Abstract
Coinfection with Plasmodium falciparum malaria and Epstein-Barr virus (EBV) is a major risk factor for endemic Burkitt lymphoma (eBL), still one of the most prevalent pediatric cancers in equatorial Africa. Although malaria infection has been associated with immunosuppression, the precise mechanisms that contribute to EBV-associated lymphomagenesis remain unclear. In this study, we used polychromatic flow cytometry to characterize CD8+ T-cell subsets specific for EBV-derived lytic (BMFL1 and BRLF1) and latent (LMP1, LMP2, and EBNA3C) antigens in individuals with divergent malaria exposure. No malaria-associated differences in EBV-specific CD8+ T-cell frequencies were observed. However, based on a multidimensional analysis of CD45RO, CD27, CCR7, CD127, CD57, and PD-1 expression, we found that individuals living in regions with intense and perennial (holoendemic) malaria transmission harbored more differentiated EBV-specific CD8+ T-cell populations that contained fewer central memory cells than individuals living in regions with little or no (hypoendemic) malaria. This profile shift was most marked for EBV-specific CD8+ T-cell populations that targeted latent antigens. Importantly, malaria exposure did not skew the phenotypic properties of either cytomegalovirus (CMV)-specific CD8+ T cells or the global CD8+ memory T-cell pool. These observations define a malaria-associated aberration localized to the EBV-specific CD8+ T-cell compartment that illuminates the etiology of eBL.
INTRODUCTION
First described in 1958 (1), endemic Burkitt lymphoma (eBL) remains one of the most prevalent childhood cancers in equatorial Africa. The average annual incidence is 2 per 100,000 children, with a peak age range of 5 to 9 years (2–4). In 1964, Epstein-Barr virus (EBV) was discovered in a tumor sample obtained from a patient with eBL (5), and EBV DNA has subsequently been detected in tumor cells from 95% of eBL cases (6). Thus, EBV was identified as the first human tumor virus, with ensuing studies revealing the virus-mediated oncogenic processes (7). However, eBL is most common in children residing in areas with the highest malaria transmission intensities (3, 8–10), an enigmatic observation that leaves the malaria-associated mechanisms involved in the etiology of eBL insufficiently established by comparison.
Infection with EBV occurs early in most African populations, and almost 100% of children are EBV seropositive by 3 years of age (11, 12). Primary infection during childhood is typically asymptomatic, whereas infection in young adults can result in acute infectious mononucleosis (AIM), a self-limited lymphoproliferative disorder. To date, most immunologic studies of EBV infection are based on healthy seropositive adults or cases of AIM among adolescents in Europe or the United States (13). Collectively, these studies show that CD8+ cytotoxic T lymphocytes (CTL) are necessary for immune surveillance and control of persistent EBV infection (14, 15). The CTL response to EBV is directed against an array of antigens expressed during the lytic and latent phases of the viral life cycle (13–15), and control is associated with HLA class I-restricted gamma interferon (IFN-γ) responses (16). Previous studies have also demonstrated phenotypic and functional heterogeneity among EBV-specific CD8+ T-cell populations (17). However, little is known about these cells when primary EBV infection occurs during infancy or early childhood.
Beyond the early studies that revealed a geographic overlap between eBL and areas of intense, perennial Plasmodium falciparum malaria transmission (regions of malaria holoendemicity) (8, 9), the malaria-driven mechanisms that contribute to eBL pathogenesis remain obscure. In these regions of equatorial Africa, more than 80% of children are chronically or repeatedly infected with P. falciparum malaria by 5 years of age, and initial malaria exposure occurs within the first few months of life (18, 19). It is established that malaria parasites modulate and evade the host immune system (20). Indeed, these properties underlie the hypothesis that P. falciparum malaria suppresses immunity to EBV during coinfection. In the early 1980s, a series of seminal studies demonstrated that lymphocytes from malaria-infected individuals were unable to control the proliferation of EBV-transformed B cells in relatively crude regression assays (21, 22). Although these observations suggest that P. falciparum malaria infection disrupts EBV-specific immunity, the effector cells or mediators responsible for controlling EBV-infected B-cell growth were not identified, and overall immune competence was not assessed in the small number of individuals studied. More recently, an age-related deficiency in IFN-γ recall responses to EBV lytic and latent antigens was demonstrated in children (i.e., 5 to 9 years of age) with holoendemic malaria exposure compared to those from an area of malaria hypoendemicity (23). In addition, EBV load in African children correlates with malaria exposure (24, 25), further implicating coinfection as a risk factor for eBL tumorigenesis. However, it remains unclear how P. falciparum malaria might potentiate a deficit in EBV-specific T-cell immunity and thus contribute to eBL lymphomagenesis.
Two mutually compatible theories have been proposed to explain the relationship between EBV and P. falciparum malaria in the etiology of eBL (26). The first suggests that malaria coinfection increases the number of latently infected B cells by inducing polyclonal B-cell expansion and consequent lytic EBV reactivation (27). In turn, the greater precursor frequency of EBV-infected B cells increases the likelihood of c-myc translocation, which is a hallmark of all BL tumors (28). The second theory argues that EBV-specific T-cell responses are selectively altered during malaria coinfection, either as a cause or consequence of enhanced EBV replication, leading to impaired viral control and/or immune surveillance (29, 30).
To provide direct evidence for the role of altered EBV-specific T-cell immunity in eBL, we used polychromatic flow cytometry to characterize EBV-specific CD8+ T cells in a unique, well-characterized cohort of individuals with divergent malaria exposure. Specifically, 16-parameter flow cytometry panels were developed to quantify differentiation, exhaustion, senescence, and homeostatic potential within six distinct EBV-specific CD8+ T-cell populations and one CMV-specific CD8+ T-cell population, all restricted by HLA A*0201, and within the CD8+ T-cell compartment as a whole. By virtue of the highly multiplexed nature of our measurements, cellular characteristics could be defined in exquisite detail. Furthermore, we employed recently developed data analysis strategies to deconvolute these complex flow cytometry data sets. Accordingly, probability binning (31) and frequency difference gating (32) revealed dramatic differences between cell populations in multidimensional space that eluded conventional analytical approaches. Together, our analyses reveal an immunologic aberration confined to the EBV-specific CD8+ T-cell compartment that is associated with P. falciparum malaria exposure and further illuminate the relationship between holoendemic malaria and eBL.
