Abstract
Herpes simplex virus 2 (HSV-2) may cause frequent recurrences, highlighting its ability to evade host defense. This study tested the hypothesis that HSV-2 interferes with dendritic cell (DC) function as an escape mechanism, which may contribute to enhanced HIV replication in coinfected populations. Immature monocyte-derived human DCs were exposed to live or UV-inactivated HSV-2 or lipopolysaccharide. Little or no increase in the maturation marker CD83 was observed in response to HSV-2 and HSV-2 exposed DCs were impaired in their ability to present antigen (influenza) to T cells. Exposure to UV-inactivated virus stimulated a modest, but significant increase in CD83, suggesting that viral gene expression contributes to the block in DC maturation. The functional impairment of HSV-2-exposed DCs could be partially attributed to the induction of apoptosis. Live and inactivated HSV-2 triggered an increase in the number of early and late apoptotic cells in both the infected and bystander cell populations; apoptosis was associated with a decrease in cellular FLICE-inhibitory protein (c-FLIP). Paradoxically, HSV-2 induced Akt phosphorylation, which typically promotes DC maturation and survival. Despite these aberrant responses, live and inactivated HSV-2 induced the release of cytokines into culture supernatants, which were sufficient to activate HIV-1 replication in latently infected U1 cells. Together, these findings suggest that in the presence of overt or subclinical HSV-2, the function of mucosal DCs would be impaired. These responses may allow HSV to escape immune surveillance but may also promote HIV infection and contribute to the epidemiological link between HIV and HSV.
INTRODUCTION
Herpes simplex virus 2 (HSV-2) is one of the most common causes of genital ulcer disease worldwide and epidemiological studies consistently demonstrate a strong link between HSV-2 and the risk for HIV acquisition and transmission (1–3). The prevalence of HSV-2 infection among people with HIV from sub-Saharan African countries ranges from 50 to 90% (4). Frequent sampling by genital tract swabs indicates that 75 to 90% of infected individuals intermittently shed virus, although the majority of these episodes are asymptomatic (5, 6). HSV shedding is associated with a higher frequency of HIV detection and greater number of HIV particles in genital secretions, resulting in an increased risk of transmission (7). The molecular mechanisms underlying the increased risk for HIV acquisition and replication in the setting of HSV-2 infection have not been fully elucidated. We hypothesize that HSV may impair the function of dendritic cells (DCs) and that the induced changes may alter the genital tract mucosal immune environment to facilitate HIV infection.
DCs play a major role in mucosal defense and link innate and adaptive immune responses. Immature monocyte-derived DCs (moDCs) respond to pathogens by undergoing a maturation process induced directly through pattern recognition receptors, or indirectly through signals such as tumor necrosis factor alpha (TNF-α) that are secreted by surrounding cells. Maturation is characterized by increased surface expression of costimulatory and adhesion molecules and the release of cytokines and chemokines. These phenotypic changes facilitate migration of the DCs to the draining lymph nodes to initiate an antiviral T cell response. After the interaction of naive T cells with mature antigen-bearing DCs, T cells undergo activation and migrate back to the infection site to eliminate infected cells (8–10).
Interference with DC function is recognized as a potential viral immune evasion strategy. However, studies of the interactions between HSV and DCs have yielded conflicting results. Several studies have shown that both HSV-1 and HSV-2 can infect immature moDCs, but the consequences of infection on DC phenotype and function have varied. For example, one study found that HSV-1 infection of immature human moDCs resulted in the downregulation of costimulatory and adhesion molecules (11), whereas others found that HSV-1 induced partial maturation of both infected and bystander moDCs (12, 13). A more recent study found that DCs recognize the complex of the essential envelope viral glycoproteins—gB, gD, and gH/gL—and respond with upregulation of CD40, CD83, CD86, and HLA-DR and the production of IFN-α and interleukin-10 (IL-10), but not IL-12p70 (14).
The DC response to HSV-2 has been studied primarily in murine or nonhuman primate models. For example, intravaginal inoculation of mice with HSV-2 led to the rapid recruitment of submucosal DCs into the infected epithelium and subsequently, DCs harboring viral peptides emerged in the draining lymph nodes and stimulated IFN-γ secretion from HSV-specific CD4+ T cells (15). These findings suggest that DCs are effective and promote a T cell response in the vaginal mucosa. In contrast, exposure of immature moDCs isolated from rhesus macaques to HSV-2 stimulated weak T-cell responses in vitro and decreased the expression of costimulatory and adhesion molecules (16). Building from this framework, we evaluated the impact of HSV-2 on human immature moDCs and explored the potential consequences of observed changes for HIV infection.
MATERIALS AND METHODS
Cells and viral strains.
Peripheral blood mononuclear cells (PBMC) were isolated from human leukopacks (New York Blood Center) by density gradient centrifugation using Ficoll-Histopaque (Sigma-Aldrich, St. Louis, MO). CD14+ monocytes were isolated using CD14 magnetic cell separation (Easysep; StemCell Technologies, Vancouver, British Columbia, Canada), and immature moDCs were generated by culturing the cells for 7 to 8 days in RPMI 1640, 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml supplemented with 800 U of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF)/ml and 800 U of recombinant human IL-4/ml (R&D Systems, Inc., Minneapolis, MN). CaSki (human cervical epithelial) and Vero (monkey kidney epithelial) cells were obtained from the American Type Culture Collection (ATCC), Manassas, VA, and maintained as previously described (17). U1 cells were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (18). Viral stocks of HSV-2(G) and HSV-2(333)ZAG were propagated on Vero cells (ATCC, Manassas, VA), and virus titers were determined by plaque assay (17). The latter recombinant virus expresses green fluorescent protein (GFP) under the control of a cytomegalovirus promoter; the construct was inserted in an intergenic region between UL3 and UL4 (gift from P. Spear, Northwestern University). All viral stocks were stored at −80°C. Virus was inactivated by exposure to UV light at a distance of 10 cm from the light source for 7 min (19). Recombinant influenza virus bearing hemagglutinin and neuraminidase from the strain A/Brisbane/59/2007 (the CDC-recommended vaccine strain for the 2008/2009 and 2009/2010 seasons) and the rest of the genomic segments from A/Puerto Rico/08/1934 (BB.PR8) was kindly provided by Adolfo Garcia-Sastre. BB.PR8 was grown in 9-day-old embryonated chicken eggs (SPAFAS; Charles River Laboratories) and titrated by plaque assay on MDCK cells according to standard procedures.
Infection of DCs with HSV-2.
Immature moDCs were challenged with live or UV-inactivated HSV-2, mock infected by being exposed to RPMI with 10% FBS, or matured by exposure to 1 μg of lipopolysaccharide (LPS; InvivoGen, San Diego, CA)/ml (20). To evaluate whether virus productively infected the cells, viral inoculum was removed from DCs or CaSki cells after challenge for 1 h at 37°C, the cells washed three times with phosphate-buffered saline (PBS), and then cultured in fresh medium in the absence or presence of acyclovir (ACV; 100 μg/ml; Bedford Laboratories, Bedford, OH). Culture supernatants were harvested at 2, 12, 24, and 48 h postinfection (p.i.), and viral yields were quantified by plaque assay on Vero cells. The infection of DCs was also assessed using the GFP-expressing HSV-2(333)ZAG strain, which discriminates infected and bystander DCs. GFP expression was used as a marker of viral infection. The infected cells were analyzed by flow cytometry, after gating on the live populations using a fixable violet Live/Dead marker (Invitrogen, Carlsbad, CA). Ten thousand live events were acquired per sample.
Flow cytometry.
