Abstract
T lymphocyte dysfunction contributes to human immunodeficiency virus type 1 (HIV-1) disease progression by impairing antivirus cellular immunity. However, the mechanisms of HIV-1 infection-mediated T cell dysfunction are not completely understood. Here, we provide evidence that expansion of monocytic myeloid-derived suppressor cells (M-MDSCs) suppressed T cell function in HIV-1-infected individuals. We observed a dramatic elevation of M-MDSCs (HLA-DR−/low CD11b+ CD33+/high CD14+ CD15− cells) in the peripheral blood of HIV-1-seropositive subjects (n = 61) compared with healthy controls (n = 51), despite efficacious antiretroviral therapy for nearly 2 years. The elevated M-MDSC frequency in HIV-1+ subjects correlated with prognostic HIV-1 disease markers, including the HIV-1 load (r = 0.5957; P < 0.0001), CD4+ T cell loss (r = −0.5312; P < 0.0001), and activated T cells (r = 0.4421; P = 0.0004). Functional studies showed that M-MDSCs from HIV-1+ subjects suppressed T cell responses in both HIV-1-specific and antigen-nonspecific manners; this effect was dependent on the induction of arginase 1 and required direct cell-cell contact. Further investigations revealed that direct HIV-1 infection or culture with HIV-1-derived Tat protein significantly enhanced human MDSC generation in vitro, and MDSCs from healthy donors could be directly infected by HIV-1 to facilitate HIV-1 replication and transmission, indicating that a positive-feedback loop between HIV-1 infection and MDSC expansion existed. In summary, our studies revealed a novel mechanism of T cell dysfunction in HIV-1-infected individuals and suggested that targeting MDSCs may be a promising strategy for HIV-1 immunotherapy.
INTRODUCTION
The importance of T lymphocytes in human immunodeficiency virus type 1 (HIV-1) infection is highlighted by the existence of an elite controller population (also called long-term nonprogressors) whose ability to contain infection correlates with the presence of strong HIV-1-specific T cell responses (1, 2). In most chronically infected HIV-1 individuals, however, T cell dysfunction and depletion are common events that accelerate HIV-1 disease progression (3, 4, 5). Although the mechanisms underlying T cell dysfunction by HIV-1 infection have been studied extensively, the details remain elusive (6, 7, 8).
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of bone marrow-derived myeloid progenitors and immature myeloid cells that have been prevented from fully differentiating out of the progenitor stage under some pathological conditions, such as cancer, inflammatory diseases, and autoimmune disorders (9, 10, 11, 12, 13, 14). They migrate out of the bone marrow and accumulate in both the blood and peripheral lymphoid tissues, where they function as immune suppressors by inhibiting other immune cells. In mice, MDSCs are identified as Gr1+ CD11b+ cells. Human MDSCs are less well characterized because no uniform markers are available. However, they usually express the common myeloid markers CD33 and CD11b but lack expression of markers for mature myeloid cells, such as HLA-DR (9, 10). Due to the heterogeneous nature of these cells, MDSCs can be further divided into 2 major subsets: monocytic (M-MDSC) and granulocytic (G-MDSC). For human MDSCs, the monocytic subset contains CD14+ cells, while the granulocytic subset contains CD14− but CD15+ cells (9, 10). These 2 subtypes of MDSCs may have different biological functions and use different mechanisms for immune suppression. Characterization and functional studies of these subsets will delineate the mechanisms through which MDSCs mediate immune suppression under specific pathological conditions.
Although MDSCs have been intensively studied in cancer, their emerging roles in the pathogenesis of infectious viral diseases are just being understood (15, 16, 17, 18). It has been reported that infection with influenza A virus (IAV) in mice or human patients resulted in the expansion of MDSCs, which suppressed IAV-specific immune responses, and their function was modulated by iNKT cells. The MDSCs in IAV-infected patients have been described as immunosuppressive CD11b+ cells (16). Studies investigating hepatitis C virus (HCV) and MDSCs showed that HCV core protein-treated CD33+ mononuclear cells display a CD14+ CD11b+/low HLA-DR−/low phenotype that suppresses T cell function through upregulation of reactive oxygen species (ROS) production; chronically infected HCV patients displayed the same phenotype of MDSCs as that generated in vitro (17). In a murine model of chronic hepatitis B virus, accumulation of MDSCs was also observed in the livers of mice (15). A very recent report showed that levels of MDSCs with a CD11b+ CD33+ CD14− CD15+ phenotype, which is associated with disease progression, were elevated in HIV-1-infected individuals (18). Collectively, these reports suggest that MDSCs may represent a novel player in viral immune evasion, although MDSCs from different viral diseases may have distinct phenotypes and utilize different mechanisms for immunosuppression.
In the present study, we performed mechanistic studies to investigate MDSC expansion and its contribution to immunodeficiency in HIV-1+ subjects. In contrast to the previous reports, we observed a dramatic elevation of a monocytic subset of MDSCs (HLA-DR−/low CD11b+ CD33+/high CD14+ CD15−) in HIV-1+ subjects compared with healthy controls. The level of monocytic MDSCs correlated strongly with HIV-1 disease progression. HIV-1-derived M-MDSCs were functionally suppressive to T cell responses through induction of arginase 1 (ARG1) and required direct cell contact. Moreover, we found that direct HIV-1 infection or exposure to HIV-1-encoded protein Tat could drive MDSC generation in vitro, and MDSCs may serve as direct targets of HIV-1 infection, indicating that a positive-feedback loop existed between HIV-1 infection and M-MDSC accumulation. Taken together, our study revealed that expansion of monocytic MDSCs may represent a novel mechanism of T cell dysfunction in HIV-1-infected individuals and suggested that targeting MDSCs is potentially useful for HIV-1 immunotherapy.
MATERIALS AND METHODS
Ethics statement.
This research was approved by the Ethics Review Board of Guangzhou No. 8 People's Hospital and the Ethics Review Board of Sun Yat-Sen University. Written informed consent was provided by study participants and/or their legal guardians.
Patients and healthy donors.
HIV-1-infected patients (n = 61) were recruited at No. 8 People's Hospital (Guangzhou Infectious Disease Hospital, Guangzhou, China). For enrollment in the study, only HIV-1-infected individuals without obvious secondary infections (identified by history, clinical manifestation, and blood tests) and who had not received any therapy for at least 3 months prior to the study were included. Some enrolled HIV-1+ patients (25/61) were followed for almost 2 years during highly active antiretroviral therapy (HAART), and blood samples were harvested at various weekly time points post-HAART. Healthy controls (n = 51) were a group of local volunteers who were seronegative for HIV-1 and had no reported history of chronic illness or intravenous drug use. The basic characteristics of HIV-1+ subjects and healthy donors are outlined in Table 1.
Table 1.
Basic characteristics of HIV-1-infected individuals and healthy donors
| Baseline characteristic | Value |
|
|---|---|---|
| HIV infected | Healthy control | |
| Subjects (n) | 61 | 51 |
| Female [no. (%)] | 29 (48) | 25 (49) |
| Male [no. (%)] | 32 (52) | 26 (51) |
| Age [yr (range)] | 38 (16–60) | 36 (18–59) |
| CD4 absolute median no. of cells/μl (range) | 167.5 (4–568) | 827 (478–1,329) |
| CD4 median % (range) | 25.5 (8.5–68.8) | 47 (32–65) |
| CD8 absolute median no. of cells/μl (range) | 753 (190–1,972) | 457 (203–767) |
| CD8 median % (range) | 30 (11.1–68.9) | 28 (17–36) |
| HIV RNA median copies/ml (range) | 2.497 × 104 (4 × 102–1.06 × 106) | NAa |
NA, not applicable.
PBMC isolation and flow cytometric analysis.