MATERIALS AND METHODS
Study participants.
Approval for this study was obtained from the Ethical Review Committee at the Kenya Medical Research Institute (KEMRI) and the Institutional Review Board for Human Studies, University Hospitals of Cleveland, Case Western Reserve University; the latter was the institutional affiliation for A.M.M. at the time of this study. Written informed consent was obtained in all cases from study participants or parents of minors.
Participant recruitment and sample collection were conducted in two epidemiologically distinct areas of western Kenya: (i) Nyanza Province, Kisumu District, in the sublocation of Kanyawegi; (ii) Rift Valley Province, Nandi District, in the sublocation of Kipsamoite. The first study site is situated on the shore of Lake Victoria, 10 km west of Kisumu; malaria transmission in this area is holoendemic (i.e., intense and perennial). The second study site is located in the highlands, 150 km northeast of Kisumu; malaria transmission in this area is hypoendemic (i.e., sporadic with periodic outbreaks of malaria morbidity in a population with low parasite prevalence) (33).
For this study, we collected blood samples from individuals with known HLA class I genotypes and divergent malaria exposure histories who experienced primary EBV infection prior to 3 years of age (25, 34). No evidence of fever or anemia was present in these individuals at the time of venous blood sampling; thus, any cases of malaria parasitemia were asymptomatic.
Sample collection.
Venous blood was collected in sodium-heparinized tubes and transported to the KEMRI Center for Global Health Research in Kisumu for processing within 3 h. Peripheral blood mononuclear cells (PBMCs) were separated from whole blood by Ficoll-Hypaque density gradient centrifugation, counted, and then resuspended in freezing medium comprising 90% heat-inactivated, filter-sterilized fetal bovine serum and 10% dimethyl sulfoxide (Sigma). Aliquots of 6 million cells/ml per vial were cooled overnight at a rate of −1°C per minute prior to long-term storage in vapor-phase liquid nitrogen. Samples were transported with a Centers for Disease Control and Prevention (CDC) import permit using an MVE IATA-approved vapor-phase shipper to maintain temperatures below −180°C en route.
Tetrameric peptide-HLA A*0201 complexes.
Soluble, biotinylated peptide-HLA A*0201 (pHLA A*0201) monomers were produced and tetramerized with fluorochrome-conjugated streptavidin as described previously (35). The following peptides (>95% purity; BioSynthesis) were used for monomer production: (i) GLCTLVAML (GLC; EBV BMLF1, residues 280 to 288), (ii) YVLDHLIVV (YVL; EBV BRLF1, residues 109 to 117), (iii) YLLEMLWRL (YLL; EBV LMP1, residues 125 to 133), (iv) FLYALALLL (FLY; EBV LMP2, residues 356 to 364), (v) CLGGLLTMV (CLG; EBV LMP2, residues 426 to 434), (vi) LLDFVRFMGV (LLD; EBV EBNA3C, residues 284 to 293), and (vii) NLVPMVATV (NLV; CMV pp65, residues 495 to 503).
Flow cytometry and data analysis.
Samples were thawed and then stained with pHLA A*0201 tetramers and a panel of fluorochrome-conjugated monoclonal antibodies as described previously (36). Data were analyzed using FlowJo version 9.2 (Treestar Inc.), which contains platforms for probability binning/frequency difference gating and SPICE (31, 32). Adjustments for multiple comparisons were not performed due to considerable overlap between cell subsets (37). Instead, a more stringent threshold for significance was set (P < 0.01).
RESULTS
Development and validation of polychromatic flow cytometry panels for the evaluation of EBV-specific CD8+ T-cell immunity.
To evaluate the effects of P. falciparum malaria on EBV-specific CD8+ T-cell immunity, we conducted a cross-sectional study of children and adults from regions where malaria is either holoendemic or hypoendemic (34). Peripheral blood samples were examined using two 16-parameter flow cytometry panels to determine antigen specificity and molecular coexpression patterns at the single-cell level (Fig. 1A and B). Reagents and staining panels were developed as described previously (36). Assay procedures were validated for rare event analysis using a set of test samples (n = 28), with previously defined epitope-specific CD8+ T-cell responses corresponding to the HLA A*0201-restricted specificities examined in this study. All pHLA A*0201 tetramers were quality controlled using PBMCs from HLA A*0201− donors and HLA A*0201+ donors with no evidence of CMV or EBV infection; no nonspecific staining was observed in either setting. Individual samples were also evaluated repeatedly to ensure procedural reproducibility. This approach enabled us to interrogate EBV-specific CD8+ T-cell populations with unprecedented precision and depth.
Fig 1.
Gating strategy and flow cytometric staining patterns. (A) Gating strategy for identification of CD8+ T cells. Cell doublets were excluded from the analysis on the basis of forward scatter area and height characteristics (FSC-A and FSC-H, respectively), and live (ViViD−) CD3+ lymphocytes were selected. Fluorochrome aggregates were then excluded (not shown) prior to the selection of CD4− CD8+ T cells for further analysis. (B) Representative staining patterns for immunophenotypic markers within the total CD8+ T-cell compartment (top row), and the identification of EBV-specific CD8+ T-cell populations using pHLA A*0201 tetramers (middle and bottom rows). Low-resolution plots are used for EBV-specific CD8+ T-cell events to aid visualization of low-frequency populations. In the individual depicted here, clear CD8+ T-cell populations are observed for all specificities with the exception of YLL.
Immunodominance profiles among HLA A*0201-restricted EBV-specific CD8+ T-cell populations.
