Abstract
The present study describes the generation of a new Orf virus (ORFV) recombinant, D1701-V-RabG, expressing the rabies virus (RABV) glycoprotein that is correctly presented on the surface of infected cells without the need of replication or production of infectious recombinant virus. One single immunization with recombinant ORFV can stimulate high RABV-specific virus-neutralizing antibody (VNA) titers in mice, cats, and dogs, representing all nonpermissive hosts for the ORFV vector. The protective immune response against severe lethal challenge infection was analyzed in detail in mice using different dosages, numbers, and routes for immunization with the ORFV recombinant. Long-term levels of VNA could be elicited that remained greater than 0.5 IU per ml serum, indicative for the protective status. Single applications of higher doses (107 PFU) can be sufficient to confer complete protection against intracranial (i.c.) challenge, whereas booster immunization was needed for protection by the application of lower dosages. Anamnestic immune responses were achieved by each of the seven tested routes of inoculation, including oral application. Finally, in vivo antibody-mediated depletion of CD4-positive and/or CD8-posititve T cell subpopulations during immunization and/or challenge infection attested the importance of CD4 T cells for the induction of protective immunity by D1701-V-RabG. This report demonstrates another example of the potential of the ORFV vector and also indicates the capability of the new recombinant for vaccination of animals.
INTRODUCTION
Rabies is a highly prevalent zoonotic disease and a public health threat worldwide, leading to 55,000 human deaths annually. Most of them occur in Asia and Africa and are primarily elicited by rabid domestic dogs or other canids (1). The majority of rabies cases is found in wild animals, like raccoons, skunks, bats, and foxes. The causative agent of rabies is the neurotropic Rabies virus (RABV) belonging to the genus Lyssavirus of Rhabdoviridae. After peripheral infection, RABV invades the central nervous system, resulting in progressive fatal encephalomyelitis in almost all cases (2). The single-stranded, negative-sense RNA genome of RABV encodes five structural proteins designated N (nucleoprotein), P (phosphoprotein), M (matrix protein), G (glycoprotein), and L (RNA-dependent RNA polymerase) (reviewed in reference 3). The glycoprotein, here referred to as RabG, represents the major antigen of RABV and is responsible mainly for the induction of protective immunity (4–7). RabG is expressed as a trimer transmembrane protein forming projections on the surface of RABV or of the infected cells and is the target for binding virus-neutralizing antibodies (VNA). Protection against rabies correlates with the presence of VNA, which persists for many years, and VNA titers greater than 0.5 IU per ml serum are accepted for protection. Accordingly, rabies can be considered a T-helper type 2 cell responsive disease, and the help of CD4-positive T cells to activate B cells is crucial for protection (8).
Controling rabies mass vaccination of wildlife, and especially of dogs and cats, is considered to be the most effective strategy (1). To achieve that goal, various vaccines have been developed during the last years. However, there is still a need for the improvement of rabies vaccines, which ideally should combine attributes such as (i) effectiveness in all important target animals transmitting RABV, (ii) induction of long-lasting immunity after single administration, (iii) efficacy after oral application, (iv) innocuousness in all RABV-susceptible animals, (v) stability and convenient handling of the vaccine, and (vi) low costs. Available vaccines do not comprise all those properties and, therefore, fail to successfully control rabies. For instance, vaccination with avirulent RABV strains almost completely eradicated vulpine rabies in Europe (9) or coyote and raccoon rabies in North America (10) but failed to protect dogs, skunks, and other animals by a single oral immunization (11). In addition, the risk of possible reversion to virulence cannot be completely excluded with these vaccines.
Since the recent introduction of “reverse genetics” technology (12, 13), new live attenuated RABV can be designed, for instance, by gene mutations (see reference 14), duplication or triplication of the RabG gene (15, 16), or simultaneous expression of inserted cytokine genes to stimulate the innate immune response (17, 18). Some of them are promising candidates of rabies vaccines due to their apathogenicity and improved immunogenicity in animal models. DNA vaccines have several advantages, such as stability, low production costs, and ease of construction (reviewed in reference 19). DNA-based rabies vaccines can induce adequate RABV-specific immune responses in small rodents when optimally formulated (20). However, multiple immunizations using high DNA doses are required to achieve modest immunity against RABV in mammals, which is accompanied by the risk of tolerance. In addition, most DNA vaccines need adjuvants to improve efficacy, which raises several safety concerns (21).
Live recombinant vectors represent the most promising vaccine candidates, not only as rabies vaccines for companion animals. Various recombinant rabies vaccines have been generated using different viral vector systems, such as Newcastle disease virus (22), Sindbis virus (23), herpesvirus (24), adenovirus (for a review, see reference 14), or baculovirus (25). Moreover, numerous poxvirus-vectored vaccines expressing RabG have been constructed, as reviewed recently (26). Despite inducing excellent humoral and cellular immunity, tolerating the insertion of large foreign genes, and having a stable genome and technologies for the construction of recombinants that are well established, these vaccines also have certain weaknesses. The first licensed recombinant vaccinia virus (VACV) V-RG expressing RabG (27, 28) was successfully used for oral immunization of raccoons, red and gray foxes, and coyotes in North America and Western Europe (10, 29), but V-RG failed to induce complete protection in skunks (30) and dogs (11) by single oral administration. Moreover, V-RG has been associated with severe skin inflammation, at least in one case, and the possibility to cause systemic VACV infection in human has been reported (31). Another drawback of VACV-vectored vaccines, similar to adenovirus-vectored vaccines, represents preexisting or vaccination-induced vector immunity, which can inhibit uptake of the recombinant and prevent the generation of sufficient anti-RABV immunity or boosting anamnestic response (32). A safer, attenuated, and replication-deficient variant of VACV vector is represented by the modified vaccinia virus Ankara (MVA) strain. The RabG-expressing MVA recombinant, however, was found to be less immunogenic than V-RG and did not mediate complete protection against RABV, and dogs were not protected by oral administration (14, 33). Other poxviral vectors have been used to generate host-restricted recombinant live vaccines against rabies, such as raccoon poxvirus (34), canary poxvirus (35), fowl poxvirus (36), or capripoxvirus (37). Still, these vaccines have their limitations, particularly regarding efficacy restricted to certain species, safety concerns, and utility for pre- and postexposure vaccination.
Recently, we reported the successful use of the Parapoxvirus Orf virus (ORFV) as a novel virus vector system for expressing different foreign antigens. Its key benefits are the very restricted host range (sheep and goats), the skin tropism, and the absence of systemic virus spread even in immunocompromised individuals or after intravenous injection of high virus dose (38, 39). The short-term vector-specific immunity and the lack of serum antibodies efficiently neutralizing ORFV (38, 40) enable repeated immunizations with the same or with different ORFV recombinants. Unique immune-modulating properties of ORFV strongly stimulate the innate immunity (40–43) and rapidly generate foreign antigen-specific immune responses (44–47). The apathogenic, Vero cell culture-adapted ORFV strain D1701-V is used to generate recombinants by substituting the viral vegf-e gene with a foreign gene, which thereby removes an ORFV virulence gene and leads to further attenuation (38, 44, 48). D1701-V recombinants have been reported to mediate protective immunity against a number of different viral infections, such as rabbit hemorrhagic disease virus (49), classical swine fever (50), Borna disease virus (51), or pseudorabies virus (44, 52, 53).