The phenotype of immature moDCs was analyzed by fluorescence-activated cell sorting (FACS) 7 to 8 days after culture of CD14+ monocytes in the presence of GM-CSF and IL-4. Cells were stained (single stain per antibody) with anti-CD11c-APC, anti-HLA-DR-FITC, anti-DC-SIGN-FITC, anti-CCR5-FITC, anti-CXCR4-Pe-Cy5, and anti-HLA-ABC-APC (BD Pharmingen, San Diego, CA) for 30 min, washed twice in PBS, and fixed in 4% paraformaldehyde (BD Biosciences, Franklin Lakes, NJ). Similarly, to assess the state of maturation of the moDCs, at 4 or 8 h postexposure to HSV-2 (multiplicity of infection [MOI] = 1 to 10 PFU/cell), LPS, or medium alone, 5 × 105 cells were stained with anti-CD83-APC (BD Pharmingen, San Diego, CA) and fixed as previously described. Flow cytometry was performed on a Becton Dickinson LSR II analyzer, and analysis was carried out using the FlowJo v9.3.1 software (TreeStar, Inc., Ashland, OR). Ten thousand live events were acquired per sample.
Influenza antigen presentation.
Ten thousand moDCs were mock infected or exposed to influenza virus BB.PR8 and HSV-2(G) (live or UV-inactivated) (MOI = 1 or 5 PFU/ml) simultaneously or influenza virus BB.PR8 at 4 h after exposure to HSV-2. Viral inocula were removed; the cells were then washed in PBS and cultured with autologous CD14− T cells at a 1:10 DC/T cell ratio in the presence of ACV (200 μg/ml). Influenza antigen presentation was determined by IFN-γ release, measured 40 h postcoculture by ELISpot (MABTECH, Inc., Cincinnati, OH).
Apoptosis analysis.
DCs were exposed to staurosporine (1 μM) as a positive control, live, or UV-inactivated HSV-2 (MOI = 5 PFU/cell) or medium as a negative control (mock) and were harvested 4 and 8 h posttreatment and analyzed for early and late signs of apoptosis using an annexin V and 7-aminoactinomycin D (7AAD) staining protocol (BD Pharmingen). Briefly, 5 × 105 cells were washed twice in cold PBS and resuspended in buffer containing 10 mM HEPES (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2. The cells were then incubated with phycoerythrin (PE)-conjugated annexin V and/or 7AAD for 30 min in the dark at room temperature. Cells were analyzed by flow cytometry, as described above, acquiring 10,000 events per sample. To differentiate infected from bystander cells, moDCs were infected with HSV-2(333)ZAG.
Western blot analysis.
DCs (106 cells) were exposed to HSV-2(G) (MOI = 10 PFU/cell) and LPS (10 μg/ml) for 1 h at 37°C in the absence or presence of wortmannin (25 μM; Tocris Bioscience, Ellisville, MO) or rapamycin (0.1 μM; Tocris Bioscience). Additional controls included cells exposed to staurosporine (1 μM) for 4 h. In select experiments, DCs were infected with HSV-2(333)ZAG and sorted 4 h p.i. based on GFP expression to differentiate infected from bystander cells. The cells were harvested and lysed in 100 μl of lysis buffer containing 20 mM Tris (pH 7.5), 50 mM NaCl, 1% NP-40, and 0.05% deoxycholate and supplemented with phosphatase and protease inhibitors (Roche, Germany). Proteins were separated by SDS-PAGE and transferred to membranes for immunoblotting. Membranes were incubated according to the manufacturer's instructions with anti-phospho-Ser473 Akt1/2/3 (1:500; sc-7985-R, Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-Thr308 Akt1/2/3 (1:500; sc-16646-R; Santa Cruz Biotechnology), anti-total Akt1/2/3 (1:500; sc-8312; Santa Cruz Biotechnology), anti-gB (1:500; P1123; Virusys, Taneytown, MD), anti-caspase-3 (1:1,000; catalog no. 9662; Cell Signaling Technology, Danvers, MA), anti-caspase-8 (1:800; catalog no. 9746; Cell Signaling Technology), anti-c-FLIP (NF6) (1:500; Enzo Life Sciences, Farmingdale, NY), and anti-β-actin (1:10,000; A-5441; Sigma). The membranes were stripped between antibodies. Blots were scanned, and the band intensities were analyzed by using ImageJ (National Institutes of Health, Bethesda, MD).
Detection of cytokines and chemokines.
Culture supernatants from DCs that had been exposed to HSV-2(G) (MOI = 5 PFU/cell), LPS (1 μg/ml), staurosporine (1 μM), or medium (mock) were collected 24 h posttreatment, and the cytokine and chemokine levels were determined by using multiplex proteome bead arrays (Chemicon International and Milliplex [Millipore, Billerica, MA]). The levels were quantified by Luminex and analyzed using StarStation (Applied Cytometry Systems, Sheffield, United Kingdom). The levels of IFN-β secreted in the 24-h supernatants of exposed DCs were quantified using a VeriKine human IFN-β ELISA kit (PBL Interferon Source, Frederick, MD).
Expression of cytokines by qRT-PCR.
IFN-β expression levels in human moDCs that were exposed to medium (mock), LPS (1 μg/ml), staurosporine (1 μM), or HSV-2(G) (MOI = 5 PFU/cell) (live and UV inactivated) were quantified by quantitative real-time PCR (qRT-PCR) using iQ SYBR green Supermix (Bio-Rad, Hercules, CA) according to the manufacturer's instructions and a C1000 thermal cycler/CFX96 real-time PCR detection system (Bio-Rad). The PCR temperature profile was 95°C for 3 min, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. The mRNA level of each sample was normalized to that of the two housekeeping genes rps11 and α-tubulin using the standard 2−ΔCT method (21, 22). The primer sequences for IFN-β, rps11, and α-tubulin were previously described (23).
Induction of HIV-1 replication in U1 cells.
U1 cells, a persistently HIV-infected cell line, were seeded at 105 cells/well in 96-well plates and were then incubated with 200 μl of culture supernatants that had been harvested 24 h p.i. from mock-, LPS (1 μg/ml)-, or HSV-2(G) (MOI = 5 PFU/cell; live or UV-inactivated)-exposed DCs. Supernatants were UV irradiated to inactivate any newly synthesized virus prior to U1 culture setup. As a control, U1 cells were also incubated with TNF-α at 100 U/ml (R&D Systems), a concentration previously shown to induce HIV release (24). Cell-free supernatants were collected 1 and 4 days p.i., and HIV-1 was quantified by determining p24 content by enzyme-linked immunosorbent assay (ELISA; PBL Interferon Source).
Statistical analysis.
Responses were compared by one-way analysis of variance with Bonferroni correction or the Student paired t test, using GraphPad Prism version 4 (San Diego, CA), and a P value of <0.05 was considered significant.
RESULTS
HSV infects immature moDCs.
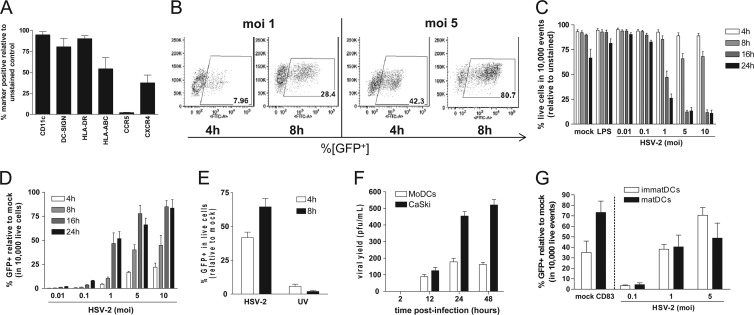
Immature moDCs were differentiated from PBMCs for 7 days in the presence of IL-4 and GM-CSF, and the phenotype was characterized by flow cytometry (Fig. 1A). To examine the susceptibility to HSV-2, immature moDCs were infected with HSV-2(333)ZAG, and infection was monitored by measuring GFP expression by flow cytometry (Fig. 1B). In parallel, cell viability was assessed. There was no significant loss in cell viability 4 or 8 h p.i., but viability was reduced by 16 h p.i. in response to MOI > 1 PFU/cell (Fig. 1C). Thus, subsequent experiments were performed 4 or 8 h p.i. with an MOI of 1 or 5 PFU/cell. Analysis within the live cell population showed that viral infection (measured by GFP expression) increased over time and in a dose-dependent manner (Fig. 1D). Approximately 64.5% ± 6.4% of the DCs expressed GFP by 8 h p.i. after exposure to 5 PFU/cell. In contrast, only 2% ± 0.8% of cells expressed GFP after exposure to UV-inactivated virus (Fig. 1E). Susceptibility to viral infection was further assessed by extracting RNA from infected cell lysates and probing for the presence of RNA representing all three classes of HSV proteins by RT-PCR; ICP0 (immediate early), viral thymidine kinase (early), and glycoprotein B (late) were detected 4, 8, and 24 h p.i. (data not shown).
