Abstract
A single intramuscular application of the live but not UV-inactivated recombinant rabies virus (RABV) variant TriGAS in mice induces the robust and sustained production of RABV-neutralizing antibodies that correlate with long-term protection against challenge with an otherwise lethal dose of the wild-type RABV. To obtain insight into the mechanism by which live TriGAS induces long-lasting protective immunity, quantitative PCR (qPCR) analysis of muscle tissue, draining lymph nodes, spleen, spinal cord, and brain at different times after TriGAS inoculation revealed the presence of significant copy numbers of RABV-specific RNA in muscle, lymph node, and to a lesser extent, spleen for several days postinfection. Notably, no significant amounts of RABV RNA were detected in brain or spinal cord at any time after TriGAS inoculation. Differential qPCR analysis revealed that the RABV-specific RNA detected in muscle is predominantly genomic RNA, whereas RABV RNA detected in draining lymph nodes is predominantly mRNA. Comparison of genomic RNA and mRNA obtained from isolated lymph node cells showed the highest mRNA-to-genomic-RNA ratios in B cells and dendritic cells (DCs), suggesting that these cells represent the major cell population that is infected in the lymph node. Since RABV RNA declined to undetectable levels by 14 days postinoculation of TriGAS, we speculate that a transient infection of DCs with TriGAS may be highly immunostimulatory through mechanisms that enhance antigen presentation. Our results support the superior efficacy and safety of TriGAS and advocate for its utility as a vaccine.
INTRODUCTION
Rabies is a zoonotic disease that continues to be an important public health problem causing an estimated 55,000 human deaths each year globally, the majority of which occur in Africa and Asia (1). In Asia, parts of America, and large parts of Africa, canines remain the principal host of rabies virus (RABV), and more than 95% of all human rabies cases are caused by exposure to infected dogs (2, 3). The most effective means to protect humans and livestock against rabies is by prophylactic immunization with an RABV vaccine. Although inactivated tissue culture RABV vaccines are safe, they induce protective immunity only when administered in multiple doses and generally require repeat booster doses to provide long-lasting protection. Repeated prophylactic immunization is cost-prohibitive and operationally unrealistic for both people and animals in developing countries. A single-dose RABV vaccine capable of inducing robust, enduring immunity would be highly advantageous in controlling rabies in dogs, livestock, and humans worldwide.
A hallmark of wild-type RABV is its neuroinvasiveness—a unique ability to invade the central nervous system (CNS) from peripheral sites of inoculation (4, 5). Important aspects of this property include the capacity to preserve the integrity of the neuronal network and the ability to evade host immunity (6, 7). One of the important mechanisms that contribute to both of these features of rabies pathogenesis is the control of viral replication, which is seen with pathogenic but not attenuated RABV strains (8, 9). Low replication levels contribute to RABV pathogenesis by conserving the structure of the neurons that are used by these viruses to spread to the CNS and by minimizing viral antigen exposure to the host immune system (10). This is particularly important for RABV glycoprotein (G protein), which is the major target of RABV-specific immunity (11, 12). In contrast to wild-type RABVs, attenuated RABV strains replicate rapidly, express large amounts of G protein, and induce strong innate and adaptive immune responses that can clear an RABV infection. These properties form the basis for the utility of attenuated RABV strains in pre- and postexposure prophylaxis against wild-type RABV infections.
Studies of antibody escape mutants revealed that the main contributor to the pathogenicity of RABV is its G protein, in that substitution of the Arg at position 333 with Glu renders an RABV considerably less pathogenic (13, 14). Reverse genetics technology has been used to construct SADB19-based recombinant RABVs with this substitution together with an Asn194-to-Ser194 mutation that stabilizes the attenuated phenotype (8, 9, 15). Although recombinant RABVs containing this doubly modified G, termed GAS, are strongly attenuated, we have found that engineering the virus to express additional GAS genes not only augments its nonpathogenic phenotype but also substantially increases its immunogenicity (11, 16–18). While single and double GAS variants are largely apathogenic for normal adult mice, they are cytopathic in vitro and have residual pathogenicity for immunocompromised animals (11, 17). Containing three GAS genes, the TriGAS RABV variant is unique in being nonpathogenic for mice that either are developmentally immunocompromised or have inherited deficits in immune functions (17, 19, 20). Evidence suggests that the higher level of attenuation of TriGAS is related to increased G expression rather than an increase in genome size (17). This is accompanied by an enhanced capacity to induce protective RABV immunity, which is best illustrated by the fact that TriGAS inoculation provides prophylactic protection to mice several hours to days after infection with a lethal dose of wild-type RABV (17).
The unique capacity of superinfection with TriGAS to prevent lethal rabies in mice infected with wild-type RABV suggests that this attenuated virus induces protective elements of immunity considerably more rapidly than other variants. RABV-neutralizing antibodies (VNAs) are the major immune effectors against RABV, and rabies G is the target for nearly all VNAs (21). As expected from their role in promoting antibody production, the activity of CD4 T cells is critical to the development of protective immunity against rabies (22–24). The presentation of RABV antigen to these cells is therefore one of several rate-limiting steps in the induction of RABV immunity. During RABV infection, the rate of RABV RNA synthesis and G expression correlate with changes in the expression levels of a variety of host genes (8, 25). This indicates that the products of RABV infection, including viral RNA and G protein, likely include pathogen-associated molecular patterns (PAMPs), which are recognized by pathogen recognition receptors (PRRs) such as RIG-I-like helicases and toll-like receptors (TLRs) (26). For example, mice lacking TLR7 exhibit a delay in the production of RABV-specific antibodies and increased mortality when infected with RABV (20). The induction of PRRs generally depends on virus replication (26, 27), and this is evidently also the case for RABV, for which infection induces a TH1-biased response, as opposed to the TH2-biased response elicited by the administration of killed vaccine (19). Thus, there are several features that suggest that the induction of RABV immunity may be superior with live vaccines, such as TriGAS, than inactivated vaccines.
Although live-attenuated rabies vaccines have been extensively used for oral immunization of wildlife, their use in domestic animals and, particularly, humans must be approached cautiously. The possibility that an attenuated RABV might spread to the CNS and replicate in CNS tissues at low levels that evade immune detection, thereby causing progressive neuronal damage, must be considered. Therefore, to provide further insight into the safety and efficacy of TriGAS, we carefully determined both the location and time course of TriGAS infection after intramuscular (i.m.) administration. We demonstrate that TriGAS infects particular immune cell types, without detectable virus propagation, in the lymph nodes draining the site of inoculation. Infection of these cells orchestrates potent, enduring immune protection against rabies.