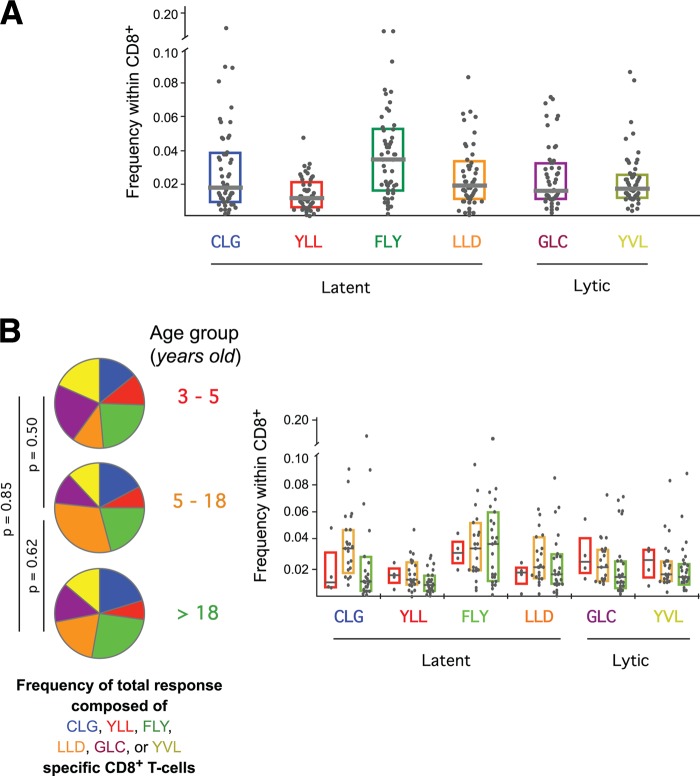
Initially, we examined the frequencies of CD8+ T cells directed against six different HLA A*0201-restricted EBV-derived epitopes. Four of these epitopes (YLL, FLY, CLG, and LLD) are derived from latently expressed proteins, and two (GLC and YVL) are derived from lytic-phase proteins. There are dramatic differences between these epitopes with respect to the milieu and kinetics of antigen presentation to the immune system. Remarkably, however, we found no significant differences between the frequencies of CD8+ T cells targeting latent and lytic epitopes (Fig. 2A). Moreover, no single epitope-specific CD8+ T-cell population was consistently immunodominant across the cohort as a whole. These observations held after stratification of study participants for malaria exposure (data not shown) and age (Fig. 2B). The similar distribution of these CD8+ T-cell populations across all age groups suggests that the nature of the EBV-specific response is set early in life and changes little over time.
Fig 2.
Frequencies of EBV-specific CD8+ T cells. (A) Frequencies of EBV-specific CD8+ T cells defined by pHLA A*0201 tetramer staining. (B) Frequencies of EBV-specific CD8+ T cells by age group (children ages 3 to 5 years, red; children/adolescents ages 5 to 18 years, yellow; adults, green). There were no significant frequency differences between age groups for any of the depicted CD8+ T-cell specificities. Horizontal bars represent median values, and boxes delineate the 25th and 75th percentiles.
Distinct phenotypes characterize different EBV-derived epitope-specific CD8+ T-cell populations.
Next, we examined the phenotypic properties of EBV-specific CD8+ T cells across the entire study population regardless of malaria exposure. Minor phenotypic differences were observed in some EBV-specific CD8+ T-cell populations by age. However, these were observed only in exceedingly rare cell populations, and there was no clear relationship between the populations that differed; for example, expression of the senescence marker CD57 was not consistently lower in the youngest individuals (data not shown). On this basis, we subsequently aggregated all study participants irrespective of age. In order to minimize the contribution of nonspecific events (“noise”), we stratified the data set according to the number of cells collected on the flow cytometer and selected only those populations that achieved a statistically rigorous minimum number of antigen-specific events (Fig. 3; see also Fig. S1 in the supplemental material).
Fig 3.

Distribution of phenotypes within each antigen-specific CD8+ T-cell population, grouped by the number of events collected on the flow cytometer. Data are shown for CD8+ T cells directed against EBV-derived latent antigens. Pies depict data for samples where 1 to 5, 6 to 10, 11 to 20, or 20+ antigen-specific events were collected. Slices within each pie represent the proportion of CD8+ T cells expressing a particular combination of the markers analyzed. Pies for each event category were compared by permutation analysis to determine if the distribution of phenotypes differed according to the number of events collected. For example, among CD8+ T cells specific for the FLY epitope, the distribution of phenotypes observed when 1 to 5 events were collected (pie 1) was significantly different from the distribution observed when 20+ events were collected (P = 0.037). Similarly, the distribution differed significantly when 6 to 10 (pie 2) and 11 to 20 (pie 3) events were collected (P = 0.074 and P = 0.002, respectively). Thus, the phenotype of FLY-specific CD8+ T cells was not considered in samples with less than 20 such events. For all other latent-phase antigens, the distribution of phenotypes was not significantly different when more than 5 events were collected; these pies are highlighted with a black border. Therefore, for these specificities, only study participants with fewer than 5 antigen-specific events were excluded from the analysis. The numbers in the center of the pie represent the number of study participants included in each group. Thus, FLY-specific CD8+ T-cell populations were analyzed in 20 of the 56 individuals (pie 4), while responses to the CLG, YLL, and LLD epitopes were analyzed in 38, 29, and 41 of the 56 individuals, respectively (sum of pies 2 to 4). Of note, although the number of antigen-specific events analyzed was low, sources of nonspecific binding were rigorously excluded, and the total number of events collected ranged from 350,000 to 950,000. Data for CD8+ T cells directed against EBV-derived lytic antigens are shown in Fig. S1 in the supplemental material.