The present study describes the generation of the ORFV recombinant D1701-V-RabG expressing the RABV glycoprotein and its successful use for mounting protective immunity against lethal RABV challenge infection of mice. Notably, even a single immunization was sufficient to induce high RABV-specific VNA titers in mice, cats, and dogs by different routes of application. Finally, in vivo antibody-mediated depletion of CD4-positive and/or CD8-posititve T cells of mice during immunization and challenge infection attested the importance of CD4-positive T cells for the induction of protective immunity by D1701-V-RabG. This report demonstrates the potential use of this new ORFV recombinant to vaccinate companion animals against rabies.
MATERIALS AND METHODS
Cells and viruses.
The ORFV recombinants were propagated, plaque purified, and titrated in Vero cells as described recently (44, 49). Rabies challenge virus standard strain CVS-11 (Friedrich-Loeffler-Institut, Germany) was titrated by fluorescence focus assay. ORFV gene expression was arrested in the early phase by cytosine arabinoside treatment (AraC; 40 μg/ml; Sigma, Germany).
Generation of D1701-V-RabG recombinant.
The RabG gene of strain PV11 (accession no. AF233275) exhibiting 97 to 98% identity to various other RABV strains, including CVS, was chemically synthesized and provided as pUC plasmid (Blue Heron Biotech). The complete G gene was isolated as an EcoRI-BamHI DNA fragment (1,582 bp) by agarose gel electrophoresis and Qiaex II gel extraction (Qiagen, Germany) followed by ligation (fast ligation kit, Promega, Germany) into EcoRI-BamHI-digested plasmid pdV-Rec1 (44). The resulting transfer plasmid pdV-RabG was DNA sequenced to verify correct insertion of the RabG gene (data not shown). Using plasmid pdV-RabG (2 μg) for nucleofection (Nucleofector kit V; Lonza, Germany) of Vero cells infected with the LacZ gene-positive recombinant D1701-VrV with a multiplicity of infection (MOI) of 0.2, the new ORFV recombinant D1701-V-RabG was selected by plaque PCR screening and produced as described previously (49). The PCR primers were purchased from Metabion (Martinsried, Germany) with the nucleotide sequences for the LacZ gene as 5′-CGA TAC TGT CGT CGT CCC CTC AA-3′ (foward) and 3′-TCA AGT CAT CAC GCC GCT CAA C-5′ (reverse), resulting in a 433-bp-sized amplicon, and for the RabG gene as 5′-GGA GTC TCT CGT TAT CAT ATC TC-3′ (forward) and 3′-GCT TTA ACT CGT GGA ACA ACA TC-5′ (reverse) to amplify a 508-bp fragment.
Antibodies.
RabG-specific antibodies, polyclonal rabbit antiserum G154-3, G-specific antipeptide antiserum, and monoclonal antibody E559 were generously provided by K. K. Conzelmann (Ludwig-Maximilians University, Munich, Germany) and by Stefan Finke (Friedrich-Loeffler-Institut, Germany).
IPMA.
Successful expression of the inserted foreign gene was first assayed by immunoperoxidase monolayer assay (IPMA), an immunohistochemical staining of recombinant virus plaques titrated in Vero cells. After the appearance of virus plaques, the medium was aspirated and the cells were dried at room temperature (RT) for 10 min. Thereafter, cells were fixed with abs. methanol at −20°C for 15 min, washed twice with ice-cold 1% (vol/vol) fetal calf serum (FCS) in phosphate-buffered saline (PBS), blocked with PBS containing 10% (vol/vol) FCS for 90 min, and incubated for 60 min at RT with the RabG-specific monoclonal antibody E559 (diluted 1:200 in 1% FCS in PBS). After being washed 3 times with PBS-T (PBS containing 0.05% [vol/vol] Tween 20), a peroxidase-coupled anti-mouse secondary antibody (1:2,000; DIANOVA, Germany) was added and slowly agitated for 60 min at RT. After being thoroughly washed with PBS-T and PBS, substrate (Vector Nova Red; Vector Laboratories, Axxora, Germany) was added as recommended by the manufacturer until red-brown positive staining became visible.
Western blot analysis was performed as described previously (49). The RabG-specific rabbit antiserum G154-3 was used diluted 1:50,000, and the peroxidase-coupled anti-rabbit secondary antibody (Jackson ImmunoResearch, Dianova, Germany) was used in a 1:20,000 dilution. Detection by enhanced chemiluminescence (ECL) was accomplished with the substrate Immobilon Western horseradish peroxidase (HRP) (Millipore, Germany) and ECL X-ray films (Pierce, Fisher Scientific, Germany).
Immunofluorescence was performed as published recently (49). Intracellular staining was achieved by permeabilization with 0.2% (vol/vol) Triton X-100 in PBS for 5 min at RT. The E559 monoclonal antibody (54) was diluted 1:1,000 and incubated for 1 h at 37°C followed by extensive washing in PBS. Secondary Alexa-555-coupled anti-mouse antibody (Fisher Scientific, Invitrogen, Germany) was diluted 1:1,000, and after 30 min at 37°C the slides were washed with PBS.
Actin staining was achieved with phalloidin CF-647 according to the instructions of the manufacturer (Biotium, Germany) followed by staining of the nucleus with 1 μg/ml DAPI (4′,6-diamidin-2′-phenylindol-dihydrochlorid; Sigma-Aldrich, Germany) for 20 to 30 min at RT in the dark. Finally, slides were thoroughly washed and embedded in Mowiol-DABCO (1,4-diazabicyclo[2.2.2]octan; Carl Roth, Germany). Fluorescence images were recorded with an Axiovert 200 microscope using Axiovision software (Zeiss, Germany).
RABV-neutralizing antibody assay.
VNA titers were determined using the rapid fluorescent focus inhibition test (RFFIT) as described previously (4) and modified for 96-well cell culture plates. The obtained VNA titers were transformed to IU using a standard human anti-rabies immunoglobulin containing known IU per ml (NIBSC, United Kingdom). The fluorescein isothiocyanate (FITC)-labeled rabies conjugate was purchased from Fujire Diagnostics (IBL, Germany). The VNA titers are represented as geometric mean titers (GMT), and mouse preimmune serum was used as a negative control.
Mouse immunization and challenge infection experiments.
C57BL/6 and BALB/c mice were bred at Friedrich-Loeffler-Institut, Institute of Immunology, Tuebingen, Germany. B-cell-deficient mice, B6.129S2-Ighmtm1Cgn/J, were purchased from Charles River Laboratories and kept in the specific pathogen-free facility at the FLI.
The indicated amounts of D1701-V-RabG were administered in 50-μl volumes intramuscularly (i.m.) into the quadriceps muscle, intravenously (i.v.), intraperitoneally (i.p.), subcutaneously (s.c.); in 20-μl volumes intranasally (i.n.) or intradermally (i.d.), or orally by instillation of 0.1 ml with an intragastric gavage. Multiple immunizations were done in 2- or 3-week intervals, and challenge infection was performed 2 to 3 weeks after the last immunization. In addition, groups of mice were i.m. immunized with 0.1 ml of the commercial human rabies vaccine Rabipur (Novartis Vaccines, Germany) or animal rabies vaccine Nobivac (Intervet, Germany) as reported (23).