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll centrifugation and analyzed immediately or cryopreserved at −80°C in 80% fetal calf serum, 10% RPMI 1640 (Invitrogen, Grand Island, NY), and 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO). The following anti-human antibodies were purchased from eBioscience (San Diego, CA): CD11b-fluorescein isothiocyanate (FITC), CD33-phycoerythrin (PE), HLA-DR–PE–Cy5, CD14-PE-Cy7, CD15-eFluor450, CD4-PE, CD8a-FITC, CD38-PE-Cy5, CD8-PE-Cy5, CD3-PE-Cy5, and their corresponding isotype controls. The following anti-human antibodies were from BD Biosciences (San Jose, CA): CD3-PE-Cy7, CD195 (CCR5)-allophycocyanin (APC)-Cy7, CD184 (CXCR4)-PE-Cy7, CD4-V-500, and APC-Cy7-IgG2 and V-500-IgG2 isotype antibodies. The cell phenotype was analyzed by flow cytometry on a flow cytometer (BD LSR II; BD Biosciences, San Jose, CA), and data were analyzed with the CellQuest program (Becton, Dickinson, Mountain View, CA). Data were acquired as the fraction of labeled cells within a live-cell gate set for 50,000 events. For the flow cytometric sorting, a BD Influx machine (BD Biosciences) was used. The strategy for MDSC sorting was HLA-DR−/low CD11b+ CD33+/high cells from live PBMCs. Depletion of MDSCs was performed by harvesting the remaining PBMCs after MDSC sorting. 4′,6-Diamidino-2-phenylindole (DAPI) (1 μg/ml; Roche, Basel, Switzerland) was used to distinguish live cells from dead cells. The experiments were performed in a biosafety laboratory.
IFN-γ ELISPOT.
Ninety-six-well plates were coated with anti-human gamma interferon (IFN-γ) antibody (U-Cytech). PBMCs were cultured on the coated plates at 2.5 × 105 cells/well and stimulated with a pool of HIV-1 gag peptides (2.5 μg/ml) or left unstimulated (negative control) in complete medium for 24 h. The cells were then washed and incubated overnight at 4°C with another biotinylated anti-IFN-γ antibody (U-Cytech, The Netherlands). Reactions were visualized using Streptavidin-alkaline phosphatase (AP) conjugate (BD PharMingen) and 5-bromo-4-chloro-3-indolylphosphate (BCIP)/nitroblue tetrazolium (NBT) substrate (Pierce, Rockford, IL). The number of spots per 106 PBMCs, which represented the number of IFN-γ-producing cells, was calculated with an enzyme-linked immunospot (ELISPOT) plate reader (Bio-Sys GmbH, Karben, Germany).
T cell proliferation assay.
T cell proliferation was evaluated by CFSE (5,6-carboxyfluorescein diacetate, succinimidyl ester) dilution. Purified T cells were labeled with CFSE (3 μM; Invitrogen), stimulated with anti-CD3/CD28 antibodies (5 μg/ml; eBioscience), and cultured alone or cocultured with autologous MDSCs at the indicated ratios for 3 days. The cells were then stained for surface marker expression with CD4-PE or CD8-PE-Cy5 antibodies, and T cell proliferation was analyzed on a flow cytometer (BD LSR II; BD Biosciences, San Jose, CA). For antigen-specific T cell responses, PBMCs were labeled with CFSE, followed by stimulation with HIV-1 gag-specific peptides.
ELISA.
IFN-γ quantification in culture supernatants was determined using an enzyme-linked immunosorbent assay (ELISA) following the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Arginase activity assay.
The activity of arginase was measured in cell lysates. Briefly, cells were lysed with 0.1% Triton X-100 for 30 min, followed by the addition of 25 mM Tris-HCl and 10 mM MnCl2. The enzyme was activated by heating for 10 min at 56°C. Arginine hydrolysis was performed by incubating the lysate with 0.5 M l-arginine at 37°C for 120 min. The urea concentration was measured at 540 nm after the addition of α-isonitrosopropiophenone (dissolved in 100% ethanol), followed by heating at 95°C for 30 min.
NO production.
The NO content in plasma was measured following the manufacturer's protocol (Biovision, Milpitas, CA). Plasma samples (150 μl) were first mixed with ZnSO4 (8 μl) and vortexed, and then NaOH (8 μl) was added, followed by centrifugation for 10 min at 14,000 rpm. One hundred microliters of the deproteinized supernatants was transferred to a clean tube, mixed with Greiss reagent, and incubated for 10 min at 60°C. The absorbance at 550 nm was measured using a microplate reader (Bio-Rad, Hercules, CA). Nitrite concentrations were determined by comparing the absorbance values for the test samples to a standard curve generated by serial dilution of 0.25 mM sodium nitrite.
Quantitative reverse transcription (qRT)-PCR.
RNA was extracted with an RNase Minikit, and cDNA was synthesized using a SuperScript III reverse transcriptase kit (Qiagen, Valencia, CA). PCR was performed as described previously (19). Briefly, reactions were done in triplicate using SYBR Green (TaKaRa, Otsu, Japan) and normalized to endogenous cyclophilin mRNA levels using gene-specific primers for each target (see Table S1 in the supplemental material). For quantification of HIV-1 RNA gag copy numbers in MDSCs or CD4 T cells, we used GAG-for and GAG-rev primers for amplification (see Table S1 in the supplemental material). Standard curves were generated from 10-fold serial dilutions of known concentrations of a synthetic HIV-1 transcript. The copy numbers of the synthetic HIV-1 standard were calculated from its quantity and molecular weight. Triplicate reactions were run, and the number of template copies was calculated based on the standard curve (20).
Nested RT-PCR.
RNA from PBMCs or MDSCs was extracted with an RNase Minikit (Qiagen) and used for amplification of viral gag or partial env genes (V1-C3 region). The first-round PCR was performed using a one-step RT-PCR kit (TaKaRa, Otsu, Japan), and second-round PCR was performed with High Fidelity Prime Star (TaKaRa, Otsu, Japan), following the manufacturer's instructions. The primers used for this experiment are listed in Table S1 in the supplemental material. The PCR products were confirmed by sequencing. The first-round primers for gag were GAL-L and GAL-E2, and the second-round primers were GUX and GDX. The first-round primers for env were enF1 and enR1, and the second-round primers were EC1FA and EC3RA.
Measurement of viral load.
Plasma HIV-1 RNA was quantified by PCR (Cobas Amplicor test; Roche Diagnostic, Basel, Switzerland).
HIV-1 production and infection.
To generate viruses, 293T cells were transfected with the HIV-1 infectious clone NL4-3 (X4) or YU-2 (R5) using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Culture supernatants were harvested at 48 h posttransfection. To normalize viral inputs, the amount of p24 was measured by HIV-1 p24 ELISA (Abnova, Walnut, CA). The target cells (1 × 106) were infected with the equivalent of 10 ng or 20 ng HIV-1 p24 in 1 ml for 3 h at 37°C. The virus-containing supernatants were then removed by washing 3 times with PBS. HIV-1 replication postinfection was monitored by p24 analysis.
Transwell assays.
The MDSC-T cell coculture experiments were performed with Transwells to determine the cell contact dependency of the MDSC function. For these studies, MDSCs isolated by flow cytometric sorting were cultured in Transwell inserts (0.4-μm pore; Millipore, Billerica, MA), and fresh autologous T cells were cultured in 96-well plates. For T cell proliferation assays, MDSCs were cocultured with CFSE-labeled T cells at different ratios in the presence of anti-CD3/CD28 stimuli for 3 days. Proliferation of T cells was evaluated by flow cytometry. For the effect of MDSCs on HIV-1 replication, MDSCs were cocultured with HIV-1-infected T cells at different ratios with Transwells, as described above, and culture supernatants were harvested on different days for HIV-1 p24 measurement.
Statistics.
Clinical and immunological parameters were compared by nonparametric Mann-Whitney U tests. For in vitro experiments, statistical analyses were done using paired t tests. Changes of MDSC levels in clinical patients between various time points during HAART were evaluated by one-way analysis of variance (ANOVA), followed by least significant difference (LSD) t tests for pairwise comparisons. Correlations between different parameters were analyzed using a Spearman rank test. Statistical tests were performed using GraphPad Prism version 5.0a and SPSS Statistics 17.0. P values of <0.05 were considered significant.
RESULTS
Dramatic elevation of levels of monocytic MDSCs in the peripheral blood of HIV-1-infected individuals despite prolonged HAART.