Fig 1.
Immature moDCs support low level of HSV-2 replication. (A) Phenotypic characterization of moDCs after differentiation from CD14+ monocytes, cultured in GM-CSF and IL-4 for 7-8 days. The data are means + the standard errors of the mean (SEM) obtained from a minimum of three independent donors. Immature moDCs were exposed to HSV-2(333)ZAG (MOI = 0.01 to 10 PFU/cell, as indicated) for 1 h at 37°C. (B and C) At the specified times p.i., infection was assessed by quantifying GFP expression (10,000 live event acquisition) by flow cytometry (B), and cell viability was assessed in 10,000 total events by staining with a Live/Dead marker (C). Infected (GFP+) DCs within the live population of panel C are shown in panel D. The data are representative dot plots (B) and are means + SEM (C and D) of a minimum of five independent experiments. (E) Immature moDCs were exposed to live or UV-inactivated HSV-2(333)ZAG (MOI 5 PFU/cell) for 1 h at 37°C. At the indicated times p.i., infection was assessed by quantifying GFP expression (10,000 live event acquisition) by flow cytometry. The data are means + the SEM of seven independent experiments. (F) Immature moDCs and CaSki cells were infected with HSV-2(G) (MOI = 1 PFU/cell). At the indicated times p.i., the culture supernatants were collected, and viral yields were quantified by performing plaque assays on Vero cells. The results are presented as PFU/ml and are means + the standard deviations (SD) obtained from five independent experiments conducted in duplicate. (G) Immature or LPS-matured DCs were evaluated for CD83 expression (left) and then infected with HSV-2(333)ZAG at the indicated MOIs and at 4 h p.i. were analyzed for GFP expression as a marker of infection by flow cytometry (10,000 live event acquisition). The results are presented as means + the SEM of three independent experiments.
To determine whether the DCs were productively infected, immature moDCs cells were exposed to HSV-2(G) (MOI = 1 PFU/cell) and release of viral progeny was monitored by collecting culture supernatants and quantifying the released virus by plaque assay. For comparison, CaSki cells, a human cervical epithelial cell line, were infected in parallel. HSV-2 productively infected immature moDCs, as indicated by the increase in infectious virus released into the culture supernatants over 24 h, although no further increase was observed between 24 and 48 h, presumably reflecting decreased cell viability. Viral yields were lower in moDCs compared to CaSki cells (Fig. 1F).
We further assessed the susceptibility to HSV-2 infection in mature compared to immature cell cultures. DCs were allowed to mature in the presence of LPS (1 μg/ml) for 24 h, and maturation was monitored by assessing CD83 expression (Fig. 1G, left). Immature and mature DCs were exposed to increasing MOIs of HSV-2(333)ZAG; no significant difference in susceptibility to infection was observed between mature and immature DCs (Fig. 1G, right).
HSV blocks DC maturation.
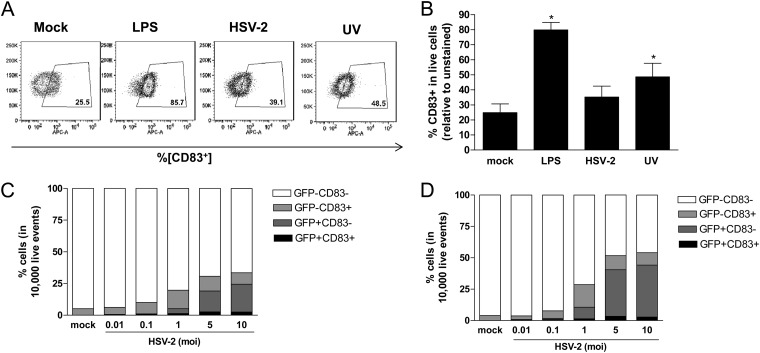
DC maturation is characterized by an increase in the surface expression of costimulatory molecules including CD83 (9). To evaluate the response to HSV, immature moDCs were exposed to HSV-2(G) and treated with LPS as a positive control or with medium alone, and the expression of CD83 was assessed by flow cytometry. LPS triggered phenotypic maturation of the cells characterized by a significant increase in the expression of CD83 from 25.5 to 85.7%, whereas little increase in CD83 expression was observed 4 h postinfection in HSV-2-exposed cells (39.1%), and there was no further increase in CD83 expression at later time points (Fig. 2A and B). Simultaneous exposure of DCs to HSV-2 and LPS also prevented DC maturation, suggesting that HSV blocks DC maturation (not shown). To further assess whether viral gene expression blocked DC maturation, additional studies were conducted with UV-inactivated virus. UV inactivation prevents viral gene expression (including GFP expression; Fig. 1E) after viral entry (19). Exposure to UV-inactivated virus induced a modest, but significant increase in CD83 expression relative to mock-treated cells (48.5% versus 25.5%) (P = 0.05) (Fig. 2A and B).
Fig 2.
LPS, but not HSV-2, triggers DC maturation. (A and B) Immature moDCs were exposed to LPS (1 μg/ml) or HSV-2(G) (live or UV inactivated) (MOI = 5 PFU/cell) for 1 h at 37°C. The cells were analyzed by flow cytometry for surface marker CD83 expression at 4 h posttreatment. The data are representative dot plots (A) or the mean percent % CD83+ cells + the SEM from six independent experiments (B). (C and D) Further analysis of CD83 expression within infected (GFP+; darkest two segments) or bystander (GFP−; lightest two segments) populations following exposure of DCs to LPS or HSV-2(333)ZAG (MOI = 0.01 to 10 PFU/cell as indicated) at 4 and 8 h postexposure (C and D, respectively). The results are presented as the percentage of 10,000 live events acquired and are means obtained from four independent experiments. Asterisks indicate significant differences relative to mock-treated DCs (P = 0.05).
To evaluate whether HSV blocked DC maturation in infected and bystander cells, we took advantage of HSV-2(333)ZAG, which allows for discrimination between infected (GFP+) and bystander (GFP−) DCs. The proportion of infected DCs increased in a dose (MOI)- and time (4 and 8 h p.i.)-dependent manner; however, the majority of GFP+ cells remained CD83− (Fig. 2C and D). In contrast, there was a modest and significant increase in CD83+ cells in the bystander (GFP−) population after HSV-2 exposure compared to mock-exposed cells. For example, after exposure to an MOI of 5 PFU/cell, the proportion of GFP−/CD83+ cells increased significantly from 5% ± 0.9% to 11.7% ± 3.3% (relative to mock-exposed cells) (P = 0.03), but there was no increase in GFP+/CD83+ cells 4 h p.i.
HSV-2-exposed DCs are functionally anergic.