Sex-matched 6- to 8-week-old mice (before immunization) were challenge infected under anesthesia intracerebrally (i.c.) into the left hemisphere with the indicated 50% lethal dose (LD50) in a 20-μl volume. Intracerebral challenge infection was chosen to test the protective potency under very stringent conditions basically according to the NIH potency test (20, 23). The animals were inspected three times daily and scored for the appearance of slight neurological signs as beginning ataxia and slightly reduced motility (level 1); for increased neurological signs, such as trembling and/or disorientation after tail spinning (level 2); and for severe signs of disease (level 3) showing ruffled fur, hunched position, and complete paralysis. Animals scored twice at level 3 were immediately sacrificed. The experiment was terminated 28 days after virus challenge. All mice experiments were approved by the local authorities according to the German Animal Protection law.
In vivo depletion of T cell subpopulations.
Monoclonal antibodies used for depletion analysis directed against murine CD4 (monoclonal antibody [mAB] YTS 191.1) or CD8 (mAB YTS 169.4) (55) were kindly provided by L. Stitz, (Friedrich-Loeffler-Institut, Germany). The antibodies were diluted 1:25 in PBS, and 0.2 ml was administered i.p. per mouse at the indicated times as described in the legend to Fig. 7. For simultaneous depletion of CD4- and CD8-positive T cells, the 1:25 diluted antibodies were combined in 0.2 ml. Absence of the T cell subsets and their reconstitution after 10 to 14 days has been reported earlier (56) and was controlled by fluorescence-activated cell sorting (FACS) analysis (data not shown).
Fig 7.
Relevance of T cells for D1701-V-RabG-induced protective immunity. CD4-, CD8-, or CD4- and CD8-positive T cell subsets were selectively eliminated in vivo by antibody-mediated depletion as described in Materials and Methods. At the bottom of the figure, the treatment protocol is schematically depicted, indicating the days of antibody i.p. injection as black boxes. Immunization with 107 PFU of D1701-V-RabG was performed at day 0 (arrow), and all animals were i.c. challenge infected at day 15 (arrow). The most pronounced effects on the survival rate were found by T cell depletion before prime immunization (A) or depleting the T cell subsets during the entire experiment (C), whereas removal of the T cell populations before the time point of challenge infection had the least effect on the survival rate (B).
Vaccination of dogs.
Beagles (6 months of age) were randomized into three groups: group 1 (n = 5 dogs) received D1701-V-RabG s.c. between the shoulder blades, group 2 (n = 5 dogs) received D1701-V-RabG i.m. into semimembranous muscle, and group 3 (n = 2 dogs) was the PBS-vaccinated control group (1 animal s.c. and 1 animal i.m. immunized). Booster immunization was performed 4 weeks after prime immunization. The administered amount of D1701-V-RabG was 107.57 50% tissue culture infective dose (TCID50). Blood samples for rabies serology were collected on day 0 prevaccination and thereafter weekly over a period of 7 weeks post-prime immunization. Serum samples were tested for RABV-specific VNA by RIFFT (Biobest Laboratories Ltd., Edinburgh, United Kingdom). The general health of the animals was observed daily, focused on the injection site for visible, palpable, or painful reactions and on rectal temperatures.
Immunization of cats.
Forty-five domestic short-hair, specific pathogen-free cats (11 weeks of age at day 0) were randomized into six groups and were immunized s.c. either with serial 10-fold dilutions of D1701-V-RabG (108 to 105 TCID50), with the commercial live canarypox vector PureVax feline rabies (Merial Animal Health Inc., Duluth, MN) according to the manufacturer's recommendation, which corresponds to 106.8 50% fluorescent assay infectious dose (FAID50), or with PBS as the control. Blood samples for rabies serology were collected on day −1 (prevaccination) and thereafter weekly over a period of 7 weeks postimmunization. Serum samples were tested for RABV-specific VNA by RFFIT and by enzyme-linked immunosorbent assay (ELISA) (Platelia Rabies II; Bio-Rad Laboratories, Hercules, CA). Nasal swab samples were collected on days 1 to 7 postvaccination and analyzed for the presence of infectious D1701-V-RabG by virus isolation. The general health of the animals was observed daily, focusing on the injection site for hair loss, swelling, pain, self-trauma (licking, biting, rubbing), hyperthermia, and depression.
RESULTS
In vitro characterization of D1701-V-RabG.
Recombinant viruses were selected, plaque purified, and propagated as described in Materials and Methods and published recently (44, 49). Virus plaque isolates negative for the LacZ gene were subjected to 4 additional rounds of plaque purification to receive genetically homogenous RabG-containing recombinant D1701-V-RabG.
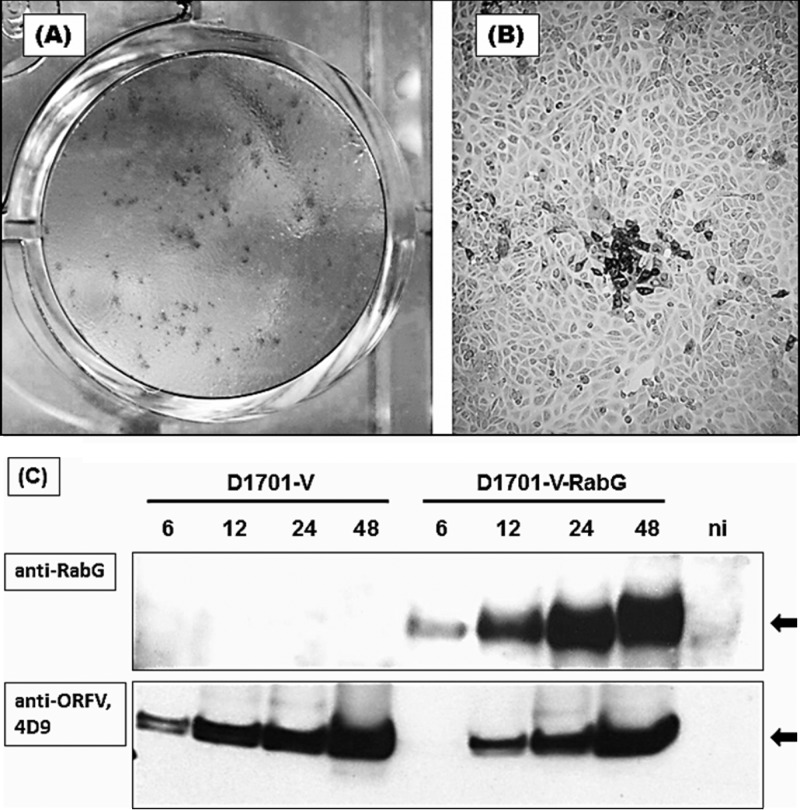
Expression of RabG protein in individual recombinant virus plaques was confirmed using immunoperoxidase monolayer assay (IPMA) as described in Materials and Methods. A representative result is shown in Fig. 1A and B, demonstrating the specific brownish staining of individual virus plaques positive for the RABV glycoprotein. Control staining of parental virus-infected or noninfected cells remained negative (not shown). In vitro expression of the RABV glycoprotein was demonstrated by Western blot analysis (Fig. 1C). Protein lysates were prepared 6, 12, 24, and 48 h after infection (hpi) of Vero cells with the parental D1701-V or with the recombinant D1701-V-RabG. The RabG with an apparent molecular weight of approximately 62 kDa could be detected already 6 hpi, with increasing amounts at later time points, but not in parental D1701-V-infected or noninfected cell lysates (Fig. 1C). The major virion protein of ORFV was detectable in all infected cells, whereas noninfected (NI) cells remained negative at the same time points. Comparison of in vitro single-step growth curves showed no significant differences in burst size or growth kinetics between D1701-V and the recombinant D1701-V-RabG (data not shown). Correct insertion of the RabG gene into the vegf-e gene locus of ORFV was verified by Southern blot hybridization (not shown). Specific RabG gene transcription controlled by the strong early vegf-e promoter of ORFV (PVEGF) was also ascertained by Northern blot hybridization (data not shown).