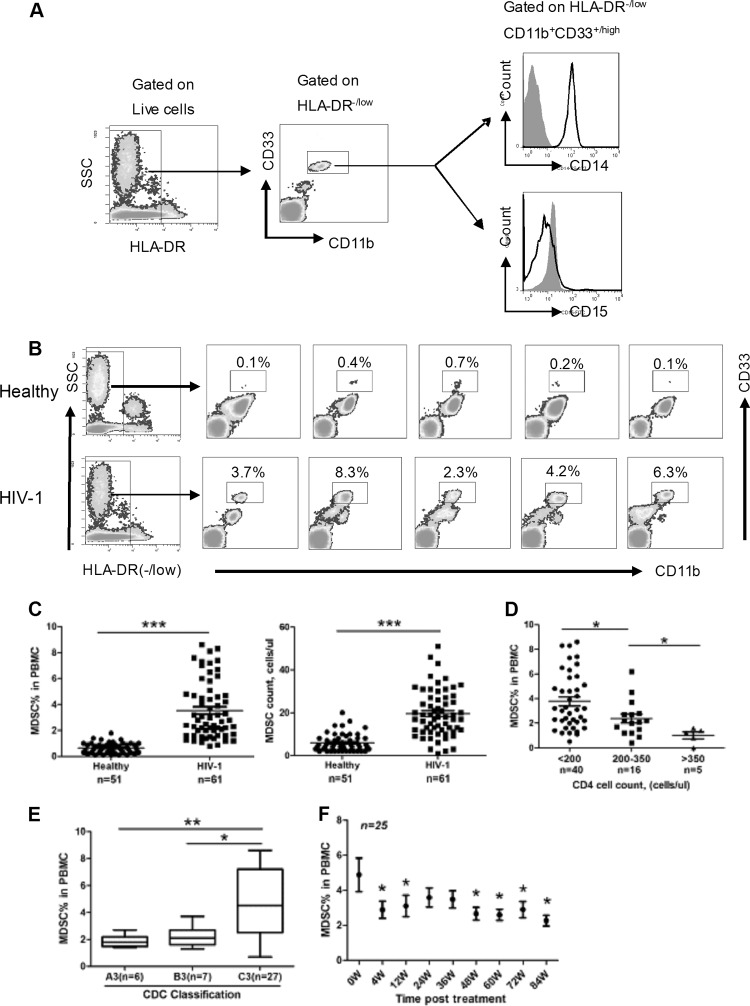
To determine whether MDSCs play a role in HIV-1 disease pathogenesis, we first compared the MDSC frequencies and absolute cell counts in the peripheral blood from HIV-1-seropositive subjects (n = 61) with those of healthy subjects (n = 51). The characteristics of the enrolled HIV-1+ subjects and healthy donors are summarized in Table 1. We collected the PBMCs from healthy and HIV-1-infected subjects and analyzed MDSC frequency by flow cytometry. We observed a clear population of HLA-DR−/low CD11b+ CD33+/high MDSCs in the samples we analyzed. These cells were CD14+ but lacked expression of CD15, supporting the idea that they were a monocytic subset (Fig. 1A). There was no difference in CD14 expression in this subset between healthy donors and HIV-1+ subjects (data not shown). Importantly, the monocytic MDSCs that we observed were distinct from another granulocytic subset (CD11b+ CD33+ CD14− CD15+) of MDSCs that was reported by another group in HIV-1-infected individuals (18). In support of the difference in the identities of these 2 subsets (21), there were clearly 2 subpopulations among CD11b+ CD33+ cells from PBMCs of HIV-1+ subjects: one was CD14+, and the other was CD14− (see Fig. S1 in the supplemental material). We found a dramatic elevation of monocytic-MDSC frequency and absolute cell counts in HIV-1+ subjects compared with healthy controls, as shown by the representative flow cytometric data in Fig. 1B and the quantified data across all samples in Fig. 1C. Meanwhile, no considerable change was observed for the granulocytic subset (CD11b+ CD33+ CD14− CD15+) in HIV-1+ donors in our study (data not shown). When we further examined the M-MDSC levels in the HIV-1+ subjects with different baseline CD4+ T cell count categories, we found that the percentage of M-MDSCs was inversely correlated with CD4+ T cell counts, as subjects in the category with CD4 counts of <200 cells/μl displayed significantly higher M-MDSC percentages than those in the category with CD4 counts of >200 cells/μl (Fig. 1D). We also compared the M-MDSC frequencies in HIV-1+ subjects at different clinical stages, as defined by the U.S. Centers for Disease Control and Prevention (CDC) classification system, which is based on CD4+ T cell counts and symptoms indicative of immune dysfunction (22). HIV-1-infected subjects in advanced stages (C3, with a CD4 count of <200 cells/μl and demonstrating AIDS symptoms) had higher M-MDSC levels than those in early stages (A3 and B3, with a CD4 count of <200 cells/μl and an asymptomatic condition [A3] or non-AIDS symptoms [B3]) (Fig. 1E), further supporting the idea that M-MDSC frequency in HIV-1+ subjects is closely associated with disease progression.
Fig 1.
Expansion of monocytic MDSCs in HIV-1-infected individuals. (A) Gating strategy of M-MDSCs by flow cytometry analysis. HLA-DR−/low cells were first selected from live PBMCs, and the CD11b+ CD33+/high population was further gated as M-MDSCs. The expression of cell surface markers CD14 and CD15 on this population was subsequently evaluated. Black, HIV-1; gray, isotype. SSC, 1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate. (B) Representative flow cytometry data for M-MDSCs from HIV-1+ donors and healthy controls. The boxed areas represent the percentage of HLA-DR−/low CD11b+ CD33+/high cells in PBMCs. (C) Statistical analysis of M-MDSC frequency (left) and absolute cell counts (right) in the peripheral blood from HIV-1+ donors (n = 61) and healthy controls (n = 51). ***, P < 0.0001. (D and E) Statistical analysis of M-MDSC frequency in PBMCs from HIV-1+ donors with different CD4+ T cell counts (D) or at different clinical stages (E) according to the CDC classification system (A3, B3, and C3, CD4 counts of <200 cells/μl, from early to advanced stages: A3, asymptomatic condition; B3, non-AIDS symptoms; C3, AIDS symptoms). (C and D) Horizontal bars represent the mean and standard error of the mean (SEM). (E) The boxes' bars represent the maximum and minimum values of MDSC% in PBMC. The upper and lower portions of these boxes represent the interquartile range. Horizontal lines represent the median in the boxes. (F) Changes in M-MDSC frequencies in HIV-1-infected individuals undergoing HAART (n = 25). Symbols and bars represent median and interquartile range (D to F) *, P < 0.05; **, P < 0.01 (statistically significant difference from controls).
HAART is currently the standard clinical treatment to suppress viral replication in HIV-1-infected individuals (23, 24). Since HAART lowers viral titers, subsequently allowing CD4+ T cell counts to rise, we asked whether M-MDSC levels were normalized by HAART administration in HIV-1+ subjects. PBMCs were collected from a group of HIV-1+ patients undergoing HAART (n = 25) at various time points over a period of 84 weeks to monitor MDSC levels. Each patient in the study experienced complete viral suppression and gained CD4 counts in response to HAART (see Fig. S2 in the supplemental material). The data revealed that the M-MDSC frequency clearly decreased from baseline levels at week 4; M-MDSC levels then fluctuated but maintained a lower level than before HAART (week 0) during the entire period of therapy (Fig. 1F). The remarkable decrease in MDSC frequency at week 4 coincided with the dramatic decline in plasma viremia at that time, indicating a potential direct link between M-MDSC expansion and HIV-1. Patients at week 84 post-HAART still displayed a significantly higher frequency of M-MDSCs than healthy controls (see Fig. S3 in the supplemental material), suggesting that HAART administration failed to normalize M-MDSC levels.
