daughter cells, the observations made with 53BP1 are very similar to those obtained here with senataxin. We therefore asked whether 53BP1 and senataxin colocalize within such nuclear bodies. 53BP1 and senataxin were found to colocalize perfectly within the large foci that arise in G1 cells and also in the numerous small foci found in S/G2 cells (Fig. 4c). Moreover, both proteins colocalized in foci that formed in response to aphidicolin treatment. Like 53BP1, senataxin colocalized with γH2AX in G1 and S/G2 cells (Fig. 4d). These results show that senataxin colocalizes with 53BP1/γH2AX, which are known to mark sites of spontaneous DNA lesions and, by inference, that senataxin colocalizes with 53BP1/γH2AX in the large, transcriptionally active nuclear bodies known as OPT (Oct1, PTF, transcription) domains that form at fragile sites in G1 cells (29–31).
To determine whether the induction and colocalization of senataxin/53BP1 foci were a general response to DNA damage-induced replication stress, cells were treated with a variety of DNA-damaging agents, including hydroxyurea (HU), mitomycin C (MMC), cisplatin (CPT), and phleomycin (Phleo). Exposure to all four agents increased the number of senataxin foci compared with the untreated control (Fig. 5a). Phleomycin was found to be the most effective for inducing senataxin foci, which again colocalized well with both 53BP1 and γH2AX (Fig. 5b). Like 53BP1, senataxin also colocalized with a variety of DNA damage signaling and repair factors, including MDC1, FANCD2, XPA, ATRIP, and BRCA1 (Fig. 5c). Since ATM kinase and DNA-PK are integral components of the DNA damage response, we analyzed the effect of the ATM/ATR inhibitor caffeine, the ATM inhibitor KU55933, and the DNA-PK inhibitor NU7441 on the levels of senataxin foci in S/G2 cells. We found that all three treatments led to a reduction in the number of senataxin foci (Fig. 5d).
Fig 5.
Colocalization of senataxin with factors involved in the DNA damage response. (a) HeLa cells were treated with hydroxyurea (HU), mitomycin C (MMC), cisplatin (CPT), or phleomycin (Phleo) for 4 h prior to fixation and immunostaining. DMSO was used as a control. The graph shows the percentage of cells (n = 230) with >5 senataxin foci. (b) Phleomycin-treated cells were immunostained with antibodies against senataxin, 53BP1, or H2AX. (c) HeLa cells were synchronized using a double thymidine block, and 7 h after release, the cells in G2 were immunostained for senataxin and the indicated repair factors. (d) HeLa cells were treated for 24 h with caffeine, the DNA-PK inhibitor NU7441, the ATM inhibitor KU55933, or DMSO (control) prior to fixation and immunostaining for senataxin and cyclin A. The graph shows the percentage of cyclin-A positive (S/G2) cells (n = 235) that had >5 senataxin foci.
Senataxin nuclear foci are linked to transcription-induced RNA/DNA hybrids.
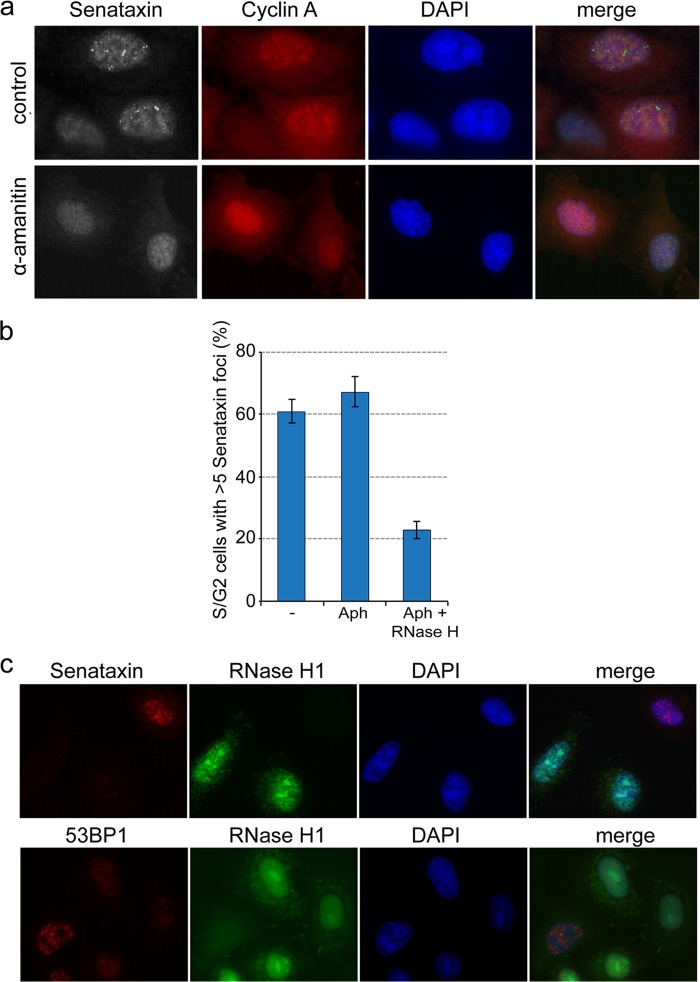
Our observations of potential links between senataxin, transcription, replication stress, and the DNA damage response next led us to investigate whether senataxin foci might form as a consequence of collisions between the replisome and the transcription machinery. We therefore treated cells for 24 h with α-amanitin, which inhibits RNA polymerase II-mediated transcription (32). We found that α-amanitin treatment suppressed the formation of senataxin foci in S/G2 cells compared with the untreated control cells (Fig. 6a). Since we did not observe any changes to the cellular levels of senataxin protein (data not shown), these results indicate that the formation of senataxin foci is dependent upon transcription.