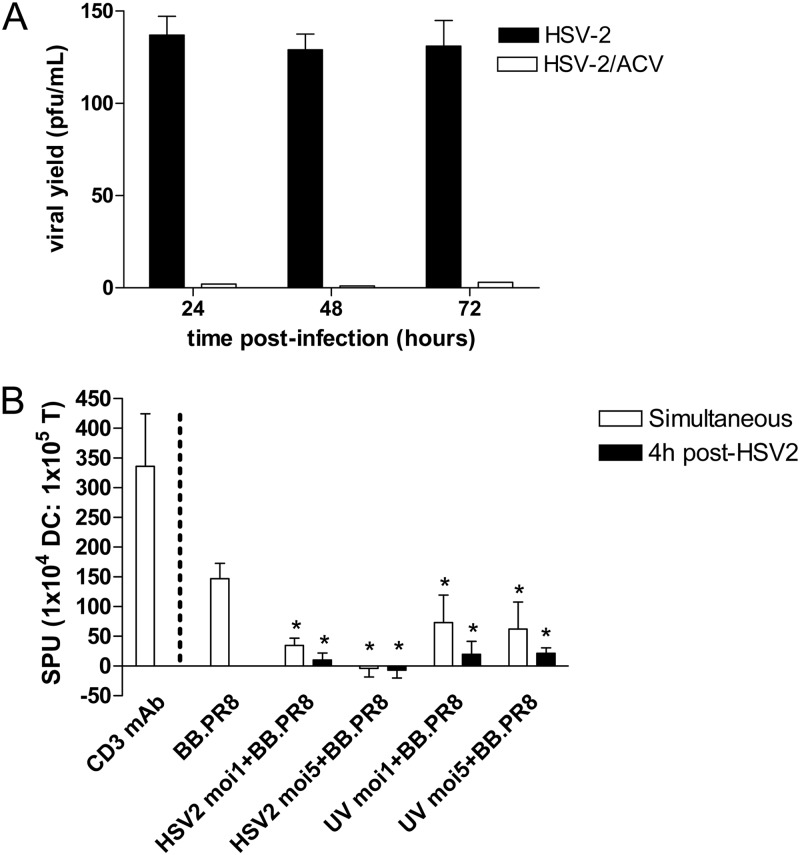
The observation that HSV-2 failed to trigger CD83 expression in the infected cell population suggests that the HSV-exposed cells are functionally impaired. To further address this, we compared the ability of HSV exposed DCs to present influenza virus to T cells. It has previously been shown that influenza virus infects DCs, evidenced by an increase in viral RNA and protein levels (23, 25, 26). Immature moDCs were either exposed to HSV-2 (live or UV inactivated) and influenza virus simultaneously or to influenza virus at 4 h post-HSV infection. ACV (200 μg/ml) was added to the cultures to prevent HSV replication. In pilot studies with HSV-2 (MOI = 1 PFU/cell), we observed that ACV inhibited viral replication (Fig. 3A) and blocked HSV-induced DC death (data not shown). After the viral challenges, the DCs were cocultured with autologous CD14− T cells, and T cell responses were monitored by ELISpot assay for IFN-γ production. Positive controls included moDCs exposed to influenza virus alone prior to coculture with T cells and T cells stimulated with a CD3 MAb. Preexposure of DCs to HSV-2 or simultaneous exposure to HSV-2 and influenza virus significantly blocked the IFN-γ response to influenza virus (P < 0.05) (Fig. 3B). There was a small (but statistically nonsignificant) increase in the IFN-γ response to influenza virus when DCs were exposed to UV-inactivated HSV-2 compared to live HSV-2, although the IFN-γ response remained significantly less than the response to influenza virus alone.
Fig 3.
HSV-2-infected moDCs are impaired in antigen presentation. (A) Immature moDCs or CaSki cells were infected with HSV-2(G) (MOI = 1 PFU/cell) in the absence or presence of acyclovir (ACV) at 100 μg/ml. At the indicated times p.i., culture supernatants were collected, and viral yields were quantified by performing plaque assays on Vero cells. The results are presented as PFU/ml and are means + the SD obtained from five independent experiments conducted in duplicate. (B) 104 human moDCs were exposed to influenza virus BB.PR8 and HSV-2(G) simultaneously or to influenza virus BB.PR8 at 4 h post-HSV-2(G) challenge and then cultured with autologous CD14− T cells at a 1:10 DC/T cell ratio in the presence of ACV (200 μg/ml). IFN-γ release was measured at 40 h postcoculture. T cells stimulated with a CD3 MAb served as a positive control for the ELISpot assay (far left). The results are presented as means + the SEM spot-forming units (SPU) obtained in five independent experiments, each performed in triplicate. The asterisks indicate significant differences relative to influenza virus-challenged DCs (P < 0.05).
HSV-2 triggers DC apoptosis.
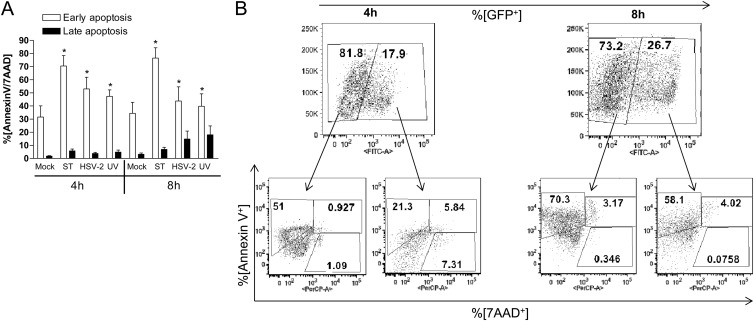
It is possible that the functional impairment of HSV-exposed DCs could be attributed, at least in part, to apoptosis. Although HSV-2 has evolved several strategies to prevent apoptosis in epithelial cells as a survival mechanism, it has been suggested that virus may promote apoptosis in DCs and T cells in an effort to limit the immune response (27, 28). The extent of apoptosis has varied from study to study, and most work has been conducted with HSV-1 and/or nonhuman DCs (16, 29). To determine whether HSV-2 induced apoptosis in human moDCs, the cells were infected with HSV-2(333)ZAG (live or inactivated), mock infected (negative control), or treated with staurosporine (1 μM) (positive apoptosis control) and analyzed for early and late signs of apoptosis using annexin V and 7AAD markers. A significant increase in early apoptotic (annexin V+ 7AAD−) cells was observed 4 and 8 h p.i. after exposure to live or UV-inactivated HSV-2 (MOI = 5 PFU/cell) compared to mock-infected cells (P < 0.05); staurosporine also induced a significant increase in early apoptosis at both time points. This progressed to a modest increase in late apoptosis (annexin V+ 7AAD+) (Fig. 4A). Subsequent analysis of subpopulations showed that virus induced apoptosis in both GFP+ (infected) and GFP− (bystander) DCs (Fig. 4B).
Fig 4.
HSV-2 induces apoptosis in infected and bystander moDCs. (A) Immature moDCs were mock infected, exposed to staurosporine (ST; 1 μM) or to live and UV-inactivated HSV-2(333)ZAG (MOI = 5 PFU/cell), and analyzed by flow cytometry for apoptosis at 4 h and 8 h p.i. The results are presented as the percentages of cells that are annexin V+ 7AAD− (early apoptosis) and annexin V+ 7AAD+ (late apoptosis) and are means + the SEM of a minimum of three independent experiments. The asterisks indicate significant differences relative to mock-treated DCs (P < 0.05). (B) Dot blots of subsequent analysis of infected (GFP+) and bystander (GFP−) DC populations 4 and 8 h p.i. The results are representative data obtained from five independent experiments.
HSV-2 decreases the expression of cellular FLIP.
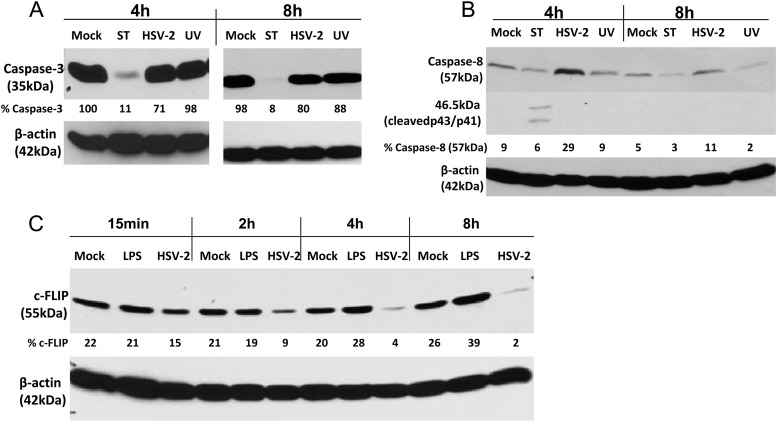
To explore the mechanism by which HSV-2 induced apoptosis, moDCs were exposed to live or UV-irradiated HSV-2 and assessed at different times p.i. for their expression of caspase-3, caspase-8, and c-FLIP, a potent inhibitor of apoptosis (30, 31). Staurosporine or LPS were included as controls. HSV induced a small decrease in caspase-3 (Fig. 5A) and, conversely, a modest increase in caspase-8 expression (Fig. 5B). The UV-inactivated virus also triggered a small decrease in caspase-3 but had no effect on caspase-8. HSV (but not LPS) significantly reduced the expression of c-FLIP (Fig. 5C). The downmodulation of c-FLIP is consistent with prior studies with HSV-1 (30, 31). As expected, staurosporine triggered cleavage of caspases, as evidenced by a marked reduction in caspase-3 and the presence of cleaved forms of caspase-8 (Fig. 5A and B, respectively) (32).