MATERIALS AND METHODS
Viruses.
The recombinant RABV SPBAANGAS-GAS-GAS (TriGAS) was generated as described elsewhere (17). The pathogenic RABV strain DOG4 was propagated in NA cells (C1300 mouse neuroblastoma, clone NA) as described previously (17). TriGAS and CVS-11 RABV strains were propagated in BSR cells, a derivative of BHK-21 cells (28). To determine virus titers, NA cells were grown for 2 days, and the monolayers were infected with virus in 10-fold serial dilutions. Forty-eight hours postinfection (p.i.), the cells were fixed with 80% acetone and stained with fluorescein isothiocyanate (FITC)-labeled RABV nucleoprotein (N)-specific antibody (Fujirebio Diagnostics, Malvern, PA). Virus titers in triplicate samples were determined using a fluorescence microscope.
Vaccination and challenge of mice.
Eight- to 10-week-old female Swiss Webster mice were purchased from Taconic Farms (Hudson, NY). To determine immunological memory induced by TriGAS, three groups of 10 mice were immunized intramuscularly (i.m.) into the gastrocnemius with 100 μl containing 1 × 105 focus-forming units (FFU) of live TriGAS, three groups were immunized with UV-inactivated TriGAS, and three groups were immunized with phosphate-buffered saline (PBS). Three weeks or three or six months later, mice were challenged with 10 i.m. 50% lethal doses (LD50s) of the highly pathogenic RABV strain DOG4. The mice were then observed daily for at least 40 days for clinical signs of rabies, and body weights and survival were recorded. Moribund mice were euthanized. All procedures were conducted in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals under protocols approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University (Animal Welfare Assurance no. A3085-01).
Measurement of serum antibody titers.
Mouse sera were tested for the presence of RABV-specific virus neutralizing antibody (VNA) by the rapid fluorescent focus inhibition test (RFFIT) as described previously (29). Briefly, RABV CVS-11 was treated with 3-fold serially diluted mouse sera and then added to 2-day-old 90% confluent NA cells. Twenty-four hours after infection, the cells were fixed with 80% acetone and stained with fluorescein isothiocyanate (FITC)-labeled RABV N-specific antibody (Fujirebio Diagnostics). Neutralization titers, defined as the inverse of the highest serum dilution that neutralizes 50% of the infected virus, were normalized to international units (IU) using the World Health Organization anti-RABV antibody standard. Levels of RABV-specific total IgG, IgG1, IgG2a, IgG2b, and IgM in sera were assessed by enzyme-linked immunosorbent assay (ELISA) as described elsewhere (18). Briefly, 96-well plates were coated with purified RABV G protein (0.67 mg/ml), diluted in coating buffer (50 mM Na2CO3, pH 9.6). Nonspecific binding was reduced with 1% bovine serum albumin (BSA) (total IgG) or 1% normal horse serum (IgG subtypes) in PBS (1 h, 37°C). Antibodies from serially diluted sera were detected using alkaline phosphatase-conjugated anti-mouse whole IgG (1:1,000; MP Biomedicals, Santa Ana, CA) or biotinylated anti-mouse IgG1, IgG2a, IgG2b, or IgM (1:1,000; Southern Biotech, Birmingham, AL) (2 h, room temperature). For IgG subtypes, Vectastain ABC reagent (Vector Laboratories, Burlingame, CA) was added (0.1% PBS-Tween, 30 min, room temperature). p-Nitrophenyl phosphate (pNPP) (Sigma-Aldrich, St. Louis, MO) was utilized as a substrate in 0.1 M glycine, pH 10.4 (total IgG), or 0.1 M sodium bicarbonate, pH 9.5 (IgG subtypes) (30 min, 37°C). Absorbance was measured at 405 nm for phosphatase activity in a microplate spectrophotometer (Bio-Rad 680; Bio-Rad, Hercules, CA).
Real-time PCR analysis.
Eight- to 10-week old female Swiss Webster mice served as uninfected controls or were infected i.m. with 1 × 105 or 1 × 107 FFU of TriGAS, and at various times later, mice were cardiac perfused, tissues were removed, and total RNA was isolated using the RNeasy minikit (Qiagen, Valencia, CA) according to the manufacturer's instructions. For quantification of total RABV RNA, genomic RABV RNA, and RABV mRNA, reverse transcription (RT) and quantitative PCR (qPCR) were performed on each tissue RNA as described previously (17, 18, 30). Briefly, cDNA was synthesized by reverse transcription using the IScript cDNA synthesis kit (Bio-Rad) using random hexamer primers that do not discriminate between messenger and genomic RNAs. To determine the quantities of genomic RABV RNA and RABV mRNA, mRNA-specific [oligo(dT)] or RABV genome-specific (8) primers were used to generate cDNA using the Omniscript RT kit (Qiagen). qPCR was performed on the iCycler iQ real-time detection system (Bio-Rad) using iQ Supermix (Bio-Rad). Gene-specific probes and primers for CD4, CD8, and CD19 were described previously (30), and probes and primers for CD11c were purchased from Invitrogen (Grand Island, NY). The RNA copy numbers for a particular gene were normalized to the copy numbers of the housekeeping gene L13 in each sample as detailed elsewhere (30). Statistical significance of the differences between groups was determined using the Mann-Whitney test.
Isolation of B, T, and dendritic cells from lymph nodes.
Swiss Webster mice were infected i.m. with 107 FFU of TriGAS. Forty-eight hours later, mice were cardiac perfused and lymph nodes were harvested and placed in ice-cold RPMI supplemented with 5% fetal bovine serum (FBS). Single-cell suspensions were obtained by teasing the tissues through a stainless steel mesh. Cell subsets were isolated by positive selection using magnetically activated cell sorting (MACS) technology with the CD19+ B cell isolation kit or the CD11c+ dendritic cell (DC) isolation kit or by negative selection with the CD3ε T cell isolation kit (Miltenyi Biotec, Cambridge, MA). RNA was isolated from each cell type, and cDNA was reverse transcribed as outlined above.
RESULTS
Intramuscular immunization with a single dose of live but not UV-inactivated TriGAS confers long-lasting protective immunity against rabies in mice.
