Abstract
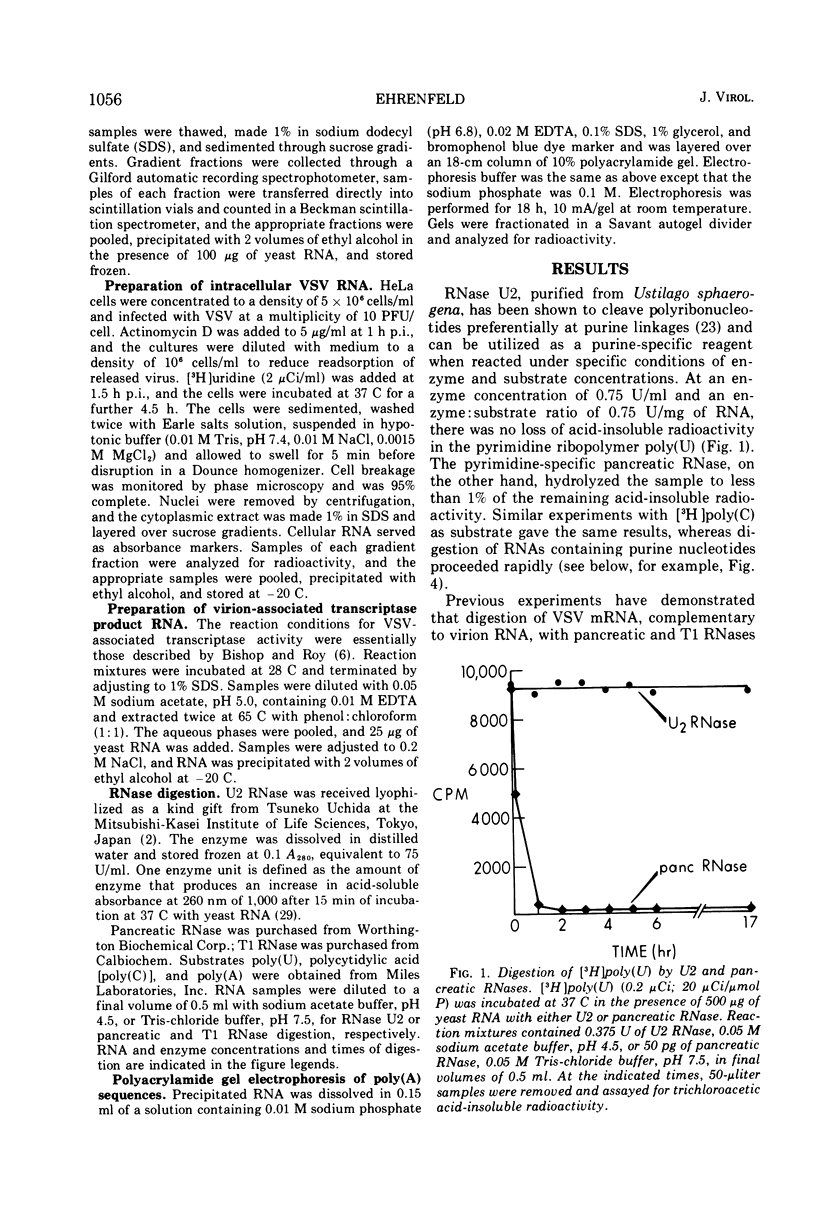
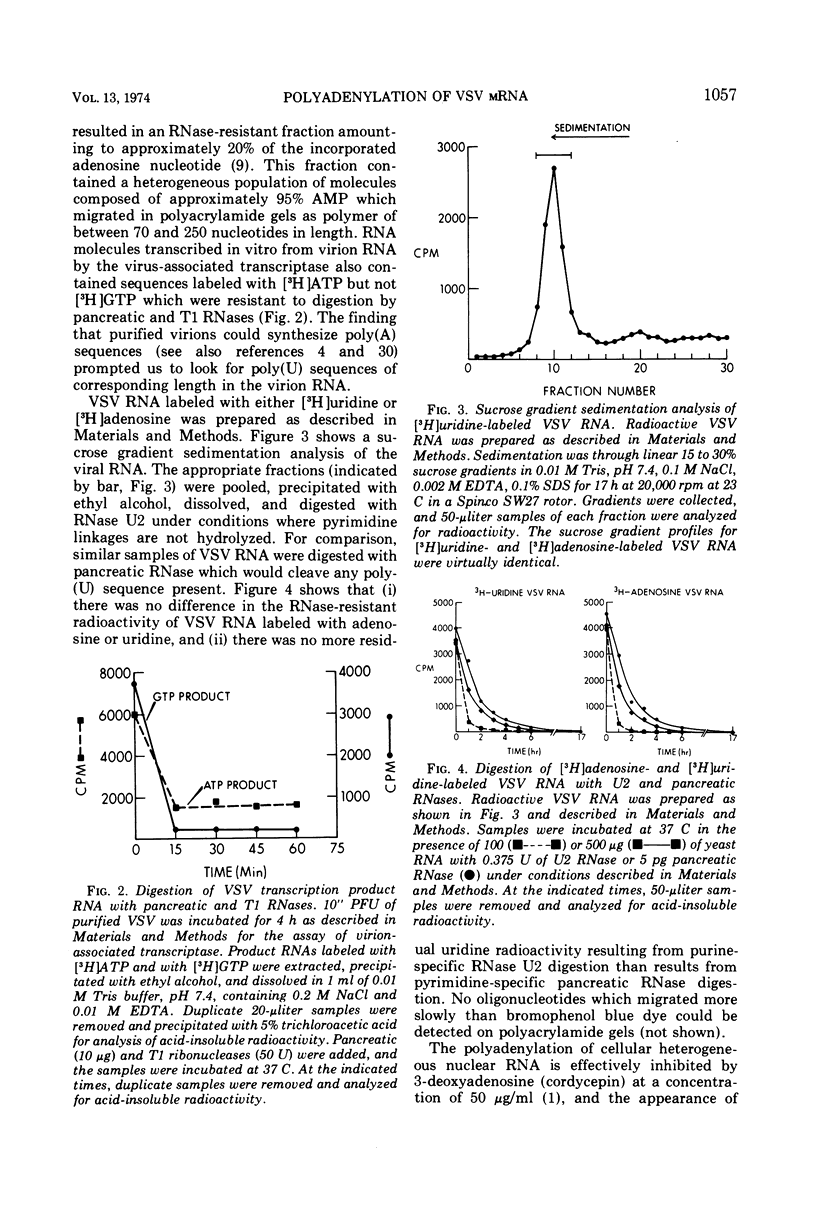
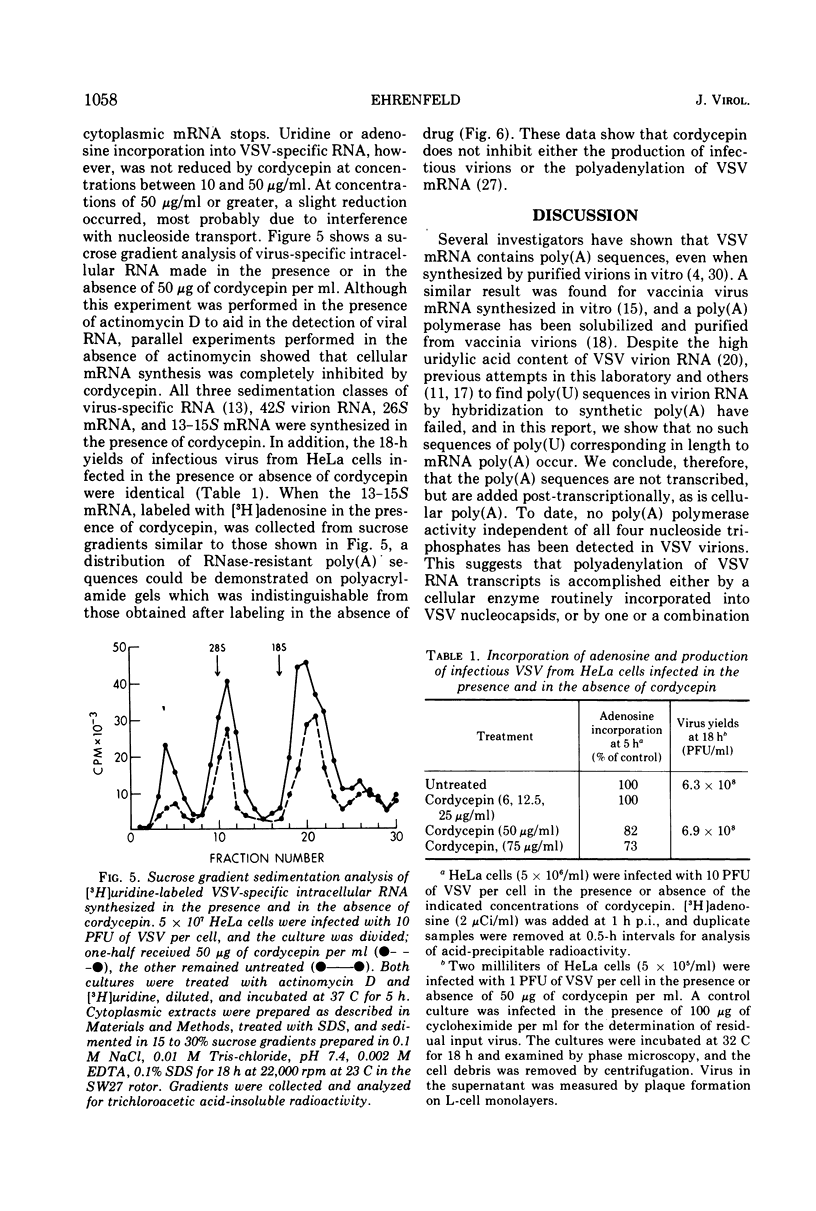
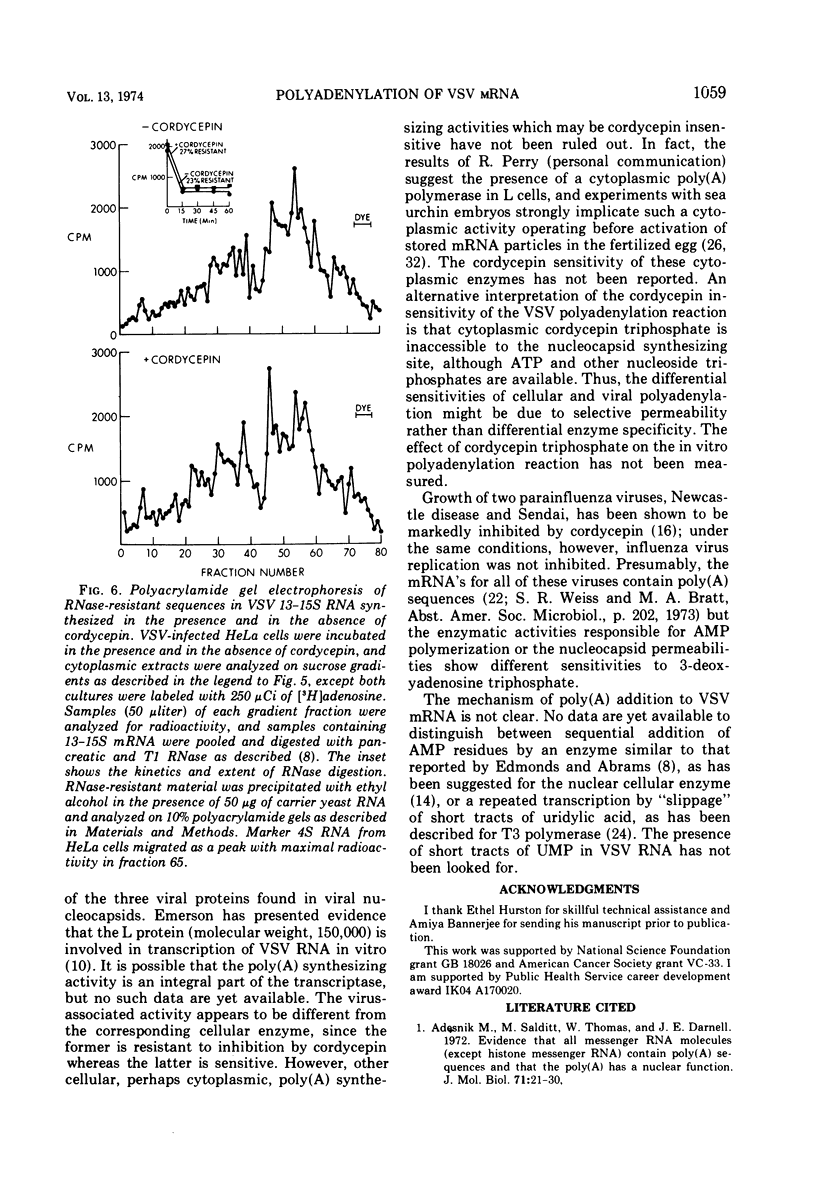
Vesicular stomatitis virus (VSV) mRNA isolated from infected cell polysomes contains polyadenylic acid [poly(A)] sequences. Detergent-activated purified virions in vitro can transcribe complementary RNA, which has sedimentation properties similar to mRNA, and this RNA also contains poly(A) sequences. Digestion of virion RNA with U2 RNase under conditions where hydrolysis is specific for purine linkages leaves no sequences of polyuridylic acid corresponding in length to the poly(A) on the transcripts. Growth of infectious virus is not inhibited by 3-deoxyadenosine (cordycepin) under conditions in which it inhibits polyadenylation of cellular mRNA. The virus-specific mRNA produced in the presence of cordycepin has poly(A) sequences of the same size distribution as that synthesized in the absence of cordycepin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Arima T., Uchida T., Egami F. Studies on extracellular ribonucleases of Ustilago sphaerogena. Purification and properties. Biochem J. 1968 Feb;106(3):601–607. doi: 10.1042/bj1060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K., Rhodes D. P. In vitro synthesis of RNA that contains polyadenylate by virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3566–3570. doi: 10.1073/pnas.70.12.