Fig 5.
HSV-2-induced apoptosis is associated with reduced expression of cellular-FLICE inhibitory protein. Immature moDCs were mock infected or exposed to staurosporine (1 μM) or live or UV-inactivated HSV-2(G) (MOI = 10 PFU/cell). At the indicated times postexposure, the cells were lysed and analyzed by Western blotting for the expression of caspase-3 (A) or caspase-8 (B) and β-actin as a loading control. A blot representative of results obtained from two independent experiments is shown. (C) Alternatively, cells were infected with HSV-2(G) (10 PFU/cell), medium (mock), or LPS (10 μg/ml) and, at the indicated times postexposure, the cells were lysed and blotted for expression of c-FLIP and β-actin. The blots were scanned, and the percentages of caspase-3 (A), caspase-8 (B), and c-FLIP (C), relative to β-actin, are indicated below each lane.
HSV-2 triggers persistent Akt phosphorylation.
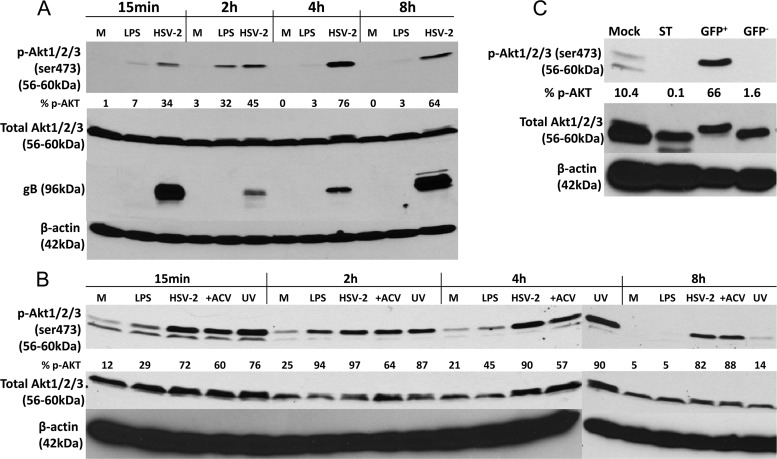
DC maturation and antiapoptotic responses are associated with activation of Akt signaling and pharmacological blockade of Akt signaling impairs DC maturation and induces apoptosis (33). Thus, we predicted that LPS, but not HSV-2, would trigger the phosphorylation of Akt. Paradoxically, we found that HSV-2 induced a sustained increase in phosphorylated Akt (p-Akt) as early as 15 min p.i., which persisted up to 8 h p.i. (Fig. 6A). Similar results were obtained with either an anti-phospho-Ser473 or anti-phospho-Thr308 antibody (not shown). Infection was confirmed by probing the blots for glycoprotein B (gB); the presence of gB 15 min p.i. reflects input virus and the increased expression over time reflects newly synthesized viral glycoprotein. LPS, in contrast, induced a more transient response with phosphorylated forms of Akt (p-Akt) peaking by 2 h postexposure (Fig. 6A). UV-inactivated virus and infection in the presence of ACV also triggered the phosphorylation of Akt, although the response to inactivated virus was no longer detected 8 h p.i. (Fig. 6B).
Fig 6.
HSV-2 triggers persistent Akt activation, which does not require viral gene expression or viral replication. (A) Immature moDCs were mock infected (M) or exposed to LPS (10 μg/ml) or HSV-2(G) (MOI = 10 PFU/cell), and the cells were harvested at the indicated times posttreatment and analyzed by Western blotting for expression of phosphorylated Akt (p-Akt), total Akt, viral glycoprotein B (gB), and β-actin expression. (B) Immature moDCs were mock infected (M) or exposed to LPS (10 μg/ml) or HSV-2(G) (live and UV inactivated) (MOI = 10 PFU/cell) in the absence or presence of ACV (100 μg/ml). At the indicated times posttreatment, the cells were harvested, lysed, and analyzed by Western blotting for the expression of p-Akt, total Akt, and β-actin expression. (C) Immature moDCs were exposed to HSV-2(333)ZAG and at 4 h p.i. were sorted for GFP expression, and 106 GFP+ and GFP− cells were lysed and analyzed by immunoblotting using antibodies to p-Akt, total Akt, and β-actin. Staurosporine (1 μM)-treated cells were included as an apoptosis control. The blots were scanned, and the protein levels were quantified by using ImageJ. They are representative of three (A and C) or two (B) independent experiments, and the percentages of p-Akt relative to total Akt after scanning are indicated below each lane.
To evaluate whether induction of Akt phosphorylation was directly linked to viral infection, HSV-2-exposed mDCs were sorted for GFP expression 4 h p.i., lysed, and probed for p-Akt. Only the infected (GFP+) DCs exhibited an increase in p-Akt levels. The proapoptotic drug staurosporine was associated with a reduction in p-Akt (Fig. 6C).
Induction of apoptosis and blockade of DC maturation are independent of Akt phosphorylation.
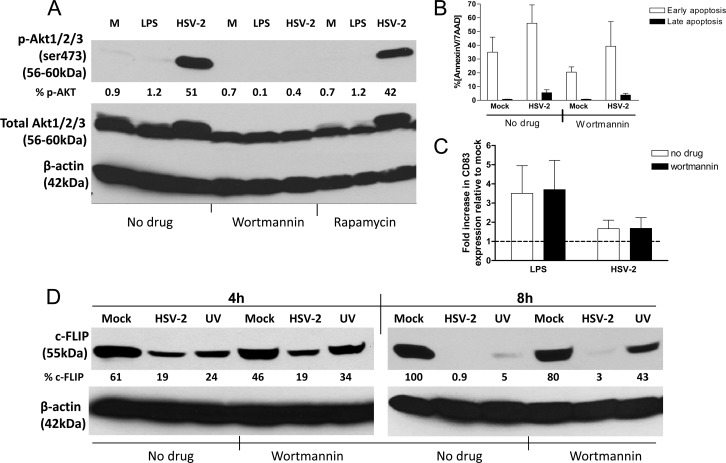
The observation that live (and UV-inactivated) HSV-2 triggered a sustained increase in Akt phosphorylation in the infected cells but not in the bystander cells, whereas early apoptosis was induced in both populations, suggests that these responses may be independent. To explore this notion, immature moDCs were exposed to HSV-2 in the presence of wortmannin, a pharmacological inhibitor of PI3K that blocks Akt phosphorylation, or rapamycin, which acts downstream of Akt but may induce negative-feedback activation of Akt signaling (34). Exposure of cells to wortmannin, but not rapamycin, prevented the virus-induced phosphorylation of Akt (Fig. 7A). However, wortmannin did not prevent virus-induced apoptosis (Fig. 7B), overcome the block to DC maturation as measured by CD83 expression (Fig. 7C), or prevent the downmodulation of c-FLIP (Fig. 7D). Consistent with the finding that UV-inactivated virus also induced apoptosis, there was a reduction in c-FLIP detected by Western blotting in DCs exposed to UV-inactivated HSV-2 compared to mock-treated cells both in the absence and presence of wortmannin (Fig. 7D).
Fig 7.