Given the large number of measured parameters and the challenges associated with the interpretation of such complex multivariate data, our initial analyses focused on the differentiation stage of EBV-specific CD8+ T cells, defined by the coordinate expression of CD45RO, CCR7, CD27, CD127, and CD57 (see Fig. S2 in the supplemental material). Although CD57 typically defines senescent cells (38), we have observed in previous analyses that expression is not exclusively linked to advanced differentiation (39), which complicates the categorization and nomenclature of maturational subsets. In this light, we examined other phenotypic categories separately within the CD57+ and CD57− subsets. This analysis showed that many of the CD57− cells directed against both latent and lytic EBV-derived epitopes were central memory-like cells, expressing various combinations of CD45RO, CCR7, CD27, and CD127. Notably, EBV-specific CD8+ T cells with a naïve-like phenotype (CD45RO− CCR7+ CD27+ CD127+ CD57−) were frequently observed, in line with previous reports in other systems (40, 41). This is consistent with the recent description of stem cell-like memory T cells, which express many other surface markers typically associated with antigen-inexperienced naïve cells and likely represent a very early stage of memory T-cell differentiation (42).
In further analyses, we examined the phenotypic properties of EBV-specific CD8+ T-cell populations in terms of homeostatic potential (CD127), exhaustion (PD-1), and senescence (CD57). Clear phenotypic differences were observed between CD8+ T-cell populations that targeted distinct EBV-derived antigens (Fig. 4). Thus, substantial diversity exists within the CD8+ T-cell response to EBV, which likely reflects the complexity of antigen expression and immunosurveillance. Despite this complexity, however, CD8+ T cells directed against the latent antigens FLY, CLG, and YLL generally expressed markers consistent with the retention of homeostatic potential in the relative absence of exhaustion/senescence. In contrast, CD8+ T cells directed against the lytic antigens GLC and YVL more frequently displayed an exhausted/senescent phenotype lacking homeostatic potential. Interestingly, CD8+ T cells specific for the latent epitope LLD (EBNA3C) exhibited similar proportions of exhausted/senescent cells compared to the antilytic populations, thereby suggesting that the biology of EBNA3C expression may differ from that of other latent antigens.
Fig 4.
Relative frequencies of EBV-specific CD8+ T cells expressing various combinations of CD45RO, CD57, CD127, and PD-1 within each tetramer+ population. Data are arranged in order of increasing differentiation, such that the bar charts on the left side of the figure depict CD127+ events and the bar charts on the right side of the figure depict CD127− events. Within each section, increasingly exhausted or senescent populations are found to the right. For example, CD8+ T cells expressing CD127 without markers of exhaustion or senescence are represented on the far left; these are most evident within the populations specific for YLL, LLD, GLC, and YVL. In contrast, exhausted or senescent CD8+ T cells without homeostatic potential are represented on the far right; these are most evident within the populations specific for LLD, GLC, and YVL. Bars represent median values for the depicted cell types (x axis key) within CD8+ T-cell populations specific for the listed EBV-derived epitopes (right y axis labels). Hatched green (latent) and red (lytic) lines connect the medians for each distinct phenotype to aid visual comparison within each specificity.
Phenotypic differences between EBV-specific CD8+ T cells associated with malaria endemicity.
Next, we stratified study participants by malaria exposure and examined the phenotype of EBV-specific, CMV-specific, and total CD8+ T-cell populations. Malaria exposure was determined as described previously (43), based on well-defined epidemiologic parameters, and confirmed with clinical or laboratory data. Specifically, blood-stage infection in children occurs with <10% prevalence in regions where malaria is hypoendemic, whereas childhood infection rates in regions of malaria holoendemicity are consistently >50% throughout the year (44). Individuals from regions with strikingly divergent cumulative malaria exposure (hypoendemic versus holoendemic) were compared for this study, and two distinct approaches to data analysis were employed.
In the first approach, the proportion of CD8+ T cells that expressed each single marker was compared across both study groups and any statistically significant differences were noted. Subsequently, the proportion of CD8+ T cells that expressed every combination of two, three, four, or five markers was tested across the study groups. These analyses were conducted for each EBV-specific CD8+ T-cell population and for the CMV-specific and total CD8+ T-cell populations. Figure 5 shows examples of the differences observed when dual-marker expression was examined within the EBV-derived GLC epitope-specific CD8+ T-cell population. Study participants from regions where malaria is hypoendemic (blue) had more CD45RO+ CD127+ and CD45RO+ CD57− T cells than individuals from holoendemic (red) areas (P = 0.003 and P = 0.009, respectively) and fewer CD45RO−CD127− T cells (P = 0.008).
Fig 5.
Representative examples of phenotypic patterns within EBV-derived GLC-specific CD8+ T-cell populations that differed by malaria exposure. Horizontal bars represent median values, and boxes delineate the 25th and 75th percentiles. Box colors depict malaria endemicity (hypoendemic, blue; holoendemic, red).
Similar analyses were performed for all combinations of phenotypic markers. Tables 1 and 2 list the phenotypically defined CD8+ T-cell subsets across all specificities that were significantly elevated in study participants from regions where malaria is hypoendemic and holoendemic, respectively. In general, the numbers of cell types associated with less differentiated memory T-cell populations (e.g., CD127+ cells) were elevated in individuals from regions with hypoendemic malaria, whereas the numbers of more differentiated (e.g., CD27−, CCR7−), exhausted (PD-1+), or senescent (CD57+) cells were elevated in those exposed to holoendemic malaria. Although no adjustments were made for multiple comparisons, several comparisons were highly significant (P < 0.01), and taken together, the data present a uniform picture. Specifically, individuals with chronic/recurrent malaria exposure have elevated levels of highly differentiated EBV-specific CD8+ T cells compared to those of individuals with little or no malaria exposure. Importantly, no statistically significant phenotypic differences between study groups were observed within either the CMV-specific or the total CD8+ T-cell populations (Tables 1 and 2). Thus, malaria exposure uniquely affects the EBV-specific CD8+ T-cell compartment.
Table 1.