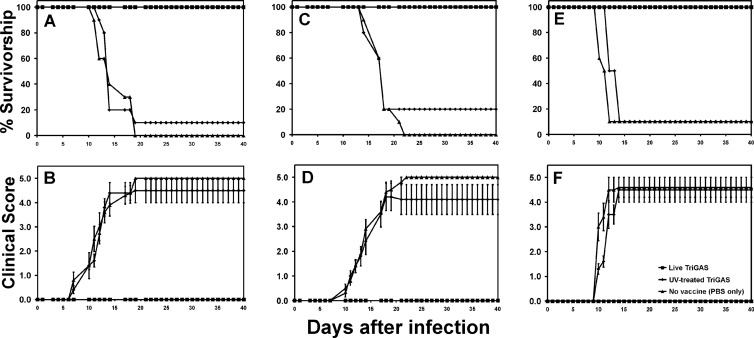
As a consequence of interaction with PRRs and increased antigen load, it is expected that a single dose of a live-attenuated vaccine would have immunogenicity superior to that of the same dose of the vaccine when inactivated. This proves to be the case for TriGAS. Figure 1A shows that mice given a single dose of 105 FFU live TriGAS 3 weeks previously are fully protected against an RABV challenge that is 90 to 100% lethal for nonimmune mice and mice that had received 105 FFU of UV-inactivated TriGAS. More importantly, mice immunized with live TriGAS are fully protected when DOG4 challenge infection is performed 3 months (Fig. 1C) and as late as 6 months (Fig. 1E) after immunization. While none of the mice immunized with live TriGAS exhibited clinical RABV signs following challenge (see Fig. 3B, D, and F), the large majority (80% to 90%) of mice that had received UV-inactivated TriGAS or were unvaccinated succumbed to rabies.
Fig 1.
Immunization with live but not UV-inactivated TriGAS confers long-lasting protective immunity against an infection with wild-type RABV. Groups of Swiss Webster mice were immunized i.m. with 105 FFU of live or UV-inactivated TriGAS or received only PBS. At 3 weeks (A and B), 3 months (C and D), or 6 months (E and F) after immunization, 10 mice of each group were infected i.m. with 10 LD50s of DOG4 RABV, and survivorship (A, C, and E) and clinical scores (B, D, and F) were recorded daily for 40 days.
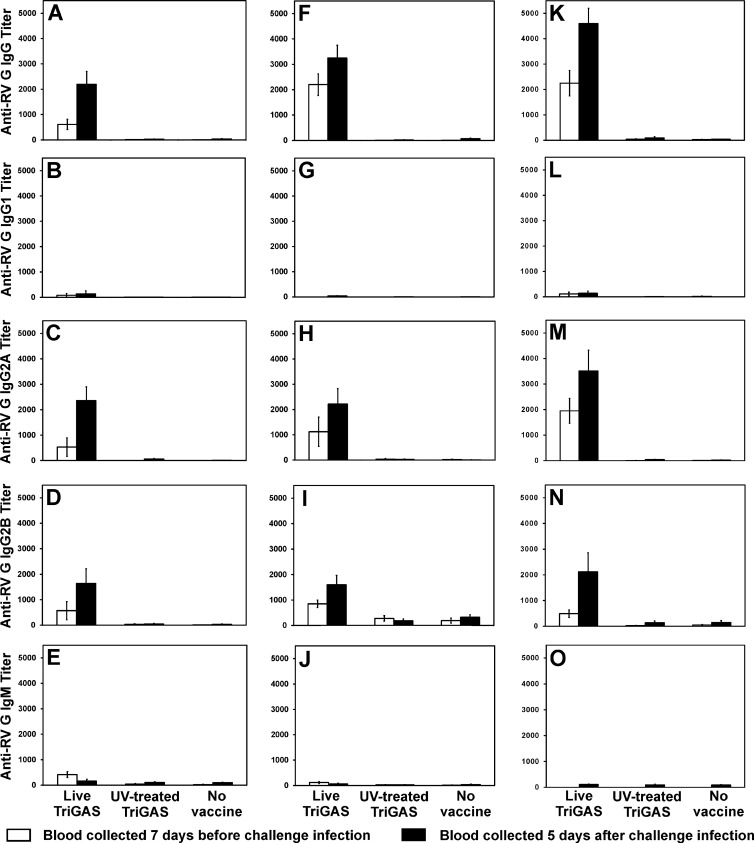
Fig 3.
RABV G-specific antibody isotypes produced at different times after immunization before and after virus challenge. Groups of Swiss Webster mice immunized i.m. with 105 FFU of live or UV-inactivated TriGAS or only PBS and infected 3 weeks (A to E), 3 months (F to J), or 6 months (K to O) later i.m. with 10 LD50s of DOG4 RABV were bled 7 days before and 5 days after virus challenge. Isotypes of RABV G-specific antibody were determined by ELISA as described in Materials and Methods. Values are mean titers (± the standard error [SE]) calculated from 10 mice per group.
Vaccination with live TriGAS is associated with the robust and sustained production of VNA of predominantly IgG2a and IgG2b isotypes.
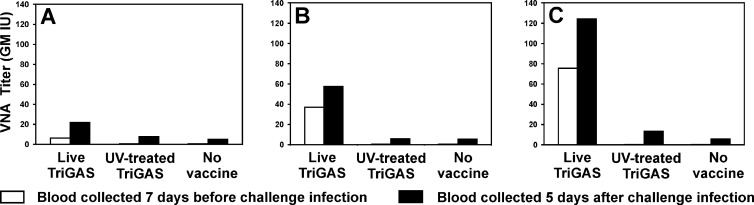
VNA, the essential antigen-specific effectors of RABV immunity, are found at significant levels in sera 2 weeks after immunization of mice with live TriGAS (geometric mean titer [GMT], >7 IU) (Fig. 2A). No VNA were detected in the sera of mice 2 weeks after inoculation with the same amount of UV-inactivated TriGAS. When challenged with DOG4 3 weeks after receiving inactivated or live TriGAS, only the mice immunized with live TriGAS showed evidence of an anamnestic immune response and survived (Fig. 2A). At 5 days after DOG4 challenge, serum VNA levels were strongly elevated in the mice previously immunized with live TriGAS. While none of the mice survived the challenge, VNA were detected 5 days postchallenge in the sera of mice that had received inactivated TriGAS as well as previously nonimmune controls. However, there was no increase in VNA titer resulting from prior administration of inactivated TriGAS. Serum VNA titers increased more than 5-fold between 2 weeks and 3 months after immunization with live TriGAS and remained at high levels for at least 6 months (Fig. 2). In contrast, VNA were not detected in the sera of mice at any time after receiving inactivated TriGAS (Fig. 2). With the exception of increasing serum VNA levels in mice given live TriGAS, similar patterns of survival and VNA responses emerged for the three groups of mice when challenged at 3 (Fig. 2B) and 6 (Fig. 2C) months following vaccination. At 5 days postchallenge, serum VNA were detected at equivalent levels in mice initially treated with inactivated TriGAS and previously naïve controls while serum VNA titers were elevated from already high levels in mice immunized with live TriGAS. Only mice immunized with live TriGAS survived.