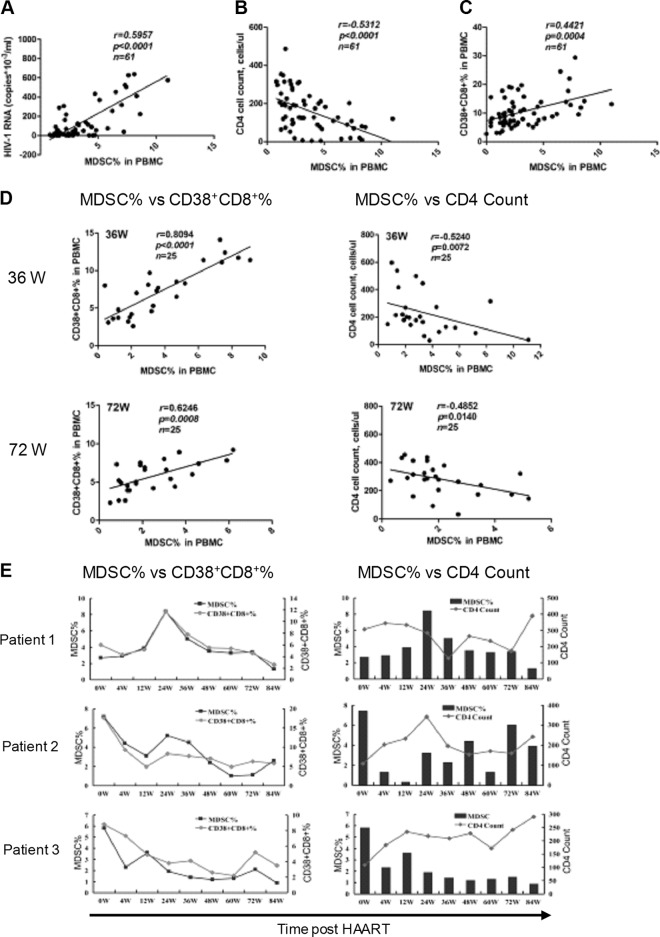
M-MDSC frequency correlated with HIV-1 disease markers.
To further investigate the clinical significance of M-MDSCs in HIV-1 pathogenesis, we evaluated the correlation between M-MDSC levels and HIV-1 disease prognostic markers, including the plasma viral load and CD4+ T cell counts in HAART-naïve HIV-1+ subjects (n = 61). The results showed that the M-MDSC levels correlated well with plasma HIV-1 RNA (r = 0.5957; P < 0.0001) (Fig. 2A) while inversely correlating with CD4+ counts (r = −0.5312; P < 0.0001) (Fig. 2B). Consistent with previous reports (25) and supporting the validity of our studies, we found and confirmed a negative correlation between plasma HIV-1 RNA and CD4+ counts (data not shown). In addition to the HIV-1 load and CD4+ T cell counts, chronic immune activation—mainly caused by translocation of microbial products from damaged mucosa into the bloodstream—is another factor known to be associated with clinical progression of HIV-1 (26, 27). The typical readout for this abnormal immune activation is detection of CD38+ CD8+ activated T cells in PBMCs (28, 29). As expected, the CD38+ CD8+ T cell frequency was significantly higher in HIV-1+ subjects than in controls and correlated with CD4+ T cell loss and higher plasma HIV-1 RNA levels (data not shown). When we evaluated whether M-MDSCs also correlated with this readout of chronic immune activation, we found a moderate but significant correlation between M-MDSCs and CD38+ CD8+ T cells (r = 0.4421; P = 0.0004) (Fig. 2C).
Fig 2.
Clinical significance of M-MDSCs in HIV-1 disease progression. (A to C) Correlation between M-MDSCs and HIV-1 prognostic markers, including plasma HIV-1-RNA (A), circulating CD4+ T cell counts (B), and CD38+ CD8+ activated T cells (C). (D) Correlation between M-MDSCs and HIV-1 markers in patients at 36 and 72 weeks (W) post-HAART (n = 25). (E) Dynamic changes in M-MDSCs and CD38+ CD8+ activated T cells and CD4+ T cell counts in 3 representative HIV-1+ donors post-HAART. (A to D) Correlation was evaluated by Spearman analysis. The lines in the graphs represent the regression line.
Based on the observation that M-MDSC frequency was correlated with important HIV-1 disease markers, we asked whether HAART could abrogate these associations. The results showed that the significant correlation between M-MDSCs and HIV-1 prognostic markers was maintained at both the 36- and 72-week time points post-HAART (Fig. 2D). Interestingly, the r value reflecting the correlation between MDSCs and CD38+ CD8+ T cells was even higher post-HAART than at baseline. Examination of individual patients showed dynamic changes in MDSC levels, followed closely by changes in activated CD38+ CD8+ T cells and circulating CD4+ T cell counts in response to HAART (Fig. 2E). Thus, our studies demonstrated that MDSC frequency in HIV-1+ subjects correlated well with prognostic HIV-1 disease markers both before and after HAART administration.
M-MDSCs from HIV-1+ subjects suppressed T cell responses.
MDSCs are known to suppress T cell immune responses under some pathological conditions (9, 10). Therefore, we next determined whether HIV-1-derived M-MDSCs were functionally suppressive toward T cells by performing IFN-γ ELISPOT assays for quantification of HIV-1-specific T cell responses. PBMCs harvested from HIV-1+ subjects (n = 8), with or without M-MDSC depletion, by flow cytometric cell sorting were subjected to antigen-specific stimulation with a pool of HIV-1 gag peptides, and production of IFN-γ was quantified by ELISPOT assay. In order to eliminate the effects of MDSC depletion on T cell concentrations in PBMCs, we calculated the number of spots formed based on absolute T cell numbers, in addition to PBMCs. The data showed that M-MDSC depletion significantly enhanced HIV-1-specific T cell responses from HIV-1+ subjects (Fig. 3A), suggesting that the presence of M-MDSCs actively repressed T cell function. Measurement of T cell proliferation by CFSE dilution in MDSC-replete or MDSC-depleted PBMCs stimulated with HIV-1-specific peptides gave consistent results in 5 individuals (Fig. 3B).
Fig 3.
M-MDSCs from HIV-1+ subjects suppressed T cell responses. (A and B) Depletion of M-MDSCs enhanced HIV-1-specific T cell responses. PBMCs or PBMCs with M-MDSC depletion (PBMC-MDSC) from HIV-1+ donors were stimulated with HIV-1 gag-specific peptides. (A) Production of IFN-γ was determined by ELISPOT assay in samples from 8 individuals, based on PBMCs (left) or absolute T cell numbers (right). SFU, spot-forming units. (B) Proliferation of T cells was examined by CFSE dilution. (Left) Representative flow cytometry data from 1 individual. (Right) Results for stimulated samples from 5 individuals. (C to E) Suppressive effects of M-MDSCs on T cell function in coculture experiments. (C) CD3+ T cells from HIV-1+ donors were stimulated with anti-CD3/CD28 antibodies, cocultured with M-MDSCs from the same donors at different ratios for 3 days, and evaluated for T cell proliferation by CFSE labeling. Unstimulated T cells were used as a negative control. (Left) Representative flow cytometry data from 1 individual. (Right) Results from 4 individuals. (D) Production of IFN-γ by T cells in supernatants from panel C was measured by ELISA. Means and SD are shown; n = 4. (E) MDSC-T cell coculture experiments as in panel C were performed with Transwells to determine the cell contact dependency of MDSC function. (Top) Results from 4 individuals. (Bottom) Percent inhibition of T cell proliferation by MDSCs in the presence or absence of Transwells (T cell/MDSC ratio, 2:1). The data represent means and SD for 4 donors. (A to E) *, P < 0.05, and **, P < 0.01, compared with the corresponding controls.
The suppressive effect of M-MDSCs on T cells was further confirmed in the MDSC-T cell coculture system, in which M-MDSCs purified by flow cytometric sorting from HIV-1+ subjects efficiently inhibited T cell responses to anti-CD3/CD28 stimulus, including cell proliferation (Fig. 3C) and IFN-γ production (Fig. 3D), for both CD4+ and CD8+ T cells in a dose-dependent manner. This result was consistent among M-MDSCs from 4 individual HIV-1+ subjects. In contrast, M-MDSCs from healthy subjects exhibited no suppressive effect (see Fig. S4 in the supplemental material), which is consistent with previous reports showing that MDSCs are nonsuppressive under physiological conditions (9). In order to determine whether the suppressive effects of HIV-1-derived M-MDSCs on T cells require direct cell-cell contact, MDSC-T cell coculture experiments were performed in the context of Transwells. The results showed that separation of M-MDSCs and T cells with Transwells completely eliminated the suppressive effects of M-MDSCs (Fig. 3E). Our observations from this experimental series demonstrated that M-MDSCs present in HIV-1+ subjects actively suppressed T cells in a cell contact-dependent manner, which contributes to the T cell hyporesponsiveness characteristic of HIV-1 infection.