Phenotypically defined CD8+ T-cell subsets that were significantly elevated in regions with hypoendemic malaria
| Epitope |
P value for indicated phenotype |
||
|---|---|---|---|
| CD45RO+ CD127+ | CD45RO+ CD57− | CD45RO+ CCR7− CD57− CD127+ | |
| CLG | |||
| GLC | 0.003 | 0.009 | |
| LLD | |||
| YLL | 0.031 | ||
| YVL | |||
| CMV | 0.808 | 0.151 | 0.230 |
| CD8 | 0.151 | 0.837 | 0.250 |
Table 2.
Phenotypically defined CD8+ T-cell subsets that were significantly elevated in regions with holoendemic malaria
| Epitope |
P value for indicated phenotype |
||||||
|---|---|---|---|---|---|---|---|
| CD45RO+ CD127− | CD45RO+ CCR7− | CD45RO+ CCR7− CD127− PD1+ | CD45RO− CD127− | CD27− CD57+ CD127− | CD45RO− | CD27+ CD127− | |
| CLG | 0.015 | 0.087 | 0.016 | ||||
| GLC | 0.008 | 0.003 | |||||
| LLD | 0.010 | ||||||
| YLL | |||||||
| YVL | 0.013 | ||||||
| CMV | 0.566 | 0.621 | 0.791 | 0.116 | 0.296 | 0.650 | 0.988 |
| CD8 | 0.520 | 0.710 | 0.264 | 0.446 | 0.680 | 0.608 | 0.192 |
Malaria-associated phenotypic differences in EBV-specific CD8+ T-cell populations pinpointed in multidimensional space.
The manual gating approach described above has two significant limitations. First, any population differences that are only manifest in multiple dimensions will be more difficult to resolve, and appropriate gating strategies that discriminate such subsets optimally may not be evident from examination of the dual-parameter histograms. Second, a large number of phenotypic populations are necessarily identified, leading to loss of significance when correcting for multiple comparisons. To overcome these limitations, we adopted a second approach to confirm and extend our observations. Specifically, we used frequency difference gating (32), a method that does not rely on serial bivariate analysis and therefore has the potential to reveal population differences that occur across multidimensional space.
Using this approach, we initially examined EBV-derived GLC epitope-specific CD8+ T cells (Fig. 6). The results confirmed and extended our previous analysis, in which CD8+ T cells with a central memory-like phenotype (CD45RO+ CD127+ and CD45RO+ CD57−) were associated with hypoendemic malaria exposure (Table 1 and Fig. 5). Frequency difference gating revealed a phenotypic space defined by the CD45RO+ CD27+ CCR7+ CD127+ CD57− PD-1+ expression pattern that contained 9.9% of GLC-specific CD8+ T cells from individuals with hypoendemic malaria exposure and only 2.9% of the corresponding CD8+ T-cell population from individuals with holoendemic malaria exposure (P < 0.05). Thus, as in the previous analysis, highly differentiated cells were enriched within the GLC-specific CD8+ T-cell compartment in individuals with holoendemic malaria exposure.
Fig 6.
Frequency difference gating reveals CD8+ T-cell subsets that are altered by malaria exposure. A subset of CD45RO+ CD27+ CCR7+ CD127+ CD57− PD-1+ (central memory-like) EBV-derived GLC-specific (top) and FLY-specific (middle) CD8+ T cells is diminished in individuals from regions with holoendemic malaria. In the pooled data from all CD8+ T cells that target latent antigens, a subset that includes central memory-like cells is dramatically reduced in individuals with holoendemic malaria exposure.
Examination of the other specificities revealed more striking differences (Fig. 6). In individuals with hypoendemic malaria exposure, frequency difference gating identified an EBV-derived FLY epitope-specific CD8+ T-cell population that was highly enriched for central memory-like cells compared to the holoendemic group (27.7% versus 5.7%, P < 0.0001). This difference was not apparent with the first approach to data analysis. Furthermore, we found that individuals with hypoendemic malaria exposure also had higher levels of central memory-like cells when responses to any latent epitope were pooled (P < 0.001). Differences according to malaria endemicity across pooled CD8+ T-cell populations specific for EBV-derived latent epitopes are summarized in Fig. S3 in the supplemental material. No statistically significant phenotypic differences between study groups were observed for pooled EBV-derived lytic epitope-specific, CMV-specific, or total CD8+ T cells.
DISCUSSION
This is the first comprehensive study of EBV-specific CD8+ T-cell immunity in individuals, including children, who reside in a region of malaria endemicity that imparts a significantly elevated risk for the development of eBL. Accordingly, the results presented here reveal previously unappreciated characteristics of the human immune response to this common, chronic viral infection and the potential role of altered immunity in susceptibility to cancer.
For epitopes presented by HLA A*0201, we found that EBV-specific CD8+ T cells directed against lytic and latent antigens were equally represented across the study population as a whole, even though the proteins are presumably expressed with different kinetics and under different conditions in vivo. We also found that the signatures of EBV-specific T-cell immunity are largely set following primary infection and appear to change little over time, as no phenotypic differences were linked to age. Furthermore, although the immune system is thought to “see” latent antigens constantly, CD8+ T cells directed against these epitopes generally retained homeostatic potential and were rarely exhausted/senescent. In contrast, CD8+ T cells directed against lytic antigens, the expression of which is thought to be episodic and infrequent, were more likely to display an exhausted/senescent phenotype lacking homeostatic potential. Given the prevalent hypothesis that such differences are related to antigen exposure, these observations suggest the occurrence of frequent EBV reactivations in the overall study population.
Interestingly, CD8+ T cells directed against the latent epitope LLD (EBNA3C) displayed phenotypic characteristics similar to those observed for the corresponding lytic antigen-specific populations; this finding is in keeping with previous studies that detail the typical immunodominance of EBNA3C (13) and suggests quantitatively enhanced or qualitatively distinct presentation of this protein among the latent antigens studied.