Fig 6.
Senataxin foci correlate with the formation of transcription-induced RNA/DNA hybrids. (a) HeLa cells were treated for 24 h with or without the transcription inhibitor α-amanitin (50 μM) prior to fixation and immunostaining. (b) HeLa cells, grown on a coverslip, were treated with or without aphidicolin for 24 h and permeabilized for 10 min with Tween 20 at room temperature, followed by incubation with or without RNase H1 for 10 min. Cells were fixed and immunostained for senataxin and cyclin A. The percentage of S/G2 cells (n = 250) with >5 senataxin foci was quantified for each condition. (c) GFP-tagged RNase H1 was transiently expressed in HeLa cells for 24 h before the cells were fixed and immunostained for senataxin, 53BP1, and GFP antibodies.
It has been suggested that Sen1/senataxin is required for the processing of RNA/DNA hybrids (R loops) formed at transcription pause sites and that the persistence of these structures leads to genome instability (1, 6, 8, 18). We therefore analyzed the relationship between senataxin foci and RNA/DNA hybrid formation by first treating cells with aphidicolin and then permeabilizing them for treatment with purified RNase H1 in order to resolve any R loops that might be present. This treatment led to a decrease in the number of senataxin foci (Fig. 6b). These results were corroborated by the transient expression of GFP-tagged human RNase H1 in HeLa cells, which resulted in the loss of senataxin foci in cells that expressed GFP-tagged RNase H1. Cells that failed to express RNase H1 exhibited normal levels of senataxin foci (Fig. 6c, top). Similar results were obtained with 53BP1 foci, which were seen only in cells that failed to express GFP-tagged RNase H1 (Fig. 6c, bottom).
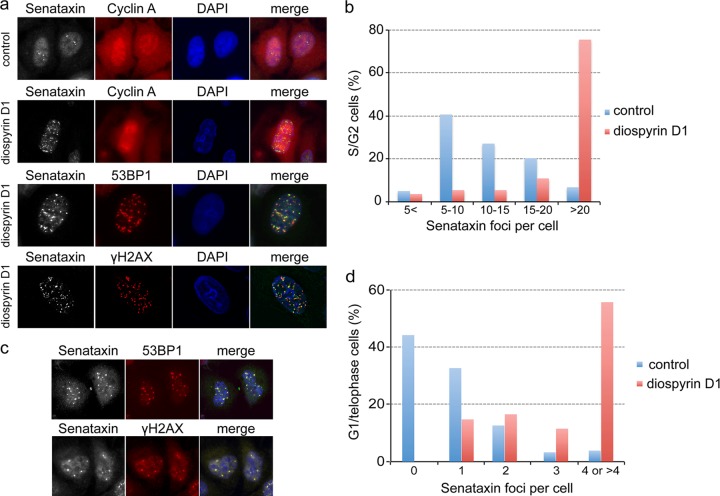
The results described above indicate that senataxin foci, like 53BP1 foci (30, 31, 33), are likely to be associated with the formation of RNA/DNA hybrids and/or R-loop-dependent genome instability. To confirm this notion, cells were treated with diospyrin D, an inhibitor of topoisomerase I (34), which is known to increase the number of RNA/DNA hybrids by inhibiting the ASF/SF2-dependent assembly of mRNPs (35–37). Treatment with diospyrin for 24 h resulted in a large increase in the number of senataxin foci per cell (Fig. 7a, top). Quantification revealed that the majority of the control S/G2-phase cells had between 5 and 10 foci per cell, whereas after diospyrin treatment, 75% of the S/G2 cells contained more than 20 foci (Fig. 7b). The increase in senataxin foci was mirrored by a similar increase in both 53BP1 and γH2AX foci, both of which colocalized with senataxin (Fig. 7a, bottom). Diospyrin treatment also increased the number of unresolved senataxin foci in mitosis, which later segregated into the daughter cells and were visualized as multiple G1 foci, where they again colocalized with 53BP1 and γH2AX (Fig. 7c and d). Taken together, these results confirm that senataxin foci identify transcription-directed RNA/DNA hybrids that form in response to replication stress. 53BP1 and other DNA repair proteins also colocalize to these sites, establishing a direct link between transcription problems and the DNA damage response.