Fig 1.
Expression of RABV G protein in D1701-V-RabG-infected Vero cells. Panels A and B demonstrate IPMA staining of recombinant ORFV plaques expressing RABV glycoprotein. Two days after infection, IPMA shows dark (brownish)-stained positive virus plaques expressing RabG (A). Cells expressing RabG can be easily discriminated from negative cells (panel B, 40-fold microscopic magnification). (C) Western blot analysis to detect the expressed RabG. Protein lysates were prepared at the indicated time points after infection with D1701-V as negative controls, with D1701-V-RabG (MOI of 1.0), or from noninfected cells (ni). The RabG protein 62 kDa in size (arrow) was specifically detected with the polyclonal antiserum G154-3 (1:10,000 diluted).
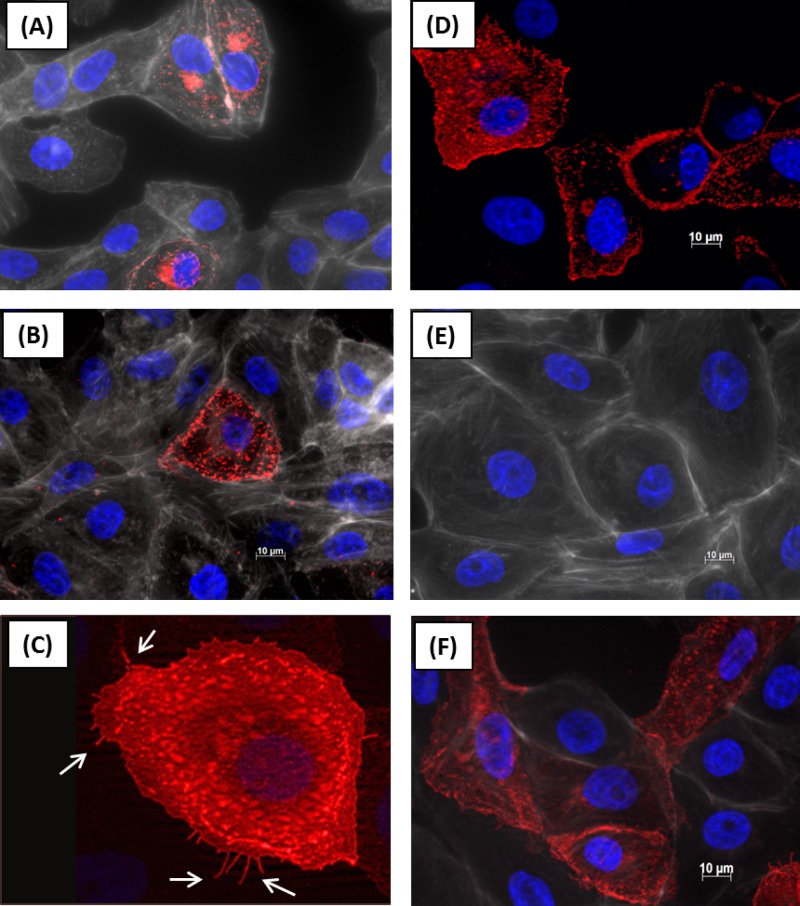
Finally, RabG expression was studied by immunofluorescence in D1701-V-RabG-infected cells by the use of the G-specific monoclonal antibody E559. Already at 4 hpi, specific intracellular (Fig. 2A) and surface expression (Fig. 2B and C) of RabG was detectable, prolonging until at least 24 h after infection (Fig. 2D). Noninfected (Fig. 2E) or D1701-V-infected (data not shown) control cells remained negative. Strong early expression of RabG was also found in the presence of AraC, which inhibits the replication of ORFV (Fig. 2F). The RabG expression in ORFV recombinant-infected cells was very similar to that seen in RABV-infected cells (54), constituting RabG spikes that project from the cell surface (Fig. 2C, arrows) and are responsible for in vivo binding of VNA. Collectively, these results show the successful generation of a new ORFV recombinant expressing the RABV glycoprotein correctly on the surface of infected cells without the need of replication or production of infectious recombinant ORFV.
Fig 2.
Detection of the RABV G protein by immunofluorescence. Vero cells were infected with D17101-V-RabG (MOI of 0.5) in chamber slides and processed for immunofluorescence as described in Materials and Methods. RabG (red staining) was detectable with the monoclonal antibody E559 (diluted 1:1,000) already 4 h after infection within permeabilized cells (A) or on the cell surface of nonpermeabilized infected cells (B, C) and 24 h after infection (D). Control staining of noninfected cells is shown in panel E. RabG expression was also found in AraC-treated cells blocked for early ORFV gene expression and inhibiting ORFV DNA replication (F).
Protection mediated by immunization with D1701-V-RabG.
The capacity of the new ORFV recombinant to mediate a protective immune response was first evaluated in mice. In a pilot experiment (data not shown), groups (n = 5 mice) were i.m. immunized once, twice, or thrice with 107 PFU of D1701-V-RabG in 2-week intervals. Two other groups were vaccinated three times with 106 PFU either i.m. or s.c. Two weeks after the last immunization, all mice were i.c. challenged with 3,000 mouse i.c. LD50 of the highly pathogenic RABV CVS-11 strain. The challenged mock-immunized animals suffered from RABV-specific clinical symptoms and had to be sacrificed between day 6 and day 8 postchallenge. All i.m. immunized animals, receiving one, two, or three inoculations of 107 PFU or three doses of 106 PFU, were completely protected, whereas 4 out of the 5 s.c. immunized animals survived the challenge. The D1701-V-RabG-immunized and protected mice did not reveal any harmful reaction, loss of body weight, or RABV-specific clinical signs.