An intriguing observation from our data is the identification of EBV-specific CD8+ T cells with a naïve-like (CD45RO− CD27+ CCR7+ CD127+ CD57−) phenotype. Within the context of this study, the functional capacity of these EBV-experienced naïve-like CD8+ T cells is as yet unknown. It is likely that such cells populate a very early differentiated memory pool (41) and perhaps even constitute an antigen-specific “stem cell-like memory” population (42). However, further work is required to clarify the biological significance of this observation.
The features of EBV-specific CD8+ T-cell immunity described above were independent of malaria exposure yet were observed in individuals who typically acquire the virus during early childhood (25). Nonetheless, malaria coinfections were associated with additional phenotypic differences between CD8+ T-cell populations specific for latent epitopes and the lytic epitope GLC (BMLF1), which displayed characteristics of a more differentiated stage in individuals from regions of malaria holoendemicity. Thus, despite the fact that all study participants were healthy, distinct malaria-associated irregularities in EBV-specific CD8+ T-cell immunity were apparent. Overall, these results support a combined role for early primary EBV infection and cumulative malaria exposure in EBV-specific T-cell immune dysregulation.
Due to the cross-sectional nature of this study and the relatively low incidence of eBL, we were not able to address directly the causal relationship between EBV, P. falciparum malaria, and eBL. However, our data offer new evidence that addresses two long-standing questions in the field.
First, as with any immunologic study in humans that attempts to inform pathogenic mechanisms, it is critical to understand whether the observed deficits represent cause or effect in the disease process. Our previous study revealed a deficiency in IFN-γ production by EBV-specific CD8+ T cells in children residing in a region with holoendemic malaria (23). However, the events that precede this immune deficiency are unclear. On that basis, we postulated that more detailed studies of EBV-specific CD8+ T-cell immunity in areas with divergent malaria endemicity might provide a better window into the complex etiology of eBL. Given the remarkable diversity of T cells, simple immunophenotypic analyses could miss fine subsets critical to the understanding of this relationship. In addition, although EBNA1 is the only EBV-derived protein expressed in eBL (45), the risk for disease may be set when T cells specific for a wide variety of lytic and latent antigens are generated. For these reasons, we used a sophisticated flow cytometric approach optimized for rare event analysis to demonstrate that a loss of central memory-like EBV-specific CD8+ T cells is associated with malaria exposure. Such immunologic perturbations within the EBV-specific CD8+ T-cell compartment could predispose to the functional deficits that accompany eBL (46).
Second, our study addresses the long-standing question of whether eBL is associated with a generalized, malaria-induced suppression of T-cell immunity (21, 22, 47). Our data demonstrate that EBV-specific, but not CMV-specific or total, CD8+ T-cell populations show significant differences associated with malaria exposure. These findings are consistent with previous studies showing that T-cell responses to malaria antigens and nonspecific mitogens are equally robust across study populations and age groups (46). Thus, malaria exposure uniquely impacts the EBV-specific CD8+ T-cell compartment, which argues against a role for generalized immune suppression in the pathogenesis of eBL.
In the context of a cross-sectional study, however, it is not possible to discern how the observed immunologic differences arise. Recently, a malarial antigen capable of inducing the reactivation of latent EBV was identified (48), possibly providing a mechanistic explanation for our findings. In this scenario, repeated malaria infections induce EBV reactivation, thereby seeding new, potentially highly activated and dividing B cells (27), which consequently increase the risk of B-cell transformation. As EBV-specific CD8+ T cells attempt to control these frequent reactivations, their differentiation is accelerated and the pool of central memory cells for such specificities is selectively depleted. This possibility is supported by seroprofiling studies, which demonstrate that elevated antibody titers to viral capsid antigen (VCA) and the Z Epstein-Barr replication activator (ZEBRA) protein are associated with holoendemic malaria exposure (49). An elevation in these antibody titers signifies viral reactivation (50), and higher VCA antibody titers were observed prior to the development of eBL in a study of Ugandan children (51, 52). In addition, malaria infection in concert with EBV reactivation may modulate elements of the innate immune system that influence T-cell maturation and differentiation (53, 54).
In summary, we have demonstrated that a select set of EBV-derived antigen-specific CD8+ T cells is altered in individuals coinfected with P. falciparum malaria and at increased risk for eBL. These findings inform our studies of eBL pathogenesis and suggest that EBV-targeted immune interventions in the setting of holoendemic malaria might hold promise for the prevention of this devastating pediatric malignancy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants K08AI51565 and R01CA134051 from the National Institutes of Health (A.M.M., J.V., K.C., P.B.E., P.O.S.), grant 1D43TW006576 from the Fogarty International Center (K.C., P.O.S.), the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (M.R., P.K.C., T.M.B.), and the Medical Research Council (D.A.P., E.G., K.L.). D.A.P. is a Medical Research Council Senior Clinical Fellow.
The manuscript was approved for publication by the director of KEMRI.
The authors have no conflicting interests to declare.
Footnotes
Published ahead of print 21 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02158-12.