HSV-2-induced apoptosis and maturation block is independent of Akt phosphorylation. (A) DCs were treated with wortmannin (25 μM), rapamycin (0.1 μM), or no drug and were mock infected (M), LPS (10 μg/ml) treated, or infected with HSV-2(G) (MOI = 10 PFU/cell). Cells were harvested 4 h p.i. and analyzed by Western blotting for the expression of p-Akt, total Akt, and β-actin expression. The blot is a representative of two independent experiments. The blots were scanned, the protein levels were quantified, and the percentages of p-Akt relative to total Akt are indicated below each lane. (B and C) In parallel, DCs were challenged with HSV-2(G) (MOI = 5 PFU/cell) in the presence of wortmannin and at 4 h p.i. were analyzed by FACS for apoptosis (B) and maturation (C) (as quantified by CD83 expression). The results are presented as the percent positive for annexin V+ 7AAD− (early apoptosis) and annexin V+ 7AAD+ (late apoptosis) (B) and the fold increase in CD83 expression relative to mock-treated cells (C). The data are means + the SEM of three independent experiments. (D) DCs were treated with wortmannin or no drug and were mock infected or exposed to live or UV-inactivated HSV-2(G) (MOI = 10 PFU/cell) and, at the indicated times postexposure, the cells were lysed and analyzed by Western blotting for the expression of c-FLIP and β-actin. A blot representative of results obtained from two independent experiments is shown, and the percentage of c-FLIP relative to β-actin is indicated below each lane.
HSV-2 triggers the release of proinflammatory cytokines.
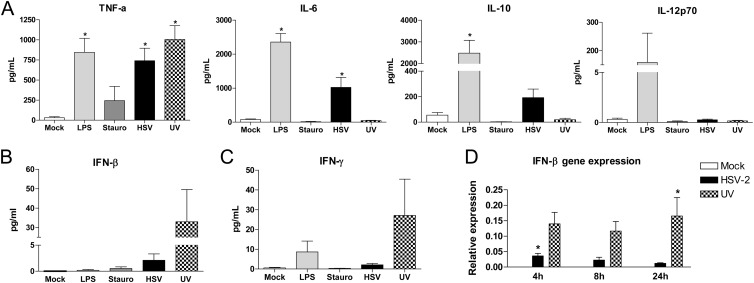
DC maturation is typically associated with release of cytokines, which facilitate the generation of effector T cell responses. Although HSV-2 failed to increase the expression of cell surface markers associated with maturation, virus did trigger significant increases in TNF-α and IL-6 compared to mock-exposed cells in 24 h culture supernatants, and the response was similar to that observed after treatment of DCs with LPS (Fig. 8A). However, in contrast to LPS, HSV-2 failed to stimulate IL-12p70 and induced a more modest IL-10 response, suggesting that HSV-exposed DCs may be less capable of generating T cell responses. UV-inactivated virus only induced the release of TNF-α, suggesting that release of other cytokines requires viral gene expression. However, UV-inactivated virus caused a greater increase in the levels of type I (IFN-β) and II (IFN-γ) IFNs released into the culture supernatants compared to live virus (Fig. 8B and C) and was associated with persistent upregulation of IFN-β gene expression by qRT-PCR, significantly higher than live virus or mock 24 h p.i. (P < 0.05) (Fig. 8D). These findings are consistent with earlier studies with HSV-1 and other cell types, indicating that immediate-early viral genes are required to block the IFN response to HSV (35–37).
Fig 8.
Live HSV-2 triggers the release of TNF-α and IL-6, whereas UV-inactivated virus triggers an IFN response. (A) Immature moDCs were mock infected, treated with LPS (1 μg/ml) or staurosporine (1 μM), or infected with live or UV-inactivated HSV-2(G) (MOI = 5 PFU/cell), and culture supernatants were harvested at 24 h p.i. and analyzed by Luminex for cytokine levels. The results are means + the SEM obtained from duplicate wells from a minimum of nine independent experiments. (B and C) Immature moDCs were exposed to the same stimuli and, 24 h postexposure, culture supernatants were assessed for the release of IFN-β by ELISA (B) or the release of IFN-γ by Luminex (C). (D) The cells were also lysed at the indicated times for the measurement of IFN-β gene expression by qRT-PCR. The data are presented as pg/ml released (A, B, and C) and gene expression (relative to the housekeeping α-tubulin gene) (D). The data are means + the SEM from three independent experiments. The asterisks indicate significant differences relative to mock-treated DCs (P < 0.05).
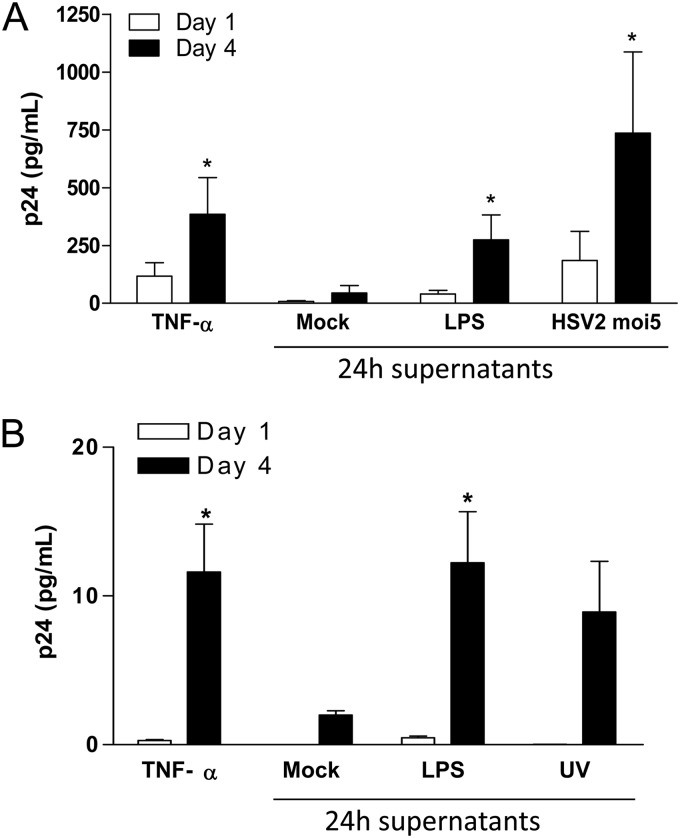
HIV-1 replication is induced by culture supernatants harvested from HSV-2-infected moDC.
U1 cells, which are persistently infected with HIV but replicate virus at low levels unless the viral LTR is activated, were treated with UV-irradiated (to inactivate any HSV) culture supernatants harvested from DCs at 24 h postexposure to HSV-2(G), LPS, or medium (negative control). Culture supernatants from LPS and HSV-2-exposed DCs significantly increased HIV replication compared to the negative control, as evidenced by an increase in p24 levels. The response was at least as great as that observed when U1 cells were treated with recombinant TNF-α (100 U/ml) (Fig. 9A). Culture supernatants from moDCs exposed to UV-inactivated virus also increased HIV-1 replication in U1 cells (Fig. 9B). Together, these data suggest that the microenvironment of the genital mucosa in response to HSV-2 infection may favor the replication of HIV-1.
Fig 9.
HSV-2-exposed DC culture supernatants induce HIV-1 replication in U1 cells. Human immature moDCs were mock exposed, exposed to LPS (1 μg/ml), and HSV-2G (MOI = 5 PFU/cell) (A) or UV-inactivated HSV-2G (MOI = 5 PFU/cell) (B) for 1 h at 37°C. U1 cells were cultured for 4 days in (UV-inactivated) DC supernatants harvested at 24 h post-HSV-2 exposure. TNF-α (100 U/ml) was included as a positive control for induction of HIV-1 replication. HIV release into the U1 cell culture supernatants was monitored by p24 ELISA on days 1 and 4. The results are means + the SEM obtained from six (A) and four (B) independent experiments where each condition was tested in triplicate. The asterisks indicate a significant increase in p24 relative to mock-treated cells (P < 0.05).
DISCUSSION
Several immune evasion strategies that may contribute to the ability of HSV to establish a lifelong infection and, in some patients, to cause recurrent clinical disease have been identified. These include inhibition of complement and antibody binding, mediated by the viral envelope glycoproteins gC and gE/gI, respectively, inhibition of major histocompatibility complex (MHC) class I-mediated antigen presentation through binding of ICP47 to the TAP, which blocks the translocation of processed peptide complexes to the cell surface and prevents CD8+ T cell recognition, and downregulation of the MHC class-I-like antigen presenting molecule, CD1, to restrict natural killer T (NKT) cell function (38, 39). The results of these studies support and extend prior observations demonstrating another immune evasion strategy: HSV blocks human moDC maturation and triggers DC apoptosis to prevent effector T cell responses (12, 13, 15, 16). Specifically, we observed that DCs exposed simultaneously (or 4 h earlier) to live HSV-2 were unable to present antigen (influenza virus) to autologous T cells despite the maturation of some of the bystander DCs in the cultures (Fig. 2) and the induction of a TNF-α and IL-6 cytokine response to virus (Fig. 8).