Induction of arginase 1 was essential for M-MDSC-mediated immune suppression in HIV-1+ subjects.
Based on the observation that M-MDSCs from HIV-1+ subjects could suppress T cell responses, we further explored the underlying mechanisms controlling M-MDSC-mediated T cell suppression. l-Arginine metabolism and its metabolic products, which serve as immune mediators, are essential for MDSC-mediated immune suppression (9, 30). We therefore compared the levels of the l-arginine metabolic products arginase, NO, and ROS in the peripheral blood from HIV-1+ subjects and controls. A dramatic increase in arginase activity was observed in PBMCs from HIV-1+ subjects compared with healthy controls (Fig. 4A); in contrast, the NO content in plasma was decreased in HIV-1+ subjects compared to healthy controls (Fig. 4B). No obvious difference was found in ROS content between HIV-1+ subjects and healthy controls (data not shown). The significant changes we observed in arginase and NO levels were consistent with previous reports (31, 32). These results suggested that HIV-1 infection may change the l-arginine metabolic pathway from NO dominant to arginase dominant.
Fig 4.
Suppressive activity of M-MDSCs from HIV-1+ donors is arginase 1 dependent. (A and B) Arginase activity in PBMCs (A) and NO content in plasma (B) from HIV-1+ donors (n = 7) and healthy controls (n = 7). (A and B) Horizontal bars represent the mean and SEM. (C) Arginase activity in PBMCs and M-MDSC-depleted PBMCs (PBMC-MDSC) from HIV-1+ subjects (n = 7). (D) Expression of Arg1 and iNos in different PBMC subpopulations was examined by qRT-PCR. N, healthy donors; H, HIV-1+ donors. The values represent means and SD for 5 individuals. (E) Arg1 induction in M-MDSCs was dependent on HIV-1 infection. M-MDSCs from healthy donors were infected with different amounts of HIV-1 viral stock (containing 10 ng/ml or 20 ng/ml p24 antigen) (left) or HIV-1 stock (20 ng/ml p24) (right) in the presence of the nucleoside reverse transcriptase inhibitor Stavudine (20 μg/ml or 50 μg/ml) or vehicle control DMSO for 48 h, and the expression of Arg1 was measured by qRT-PCR. Corresponding p24 values are indicated. The values represent means and SD for 4 individuals. (F) Effect of arginase inhibitor NOHA or l-arginine supplementation on M-MDSC function. T cells from HIV-1+ subjects were stimulated with anti-CD3/CD28, cocultured with MDSCs from the same donor at a 2:1 ratio with treatments as indicated, and evaluated for T cell proliferation by CFSE labeling. l-NMMA, NOS inhibitor; NAC, ROS inhibitor. T cells alone were used as a positive control. (G) Production of IFN-γ by T cells in supernatants from panel F was measured by ELISA. (F and G) The data represent means and SD for 4 subjects. (A to G) *, P < 0.05, and **, P < 0.01, compared with the controls.
We next investigated whether the increased arginase activity in PBMCs from HIV-1+ subjects was due to the abnormal expansion of M-MDSCs. To test this, M-MDSCs were depleted from PBMCs by flow cytometric cell sorting, and arginase activity was measured. The data showed that M-MDSC depletion dramatically reduced arginase activity in PBMCs from HIV-1+ subjects (Fig. 4C), indicating that M-MDSCs are one of the major contributors to higher arginase activity in HIV-1+ subjects. ARG1 and inducible nitric oxide synthase (iNOS) are key enzymes responsible for arginase activity and production of NO, respectively, and they share the same substrate, l-arginine (33). We therefore examined the gene expression of these enzymes by qRT-PCR in different PBMC subpopulations from HIV-1+ subjects compared to healthy controls. Consistent with biochemical assays, higher Arg1 and lower iNos expression were found in M-MDSCs from HIV-1+ subjects than in those from healthy controls (Fig. 4D). We then asked whether the induction of Arg1 in M-MDSCs was caused by HIV-1 infection. In order to answer this question, purified M-MDSCs from healthy subjects were exposed to different doses of HIV-1 stocks based on p24 antigen content; the cells were then washed with PBS and cultured for 48 h in vitro. The infection efficiency was determined by p24 production in culture medium by ELISA, and expression of Arg1 was analyzed by qRT-PCR. The data showed that HIV-1 infection clearly induced Arg1 expression in M-MDSCs in a p24 dose-dependent manner. Administration of the nucleoside reverse transcriptase inhibitor Stavudine dramatically blocked HIV-1-induced Arg1 expression when viral replication was suppressed, as expected (Fig. 4E). These observations support the idea that HIV-1 infections could induce Arg1 expression in M-MDSCs.
Since l-arginine is essential for T cell proliferation, we were interested in knowing whether HIV-1-induced Arg1 expression in M-MDSCs was associated with their suppressive effects on T cell function. In order to test this possibility, MDSC-T cell coculture experiments were performed in the presence of different inhibitors of l-arginine-metabolizing enzymes. The results showed that T cell proliferation (Fig. 4F) and IFN-γ production (Fig. 4G) suppressed by the presence of M-MDSCs were almost completely recovered after administration of the arginase inhibitor Nω-hydroxy-nor-l-arginine (NOHA) or an l-arginine supplement, while no effect was observed for the nitric oxide synthase inhibitor l-NMMA or the ROS inhibitor N-acetylcysteine (NAC).
Direct HIV-1 infection promoted MDSC expansion.
The clinical and immunological significance of M-MDSCs in the pathogenesis of HIV-1 disease prompted us to investigate the mechanisms of MDSC expansion in HIV-1+ subjects. First, we explored whether direct HIV-1 infection could lead to the accumulation of MDSCs in vitro. The cytokines interleukin 6 (IL-6) and granulocyte-macrophage colony-stimulating factor (GM-CSF) have been shown to efficiently induce the suppressive MDSCs in vitro from precursors present in the PBMCs of healthy donors (34). Therefore, we utilized the system to study the effect of HIV-1 on MDSC generation. PBMCs were harvested from healthy subjects and infected with HIV-1 strain NL4-3 (X4) or YU-2 (R5). Mitogens and/or IL-2 was not administered in order to avoid the stimulation and infection of CD4+ T cells in PBMCs. The infected cells were then cultured for 6 days in the presence of GM-CSF and IL-6, followed by flow cytometry analysis for the evaluation of MDSCs. Our results showed that HIV-1 infection led to a marked increase in HLA-DR−/low CD11b+ CD33+ cells in infected PBMC cultures, which were CD14+ CD15− and shared the same MDSC phenotype found in HIV-1-infected individuals (Fig. 5A). Functional studies were further performed to verify that MDSCs generated in vitro were immunosuppressive to T cells (Fig. 5B).
Fig 5.
Direct HIV-1 infection or exposure to the HIV-1 protein Tat promoted MDSC expansion. (A) HIV-1 infection of PBMCs promoted MDSC generation in vitro. PBMCs from healthy donors were infected with HIV-1 strain NL4-3 (X4) or YU-2 (R5) and then cultured in 10 ng/ml GM-CSF and IL-6 for 6 days. (Top) Gating strategy for MDSCs. HLA-DR−/low cells were first selected from live PBMCs, and the CD11b+ CD33+ population was further gated and defined as MDSCs. The expression of CD14 and CD15 on MDSCs was subsequently evaluated. Black, HIV-1; gray, isotype. (Bottom) MDSC frequency in HIV-1-infected PBMCs or controls. (Left) Representative example from 1 individual. (Right) The data represent means and SD from 4 individuals. (B) Suppressive functions of in vitro-generated MDSCs. PBMCs from healthy donors were infected with HIV-1 strains and then cultured for 6 days as described for panel A. MDSCs were purified by flow cytometric sorting and then cocultured with autologous T cells for 3 days with anti-CD3/CD28 stimulation. T cell proliferation was subsequently evaluated by flow cytometry. The data were obtained from 4 healthy donors. (C) The HIV-1-encoded protein Tat enhanced MDSC generation. PBMCs from healthy donors were cultured in GM-CSF and IL-6 in the presence of recombinant Tat protein or the solvent PBS, and MDSC frequency was analyzed by flow cytometry. Denatured Tat protein was used as a control. (Left) Representative example from a single healthy donor. (Right) Cumulative results from 3 healthy individuals. (A to C) *, P < 0.05; **, P < 0.01; statistically different from controls.