Fig 7.
Increased RNA/DNA hybrid formation stimulates the accumulation of senataxin foci. (a) HeLa cells grown on a coverslip were treated with or without 10 μM diospyrin for 24 h, fixed, and immunostained for the indicated proteins. (b) Control and diospyrin-treated cells were immunostained, and the number of senataxin foci present in S/G2 cells (n = 140) was measured. (c) Diospyrin-treated cells in G1 were immunostained for the indicated proteins. (d) Senataxin foci were quantified in G1/telophase cells (n = 120) following treatment with diospyrin.
DISCUSSION
In this work, we have shown that senataxin, a protein that interacts directly with RNA Pol II (17) and is involved in the termination of transcription at pause sites (18), forms distinct nuclear foci in S/G2-phase cells. These foci increase in number following replication stress and colocalize with 53BP1, a protein that plays a key role in the DNA damage response. Treatment of cells with α-amanitin (which inhibits transcription) or transient expression of RNase H1 (which digests RNA/DNA hybrids) reduced the number of senataxin foci, indicating that their formation is dependent upon transcription and involves the formation of R-loop structures that most likely arise when replication forks collide with the transcription apparatus. In support of this concept, the number of senataxin foci increased significantly when cells were treated with the topoisomerase I inhibitor diospyrin D, which increases the number of RNA/DNA hybrid structures during transcription. Taken together, these results show that senataxin lies at the interface of replication stress, transcription, and the DNA damage response.
Previous studies have shown that replication is required for R-loop formation and that persistent R loops can lead to genome instability (2, 6, 18, 38). Our work supports the concept that R loops arise as a result of impaired replication fork progression, and in particular that collisions between the replisome and the transcription apparatus lead to the accumulation of R-loop structures that need to be resolved in order to maintain genome stability. R-loop formation is favored at certain genomic loci where replication fork progression is problematic, such as common fragile sites, telomeres, and repetitive sequences. Transcription at such sites is often slow due to long gene length or the presence of long introns, leading to collisions between the transcription apparatus and the replisome. These sequences therefore pose physiological barriers to replication in G2 and have the potential to induce genome instability unless processed efficiently. The localization of senataxin to such sites, where it colocalizes with the damage response proteins 53BP1 and γH2AX, increases upon replication stress or treatment with DNA-damaging agents. Senataxin, through its interaction with RNA Pol II, would therefore be ideally situated to utilize its putative RNA/DNA helicase activity in order to promote R-loop resolution.
Cells about to undergo mitosis were found to contain two large senataxin foci that divided equally between the daughter cells, in a localization pattern that is very similar to that observed with 53BP1. The 53BP1 foci that arise in G1 cells are thought to be due to the mitotic transmission of incompletely replicated DNA and/or unresolved replication intermediates (30, 31). Also known as OPT domains, these regions accumulate 53BP1 and γH2AX, increase in number following aphidicolin-induced replication stress, and correspond to common fragile sites (30, 31). Our observations showing that senataxin and 53BP1 colocalize to these G1 nuclear bodies imply that senataxin, in coordination with a variety of DNA repair factors, is likely to be important for the processing of chromosome lesions following their transmission to daughter cells.