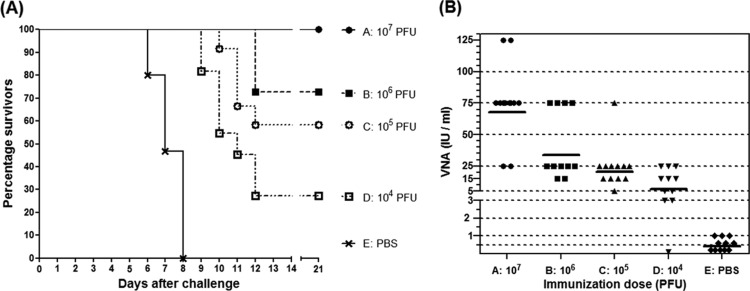
Next we asked the question, which dose of D1701-V-RabG was needed to protect mice after only a single vaccination (Fig. 3). Groups of mice (A to D; n = 11 or 12) received one i.m. dose of serial 10-fold dilutions of D1701-V-RabG ranging from 107 PFU to 104 PFU, and control animals (group E) were mock immunized with PBS. Challenge was performed 17 days later with 1,000 LD50 of RABV strain CVS-11. The results demonstrated that the protection rate was dependent on the immunization dose (Fig. 3A). All mice of group A were completely protected against the challenge, 8 out of 11 mice (73%) of group B survived, whereas 7 out of 12 mice (58%) of group C and only 3 out of 11 (27%) animals of group D survived the challenge. All PBS-immunized animals suffering from serious clinical symptoms had to be sacrificed between days 6 and 8 (Fig. 3A). The mean time to death (MTD) was extended in all immunized groups (Fig. 3A). The level of the induced serum VNA titers correlated with the dosage of the administered D1701-V-RabG. As seen in Fig. 3B, increasing amounts of the recombinant stimulated gradually rising serum antibody titers; however, a distinct correlation between the magnitude of the VNA titer and protection against challenge could not be found. Conclusively, solid protection against i.c. RABV challenge could be achieved by a single i.m. administration of 107 PFU of D1701-V-RabG.
Fig 3.
Immunogenicity induced by a single application of different amounts of D1701-V-RabG. (A) Groups of mice (n = 11/12) were i.m. immunized once with the indicated amounts (PFU) of D1701-V-RabG or control immunized (PBS) and i.c. challenge infected with 1,000 MICLD50 of RABV CVS strain at day 17. The Kaplan-Meyer survival curves demonstrate that a single i.m. vaccination with 107 PFU of the RabG recombinant was sufficient to protect all mice from challenge. In addition, the mean time to death was prolonged in comparison to that of the control-vaccinated animals. (B) The serum VNA levels of the individual animals of the indicated immunization groups induced 10 days after vaccination are depicted, and lines mark the GMT of VNA. The results imply that the VNA titer levels correlate with the applied amount of D1701-V-RabG.
Humoral immune response induced by the ORFV recombinant.
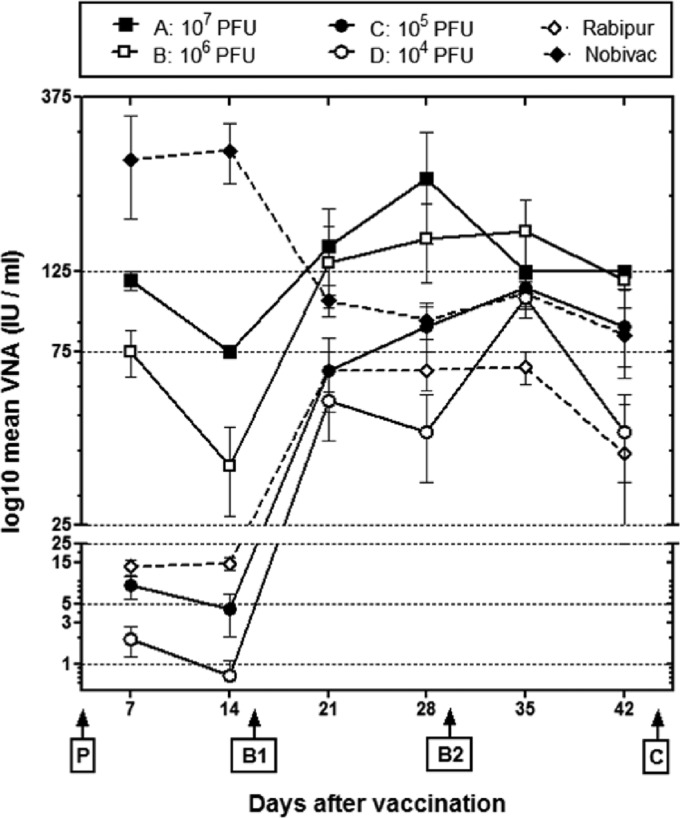
In order to determine the dose dependency of D1701-V-RabG on the development of RABV-neutralizing serum antibodies, groups of mice were i.m. immunized with 10-fold dilutions of the recombinant from 107 PFU up to 104 PFU (groups A to D). Mice were immunized three times in 2-week intervals, and sera were collected weekly over a period of 42 days for comparison of the induced VNA titers. In groups A and B, seroconversion could be detected in all animals 7 days after prime immunization with VNA mean titers of 118 IU and 75 IU, respectively. In groups C and D that received 105 PFU and 104 PFU, respectively, only 4 out of 7 or 3 out of 8 animals seroconverted within the first 14 days and displayed low VNA mean titers of 4 or 0.75 IU (Fig. 4).
Fig 4.
VNA response elicited by 3 i.m. immunizations with different dosages. Groups of mice (n = 7) were three times i.m. immunized with the indicated amounts (PFU) of D1701-V-RabG or the inactivated commercial vaccines Nobivac and Rabipur in 2-week intervals (P, prime; B1, boost 1; B2, boost 2; C, challenge infection). The course of the VNA mean titers calculated as IU per ml serum is depicted. The bars indicate standard errors of the mean (SEM). It can be seen that the boost (B1) 14 days after prime immunization (P) stimulated the titer levels, whereas a second boost (B2) did not substantially increased the VNA titers.
Booster immunization (B1) increased the VNA titers in all cases, demonstrating in group A VNA mean titers of 146 IU and in group B of 132 IU at day 21 and of 225 IU (group A) and 154 IU (group B) at day 28. Also all animals of group C and group D seroconverted to adequate VNA titers 2 weeks after the second immunization (Fig. 4). A second boost (B2) did no more stimulate the VNA titers, except of group D. Collectively, these results show a correlation between the immunization dose of D1701-V-RabG and the level of circulating VNA. The normal antibody decline was seen after prime and booster immunization, leaving the VNA titers beyond the protective threshold of 0.5 IU/ml after booster.
Additionally, we compared the immunogenicity of D1701-V-RabG with that of the commercial inactivated vaccines Rabipur and Nobivac, respectively. After 3 i.m. inoculations (0.1 ml each), the induced VNA titers were determined. Mice primed with Nobivac developed the highest VNA mean titers of 254 IU and 268 IU at days 7 and 14, which, however, after a booster vaccination, decreased continuously to 104 IU at day 21 and to 92 IU at day 28 and were comparable to the titer levels induced by 105 PFU of D1701-V-RabG. The lowest VNA titers among all groups were stimulated by Rabipur not only after prime but also after booster immunization (Fig. 4) and resembled the response induced by 104 PFU of the ORFV recombinant (Fig. 4). Comparing the protective effect, the D1701-V-RabG-immunized mice showed better survival rates than the animals vaccinated with Rabipur or Nobivac. All animals were protected by immunization with 106 PFU of D1701-V-RabG, but only 1 of 7 mice survived, or with Nobivac, protecting only 3 out of 5 animals. The application of Nobivac seemed to prime a pronounced but short-lived humoral response in mice, which could not be boosted by an additional application of the inactivated vaccine. Although only a limited number of animals per group were available for this experiment, the results indicate good potency of the live ORFV recombinant compared to the two inactivated vaccines.