Viral interference with DC function has been observed with several different viruses, suggesting that this may be a common immune evasion strategy. Similar to the results obtained in the present study, infection of immature DCs by the murine gammaherpesvirus 68 (MHV68) did not trigger increases in expression of CD80 and CD86, nor did it induce DC migration. Moreover, subsequent stimulation of MHV68-infected DCs with LPS failed to trigger an increase in expression of costimulatory molecules, indicating that the MHV68-infected cells were anergic (40). Interference with DC function has also been observed with nonherpesviruses. For example, human DCs infected with influenza A virus have lower allospecific Th1-cell stimulatory abilities than DCs activated by other stimuli, such as LPS and Newcastle disease virus infection. Specifically, influenza virus NS1 protein interfered with DC maturation, migration, and T-cell stimulatory activity (41).
Not only did HSV-2 fail to trigger DC maturation, but it also induced apoptosis in both infected and bystander cells, which likely contributes to immune evasion. These results are consistent with several other studies with murine (27), macaque (16) and human (29) DCs. However, in contrast to earlier studies (16, 27, 42), we found that UV-inactivated virus also induced apoptosis and decreased c-FLIP expression. The differences may reflect methods of inactivation (UV versus heat inactivation [16]), time points (overnight exposure [16] versus 4 h), or viral strain differences. Our findings suggest that viral gene expression is not required to induce apoptosis but may block DC maturation since UV-inactivated virus triggered an increase in CD83 expression (albeit less than observed in response to LPS) (Fig. 1). The mechanisms by which live and UV-inactivated HSV-2 induce DC apoptosis are likely mediated by activation of cytotoxic death ligands such as TNF-α (Fig. 8) and reduction in the levels of c-FLIP protein (Fig. 5 and 7), a prosurvival protein, which interferes with caspase-8 within the death-inducing signaling complex (DISC) (43–45). Our findings are consistent with recent studies with HSV-1 (30, 31). However, we found that c-FLIP expression was reduced as early as 15 min postinfection with little expression detected by 4 h p.i., whereas the response to HSV-1 was evident at later time points (12 and 18 h post-HSV-1 infection) (30, 31). We also observed an increase in caspase-8 in HSV-exposed cells, which further supports the notion that HSV-2 activates this apoptotic pathway (44, 46); there was little change in caspase-3 expression.
HSV-2 has also been shown to induce apoptosis in T cells (Jurkat and primary human CD4+ cells) through intrinsic apoptosis pathways (28). Notably, HSV antigens and activated caspase-3 were rarely detected in the same T cell, suggesting that apoptosis may involve a bystander effect. We also observed that HSV-2 triggered apoptosis in both infected (GFP+) and bystander (GFP−) moDCs, possibly through the release of TNF-α and other death ligands. However, further studies are needed to delineate the mechanism of apoptosis in the bystander DC population.
Surprisingly, despite the induction of apoptosis, HSV-2 triggered the rapid and persistent phosphorylation of Akt, suggesting that HSV-2 may interfere with the antiapoptotic and promaturation functions of Akt (33). These findings are in contrast to prior studies in epithelial cells, where it was shown that HSV-2 transiently activates Akt 4 h post-HSV infection to prevent epithelial cell apoptosis. By 8 h p.i., the phosphorylated forms of Akt were no longer detected in the epithelial cells, possibly reflecting the accumulation of viral US3 protein kinase, which provides antiapoptotic activity (42). The prosurvival function of Akt can be converted to a proapoptotic function upon prolonged hyperactivation of the Akt kinase activity or by nuclear retention or unbalanced phosphorylation of the Akt protein (47). However, pharmacological blockade of Akt phosphorylation with wortmannin failed to abrogate the virus-induced apoptosis response (or prevent reduction in c-FLIP levels), suggesting that the apoptotic response to viral infection is independent of the accumulation of p-Akt. Wortmannin treatment also did not overcome the block to DC maturation (CD83 expression). Further studies are needed to precisely delineate the role of the persistent Akt phosphorylation in response to HSV.
Despite the inability to induce maturation, live and UV-inactivated HSV-2 paradoxically triggered the release of proinflammatory cytokines, most notably TNF-α, although, as expected, only the UV-inactivated virus induced an IFN response. The cytokine response (and possibly other immune mediators released into culture supernatants after viral exposure) to live and inactivated HSV-2 was sufficient to increase HIV replication in the chronically infected U1 cell line. These mediators could potentially activate T cells recruited to sites of HSV infection or reactivation, which could serve as targets for HIV infection, thus further contributing to the epidemiological synergy between HSV and HIV. The ability of HSV to induce a state of functional anergy and to induce apoptosis in both HSV-infected and bystander DC populations would render the DCs incapable of presenting HIV or HSV antigens to the T cells, thus preventing the host from mounting an effective cellular immune response against either virus. An additional mechanism that may contribute to the ability of HSV to fuel HIV infection was suggested by a recent study that reported that HSV induces the release of retinoic acid (RA) from DCs. RA expands α4β7high CD4+ T cell subset, which may be highly susceptible to HIV, thus further enhancing HIV infection, particularly in the gut (48).
How well the results observed in these studies with isolated human moDCs translate to what happens clinically, where cross talk between different cell types may modulate responses, requires further study. It will also be important to assess how other human DC populations (e.g., primary myeloid DCs, plasmacytoid DCs, or Langerhans cells) respond to HSV. Sensitive PCR assays demonstrate frequent and rapid reactivation and clearance of HSV-2 in genital tissue, which suggests that most of the time the mucosal immune response controls viral infection (5). However, in the setting of clinical recurrences, we speculate that the interference with DC function helps the virus to escape the immune system. Moreover, both in the setting of subclinical and clinical HSV recurrences and even in the presence of suppressive acyclovir therapy, the DC responses to HSV may promote HIV replication through multiple mechanisms. The results obtained may also have implications for mucosal vaccine strategies since HSV may dampen immune response to mucosal immunization.
ACKNOWLEDGMENTS
This study was supported by grants AI061679 (B.C.H.), AI65309 (B.C.H.), HHSN266200700010C (A.F.-S.) from the National Institutes of Health and by Child Health Research Career Development grant K12 HD052890 (V.M.C.).