It has been shown that in yeast, mutations in Sen1 lead to increased genome instability linked to the presence of RNA/DNA hybrids (8). R loops that persist in Sen1-defective cells are processed into DNA double-strand breaks that are subsequently repaired by homologous recombination (HR). In human cells, particularly in G1, nonhomologous end joining (NHEJ) rather than HR provides the favored mechanism for DSB repair. However, excessive use of NHEJ, as might occur when cells are subjected to replication stress, can lead to gene rearrangements, deletions, and mutations, typified by the rearrangements found at common fragile sites. It is thought that the large 53BP1 foci in G1 cells serve to protect DNA breaks from NHEJ-mediated repair until HR can occur in the subsequent S phase and in so doing avoid the potential for DNA rearrangements and genome instability (39).
Loss of senataxin activity would be expected to lead to an increased persistence of R-loop structures, which in turn would be processed into DSBs that will be repaired by NHEJ or HR, depending on the timing in the cell cycle. Consistent with this proposal, small interfering RNA (siRNA)-mediated depletion of senataxin leads to an increase in the number of 53BP1 foci (K. Radhakrishnan and S. C. West, unpublished data). We therefore suggest that senataxin may play a key role in stabilizing fragile site sequences from inappropriate processing reactions that lead to genome instability. In AOA-2, where the disease is primarily manifested in postreplicative neurons, there would be little opportunity for HR (due to the lack of replication), and repair would occur by NHEJ, increasing the chances for genome instability. Moreover, the increased number of persistent unresolved RNA/DNA hybrids, or DNA breaks, in senataxin-deficient neurons would act as transcription-blocking lesions, as suggested previously for the 5′-adenylates that persist in aprataxin-defective AOA-1 cells (40, 41). Senataxin might therefore be regarded as a DNA repair enzyme that selectively dissociates RNA/DNA hybrids that would otherwise have the potential to cause genome instability.
There are many examples of links between defects in DNA repair proteins and neurodegeneration syndromes (42). For example, ataxia telangiectasia is caused by mutations in the DNA damage signaling kinase ATM, whereas ataxia telangiectasia-like disorder (ATLD) and Nijmegen breakage syndrome (NBS) are caused by mutations in two components of the MRN (Mre11/Rad50/Nbs) complex, MRE11 and NBS1, respectively. These proteins all play important roles in DSB repair. Defects in proteins important for single-strand break repair also lead to neurodegenerative disease, such as that seen in individuals with AOA-1, spinocerebellar ataxia with axonal neuropathy (SCAN1), or xeroderma pigmentosum (XP). Defective DNA repair in mature neural tissues has also been linked to ageing, as well as to neurodegenerative diseases such as Parkinson's disease and Alzheimer's disease. In this regard, the role that senataxin may play in limiting RNA/DNA hybrid formation in long genes may be particularly important, since the transcription of some fragile-site genes, such as the WWOX, gene which spans the second most common fragile site, FRA16D (43), is known to be downregulated in neurological disorders such as Alzheimer's disease (44).
Supplementary Material
ACKNOWLEDGMENTS
We thank Robert Crouch (NIH) for the GFP-RNase HI expression construct, Tim Hunt (LRI) for cyclin A antibodies, Tony Hyman for the BAC tagging cassettes, Ross Chapman for the anti-MDC1 antibody, and Joao Matos, Kanagaraj Radhakrishnan, Hannah Mischo, and Jesper Svejstrup for their input. We are grateful to Sarah Maslen and Mark Skehel in the CRUK Protein Analysis and Proteomics unit for mass spectrometry analysis and the CRUK Cell Services and Fermentation units for their help.
This work was supported by Cancer Research UK, the European Research Council, the Louis-Jeantet Foundation, and the Swiss Bridge Foundation. O.Y. was a recipient of an EMBO long-term fellowship.
Footnotes
Published ahead of print 12 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01195-12.