Duration of VNA response.
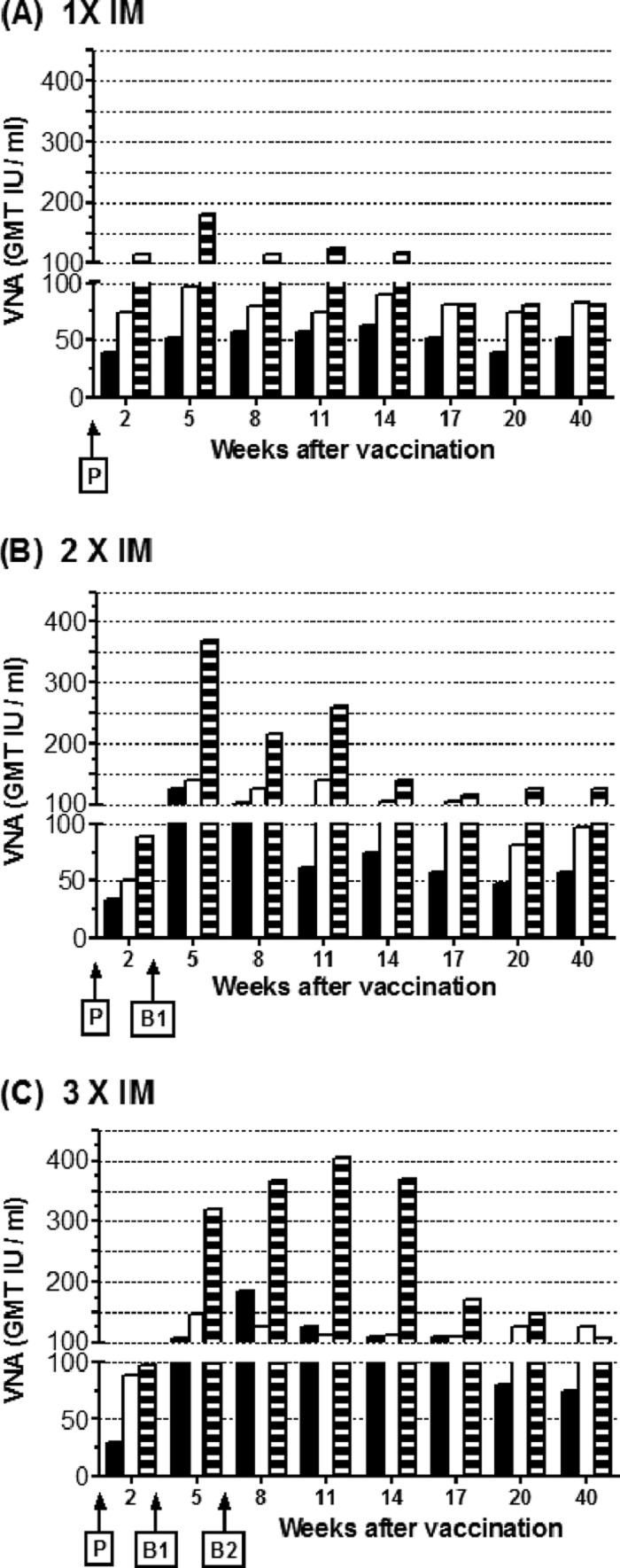
To evaluate the persistence of the serum VNA response and also the long-term protective immunity, mice (n = 7 or 8) received up to 3 i.m. immunizations of 105 PFU, 106 PFU, or 107 PFU of D1701-V-RabG. Sera were collected at 2, 5, 8, 11, 14, 17, 20, and 40 weeks after prime immunization, and mice were i.c. challenged with 100 LD50 at week 43 and observed for an additional 28 days. The development of the antibody response monitored during 40 weeks is depicted as GMT of VNA per ml serum in Fig. 5. Two weeks after prime immunization with 105 PFU, the VNA titers ranged between 30 to 39 IU, increasing to 50 to 89 IU and 89 to 115 IU after immunization with 106 PFU and 107 PFU, respectively (Fig. 5A). A second immunization 3 weeks later enhanced the antibody response to VNA titers of 108 to 125 IU (105 PFU), 138 to 146 IU (106 PFU), or 321 to 370 IU (107 PFU). Highest VNA titers were obtained by administration of the highest dose (107 PFU) of the recombinant ORFV; however, the most pronounced boosting effect was achieved with the lowest dose (105 PFU) of D1701-V-RabG (Fig. 5B). Furthermore, previous results claiming the negligibility of a third vaccination with the same doses of D1701-V-RabG were confirmed, because the VNA response was not substantially enhanced, except for the animals that received the low dose of D1701-V-RabG (105 PFU), which displayed VNA titers increasing short-term from 108 IU to 184 IU (Fig. 5C). Inspecting the development of the VNA response, it became obvious that the VNA titers of mice immunized with 106 PFU or 107 PFU declined and similar titers of approximately 100 IU per ml persisted at later times (weeks 17 to 40) regardless of the number of immunizations. Interestingly, 8 to 11 weeks after second vaccination (Fig. 5B, B1) as well as after third vaccination (Fig. 5C, B2), a similar decrease of VNA titers was observed. Although a second booster immunization (B2) with the higher dose of D1701-V-RabG (107 PFU) was not needed to enhance the VNA titers substantially, the VNA response seemed to sustain at higher titers. Notably, this effect was not obtained when using the lower immunization doses, which also did not boost the VNA response to high titers comparable to those seen after the high immunization dose.
Fig 5.
Duration of immunity mediated by D1701-V-RabG. Mice were i.m. immunized once (A), twice (B), or (C) thrice in 3-week intervals (P, prime; B1, boost 1; B2, boost 2) with the indicated amounts (PFU) of D1701-V-RabG. At the indicated weeks after vaccination, the serum VNA titers were determined and are depicted as GMT IU per ml serum. Results after immunization with 105 PFU (black columns), 106 PFU (white columns), and 107 PFU (striped columns) are depicted.
All three immunization doses mediated long-lasting protection against a comparably stringent i.c. challenge infection almost 11 months after the prime vaccination. As detailed in Table 1, the survival rate was primarily dependent on the injection dose of D1701-V-RabG, but also the number of immunizations contributed to the protective immunity. Best protection resulted for the groups immunized with the highest dose of D1701-V-RabG, wherein all mice receiving at least one booster immunization survived and only two of six mice (67%) had to be sacrificed in the group immunized only once.
Table 1.