Footnotes
Published ahead of print 14 November 2012
REFERENCES
- 1. Corey L. 2007. Synergistic copathogens: HIV-1 and HSV-2. N. Engl. J. Med. 356:854–856 [DOI] [PubMed] [Google Scholar]
- 2. Nagot N, Ouedraogo A, Defer MC, Vallo R, Mayaud P, Van de Perre P. 2007. Association between bacterial vaginosis and Herpes simplex virus type-2 infection: implications for HIV acquisition studies. Sex Transm. Infect. 83:365–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ouedraogo A, Nagot N, Vergne L, Konate I, Weiss HA, Defer MC, Foulongne V, Sanon A, Andonaba JB, Segondy M, Mayaud P, Van de Perre P. 2006. Impact of suppressive herpes therapy on genital HIV-1 RNA among women taking antiretroviral therapy: a randomized controlled trial. AIDS 20:2305–2313 [DOI] [PubMed] [Google Scholar]
- 4. Corey L, Wald A, Celum CL, Quinn TC. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 35:435–445 [DOI] [PubMed] [Google Scholar]
- 5. Mark KE, Wald A, Magaret AS, Selke S, Olin L, Huang ML, Corey L. 2008. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J. Infect. Dis. 198:1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, Krieger JN, Corey L. 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 342:844–850 [DOI] [PubMed] [Google Scholar]
- 7. Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, Defer MC, Djagbare D, Sanon A, Andonaba JB, Becquart P, Segondy M, Vallo R, Sawadogo A, Van de Perre P, Mayaud P. 2007. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N. Engl. J. Med. 356:790–799 [DOI] [PubMed] [Google Scholar]
- 8. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811 [DOI] [PubMed] [Google Scholar]
- 9. Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252 [DOI] [PubMed] [Google Scholar]
- 10. Palucka K, Banchereau J. 2002. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 14:420–431 [DOI] [PubMed] [Google Scholar]
- 11. Mikloska Z, Bosnjak L, Cunningham AL. 2001. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J. Virol. 75:5958–5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pollara G, Jones M, Handley ME, Rajpopat M, Kwan A, Coffin RS, Foster G, Chain B, Katz DR. 2004. Herpes simplex virus type-1-induced activation of myeloid dendritic cells: the roles of virus cell interaction and paracrine type I IFN secretion. J. Immunol. 173:4108–4119 [DOI] [PubMed] [Google Scholar]
- 13. Pollara G, Speidel K, Samady L, Rajpopat M, McGrath Y, Ledermann J, Coffin RS, Katz DR, Chain B. 2003. Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J. Infect. Dis. 187:165–178 [DOI] [PubMed] [Google Scholar]
- 14. Reske A, Pollara G, Krummenacher C, Katz DR, Chain BM. 2008. Glycoprotein-dependent and TLR2-independent innate immune recognition of herpes simplex virus-1 by dendritic cells. J. Immunol. 180:7525–7536 [DOI] [PubMed] [Google Scholar]
- 15. Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. 2003. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 197:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peretti S, Shaw A, Blanchard J, Bohm R, Morrow G, Lifson JD, Gettie A, Pope M. 2005. Immunomodulatory effects of HSV-2 infection on immature macaque dendritic cells modify innate and adaptive responses. Blood 106:1305–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herold BC, WuDunn D, Soltys N, Spear PG. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 65:1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Folks TM, Justement J, Kinter A, Dinarello CA, Fauci AS. 1987. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science 238:800–802 [DOI] [PubMed] [Google Scholar]
- 19. Fakioglu E, Wilson SS, Mesquita PM, Hazrati E, Cheshenko N, Blaho JA, Herold BC. 2008. Herpes simplex virus downregulates secretory leukocyte protease inhibitor: a novel immune evasion mechanism. J. Virol. 82:9337–9344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. 1997. Proinflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol. 27:3135–3142 [DOI] [PubMed] [Google Scholar]
- 21. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 22. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramos I, Bernal-Rubio D, Durham N, Belicha-Villanueva A, Lowen AC, Steel J, Fernandez-Sesma A. 2011. Effects of receptor binding specificity of avian influenza virus on the human innate immune response. J. Virol. 85:4421–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poli G, Kinter AL, Justement JS, Bressler P, Kehrl JH, Fauci AS. 1991. Transforming growth factor beta suppresses human immunodeficiency virus expression and replication in infected cells of the monocyte/macrophage lineage. J. Exp. Med. 173:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haye K, Burmakina S, Moran T, Garcia-Sastre A, Fernandez-Sesma A. 2009. The NS1 protein of a human influenza virus inhibits type I interferon production and the induction of antiviral responses in primary human dendritic and respiratory epithelial cells. J. Virol. 83:6849–6862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phipps-Yonas H, Seto J, Sealfon SC, Moran TM, Fernandez-Sesma A. 2008. Interferon-beta pretreatment of conventional and plasmacytoid human dendritic cells enhances their activation by influenza virus. PLoS Pathog. 4:e1000193 c17 doi:10.1371/journal.ppat.1000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones CA, Fernandez M, Herc K, Bosnjak L, Miranda-Saksena M, Boadle RA, Cunningham A. 2003. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J. Virol. 77:11139–11149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vanden Oever MJ, Han JY. 2010. Caspase 9 is essential for herpes simplex virus type 2-induced apoptosis in T cells. J. Virol. 84:3116–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. 2005. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J. Immunol. 174:2220–2227 [DOI] [PubMed] [Google Scholar]
- 30. Kather A, Raftery MJ, Devi-Rao G, Lippmann J, Giese T, Sandri-Goldin RM, Schonrich G. 2010. Herpes simplex virus type 1 (HSV-1)-induced apoptosis in human dendritic cells as a result of downregulation of cellular FLICE-inhibitory protein and reduced expression of HSV-1 antiapoptotic latency-associated transcript sequences. J. Virol. 84:1034–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muller DB, Raftery MJ, Kather A, Giese T, Schonrich G. 2004. Frontline: induction of apoptosis and modulation of c-FLIPL and p53 in immature dendritic cells infected with herpes simplex virus. Eur. J. Immunol. 34:941–951 [DOI] [PubMed] [Google Scholar]
- 32. Belmokhtar CA, Hillion J, Segal-Bendirdjian E. 2001. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 20:3354–3362 [DOI] [PubMed] [Google Scholar]
- 33. Xie J, Qian J, Yang J, Wang S, Freeman ME, III, Yi Q. 2005. Critical roles of Raf/MEK/ERK and PI3K/AKT signaling and inactivation of p38 MAP kinase in the differentiation and survival of monocyte-derived immature dendritic cells. Exp. Hematol. 33:564–572 [DOI] [PubMed] [Google Scholar]
- 34. Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. 2007. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26:1932–1940 [DOI] [PubMed] [Google Scholar]
- 35. Cantin EM, Hinton DR, Chen J, Openshaw H. 1995. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 69:4898–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eidson KM, Hobbs WE, Manning BJ, Carlson P, DeLuca NA. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 76:2180–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Randall RE, Goodbourn S. 2008. Interferons and viruses: an interplay between induction, signaling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1–47 [DOI] [PubMed] [Google Scholar]
- 38. Rao P, Pham HT, Kulkarni A, Yang Y, Liu X, Knipe DM, Cresswell P, Yuan W. 2011. Herpes simplex virus 1 glycoprotein B and US3 collaborate to inhibit CD1d antigen presentation and NKT cell function. J. Virol. 85:8093–8104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yuan W, Dasgupta A, Cresswell P. 2006. Herpes simplex virus evades natural killer T cell recognition by suppressing CD1d recycling. Nat. Immunol. 7:835–842 [DOI] [PubMed] [Google Scholar]
- 40. Hochreiter R, Ptaschinski C, Kunkel SL, Rochford R. 2007. Murine gammaherpesvirus-68 productively infects immature dendritic cells and blocks maturation. J. Gen. Virol. 88:1896–1905 [DOI] [PubMed] [Google Scholar]
- 41. Fernandez-Sesma A, Marukian S, Ebersole BJ, Kaminski D, Park MS, Yuen T, Sealfon SC, Garcia-Sastre A, Moran TM. 2006. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol. 80:6295–6304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Benetti L, Roizman B. 2006. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of δU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J. Virol. 80:3341–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bartz SR, Emerman M. 1999. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by upregulating FLICE/caspase-8. J. Virol. 73:1956–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. 1997. Inhibition of death receptor signals by cellular FLIP. Nature 388:190–195 [DOI] [PubMed] [Google Scholar]
- 45. Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. 2001. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J. Biol. Chem. 276:20633–20640 [DOI] [PubMed] [Google Scholar]
- 46. Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M, Scaffidi C, Krammer PH, Peter ME, Tschopp J. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517–521 [DOI] [PubMed] [Google Scholar]
- 47. Benbrook DM, Masamha CP. 2011. The pro-survival function of Akt kinase can be overridden or altered to contribute to induction of apoptosis. Curr. Cancer Drug Targets 11:586–599 [DOI] [PubMed] [Google Scholar]
- 48. Martinelli E, Tharinger H, Frank I, Arthos J, Piatak M, Jr, Lifson JD, Blanchard J, Gettie A, Robbiani M. 2011. HSV-2 infection of dendritic cells amplifies a highly susceptible HIV-1 cell target. PLoS Pathog. 7:e1002109 doi:10.1371/journal.ppat.1002109 [DOI] [PMC free article] [PubMed] [Google Scholar]