REFERENCES
- 1. Helmrich A, Ballarino M, Tora L. 2011. Collisions between replication and transcription complexes cause common fragile site instability at the longest human genes. Mol. Cell 44: 966–977 [DOI] [PubMed] [Google Scholar]
- 2. Wahba L, Amon JD, Koshland D, Vuica-Ross M. 2011. RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol. Cell 44: 978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD. 2005. Activation of the DNA damage checkpoint and genome instability in human precancerous lesions. Nature 434: 907–913 [DOI] [PubMed] [Google Scholar]
- 4. Huertas P, Aguilera A. 2003. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol. Cell 12: 711–721 [DOI] [PubMed] [Google Scholar]
- 5. Tuduri S, Crabbé L, Conti C, Tourierre H, Holtgreve-Grez H, Jauch A, Pantesco V, De Vos J, Thomas A, Theillet C, Pommier Y, Tazi J, Coquelle A, Pasero P. 2009. Topoisomerase I suppresses genomic instability by preventing interference between replication and transcription. Nat. Cell Biol. 11: 1315–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gan W, Guan Z, Liu J, Shen K, Manley JL, Li X. 2011. R-loop-mediated genome instability is caused by impairment of replication fork progression. Genes Dev. 25: 2041–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim HD, Choe J, Seo YS. 1999. The Sen1(+) gene of Schizosaccharomyces pombe, a homologue of budding yeast SEN1, encodes an RNA and DNA helicase. Biochemistry 38: 14697–14710 [DOI] [PubMed] [Google Scholar]
- 8. Mischo H, Gómez-González B, Grezechnik P, Rondón AG, Wei W, Steinmetz LM, Aguilera A, Proudfoot NJ. 2011. Yeast Sen1 helicase protects the genome from transcription-associated instability. Mol. Cell 41: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ursic D, Chinchilla K, Finkel JS, Culbertson MR. 2004. Multiple protein/protein and protein/RNA interactions suggest roles for yeast DNA/RNA helicase Sen1p in transcription-coupled repair and RNA processing. Nucleic Acids Res. 32: 2441–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ursic D, Himmel KL, Gurley KA, Webb F, Culbertson MR. 1997. The yeast SEN1 gene is required for the processing of diverse RNA classes. Nucleic Acids Res. 25: 4778–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finkel JS, Chinchilla K, Ursic D, Culbertson MR. 2010. Sen1p performs two genetically separable functions in transcription and processing of U5 small nuclear RNA in Saccharomyces cerevisiae. Genetics 184: 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinmetz EJ, Warren CD, Kuehner JN, Panbehi B, Ansari AZ, Brow DA. 2006. Genome-wide distribution of yeast RNA polymerase II and its control by Sen1 helicase. Mol. Cell 24: 735–746 [DOI] [PubMed] [Google Scholar]
- 13. Rasmussen TP, Culbertson MR. 1998. The putative nucleic acid helicase Sen1p is required for formation and stability of termini and for maxima; rates of synthesis and levels of accumulation of small nucleolar RNAs in Saccharomyces cerevisiae. Mol. Cell. Biol. 18: 6885–6896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moreira MC, Klur S, Watanabe M, Nemeth AH, Le Ber I, Moniz JC, Tranchant C, Aubourg P, Tazir M, Schols L, Pandolfo M, Schulz JB, Pouget J, Calvas P, Shizuka-Ikeda M, Shoji M, Tanaka M, Izatt L, Shaw CE, M'Zahem A, Dunne E, Bomont P, Benhassine T, Bouslam N, Stevanin G, Brice A, Guimaraes J, Mendonca P, Barbot C, Coutinho P, Sequeiros J, Durr A, Warter JM, Koenig M. 2004. Senataxin, the ortholog of a yeast RNA helicase, is mutant in ataxia-ocular apraxia 2. Nat. Genet. 36: 225–227 [DOI] [PubMed] [Google Scholar]
- 15. James PA, Talbot K. 2006. The molecular genetics of non-ALS motor neuron disease. Biochim. Biophys. Acta 1762: 986–1000 [DOI] [PubMed] [Google Scholar]
- 16. Chen YZ, Hashemi SH, Anderson SK, Huang Y, Moreira MC, Lynch DR, Glass IA, Chance PF, Bennett CL. 2006. Senataxin, the yeast Sen1p orthologue: characterization of a unique protein in which recessive mutations cause ataxia and dominant mutations cause motor neuron disease. Neurobiol. Dis. 23: 97–108 [DOI] [PubMed] [Google Scholar]