Long-lasting protective immunity
| i.m. immunization (PFU) | IU/ml (at wk 40) |
No. of survivors/total no. | Survival rate (%) | |
|---|---|---|---|---|
| GMT | Range | |||
| 1 × 105 | 51.3 | 25–125 | 1/6 | 17 |
| 2 × 105 | 56.6 | 25–125 | 2/6 | 33 |
| 3 × 105 | 74.0 | 25–125 | 3/6 | 50 |
| 1 × 106 | 83.1 | 75–125 | 3/5 | 60 |
| 2 × 106 | 96.8 | 75–125 | 4/6 | 67 |
| 3 × 106 | 125.0 | 125 | 4/4 | 100 |
| 1 × 107 | 80.6 | 25–125 | 4/6 | 67 |
| 2 × 107 | 125.0 | 125 | 6/6 | 100 |
| 3 × 107 | 108.0 | 75–125 | 7/7 | 100 |
Quality of the immune response stimulated by different routes of application.
After demonstrating the potency of i.m. application, we tested the efficacy of the new recombinant after different routes of immunization. A first pilot experiment showed that a single dose of 106 PFU of D1701-V-RabG given to mice i.n., i.v., i.p., i.d., s.c., or orally induced only moderate serum VNA titers and partial protection against i.c. challenge (100 LD50) 2 weeks later (data not shown). Therefore, we investigated whether the quality and efficacy of the induced immune response could be improved by 3 injections with a higher dosage of D1701-V-RabG (107 PFU). Sera from all animals (n = 7 per group) were collected weekly for 6 weeks to monitor the development of the serum VNA titers after the different routes of immunization (Fig. 6).
Fig 6.
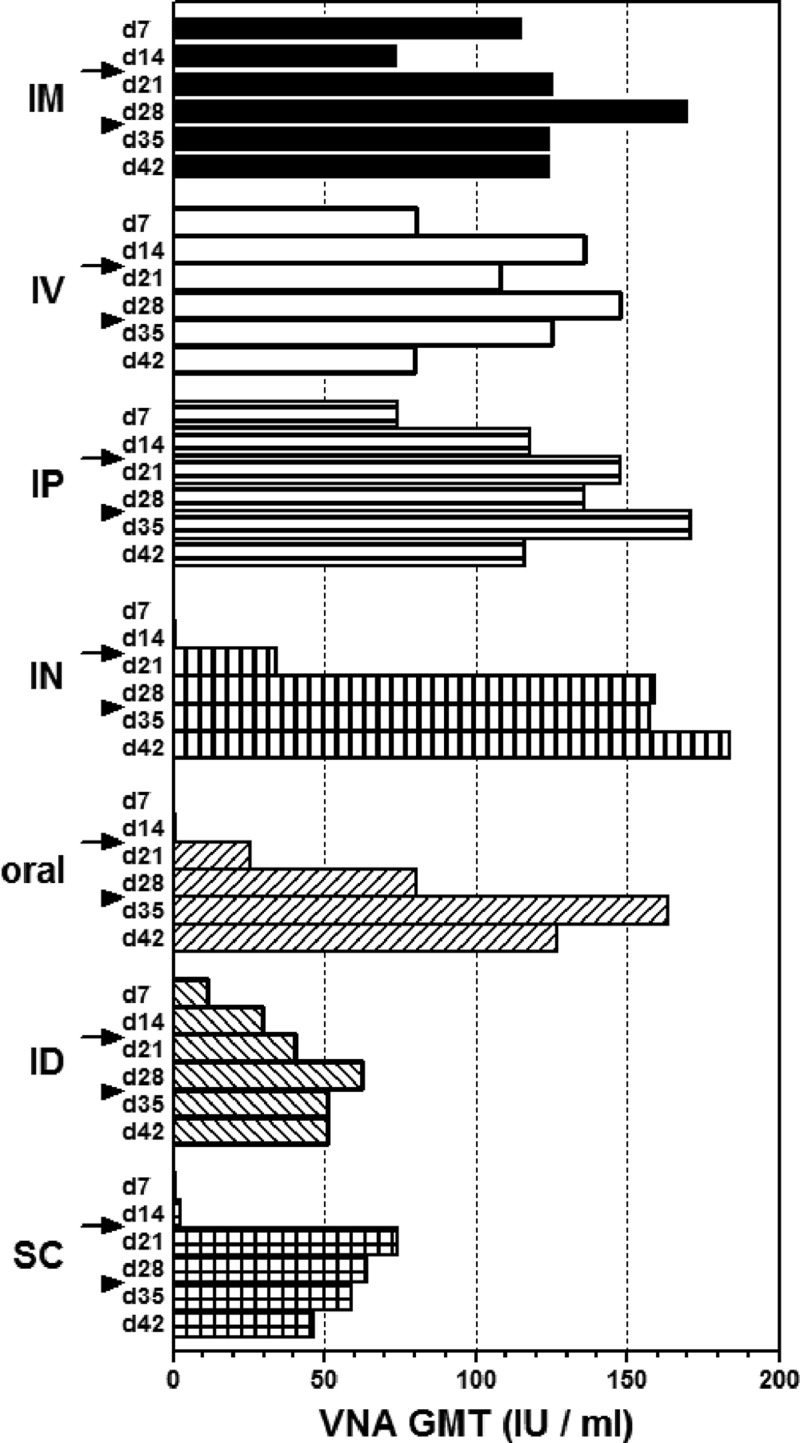
VNA response stimulated by different application routes of D1701-V-RabG. The GMT of VNA are depicted at the indicated days, 7 (d7) to 42 (d42), after the different routes of D1701-V-RabG immunization. Prime immunization was done at day 0, first boost (d15) is indicated by the arrows, and second boost (d29) by the arrowheads.
The 10-fold increased immunization dose resulted 1 week after prime vaccination in seroconversion of all i.m., i.v., and i.p. immunized animals and elicited very good VNA titers (Fig. 6). Notably, the i.d. prime injection induced lower VNA GMT than the i.m., i.v., and i.p. immunization (Fig. 6 and Table 2, d14). The weakest humoral immunity was found after prime immunization via the s.c., i.n., and oral routes (Fig. 6 and Table 2, d7). Also, only 2 (group s.c.) or 1 (groups i.n. and oral) out of 7 animals seroconverted; however, after booster immunization, all animals had seroconverted. In addition, the VNA titers of the mice of these groups were clearly elevated during 2 weeks after the second injection, mounting to GMT of 186 (i.n.), 68 (s.c.), and 116 (oral) IU per ml. Also the i.d. immunized mice now exhibited increased VNA titers (Fig. 6 and Table 2, d28). An additional slight rise in VNA GMT was also achieved by a booster immunization using i.m., i.v., and i.p. routes (Fig. 6 and Table 2, d28). Again, a third immunization did not significantly boost the VNA titers, but rather slightly reduced VNA titers were found (Fig. 6 and Table 2, d42).
Table 2.