Fig 2.
Immunization with live but not UV-inactivated TriGAS induces a sustained production of RABV-neutralizing antibody. Groups of Swiss Webster mice immunized i.m. with 105 FFU of live or UV-inactivated TriGAS or only PBS and infected 3 weeks (A), 3 months (B), or 6 months (C) later i.m. with 10 LD50s of DOG4 RABV were bled 7 days before and 5 days after virus challenge. Serum VNA titers were determined as described in Materials and Methods. VNA titers are presented as the geometric mean international units (IU) calculated from 10 mice per group.
In addition to virus neutralization, we assessed the binding to RABV G, the primary target of VNA, of the different isotypes of antibodies potentially elicited by vaccination and challenge. RABV G-specific total IgG, IgG2a, and IgG2b levels at different times after immunization and challenge largely mirror those of VNA. After immunization with live but not inactivated TriGAS, these were all significant at 2 weeks, increased at 3 months, maintained through 6 months, and boosted by challenge (Fig. 3). G-specific IgM is detectable at 2 weeks, only after immunization with live TriGAS, but disappears over the first 3 months or upon challenge (Fig. 3E, J, and O). RABV G-specific IgG1 was not evident following either live or inactivated TriGAS vaccination or after challenge of either vaccinated or naïve mice.
TriGAS RNA can be detected in muscle tissue at the site of inoculation, in draining lymph nodes, and in spleen but not in spinal cord or brain tissue.
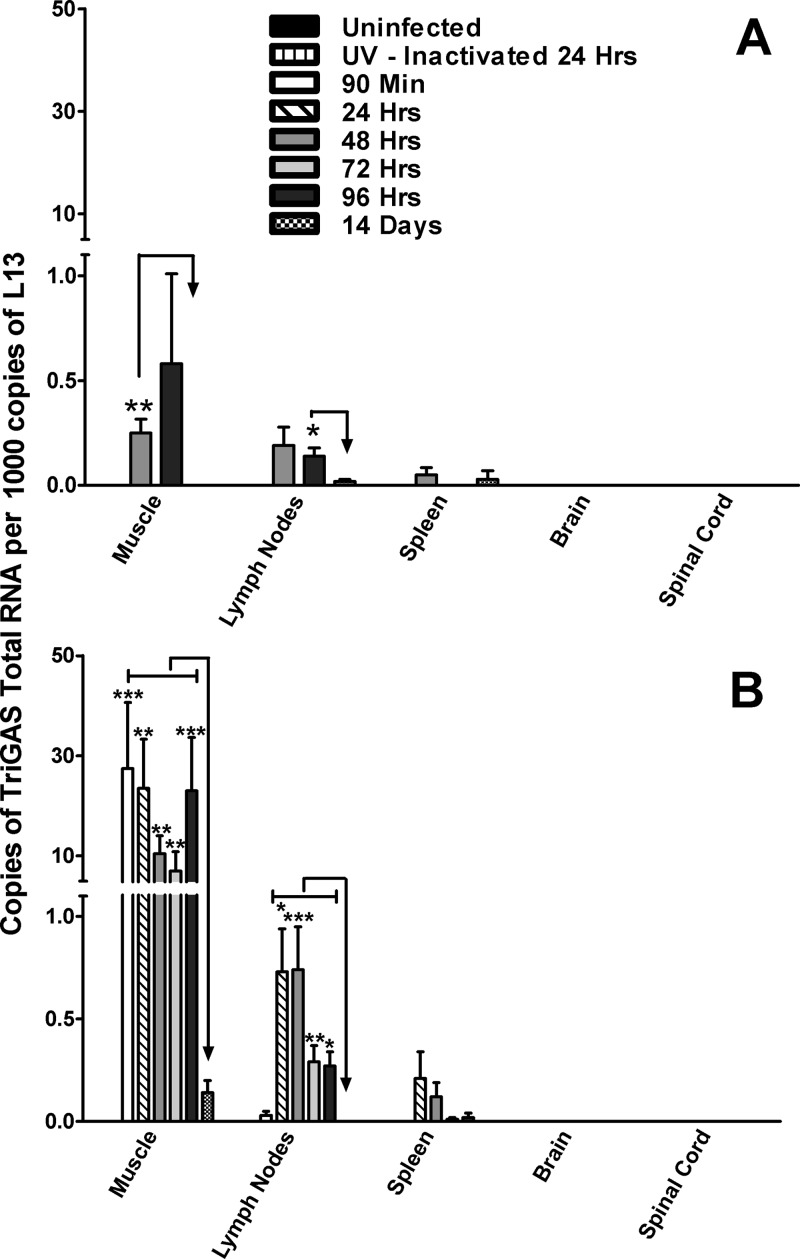
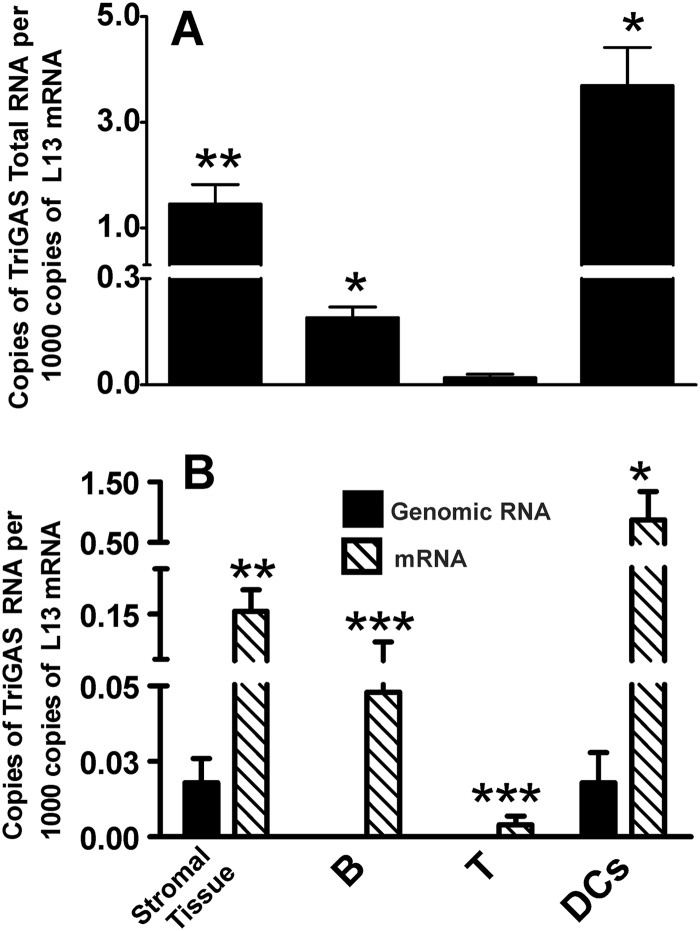
While pathogenic RABVs are generally considered to be highly neurotropic, we considered it possible that the superiority of live TriGAS over inactivated TriGAS in the single-dose triggering of a strong, long-lasting protective immune response against a lethal RABV challenge is the consequence of RABV infection of cells relevant to immune function. To test this hypothesis, we assessed gastrocnemius muscle tissue, inguinal lymph nodes, spleen, brain, and spinal cord for the presence of RABV nucleoprotein (N) RNA after the administration of 105 FFU of TriGAS into the gastrocnemius muscle. RABV N-specific total RNA was detected in muscle and lymph node between 2 and 4 days postinfection, declining to levels outside the range of accurate detection by 14 days postinfection (Fig. 4A). RABV N-specific RNA was not able to reliably be detected in spleen, brain, or spinal cord at this level of input virus. To increase the likelihood of detecting RABV N-specific RNA in the various tissues, we increased the amount of live TriGAS inoculated into the gastrocnemius muscle by 100-fold. Tissues from animals 24 h after receiving a similar dose of UV-inactivated TriGAS were also examined. While RABV RNA was not detected in any tissue after i.m. injection of 107 FFU of UV-inactivated TriGAS, following i.m. injection of 107 FFU of