- 17. Suraweera A, Lim Y, Woods YRA, Birrell GW, Nasim T, Becherel OJ, Lavin MF. 2009. Functional role for senataxin, defective in ataxia oculomotor apraxia type 2, in transcription regulation. Hum. Mol. Genet. 18: 3384–3396 [DOI] [PubMed] [Google Scholar]
- 18. Skourti-Stathaki K, Proudfoot NJ, Gromak N. 2011. Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent transcription. Mol. Cell 42: 794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lavin MF, Gueven N, Grattan-Smith P. 2008. Defective responses to DNA single- and double-strand breaks in spinocerebellar ataxia. DNA Repair 7: 1061–1076 [DOI] [PubMed] [Google Scholar]
- 20. Kawauchi J, Mischo H, Braglia P, Rondon A, Proudfoot NJ. 2008. Budding yeast RNA polymerases I and II employ parallel mechanisms of transcription termination. Genes Dev. 22: 1082–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jamonnak N, Creamer TJ, Darby MM, Schaughency P, Wheelan SJ, Corden JL. 2011. Yeast Nrd1, Nab3, and Sen1 transcriptome-wide binding maps suggest multiple roles in post-transcriptional RNA processing. RNA 17: 2011–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terzi N, Churchman LS, Vasiljeva L, Weissman JS, Buratowski S. 2011. H3K4 trimethylation by Set1 promotes efficient termination by the Nrd1-Nab3-Sen1 pathway. Mol. Cell. Biol. 31: 3569–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vasiljeva L, Kim M, Mutschler H, Buratowski S, Meinhart A. 2008. The Nrd1-Nab3-Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nat. Struct. Mol. Biol. 15: 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suraweera A, Becherel OJ, Chen P, Rundle N, Woods RA, Nakamura J, Gatei M, Criscuolo C, Filla A, Chessa L, Fusser M, Epe B, Gueven N, Lavin MF. 2007. Senataxin, defective in ataxia oculomotor apraxia 2, is involved in the defence against oxidative DNA damage. J. Cell Biol. 177: 969–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen YZ, Bennett CL, Huynh HM, Blair IP, Puls I, Irobi J, Dierick I, Abel A, Kennerson ML, Rabin BA, Nicholson GA, Auer-Grumbach M, Wagner K, de Jonghe P, Griffin JW, Fischbeck KH, Timmerman V, Cornblath DR, Chance PF. 2004. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am. J. Hum. Genet. 74: 1128–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Criscuolo C, Mancini P, Sacca F, De Michele G, Monticelli A, Santoro L, Scarano V, Banfi S, Filla A. 2004. Ataxia with oculomotor apraxia type 1 in southern Italy. Neurology 64: 2173–2175 [DOI] [PubMed] [Google Scholar]
- 27. Fogel BL, Perlman S. 2006. Novel mutations in the senataxin DNA/RNA helicase domain in ataxia with oculomotor apraxia 2. Neurology 67: 2083–2084 [DOI] [PubMed] [Google Scholar]
- 28. Duquette A, Roddier K, McNabb-Baltar J, Gosselin I, St-Denis A, Dicaire MJ, Lisel L, Labuda D, Marchand L, Mathuieu J. 2005. Mutations in senataxin responsible for Quebec cluster of ataxia with neuropathy. Ann. Neurol. 57: 408–414 [DOI] [PubMed] [Google Scholar]
- 29. Durkin SG, Glover TW. 2007. Chromosome fragile sites. Annu. Rev. Genet. 41: 169–192 [DOI] [PubMed] [Google Scholar]
- 30. Lukas C, Savic V, Bekker-Jensen S, Doil C, Neumann B, Pedersen RS, Grofte M, Chan KL, Hickson ID, Bartek J, Lukas J. 2011. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat. Cell Biol. 13: 243–253 [DOI] [PubMed] [Google Scholar]
- 31. Harrigan JA, Belotserkovskaya R, Coates J, Dimitrova DS, Polo SE, Bradshaw CR, Fraser P, Jackson SP. 2011. Replication stress induces 53BP1-containing OPT domains in G1 cells. J. Cell Biol. 193: 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindell TJ, Weinberg F, Morris PW, Roeder RG, Rutter WJ. 1970. Specific inhibition of nuclear RNA polymerase II by α-amanitin. Science 170: 447–449 [DOI] [PubMed] [Google Scholar]