We next examined whether some HIV-1-encoded proteins contributed to the observed MDSC expansion. Since a HIV-1-derived transcriptional transactivator (Tat) protein has been reported to block major histocompatibility complex class II (MHC-II) expression (35) and MDSCs express no or very low levels of the MHC class II protein HLA-DR, we reasoned that Tat may be associated with MDSC generation. In order to test this, we administered recombinant Tat protein (10 μg/ml) or the solvent phosphate-buffered saline (PBS) into the culture medium of PBMCs from healthy subjects and found that it significantly enhanced the generation of MDSCs, while denatured Tat at the same concentration failed to display any effects (Fig. 5C). These results suggested that HIV-1-derived Tat protein may play an important role in the generation of MDSCs. The impact of other HIV-1-encoded proteins on MDSC generation, however, remains to be explored.
M-MDSCs were novel targets for direct HIV-1 infection.
It has been demonstrated that some hematopoietic progenitor cells (HPCs) express HIV-1 receptor and coreceptors and are susceptible to HIV-1 infection (36, 37). MDSCs are a heterogeneous population of myeloid progenitor cells; thus, we were interested to determine whether M-MDSCs could be directly infected by HIV-1. In order to test this hypothesis, we first examined the mRNA expression of the primary HIV-1 entry receptor CD4 and entry coreceptors CCR5 and CXCR4 in purified M-MDSCs from healthy controls or HIV-1+ subjects by qRT-PCR. The data showed that M-MDSCs from both healthy controls and HIV-1+ subjects expressed moderate levels of HIV-1 receptor and coreceptors compared to CD4+ T cells (Fig. 6A). Next, we further determined whether CD4, CXCR4, and CCR5 proteins were present on the surfaces of M-MDSCs by flow cytometry analysis. The data revealed that both CXCR4 and CCR5 coreceptors were highly expressed on M-MDSCs, while the expression of CD4 was lower, in comparison with CD4+ T cells (Fig. 6B). This was confirmed with M-MDSCs from 5 healthy donors and 5 HIV-1+ subjects, as shown by the mean fluorescence index (MFI) (see Fig. S5 in the supplemental material). Staining with anti-CD3 and anti-CD4 antibodies demonstrated that the highly purified M-MDSCs were devoid of contaminating CD4+ T lymphocytes (data not shown). Our results indicated that M-MDSCs had the potential to be infected by HIV-1. Next, we determined whether HIV-1 had indeed infected the cells by examining the presence HIV-1-derived gag and env genes in sorted M-MDSCs from HIV-1+ subjects by nested RT-PCR. We observed clear positive gag and env bands in the M-MDSCs from 4/5 HIV-1+ subjects (Fig. 6C). Quantification of HIV-1 RNA in M-MDSCs was determined as the copy numbers of gag per million cells (Fig. 6D). Thus, our data supported the possibility that HIV-1 could directly infect M-MDSCs.
Fig 6.
Direct infection of M-MDSCs by HIV-1. (A) mRNA expression of the HIV-1 receptor CD4 and its coreceptors (CXCR4 and CCR5) in M-MDSCs from healthy or HIV-1+ donors was determined by qRT-PCR. The data represent means and SD for 5 individuals. (B) Expression of the HIV-1 receptor and its coreceptors on the surfaces of M-MDSCs was evaluated by flow cytometry analysis using the specific antibodies. The results are representative of 5 individuals. Solid lines, healthy donors; dashed lines, HIV-1+ subjects; gray, isotype. (A and B) CD4+ T cells from healthy donors were used as a positive control. (C) Detection of HIV-1-RNA in purified M-MDSCs from HIV-1+ donors by nested RT-PCR. Lanes: 1, PBMCs from healthy controls; 2, PBMCs from HIV-1+ donors; 3, M-MDSCs from healthy controls; 4, M-MDSCs from HIV-1+ donors; 5, PBMCs with M-MDSC depletion from HIV-1+ donors. The bands for gag and env are 1,081 bp and 845 bp, respectively. The data are representative of 4 out of 5 individuals. (D) Quantification of RNA copy numbers of HIV-1-gag in purified M-MDSCs and CD4 T cells from HIV-1+ subjects (n = 8). Horizontal bars represent the mean and SEM. (E) Detection of HIV-1-derived p24 capsid protein from in vitro-infected MDSCs. M-MDSCs from healthy donors were infected with HIV-1 strain NL4-3 (X4) or YU-2 (R5), and production of HIV-1 p24 was measured by ELISA on different days postinfection. The data represent means and SD for 3 donors. *, P < 0.05, and **, P < 0.01, compared with day 0. (F) Effect of M-MDSCs on HIV-1 replication. CD4+ T cells from HIV-1+ donors were infected with HIV-1 strains and then cocultured with M-MDSCs at 1:1 or 10:1 (T cell/MDSC) ratios. p24 production was measured on different days postinfection by ELISA. (G) Cell contact dependency of MDSC effects on HIV-1 replication. (Left) Experiments similar to that in panel F using an R5-tropic strain of HIV-1 were performed in Transwells, and HIV-1 replication was determined by p24 production in the supernatants. (Right) Percent increase of p24 production by MDSCs in the presence or absence of Transwells. (F and G) Uninfected T cells from the same donors were used as a negative control. The data represent means and SD for 3 HIV-1+ donors.
In order to test whether M-MDSCs could be infected de novo, M-MDSCs harvested from healthy subjects were exposed to HIV-1 strains in vitro and cultured in the presence of GM-CSF and IL-6 to maintain the MDSC phenotype. Production of the HIV-1-derived p24 capsid protein was measured in culture supernatants by ELISA over the 7-day culture period. We observed that both the R5 and X4 HIV-1 strains could infect and replicate in M-MDSCs from the 3 subjects we tested (Fig. 6E). These data provide more convincing evidence that HIV-1 could directly infect M-MDSCs.
We next determined whether MDSCs had any impact on HIV-1 replication in CD4 T cells, the major target of HIV-1, by performing an MDSC-T cell coculture experiment. CD4+ T cells from HIV-1+ subjects were stimulated with phytohemagglutinin (PHA) for 48 h to induce T cell activation, and they were then infected with the HIV-1 strains. We infected CD4+ T cells from HIV-1+ subjects due to the difficulty of in vitro virus outgrowth from samples from clinical patients (see the CD4 uninfected group in Fig. 6F). These infected CD4+ T cells were then cocultured with M-MDSCs harvested from the same subjects for 10 days, and the amounts of HIV-1 p24 antigen in culture supernatants were measured by ELISA to monitor viral replication. We found that the presence of M-MDSCs clearly enhanced HIV-1 replication at the higher MDSC/T cell ratios (1:1) compared to the lower ratio (1:10), indicating that the presence of M-MDSCs may enhance viral replication in CD4 T cells (Fig. 6F). Further Transwell experiments showed that the enhanced HIV-1 replication in CD4 T cells by MDSCs required direct cell-cell contact (Fig. 6G). Taken together, our observations indicated that M-MDSCs could facilitate HIV-1 infection and replication, which may represent another strategy for MDSCs to participate in HIV-1 pathogenesis.
DISCUSSION
Although MDSCs have been intensively explored in cancer, recent studies have shown an emerging role for MDSCs in the pathogenesis of infectious viral diseases (15, 16, 17, 18). However, the mechanisms for the aberrant expansion of MDSCs in viral diseases, as well as their immunological and clinical significance, are far from clear. Delineating these important issues will advance our understanding of the relationship between MDSCs and viral infection, which will benefit the development of immunotherapies to treat human viral diseases.