live TriGAS, significant copy numbers were found in gastrocnemius muscle, inguinal lymph node, and to a lesser extent, spleen (Fig. 4B). In gastrocnemius muscle, N RNA was detected beginning at 90 min, remaining for 96 h and thereafter declining to low levels by 14 days postinfection. Significant amounts of RABV N RNA were also found in lymph node, beginning at 24 h after infection but remaining elevated for only 48 h before a decline was detected. A similar pattern (with lower copy numbers) was evident in spleen. Notably, even with a substantially higher level of input virus, RABV N RNA was outside detectable limits in spinal cord and brain tissue at all times tested.
Fig 4.
Detection of total RABV N RNA in different tissues after i.m. administration of TriGAS. Groups of mice were injected into the gastrocnemius muscle with 105 FFU (A) or 107 FFU (B) of TriGAS. At the indicated times after inoculation, mice were euthanized and RNA was isolated from muscle, draining lymph node, spleen, brain, and spinal cord, and the amounts of total RABV N RNA were determined by quantitative reverse transcription-PCR (qRT-PCR) as described in Materials and Methods. Presented are the mean copy numbers (± SE) of RABV N RNA per 1,000 copies of L13 mRNA. Significant differences in expression of RABV RNA are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To provide more insight into whether the RABV RNA initially detected in muscle is likely to be due to input virus or replication, we differentiated between RABV-specific genomic RNA and mRNA in the tissue samples from mice that had received the higher dose of live TriGAS at different times after inoculation. As shown in Fig. 5A, at 90 min postinoculation in the gastrocnemius, before substantial virus replication is expected, there is 10-fold more genomic RNA than mRNA. We expect that this represents input viral RNA, as an increased ratio of mRNA to genomic RNA becomes apparent in the gastrocnemius only by 96 h after infection. In contrast, RNA detected between 24 and 48 h in draining lymph nodes is predominantly mRNA (Fig. 5B).
Fig 5.
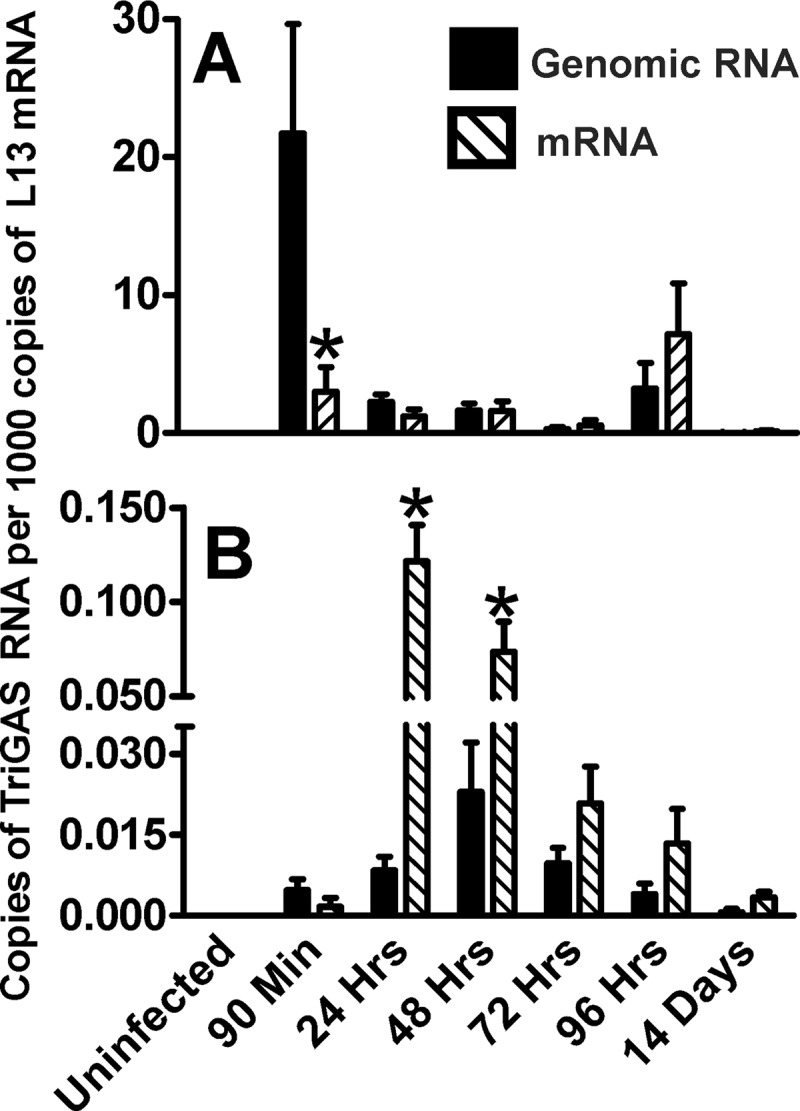
Comparison of the expressions of genomic RABV N RNA and RABV N mRNA in muscle tissue (A) and draining lymph node tissue (B) after inoculation of 107 FFU TriGAS into the gastrocnemius muscle. Muscle and lymph node tissue were collected at the indicated times after TriGAS inoculation. After isolation of total RNA from the different tissues, genomic RABV RNA and RABV N mRNA were analyzed by qRT-PCR and the amounts of RABV RNAs were normalized to the amounts of L13 mRNA detected in the different tissues as described in Materials and Methods. Expression values are presented as the means (± SE) of copy numbers of genomic RABV RNA or RABV mRNA per 1,000 copies of L13 mRNA. Significant differences in expression of genomic RABV RNA and RABV mRNA are indicated as follows: *, P < 0.05.