REFERENCES
- 1. Burkitt D. 1958. A sarcoma involving the jaws in African children. Br. J. Surg. 46:218–223 [DOI] [PubMed] [Google Scholar]
- 2. Haddow AJ. 1964. Age incidence in Burkitt's lymphoma syndrome. East Afr. Med. J. 41:1–6 [PubMed] [Google Scholar]
- 3. Mwanda OW, Rochford R, Moormann AM, Macneil A, Whalen C, Wilson ML. 2004. Burkitt's lymphoma in Kenya: geographical, age, gender and ethnic distribution. East Afr. Med. J. 8:S68–S77 [DOI] [PubMed] [Google Scholar]
- 4. Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H. 2008. Part I: cancer in indigenous Africans—burden, distribution, and trends. Lancet Oncol. 9:683–692 [DOI] [PubMed] [Google Scholar]
- 5. Epstein MA, Achong BG, Barr YM. 1964. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet i:702–703 [DOI] [PubMed] [Google Scholar]
- 6. Pagano JS. 1999. Epstein-Barr virus: the first human tumor virus and its role in cancer. Proc. Assoc. Am. Phys. 111:573–580 [DOI] [PubMed] [Google Scholar]
- 7. Thorley-Lawson DA, Allday MJ. 2008. The curious case of the tumour virus: 50 years of Burkitt's lymphoma. Nat. Rev. Microbiol. 6:913–924 [DOI] [PubMed] [Google Scholar]
- 8. Dalldorf G. 1962. Lymphomas of African children with different forms or environmental influences. JAMA 181:1026–1028 [DOI] [PubMed] [Google Scholar]
- 9. Kafuko GW, Burkitt DP. 1970. Burkitt's lymphoma and malaria. Int. J. Cancer 6:1–9 [DOI] [PubMed] [Google Scholar]
- 10. Rainey JJ, Mwanda WO, Wairiumu P, Moormann AM, Wilson ML, Rochford R. 2007. Spatial distribution of Burkitt's lymphoma in Kenya and association with malaria risk. Trop. Med. Int. Health 12:936–943 [DOI] [PubMed] [Google Scholar]
- 11. de The G, Day NE, Geser A, Lavoue MF, Ho JH, Simons MJ, Sohier R, Tukei P, Vonka V, Zavadova H. 1975. Sero-epidemiology of the Epstein-Barr virus: preliminary analysis of an international study—a review. IARC Sci. Publ. 11(Pt 2):3–16 [PubMed] [Google Scholar]
- 12. Henle G, Henle W, Clifford P, Diehl V, Kafuko GW, Kirya BG, Klein G, Morrow RH, Munube GM, Pike P, Tukei PM, Ziegler JL. 1969. Antibodies to Epstein-Barr virus in Burkitt's lymphoma and control groups. J. Natl. Cancer Inst. 43:1147–1157 [PubMed] [Google Scholar]
- 13. Hislop AD, Taylor GS, Sauce D, Rickinson AB. 2007. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 25:587–617 [DOI] [PubMed] [Google Scholar]
- 14. Khanna R, Burrows SR. 2000. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu. Rev. Microbiol. 54:19–48 [DOI] [PubMed] [Google Scholar]
- 15. Rickinson AB, Moss DJ. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15:405–431 [DOI] [PubMed] [Google Scholar]
- 16. Yang J, Lemas VM, Flinn IW, Krone C, Ambinder RF. 2000. Application of the ELISPOT assay to the characterization of CD8(+) responses to Epstein-Barr virus antigens. Blood 95:241–248 [PubMed] [Google Scholar]
- 17. Hislop AD, Gudgeon NH, Callan MF, Fazou C, Hasegawa H, Salmon M, Rickinson AB. 2001. EBV-specific CD8+ T cell memory: relationships between epitope specificity, cell phenotype, and immediate effector function. J. Immunol. 167:2019–2029 [DOI] [PubMed] [Google Scholar]
- 18. Baird JK, Snow RW. 2007. Acquired immunity in a holoendemic setting of Plasmodium falciparum and P. vivax malaria. Am. J. Trop. Med. Hyg. 76:995–996 [PMC free article] [PubMed] [Google Scholar]
- 19. ter Kuile FO, Terlouw DJ, Kariuki SK, Phillips-Howard PA, Mirel LB, Hawley WA, Friedman JF, Shi YP, Kolczak MS, Lal AA, Vulule JM, Nahlen BL. 2003. Impact of permethrin-treated bed nets on malaria, anemia, and growth in infants in an area of intense perennial malaria transmission in western Kenya. Am. J. Trop. Med. Hyg. 68:68–77 [PubMed] [Google Scholar]
- 20. Good MF, Doolan DL. 2010. Malaria vaccine design: immunological considerations. Immunity 33:555–566 [DOI] [PubMed] [Google Scholar]
- 21. Moss DJ, Burrows SR, Castelino DJ, Kane RG, Pope JH, Rickinson AB, Alpers MP, Heywood PF. 1983. A comparison of Epstein-Barr virus-specific T-cell immunity in malaria-endemic and -nonendemic regions of Papua New Guinea. Int. J. Cancer 31:727–732 [DOI] [PubMed] [Google Scholar]
- 22. Whittle HC, Brown J, Marsh K, Greenwood BM, Seidelin P, Tighe H, Wedderburn L. 1984. T-cell control of Epstein-Barr virus-infected B cells is lost during P. falciparum malaria. Nature 312:449–450 [DOI] [PubMed] [Google Scholar]
- 23. Moormann AM, Chelimo K, Sumba PO, Tisch DJ, Rochford R, Kazura JW. 2007. Exposure to holoendemic malaria results in suppression of Epstein-Barr virus-specific T cell immunosurveillance in Kenyan children. J. Infect. Dis. 195:799–808 [DOI] [PubMed] [Google Scholar]
- 24. Donati D, Espmark E, Kironde F, Mbidde EK, Kamya M, Lundkvist A, Wahlgren M, Bejarano MT, Falk KI. 2006. Clearance of circulating Epstein-Barr virus DNA in children with acute malaria after antimalaria treatment. J. Infect. Dis. 193:971–977 [DOI] [PubMed] [Google Scholar]
- 25. Moormann AM, Chelimo K, Sumba OP, Lutzke ML, Ploutz-Snyder R, Newton D, Kazura J, Rochford R. 2005. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J. Infect. Dis. 191:1233–1238 [DOI] [PubMed] [Google Scholar]
- 26. Klein E, Klein G, Levin PH. 1976. Immunological control of human lymphoma: discussion. Cancer Res. 36:724–727 [PubMed] [Google Scholar]
- 27. Donati D, Zhang LP, Chene A, Chen Q, Flick K, Nystrom M, Wahlgren M, Bejarano MT. 2004. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect. Immun. 72:5412–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taub R, Kirsch I, Morton C, Lenoir G, Swan D, Tronick S, Aaronson S, Leder P. 1982. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc. Natl. Acad. Sci. U. S. A. 79:7837–7841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moormann AM, Snider CJ, Chelimo K. 2011. The company malaria keeps: how co-infection with Epstein-Barr virus leads to endemic Burkitt lymphoma. Curr. Opin. Infect. Dis. 24:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rochford R, Cannon MJ, Moormann AM. 2005. Endemic Burkitt's lymphoma: a polymicrobial disease? Nat. Rev. Microbiol. 3:182–187 [DOI] [PubMed] [Google Scholar]