Here, we report a dramatic elevation of a monocytic subset of MDSCs in the peripheral blood of HIV-1-infected individuals. This subtype (HLA-DR−/low CD11b+ CD33+/high CD14+ CD15−) is distinct from that reported by another research group, who identified a granulocytic MDSC subset (CD11b+ CD33+ CD14− CD15+ cells) in HIV-1 patients (18). Interestingly, no considerable elevation of CD11b+ CD33+ CD14− CD15+ cells was observed in the population we studied. It has been reported that the abundance of some MDSC subsets in fresh blood samples is significantly reduced after cryopreservation (38). In order to exclude the possibility that the difference in MDSC phenotypes between our group and others was caused by sample processing, we repeated our flow cytometry analysis in both fresh and cryopreserved blood samples from enrolled subjects, and we consistently observed the elevation of M-MDSCs in HIV-1+ individuals (data not shown). Therefore, the difference in MDSC phenotypes in HIV-1-infected subjects was not caused by the sample processing. Patients with different types of cancer are known to exhibit distinct MDSC phenotypes, depending on the specific cytokine milieu produced by the tumor tissue from which the cancer is derived (9). We therefore reasoned that the difference in MDSC phenotypes in HIV-1 patients may be caused by the presence of various substrains that differ in their systemic cytokine responses, although further evaluation is needed to clarify these possibilities.
The mechanism underlying the MDSC expansion in HIV-1+ subjects is an important detail that remains to be determined. The enhanced MDSC generation in PBMCs after in vitro HIV-1 infection, as well as the strong correlation between MDSC frequency and HIV-1 viremia in clinical patients, suggested that direct HIV-1 infection may account for the MDSC expansion we observed in HIV-1+ subjects. It has been demonstrated that HPCs are susceptible to HIV-1 infection, which could cause hematopoietic defects in HIV-1+ patients, in addition to establishing latent cellular reservoirs (36, 37). Therefore, we propose that HIV-1 may conceivably infect HPCs and cause developmental defects in myeloid cells, leading to the accumulation of myeloid progenitors, such as MDSCs. The clear effect that the HIV-1-derived Tat protein had on promoting MDSC generation indicated that the Tat transcriptional transactivator may alter the transcription of key genes essential for myeloid cell development and cause accumulation of MDSCs. Interleukin 6 (Il6) and transforming growth factor-beta 1 (TGF-beta1) genes are the most likely candidates among these target genes, since they have been proven to be Tat transcriptional targets (39, 40). Additionally, the proteins are known to promote human MDSC generation in vitro (34). Elucidation of the precise mechanism mediating Tat-induced signaling leading to MDSC generation will require further exploration. Another mechanistic possibility for HIV-1-mediated MDSC expansion is that the direct infection of MDSCs by HIV-1 may lead to less apoptosis and/or a downstream block in MDSC differentiation into mature myeloid cells. Our preliminary data showed that HIV-1 infection of MDSCs did not cause significant changes in cell apoptosis or cell numbers (unpublished data), which argues against the latter possibility. However, further evidence is needed to clarify the mechanisms underlying the expansion of MDSCs in HIV-1-infected individuals.
Although the in vivo and in vitro data support the essential role of direct HIV-1 infection in driving MDSC expansion, the sustained higher MDSC frequency in clinical patients with complete viral suppression post-HAART raises the possibility that other factors may also contribute to M-MDSC accumulation in HIV-1 patients. Chronic immune activation caused mostly by microbial-product translocation through a defective mucosa into the bloodstream is known to cause systemic inflammation in HIV-1-infected individuals, even after HAART (26, 27). We argue that this inflammatory status is most likely the secondary mechanism for MDSC accumulation in HIV-1-infected individuals, since the causal relationship between inflammation and MDSC accumulation has been well established under other pathological conditions (41, 42). Furthermore, the significant correlation between MDSCs and CD38+ CD8+ activated T cells supports the latter possibility. The even higher r value, reflecting the association between M-MDSCs and activated T cells post-HAART compared with baseline, indicates that chronic immune activation and the associated inflammation could be the dominant mechanism for M-MDSC expansion when HIV-1 replication is completely suppressed by antiretroviral therapy in HIV-1 patients.
The HIV-1 infection-derived M-MDSCs suppressed T cell responses in an arginase-dependent manner. The role of arginase in HIV-1 disease has been previously described by another group that found a positive correlation between arginase activity and HIV-1 disease severity, i.e., increased arginase activity correlated with lower circulating CD4+ cell counts and higher HIV-1 viremia in HIV-1+ subjects (31). The mechanisms underlying the relationship between arginase and HIV-1 disease, however, remains unclear. Here, we showed that M-MDSC expansion was the major contributor to higher arginase levels in PBMCs from HIV-1+ subjects; this induced Arg1 expression in M-MDSCs abrogated T cell responses by depleting l-arginine, the essential amino acid for T cell proliferation, from the microenvironment (30). Thus, the data presented in the current study indicated that l-arginine supplementation or treatment with an arginase inhibitor would be potentially useful to restore T cell function in HIV-1-infected patients.
Our studies demonstrated that M-MDSCs were susceptible to HIV-1 infection in vitro. It is known that myeloid lineage cells are largely resistant to HIV-1 infection, which is associated with the higher expression levels of some restriction factors (43, 44). Therefore, it is surprising that both T-tropic and R-tropic strains could efficiently infect the myeloid progenitor MDSCs in our study. The underlying mechanisms for M-MDSC susceptibility to HIV-1 infection deserve to be explored. Our preliminary data showed that M-MDSCs from both healthy donors and HIV-1+ patients expressed significantly lower level of the myeloid restriction factors than primary monocytes from the same donors (unpublished data). This could represent a potential mechanism explaining higher susceptibility of MDSCs to HIV-1 infection. However, detailed investigations must be conducted before we can draw any conclusions. In addition to being directly infected by HIV-1, MDSCs could facilitate HIV-1 replication in CD4+ T cells in a cell contact-dependent manner. The susceptibility of MDSCs to HIV-1 infection and their enhancement of HIV-1 replication in CD4 T cells may comprise an alternative strategy for MDSCs to promote HIV-1 disease progression, in addition to their direct immunosuppressive effect on T cells. There may be a link between these 2 mechanisms, and the functional aspects of MDSCs in HIV-1 pathogenesis deserve to be explored. We are currently investigating whether HIV-1 infection of MDSCs could allow them to acquire the above-mentioned suppressive functions.
The mutual interaction between HIV-1 and MDSCs is interesting: on one hand, HIV-1 infection could promote MDSC expansion; on the other hand, MDSCs could facilitate HIV-1 infection and replication by serving as direct targets. Therefore, we speculate that a positive-feedback loop may exist between HIV-1 infection and MDSC expansion. This vicious feedback cycle may further dampen T lymphocyte function and contribute to immunodeficiency in HIV-1-infected individuals. This may represent a novel mechanism of T cell dysfunction in HIV-1 infection. However, we need to point out that even though this study suggests that direct HIV-1 infection or the HIV-1-encoded protein Tat facilitates MDSC expansion in vitro, the physiological relevance of the in vitro data to the findings in HIV-1+ subjects still needs to be carefully evaluated. Animal models, such as simian immunodeficiency virus (SIV)-infected rhesus macaques, may be used for future in vivo investigation before a causal relationship between HIV-1 infection and MDSC expansion can be fully established.
The strong correlation between the levels of M-MDSCs and HIV-1 prognostic markers has potential clinical implications. The expansion of M-MDSCs in HIV-1-infected individuals represents not only a novel important player in driving the occurrence of immune disorders, but also a potential independent biomarker to predict the progression and severity of HIV-1 disease. In conclusion, our study has shed new light on the mechanisms of T cell suppression in HIV-1 disease and suggested that targeting MDSCs may be a promising strategy for HIV-1 immunotherapy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Guangdong Innovative Research Team Program (no. 2009010058), the National Key Basic Research Program of China (973 Program, no. 2012CB524900), the Key Research Projects of the National 12th Five-Year Plan for the Prevention and Treatment of Major Infectious Diseases (no. 2012ZX10001003-003 and no. 2012ZX10001003-001), the National Natural Science Foundation of China (no. 81072397), the Natural Science Foundation of Guangdong (no. S2011020006072 and no. S2011010005587), and the Fundamental Research Funds for the Central Universities (to J.Z.).