Dendritic cells and B cells likely represent the major cell populations exhibiting RABV mRNA synthesis in lymph nodes draining the site of infection with TriGAS.
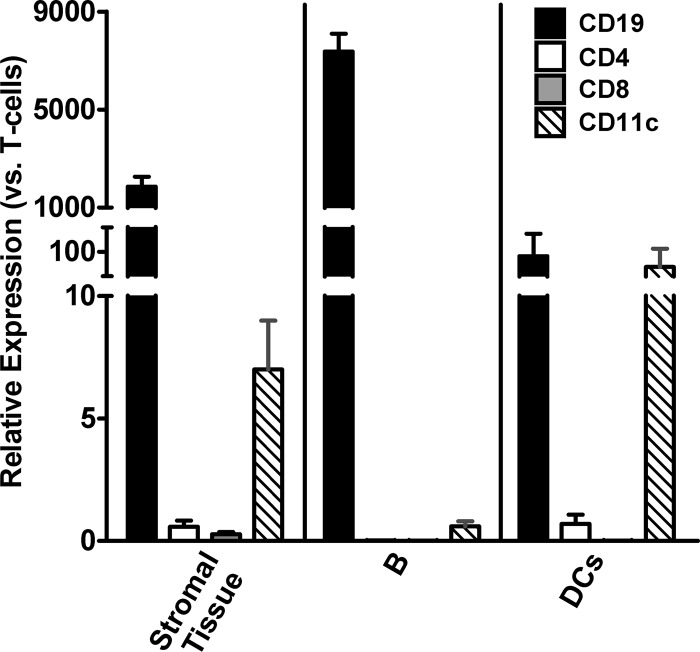
The increased ratio of messenger to genomic RNA suggests that one or more cell types in the lymph nodes draining the site of TriGAS inoculation may be transcribing viral mRNA. If this is relevant to immune stimulation, it may be expected that elevated mRNA levels are found in cells involved in antigen presentation. To identify the cells that may be infected with TriGAS, stromal tissue, B cells, T cells, and DCs were isolated from draining lymph nodes at 48 h after i.m. inoculation with 107 FFU of live TriGAS. The purity of the isolated cells was assessed by qPCR analysis of expression of mRNAs for CD4, CD8, CD11c, and CD19 (Fig. 6). qPCR analysis of RABV RNA revealed that the copy numbers of total RABV N RNA were highest in stroma, B cells, and DCs while the RABV RNA copies in T cells were at levels outside reliable detection (Fig. 7A). Furthermore, the number of RABV mRNA copies was significantly higher than the number of genomic RABV RNA copies in stroma, B cells, and DCs (Fig. 7B), indicating the transcription of viral RNA.
Fig 6.
Expression of mRNA specific for the CD19-, CD4-, CD8-, and CD11c-specific mRNA biomarkers in isolated B cells, DCs, and stroma. Forty-eight hours after inoculation of 107 FFU of TriGAS into the gastrocnemius muscle, different cell populations were isolated from the draining lymph node and the relative fold changes of mRNA levels were determined by qRT-PCR analysis using the comparative threshold cycle (CT) method. The amounts of mRNAs for the different biomarkers, which were normalized to the expression levels of L13 mRNA, are presented as the means (± SE) of fold increases in mRNA expression.
Fig 7.
Expression of RABV N RNA in isolated lymph node cells. Forty-eight hours after inoculation of 107 FFU of TriGAS into the gastrocnemius muscle, B cells, T cells, dendritic cells, and stromata were isolated from the draining lymph nodes and total RABV N RNA (A) or genomic RABV RNA and RABV N mRNA (B) were analyzed by qRT-PCR. Amounts of the different RABV N RNAs were normalized to the amount of L13 mRNA as described in Materials and Methods. Presented are the means (± SE) of RABV RNA copy numbers per 1,000 copies of L13 mRNA. Significant differences in expression of RABV RNA are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
We have recently shown that the live-attenuated TriGAS vaccine, which is nonpathogenic for mice that either are developmentally immunocompromised or have certain inherited deficits in immune function, induces immune mechanisms capable of clearing a CNS infection with pathogenic RABV (17, 18). Here we demonstrate that, in addition to its excellent postexposure efficacy, the TriGAS vaccine is able to confer long-lasting protective immunity in mice. A single intramuscular application of live but not UV-inactivated TriGAS induces the robust and sustained production of RABV G-specific IgG2a and IgG2b antibodies as well as VNA that correlate with long-term protection against challenge with an otherwise lethal dose of the wild-type RABV DOG4. This is not surprising, as 105 inactivated particles not only are a very low antigenic load but also would be ineffective at generating the pathogen-associated molecular patterns, such as de novo-synthesized RNA, required to efficiently induce an immune response.
Although it is well known that due to their ability to replicate (31), live-attenuated viral vaccines are considerably more effective than inactivated vaccines in inducing long-term protection against a variety of pathogens (32), the mechanisms responsible for the establishment of long-lasting immunity are less clear. While it has been concluded that antigen persistence plays an important role in memory maintenance and, thus, in vaccine-induced long-term protective immunity (31), other investigators demonstrated that there is no absolute requirement for continued exposure to antigen to sustain memory B cells and for the persistence of antigen-specific antibody in the serum (33). Although our finding that the TriGAS infection is cleared by 12 days after virus inoculation indicates that persistent antigen production is not necessary for the establishment of long-lived immunity, we cannot exclude the possibility that RABV antigens are stored in DCs after the infection is cleared (34). The fact that challenge infection 6 months after vaccination results in a rapid increase in serum RABV-specific IgGs confirms that memory B cells had been elicited.