- 33. Dominguez-Sánchez MS, Barroso S, Gómez-González B, Luna R, Aguilera A. 2011. Genome instability and transcription elongation impairment in human cells depleted of THO/TREX. PLoS Genet. 7: e1002386 doi:10.1371/journal.pgen.1002386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tazi J, Bakkour N, Soret J, Zekri L, Hazra B, Laine W, Baldeyrou B, Lansiaux A, Bailly C. 2005. Selective inhibition of topoisomerase I and various steps of spliceosome assembly by diospyrin derivatives. Mol. Pharmacol. 67: 1186–1194 [DOI] [PubMed] [Google Scholar]
- 35. Rossi F, Labourier E, FornÉ T, Davita G, Derancourt J, Riou JF, Antoine E, Cathala G, Brunel C, Tazi J. 1996. Specific phosphorylation of SR proteins by mammalian topoisomerase I. Nature 381: 80–82 [DOI] [PubMed] [Google Scholar]
- 36. Soret J, Gabut M, Dupon C, Kohlhagen G, Stévenin J, Pommier Y, Tazi J. 2003. Altered serine/arginine-rich protein phosphorylation and exonic enhancer-dependent splicing in mammalian cells lacking topoisomerase I. Cancer Res. 63: 8203–8211 [PubMed] [Google Scholar]
- 37. Lin SCJ, Coutinho-Mansfield G, Wang DC, Pandit S, Fu X-D. 2008. The splicing factor SC35 has an active role in transcription elongation. Nat. Struct. Mol. Biol. 15: 819–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aguilera A, Garcia-Muse T. 2012. R loops: from transcription byproducts to threats to genome stability. Mol. Cell 46: 115–124 [DOI] [PubMed] [Google Scholar]
- 39. Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, Bothmer A, Feldhahn N, Fernandez-Capetillo O, Cao L, Xu XL, Deng CX, Finkel T, Nussenzweig M, Stark JM, Nussenzweig A. 2010. 53BP1 inhibits homologous recombination in BRCA1-deficient cells by blocking resection of DNA breaks. Cell 141: 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahel I, Rass U, El-Khamisy SF, Katyal S, Clements PM, McKinnon PJ, Caldecott KW, West SC. 2006. The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature 443: 713–716 [DOI] [PubMed] [Google Scholar]
- 41. Rass U, Ahel I, West SC. 2007. Defective DNA repair and neurodegenerative disease. Cell 130: 991–1004 [DOI] [PubMed] [Google Scholar]
- 42. McKinnon PJ. 2009. DNA repair deficiency and neurological disease. Nat. Rev. Neurosci. 10: 100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ludes-Meyers JH, Bednarek AK, Popescu NC, Bedford M, Aldez CM. 2003. WWOX, the common chromosomal fragile site, FRA16D, cancer gene. Cytogenet. Genome Res. 100: 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sze CI, Su M, Pugazhenthi S, Jambal P, Hsu LJ, Heath J, Schultz L, Chang NS. 2004. Down-regulation of WW domain-containing oxidoreductase induces Tau phosphorylation in vitro. A potential role in Alzheimer's disease. J. Biol. Chem. 279: 30498–30506 [DOI] [PubMed] [Google Scholar]
- 45. Ghaemmaghami S, Huh Bower W-KK, Howson RW, Belle A, Dephoure N, O'Shea EK, Weissman JS. 2003. Global analysis of protein expression in yeast. Nature 425: 737–741 [DOI] [PubMed] [Google Scholar]
- 46. Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B. 1999. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotech. 17: 1030–1032 [DOI] [PubMed] [Google Scholar]
- 47. Poser I, Sarov M, Hutchins JR, Heriche JK, Toyoda Y, Pozniakovsky A, Weigl D, Nitzsche A, Hegemann B, Bird AW, Pelletier L, Kittler R, Hua S, Naumann R, Augsburg M, Sykora MM, Hofemeister H, Zhang Y, Nasmyth K, White KP, Dietzel S, Mechtler K, Durbin R, Stewart AF, Peters JM, Buchholz F, Hyman AA. 2008. BAC transgeneomics: a high-throughput method for exploration of protein function in mammals. Nat. Methods 5: 409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suzuki Y, Holmes JB, Cerritelli SM, Sakhuja K, Minczuk M, Holt IJ, Crouch RJ. 2010. An upstream open reading frame and the context of the two AUG codons affect the abundance of mitochondrial and nuclear RNase H1. Mol. Cell. Biol. 30: 5123–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cerritelli SM, Frolova EG, Feng C, Grinberg A, Love PE, Crouch RJ. 2003. Failure to produce mitochondrial DNA results in embryonic lethality in Rnaseh1 null mice. Mol. Cell 11: 807–815 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.