We thank Dmitry I. Gabrilovich at the H. Lee Moffitt Cancer Center and Research Institute for critical review of the manuscript. We are grateful to the volunteers who participated in this study.
Footnotes
Published ahead of print 14 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01759-12.
REFERENCES
- 1. Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Van Baarle D, Kostense S, Miedema F, McLaughlin M, Ehler L, Metcalf J, Liu SY, Connors M. 2002. HIV-specific CD8(+) T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061–1068 [DOI] [PubMed] [Google Scholar]
- 2. Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447–1450 [DOI] [PubMed] [Google Scholar]
- 3. Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097 [DOI] [PubMed] [Google Scholar]
- 4. Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4(+) T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vranjkovic A, Crawley AM, Patey A, Angel JB. 2011. IL-7-dependent STAT-5 activation and CD8(+) T cell proliferation are impaired in HIV infection. J. Leukoc. Biol. 89:499–506 [DOI] [PubMed] [Google Scholar]
- 6. Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O'Shea A, Patel N, Van Ryk D, Wei DL, Pascuccio M, Yi L, McKinnon L, Izulla P, Kimani J, Kaul R, Fauci AS, Arthos J. 2009. The integrin alpha(4)beta(7) forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. U. S. A. 106:20877–20882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eriksson EM, Milush JM, Ho EL, Batista MD, Holditch SJ, Keh CE, Norris PJ, Keating SM, Deeks SG, Hunt PW, Martin JN, Rosenberg MG, Hecht FM, Nixon DF. 2012. Expansion of CD8(+) T cells lacking Sema4D/CD100 during HIV-1 infection identifies a subset of T cells with decreased functional capacity. Blood 119:745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morou AK, Porichis F, Krambovitis E, Sourvinos G, Spandidos DA, Zafiropoulos A. 2011. The HIV-1 gp120/V3 modifies the response of uninfected CD4 T cells to antigen presentation: mapping of the specific transcriptional signature. J. Transl. Med. 9:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gabrilovich DI, Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9:162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greten TF, Manns MP, Korangy F. 2011. Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 11:802–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F, Greten TF. 2008. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology 135:871–881 [DOI] [PubMed] [Google Scholar]
- 12. Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. 2006. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J. Immunol. 176:2085–2094 [DOI] [PubMed] [Google Scholar]
- 13. Marhaba R, Vitacolonna M, Hildebrand D, Baniyash M, Freyschmidt-Paul P, Zoller M. 2007. The importance of myeloid-derived suppressor cells in the regulation of autoimmune effector cells by a chronic contact eczema. J. Immunol. 179:5071–5081 [DOI] [PubMed] [Google Scholar]
- 14. Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. 2012. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J. Immunother. 35:107–115 [DOI] [PubMed] [Google Scholar]
- 15. Chen S, Akbar SMF, Abe M, Hiasa Y, Onji M. 2011. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin. Exp. Immunol. 166:134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, Grone HJ, Platt FM, Zambon M, Cerundolo V. 2008. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J. Clin. Invest. 118:4036–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS. 2012. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology 55:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vollbrecht T, Stirner R, Tufman A, Roider J, Huber RM, Bogner JR, Lechner A, Bourquin C, Draenert R. 2012. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS 26:F31–F37 [DOI] [PubMed] [Google Scholar]
- 19. Zhou J, Cheng P, Youn JI, Cotter MJ, Gabrilovich DI. 2009. Notch and wingless signaling cooperate in regulation of dendritic cell differentiation. Immunity 30:845–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maenetje P, Riou C, Casazza JP, Ambrozak D, Hill B, Gray G, Koup RA, de Bruyn G, Gray CM. 2010. A steady state of CD4+ T cell memory maturation and activation is established during primary subtype C HIV-1 infection. J. Immunol. 184:4926–4935 [DOI] [PubMed] [Google Scholar]
- 21. Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. 2010. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 22:238–244 [DOI] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention 1992. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm. Rep. 41(RR-17):1–19 [PubMed] [Google Scholar]
- 23. Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, Gatell JM, Domingo P, Paredes R, Sharkey M, Palmer S, Stevenson M, Clotet B, Blanco J, Martinez-Picado J. 2010. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat. Med. 16:460–465 [DOI] [PubMed] [Google Scholar]
- 24. Palmisano L, Vella S. 2011. A brief history of antiretroviral therapy of HIV infection: success and challenges. Ann. Ist. Super Sanita 47:44–48 [DOI] [PubMed] [Google Scholar]
- 25. Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, Boswell SL, Mathews WC, Bangsberg DR, Martin J, Whalen CC, Sieg S, Yadavalli S, Deeks SG, Lederman MM. 2006. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA 296:1498–1506 [DOI] [PubMed] [Google Scholar]
- 26. Appay V, Sauce D. 2008. Immune activation and inflammation in HIV-1 infection: causes and consequences. J. Pathol. 214:231–241 [DOI] [PubMed] [Google Scholar]
- 27. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 28. Chun TW, Justement JS, Sanford C, Hallahan CW, Planta MA, Loutfy M, Kottilil S, Moir S, Kovacs C, Fauci AS. 2004. Relationship between the frequency of HIV-specific CD8(+) T cells and the level of CD38(+)CD8(+) T cells in untreated HIV-infected individuals. Proc. Natl. Acad. Sci. U. S. A. 101:2464–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. 1993. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J. Acquir. Immune Defic. Syndr. 6:904–912 [PubMed] [Google Scholar]
- 30. Bronte V, Zanovello P. 2005. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 5:641–654 [DOI] [PubMed] [Google Scholar]
- 31. Cloke TE, Garvey L, Choi BS, Abebe T, Hailu A, Hancock M, Kadolsky U, Bangham CR, Munder M, Muller I, Taylor GP, Kropf P. 2010. Increased level of arginase activity correlates with disease severity in HIV-seropositive patients. J. Infect. Dis. 202:374–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cairoli E, Scott-Algara D, Pritsch O, Dighiero G, Cayota A. 2008. HIV-1 induced decrease of nitric oxide production and inducible nitric oxide synthase expression during in vivo and in vitro infection. Clin. Immunol. 127:26–33 [DOI] [PubMed] [Google Scholar]
- 33. Rodriguez PC, Ochoa AC. 2008. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol. Rev. 222:180–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lechner MG, Liebertz DJ, Epstein AL. 2010. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 185:2273–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kanazawa S, Okamoto T, Peterlin BM. 2000. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity 12:61–70 [DOI] [PubMed] [Google Scholar]
- 36. Alexaki A, Wigdahl B. 2008. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. Plos Pathog. 4:e1000215 doi:10.1371/journal.ppat.1000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell J, Bixby D, Savona MR, Collins KL. 2010. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat. Med. 16:446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. 2012. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J. Immunol. Methods 381:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scala G, Ruocco MR, Ambrosino C, Mallardo M, Giordano V, Baldassarre F, Dragonetti E, Quinto I, Venuta S. 1994. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 TAT protein. J. Exp. Med. 179:961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zauli G, Davis BR, Re MC, Visani G, Furlini G, La Placa M. 1992. Tat protein stimulates production of transforming growth factor-beta 1 by marrow macrophages: a potential mechanism for human immunodeficiency virus-1-induced hematopoietic suppression. Blood 80:3036–3043 [PubMed] [Google Scholar]
- 41. Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. 2007. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 67:10019–10026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ostrand-Rosenberg S, Sinha P. 2009. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 182:4499–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T, Pancino G, Priet S, Canard B, Laguette N, Benkirane M, Transy C, Landau NR, Kim B, Margottin-Goguet F. 2012. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat. Immunol. 13:223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pion M, Granelli-Piperno A, Mangeat B, Stalder R, Correa R, Steinman RM, Piguet V. 2006. APOBEC3G/3F mediates intrinsic resistance of monocyte-derived dendritic cells to HIV-1 infection. J. Exp. Med. 203:2887–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.