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H. Complete transcription by the transcriptase of vesicular stomatitis virus. J Virol. 1971 Apr;7(4):486–490. doi: 10.1128/jvi.7.4.486-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Kinetics of RNA synthesis by vesicular stomatitis virus particles. J Mol Biol. 1971 May 14;57(3):513–527. doi: 10.1016/0022-2836(71)90106-9. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- EDMONDS M., ABRAMS R. Polynucleotide biosynthesis: formation of a sequence of adenylate units from adenosine triphosphate by an enzyme from thymus nuclei. J Biol Chem. 1960 Apr;235:1142–1149. [PubMed] [Google Scholar]
- Ehrenfeld E., Summers D. F. Adenylate-rich sequences in vesicular stomatitis virus messenger ribonucleic acid. J Virol. 1972 Oct;10(4):683–688. doi: 10.1128/jvi.10.4.683-688.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galet H., Prevec L. Polyadenylate synthesis by extracts from L cells infected with vesicular stomatitis virus. Nat New Biol. 1973 Jun 13;243(128):200–203. doi: 10.1038/newbio243200a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Comparative sedimentation coefficients of RNA extracted from plaque-forming and defective particles of vesicular stomatitis virus. J Mol Biol. 1966 Dec 28;22(2):381–384. doi: 10.1016/0022-2836(66)90143-4. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. II. Synthesis of polyriboadenylic acid. J Mol Biol. 1970 May 28;50(1):19–33. doi: 10.1016/0022-2836(70)90101-4. [DOI] [PubMed] [Google Scholar]
- Marshall S., Gillespie D. Poly U tracts absent from viral RNA. Nat New Biol. 1972 Nov 8;240(97):43–45. doi: 10.1038/newbio240043a0. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Polysomal ribonucleic acid of vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Dec;42(4):958–968. doi: 10.1016/0042-6822(70)90344-2. [DOI] [PubMed] [Google Scholar]
- Niessing J., Sekeris C. E. Synthesis of polynucleotides in nuclear ribonucleoprotein particles containing heterogeneous RNA. Nat New Biol. 1973 May 2;243(122):9–12. [PubMed] [Google Scholar]
- Pridgen C., Kingsbury D. W. Adenylate-rich sequences in Sendai virus transcripts from infected cells. J Virol. 1972 Aug;10(2):314–317. doi: 10.1128/jvi.10.2.314-317.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushizky G. W., Mozejko J. H., Rogerson D. L., Jr, Sober H. A. Characterization of enzymatic specificity of a ribonuclease from Ustilago sphaerogena. Biochemistry. 1970 Dec 8;9(25):4966–4971. doi: 10.1021/bi00827a021. [DOI] [PubMed] [Google Scholar]
- Salvo R. A., Chakraborty P. R., Maitra U. Studies on T3-induced ribonucleic acid polymerase. IV. Transcription of denatured deoxyribonucleic acid preparations by T3 ribonucleic acid polymerase. J Biol Chem. 1973 Oct 10;248(19):6647–6654. [PubMed] [Google Scholar]
- Slater I., Gillespie D., Slater D. W. Cytoplasmic adenylylation and processing of maternal RNA. Proc Natl Acad Sci U S A. 1973 Feb;70(2):406–411. doi: 10.1073/pnas.70.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria M., Huang A. S. Association of polyadenylic acid with messenger RNA of vesicular stomatitis virus. J Mol Biol. 1973 Jul 5;77(3):449–455. doi: 10.1016/0022-2836(73)90450-6. [DOI] [PubMed] [Google Scholar]
- Wild T. F. Replication of vesicular stomatitis virus: characterization of the virus-induced RNA. J Gen Virol. 1971 Nov;13(2):295–310. doi: 10.1099/0022-1317-13-2-295. [DOI] [PubMed] [Google Scholar]
- Wilt F. H. Polyadenylation of maternal RNA of sea urchin eggs after fertilization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2345–2349. doi: 10.1073/pnas.70.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]