Both attenuated and wild-type RABVs have been shown to infect human monocytes and DCs in culture (25). In vitro infection of DCs by the attenuated recombinant RABV SPBNGAS-GAS and, to a lesser extent, the wild-type RABV DOG4 was found to promote DC maturation and result in the expression of RABV G protein on the cell surface (25). Based on the current observations, we conclude that similar processes are likely to be occurring during immunization with TriGAS. After inoculation into the gastrocnemius, where a high ratio of genomic to messenger viral RNA suggests that replication is minimal, the virus either is transported or drains into the draining lymph node. In the lymph node, viral mRNA becomes considerably more abundant than genomic RNA, primarily in the stroma, B cells, and CD11c+ DCs, indicating the transcription of genomic RABV RNA. However, since we were not able to recover infectious virus from the lymph node, we assume that virus replication is abortive. Nevertheless, the temporary production of viral mRNA together with de novo-synthesized viral proteins is apparently sufficient to drive the development of a strong RABV-specific immune response that includes the generation of long-lived RABV-specific memory B cells and antibody-secreting plasma cells that form the cellular basis of immunological memory (35). We speculate that the transient TriGAS infection of DCs and certain B cells in the lymph node is highly immunostimulatory through mechanisms that enhance antigen presentation, including TLR7 activation (20, 25).
The responses to challenge infection with DOG4 in naïve mice as well as animals vaccinated with live or inactivated TriGAS provide further insight into the differences between these viruses and the dynamics of RABV infections. In mice that received live TriGAS, DOG4 challenge induces an anamnestic response, indicating that the viral antigens are recognized by memory T and B cells. However, a single dose of inactivated TriGAS has essentially no effect on the immune system, as no RABV-specific antibody is elicited and the effects of DOG4 challenge are unchanged compared to those with naïve mice. In both naïve and inactivated TriGAS-vaccinated mice, DOG4 infection is lethal. Nevertheless, a significant VNA response is detected in both groups of mice 5 days after challenge. The low copy numbers of RABV RNA that are found in draining lymph nodes at 48 h after i.m. inoculation of DOG4 (Fig. 8) suggest that, like TriGAS, DOG4 reaches peripheral lymphoid tissues and stimulates an immune response. However, based on the in vitro comparison of the effects of the attenuated SPBNGAS-GAS RABV variant and the DOG4 wild-type RABV on DCs (25), we speculate that TriGAS is much more immunostimulatory than DOG4 through its increased ability to produce RABV antigen and stimulatory signals in antigen-presenting cells. Most importantly, in contrast to DOG4, TriGAS does not replicate and spread in the CNS, an attribute which is essential for consideration as a live vaccine for use in humans or animals.
Fig 8.
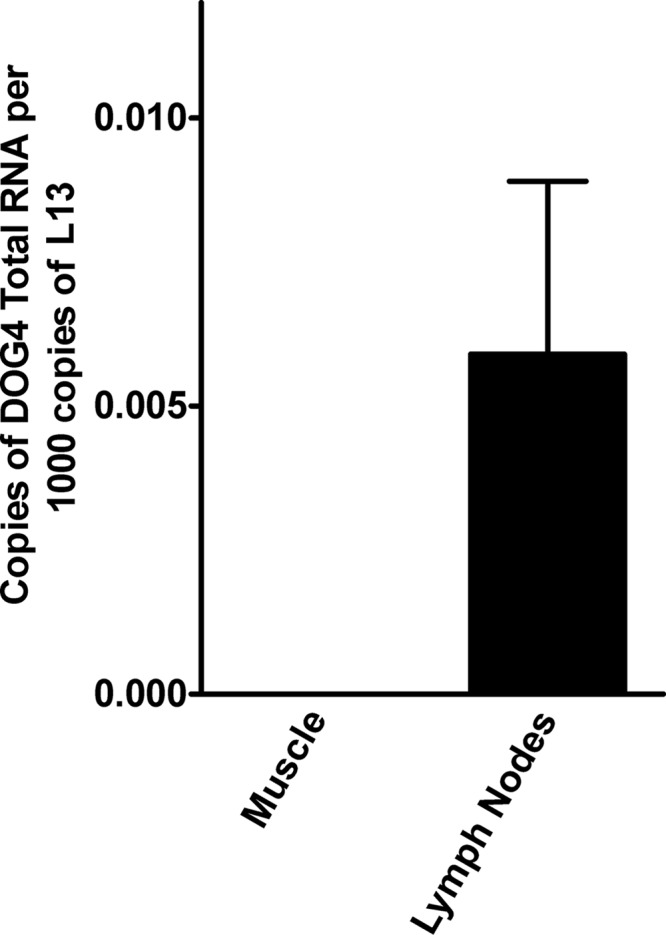
Detection of RABV N RNA in muscle and draining lymph nodes after i.m. inoculation of 106 FFU of DOG4 RABV. At 48 h after inoculation, the copy numbers of RABV N RNA present in muscle tissues and draining lymph nodes were determined by qRT-PCR as described in Materials and Methods. Presented are the mean copy numbers (± SE) of total RABV N RNA per 1,000 copies of L13 mRNA.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01AI093666 (to M.F.), R01AI060686 and 2R56AI060686-06 (to B.D.), and UO1AI083045 and R01AI093369 (to D.C.H.).