- 31. Roederer M, Moore W, Treister A, Hardy RR, Herzenberg LA. 2001. Probability binning comparison: a metric for quantitating multivariate distribution differences. Cytometry 45:47–55 [DOI] [PubMed] [Google Scholar]
- 32. Roederer M, Hardy RR. 2001. Frequency difference gating: a multivariate method for identifying subsets that differ between samples. Cytometry 45:56–64 [DOI] [PubMed] [Google Scholar]
- 33. John CC, Tande AJ, Moormann AM, Sumba PO, Lanar DE, Min XM, Kazura JW. 2008. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J. Infect. Dis. 197:519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cao K, Moormann AM, Lyke KE, Masaberg C, Sumba OP, Doumbo OK, Koech D, Lancaster A, Nelson M, Meyer D, Single R, Hartzman RJ, Plowe CV, Kazura J, Mann DL, Sztein MB, Thomson G, Fernandez-Vina MA. 2004. Differentiation between African populations is evidenced by the diversity of alleles and haplotypes of HLA class I loci. Tissue Antigens 63:293–325 [DOI] [PubMed] [Google Scholar]
- 35. Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, Sewell AK, Connors M, Douek DC. 2005. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 202:1349–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chattopadhyay PK, Roederer M, Price DA. 2010. OMIP-002: Phenotypic analysis of specific human CD8+ T-cells using peptide-MHC class I multimers for any of four epitopes. Cytometry A 77:821–822 [DOI] [PubMed] [Google Scholar]
- 37. Bender R, Lange S. 1999. Multiple test procedures other than Bonferroni's deserve wider use. Br. Med. J. 318:600–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101:2711–2720 [DOI] [PubMed] [Google Scholar]
- 39. Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, De Rosa SC. 2009. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J. Leukoc. Biol. 85:88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chelimo K, Embury PB, Sumba PO, Vulule J, Ofulla AV, Long C, Kazura JW, Moormann AM. 2011. Age-related differences in naturally acquired T cell memory to Plasmodium falciparum merozoite surface protein 1. PLoS One 6:e24852 doi:10.1371/journal.pone.0024852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, Bailer R, Graham BS, Roederer M, Koup RA. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 204:1405–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. 2011. A human memory T cell subset with stem cell-like properties. Nat. Med. 17:1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moormann AM, Embury PE, Opondo J, Sumba OP, Ouma JH, Kazura JW, John CC. 2003. Frequencies of sickle cell trait and glucose-6-phosphate dehydrogenase deficiency differ in highland and nearby lowland malaria-endemic areas of Kenya. Trans. R. Soc. Trop. Med. Hyg. 97:513–514 [DOI] [PubMed] [Google Scholar]
- 44. Hay SI, Smith DL, Snow RW. 2008. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect. Dis. 8:369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rowe M, Rowe DT, Gregory CD, Young LS, Farrell PJ, Rupani H, Rickinson AB. 1987. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 6:2743–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moormann AM, Heller KN, Chelimo K, Embury P, Ploutz-Snyder R, Otieno JA, Oduor M, Munz C, Rochford R. 2009. Children with endemic Burkitt lymphoma are deficient in EBNA1-specific IFN-gamma T cell responses. Int. J. Cancer 124:1721–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Greenwood BM, Bradley-Moore AM, Bryceson AD, Palit A. 1972. Immunosuppression in children with malaria. Lancet i:169–172 [DOI] [PubMed] [Google Scholar]
- 48. Chene A, Donati D, Guerreiro-Cacais AO, Levitsky V, Chen Q, Falk KI, Orem J, Kironde F, Wahlgren M, Bejarano MT. 2007. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog. 3:e80 doi:10.1371/journal.ppat.0030080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Piriou E, Kimmel R, Chelimo K, Middeldorp JM, Odada PS, Ploutz-Snyder R, Moormann AM, Rochford R. 2009. Serological evidence for long-term Epstein-Barr virus reactivation in children living in a holoendemic malaria region of Kenya. J. Med. Virol. 81:1088–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rahman MA, Kingsley LA, Atchison RW, Belle S, Breinig MC, Ho M, Rinaldo CR., Jr 1991. Reactivation of Epstein-Barr virus during early infection with human immunodeficiency virus. J. Clin. Microbiol. 29:1215–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de The G, Geser A, Day NE, Tukei PM, Williams EH, Beri DP, Smith PG, Dean AG, Bronkamm GW, Feorino P, Henle W. 1978. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature 274:756–761 [DOI] [PubMed] [Google Scholar]
- 52. Geser A, de The G, Lenoir G, Day NE, Williams EH. 1982. Final case reporting from the Ugandan prospective study of the relationship between EBV and Burkitt's lymphoma. Int. J. Cancer 29:397–400 [DOI] [PubMed] [Google Scholar]
- 53. Franklin BS, Parroche P, Ataide MA, Lauw F, Ropert C, de Oliveira RB, Pereira D, Tada MS, Nogueira P, da Silva LH, Bjorkbacka H, Golenbock DT, Gazzinelli RT. 2009. Malaria primes the innate immune response due to interferon-gamma induced enhancement of Toll-like receptor expression and function. Proc. Natl. Acad. Sci. U. S. A. 106:5789–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCall MB, Netea MG, Hermsen CC, Jansen T, Jacobs L, Golenbock D, van der Ven AJ, Sauerwein RW. 2007. Plasmodium falciparum infection causes proinflammatory priming of human TLR responses. J. Immunol. 179:162–171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.