Footnotes
Published ahead of print 28 November 2012
REFERENCES
- 1. Knobel DL, Cleaveland S, Coleman PG, Fevre EM, Meltzer MI, Miranda ME, Shaw A, Zinsstag J, Meslin FX. 2005. Re-evaluating the burden of rabies in Africa and Asia. Bull. World Health Organ. 83:360–368 [PMC free article] [PubMed] [Google Scholar]
- 2. Hampson K, Dushoff J, Cleaveland S, Haydon DT, Kaare M, Packer C, Dobson A. 2009. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 7:e53 doi:10.1371/journal.pbio.1000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO 2004. WHO expert consultation on rabies 2004. WHO technical report series 931. World Health Organization, Geneva, Switzerland: http://www.who.int/rabies/trs931_%2006_05.pdf [PubMed] [Google Scholar]
- 4. Dietzschold B, Schnell M, Koprowski H. 2005. Pathogenesis of rabies. Curr. Top. Microbiol. Immunol. 292:45–56 [DOI] [PubMed] [Google Scholar]
- 5. Faber M, Pulmanausahakul R, Nagao K, Prosniak M, Rice AB, Koprowski H, Schnell MJ, Dietzschold B. 2004. Identification of viral genomic elements responsible for rabies virus neuroinvasiveness. Proc. Natl. Acad. Sci. U. S. A. 101:16328–16332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lafon M. 2004. Subversive neuroinvasive strategy of rabies virus. Arch. Virol. Suppl. 2004:149–159 [DOI] [PubMed] [Google Scholar]
- 7. Wiktor TJ, Doherty PC, Koprowski H. 1977. Suppression of cell-mediated immunity by street rabies virus. J. Exp. Med. 145:1617–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faber M, Faber ML, Li J, Preuss MA, Schnell MJ, Dietzschold B. 2007. Dominance of a nonpathogenic glycoprotein gene over a pathogenic glycoprotein gene in rabies virus. J. Virol. 81:7041–7047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faber M, Faber ML, Papaneri A, Bette M, Weihe E, Dietzschold B, Schnell MJ. 2005. A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J. Virol. 79:14141–14148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dietzschold B, Li J, Faber M, Schnell M. 2008. Concepts in the pathogenesis of rabies. Future Virol. 3:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faber M, Pulmanausahakul R, Hodawadekar SS, Spitsin S, McGettigan JP, Schnell MJ, Dietzschold B. 2002. Overexpression of the rabies virus glycoprotein results in enhancement of apoptosis and antiviral immune response. J. Virol. 76:3374–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morimoto K, Hooper DC, Spitsin S, Koprowski H, Dietzschold B. 1999. Pathogenicity of different rabies virus variants inversely correlates with apoptosis and rabies virus glycoprotein expression in infected primary neuron cultures. J. Virol. 73:510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGettigan JP, Pomerantz RJ, Siler CA, McKenna PM, Foley HD, Dietzschold B, Schnell MJ. 2003. Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 Gag have greatly reduced pathogenicity but are highly immunogenic. J. Virol. 77:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mebatsion T. 2001. Extensive attenuation of rabies virus by simultaneously modifying the dynein light chain binding site in the P protein and replacing Arg333 in the G protein. J. Virol. 75:11496–11502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dietzschold ML, Faber M, Mattis JA, Pak KY, Schnell MJ, Dietzschold B. 2004. In vitro growth and stability of recombinant rabies viruses designed for vaccination of wildlife. Vaccine 23:518–524 [DOI] [PubMed] [Google Scholar]
- 16. Faber M, Dietzschold B, Li J. 2009. Immunogenicity and safety of recombinant rabies viruses used for oral vaccination of stray dogs and wildlife. Zoonoses Public Health 56:262–269 [DOI] [PubMed] [Google Scholar]
- 17. Faber M, Li J, Kean RB, Hooper DC, Alugupalli KR, Dietzschold B. 2009. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc. Natl. Acad. Sci. U. S. A. 106:11300–11305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J, Ertel A, Portocarrero C, Barkhouse DA, Dietzschold B, Hooper DC, Faber M. 2012. Postexposure treatment with the live-attenuated rabies virus (RV) vaccine TriGAS triggers the clearance of wild-type RABV from the central nervous system (CNS) through the rapid induction of genes relevant to adaptive immunity in CNS tissues. J. Virol. 86:3200–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jackson AC. 2011. Research advances in rabies, vol 79 Academic Press, London, United Kingdom [Google Scholar]
- 20. Li J, Faber M, Dietzschold B, Hooper DC. 2011. The role of toll-like receptors in the induction of immune responses during rabies virus infection. Adv. Virus Res. 79:115–126 [DOI] [PubMed] [Google Scholar]
- 21. Cox JH, Dietzschold B, Schneider LG. 1977. Rabies virus glycoprotein. II. Biological and serological characterization. Infect. Immun. 16:754–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Celis E, Wiktor TJ, Dietzschold B, Koprowski H. 1985. Amplification of rabies virus-induced stimulation of human T-cell lines and clones by antigen-specific antibodies. J. Virol. 56:426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. 1998. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J. Virol. 72:3711–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xiang ZQ, Spitalnik S, Tran M, Wunner WH, Cheng J, Ertl HC. 1994. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology 199:132–140 [DOI] [PubMed] [Google Scholar]
- 25. Li J, McGettigan JP, Faber M, Schnell MJ, Dietzschold B. 2008. Infection of monocytes or immature dendritic cells (DCs) with an attenuated rabies virus results in DC maturation and a strong activation of the NFκB signaling pathway. Vaccine 26:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997 [DOI] [PubMed] [Google Scholar]
- 27. Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. 2004. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 173:5935–5943 [DOI] [PubMed] [Google Scholar]
- 28. Sato M, Maeda N, Yoshida H, Urade M, Saito S. 1977. Plaque formation of herpes virus hominis type 2 and rubella virus in variants isolated from the colonies of BHK21/WI-2 cells formed in soft agar. Arch. Virol. 53:269–273 [DOI] [PubMed] [Google Scholar]
- 29. Wiktor TJ, Macfarlan RI, Foggin CM, Koprowski H. 1984. Antigenic analysis of rabies and Mokola virus from Zimbabwe using monoclonal antibodies. Dev. Biol. Stand. 57:199–211 [PubMed] [Google Scholar]
- 30. Phares TW, Kean RB, Mikheeva T, Hooper DC. 2006. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J. Immunol. 176:7666–7675 [DOI] [PubMed] [Google Scholar]
- 31. Gray D. 2002. A role for antigen in the maintenance of immunological memory. Nat. Rev. Immunol. 2:60–65 [DOI] [PubMed] [Google Scholar]
- 32. Mims CA, Nash A, Stephen J. 2000. Pathogenesis of infectious disease. Academic Press, London, United Kingdom [Google Scholar]
- 33. Maruyama M, Lam KP, Rajewsky K. 2000. Memory B-cell persistence is independent of persisting immunizing antigen. Nature 407:636–642 [DOI] [PubMed] [Google Scholar]
- 34. Le Roux D, Le Bon A, Dumas A, Taleb K, Sachse M, Sikora R, Julithe M, Benmerah A, Bismuth G, Niedergang F. 2012. Antigen stored in dendritic cells after macropinocytosis is released unprocessed from late endosomes to target B cells. Blood 119:95–105 [DOI] [PubMed] [Google Scholar]
- 35. Vikstrom I, Tarlinton DM. 2011. B cell memory and the role of apoptosis in its formation. Mol. Immunol. 48:1301–1306 [DOI] [PubMed] [Google Scholar]