Immunogenicity of D1701-V-RabG application by different routes
| Route | Days after prime immunizationa |
No. of seroconverted/total no. of mice |
No. of survivors/total no. (%) | MTD (days) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| d7 | d14 | d21 | d28 | d35 | d42 | d7 | d14 | d21 | |||
| i.m. | 116 (75–125)b | 75 | 126 (75–375) | 171 (75–625) | 125 | 125 | 7/7 | 7/7 | 7/7 | 6/7 (86) | 14.0 |
| i.n. | 1 (0.1–15) | 1 (0.1–15) | 34 (15–125) | 159 (75–375) | 157 (125–625) | 184 (125–625) | 1/7 | 1/7 | 7/7 | 7/7 (100) | |
| i.v. | 81 (75–125) | 136 (75–375) | 108 (75–125) | 148 (75–375) | 125 | 80 (25–125) | 7/7 | 7/7 | 7/7 | 5/7 (71) | 15.0 |
| i.p. | 74 (25–125) | 117 (75–375) | 148 (75–625) | 136 (75–375) | 171 (125–375) | 116 (75–125) | 7/7 | 7/7 | 7/7 | 5/7 (71) | 13.5 |
| i.d. | 12 (1–75) | 30 (5–75) | 41 (15–75) | 62 (15–375) | 51 (25–125) | 51 (25–125) | 5/6 | 6/6 | 6/6 | 3/6 (50) | 11.7 |
| s.c. | 1 (0.1–15) | 3 (0.1–25) | 74 (25–125) | 64 (25–75) | 59 (25–125) | 47 (25–75) | 2/7 | 5/7 | 7/7 | 3/7 (43) | 12.5 |
| Oral | 0 (0.1–25) | 1 (0.1–75) | 26 (3–75) | 81 (15–375) | 164 (125–625) | 127 (75–375) | 1/7 | 2/7 | 7/7 | 5/7 (71) | 14.0 |
| PBS | <1 | <1 | <1 | <1 | <1 | <1 | 0/7 | 0/7 | 0/7 | 0/7 (0 ) | 8.3 |
Prime immunization at day 0, first boost at day 15, second boost at day 28, and challenge at day 49.
VNA titer in IU/ml: GMT (range).
Finally, we tested the protection mediated by the different administration routes of 107 PFU of D1701-V-RabG using 100 LD50 i.c. challenge 3 weeks after the last immunization. All together, the protection rate for the different vaccination routes varied between 43% and 100% of the animals (Table 2). Remarkably, all mice receiving the 3-fold i.n. vaccination were completely protected. Due to the lack of high levels of serum VNA after priming with 106 (data not shown) or with 107 (Fig. 6) PFU, we assume that the i.n. booster vaccination was needed for protection rather than the higher vaccination dose. The finding of a weaker protective response after i.v. administration of D1701-V-RabG was in line with earlier pilot experiments, which also showed lesser protection than i.m. application. Considering MTD, our results indicate that in all cases, the vaccination led to a significant prolongation of the life span of the challenged mice, although complete protection could not be achieved in all cases (Table 2). In addition, the beginning of the clinical symptoms of the immunized but not protected mice was delayed by at least 4 to 6 days compared to the not immunized mice. Taken together, we could demonstrate that protection can be accomplished with D1701-V-RabG via different routes of application and, therefore, might be qualified for more practicable immunization routes of target animals in the field.
Contribution of T cells to the protective immunity mediated by D1701-V-RabG.
To scrutinize the contribution of CD4- and CD8-positive T cells to the protective immunity of D1701-V-RabG-vaccinated mice, both T cell subsets were selectively removed on their own or in combination by antibody-mediated depletion during different stages of immunization. The three different experimental settings outlined in the bottom part of Fig. 7 were chosen to examine (i) the importance of the T cell subsets for priming the protective anti-RABV response (Depletion-A), (ii) the necessity of their presence during RABV challenge infection (Depletion-B), and (iii) the implication of the absence of these T cell populations during immunization and challenge infection. As controls, the experiments included groups of nondepleted mice immunized (immune C) or not immunized (nonimmune C).
The impact of CD4- and/or CD8-positive T cells on the development of protective immunity after i.m. vaccination with 107 PFU D1701-V-RabG was analyzed by depleting the T cell subsets prior to immunization at days −6 and −4 (Fig. 7, Depletion-A). FACS analyses demonstrated (data not shown) that the depleted T cell populations remain absent for approximately 14 days after antibody treatment, before they become recovered, as reported earlier (55, 56). Accordingly, the mice should have regained their original immune status before challenge infection at day 15. T cell depletions prior to the immunization strongly affected the development of an adequate immunity against RABV, as demonstrated in Fig. 7A. Removal of CD4-positive T cells reduced the survival rate of the immunized mice to only 25% (2 survivors out of 8 animals), whereas the most pronounced effect was found after simultaneous depletion of CD4- and CD8-positive T cells resulting in protection of only 1 out of 8 animals (12.5%). Despite depletion of CD8-positive T cells, still 50% of the mice were protected against the lethal RABV challenge. As expected, all animals of the nonimmunized controls died between days 7 and 10 postchallenge, and 9 out of 10 immunized control animals were protected (90%).
Using the approach Depletion-B (Fig. 7), the direct contribution of the T cell subsets in protection of previously immunized animals was assessed. Mice were i.m. inoculated with the ORFV recombinant (107 PFU), and 13 days later, i.e., 2 days before RABV challenge infection (day 13), CD4-positive and/or CD8-positive T cells were depleted. Hence, the D1701-V-RabG-mediated formation of the RABV-specific immune response was not affected by the antibody treatment. To prevent restoration of the T cells during challenge infection, the antibody treatment was continued at days 15, 19, and 23 as shown in the bottom part of Fig. 7. Two days before challenge CD4-positive and/or CD8-positive T cells were selectively removed. As can be seen in Fig. 7B, the depletion of only CD8-positive T cells had virtually no influence on the protection against RABV infection. Elimination of the CD4-positive T cell subset or of both CD4- and CD8-positive T cells reduced the survival rate to 78% and 63%, respectively. The data show that in vivo depletion of the T cell subsets shortly before challenge infection only marginally influenced the previously established protective immune response.
To examine the protective efficiency of D1701-V-RabG in mice deficient for CD4-positive and/or CD8-positive T cells, animals were antibody treated at the time of vaccination and during challenge infection for a period of 24 days (Fig. 7, Depletion-C), in order to avoid the reconstitution of the T cell subsets and to ensure their absence during the complete experiment. The survival rate of immunized mice missing CD8-positive T cells was diminished to 56% (5 out of 9), whereas only 33% (3 out of 9) of the immunized mice depleted for CD4-positive T cells survived the challenge (Fig. 7C). Depletion of both T cell subsets resulted in a more pronounced decline of protection, and only 20% (2 out of 10) of the immunized animals endured the challenge (Fig. 7C). In summary, the presence of CD4-positive T cells at the time of vaccination with the recombinant D1701-V-RabG was most crucial for eliciting a solid protective immunity against RABV. Although to a lesser extent, CD8-positive T cells also contribute to protection against lethal challenge infection. In the three experimental layouts, the lack of both T cell populations impaired the survival rates most.
Finally, we demonstrated the importance of B cells for protection of D1701-V-RabG-immunized mice to RABV challenge infection. Mice genetically immune deficient for B cells, μMT mice (B6.129S2-Ighmtm1Cgn/J), were immunized three times in 2-week intervals with 107 PFU of D1701-V-RabG before i.c. challenge infection with 500 LD50 of the RABV CVS strain. None of the animals either immunized or nonimmunized resisted the challenge infection, so that all had to be sacrificed 9 days after infection due to RABV-specific clinical symptoms (data not shown). This supports the need of B cells for protection against RABV also after D1701-V-RabG immunization.
Antibody response in dogs.
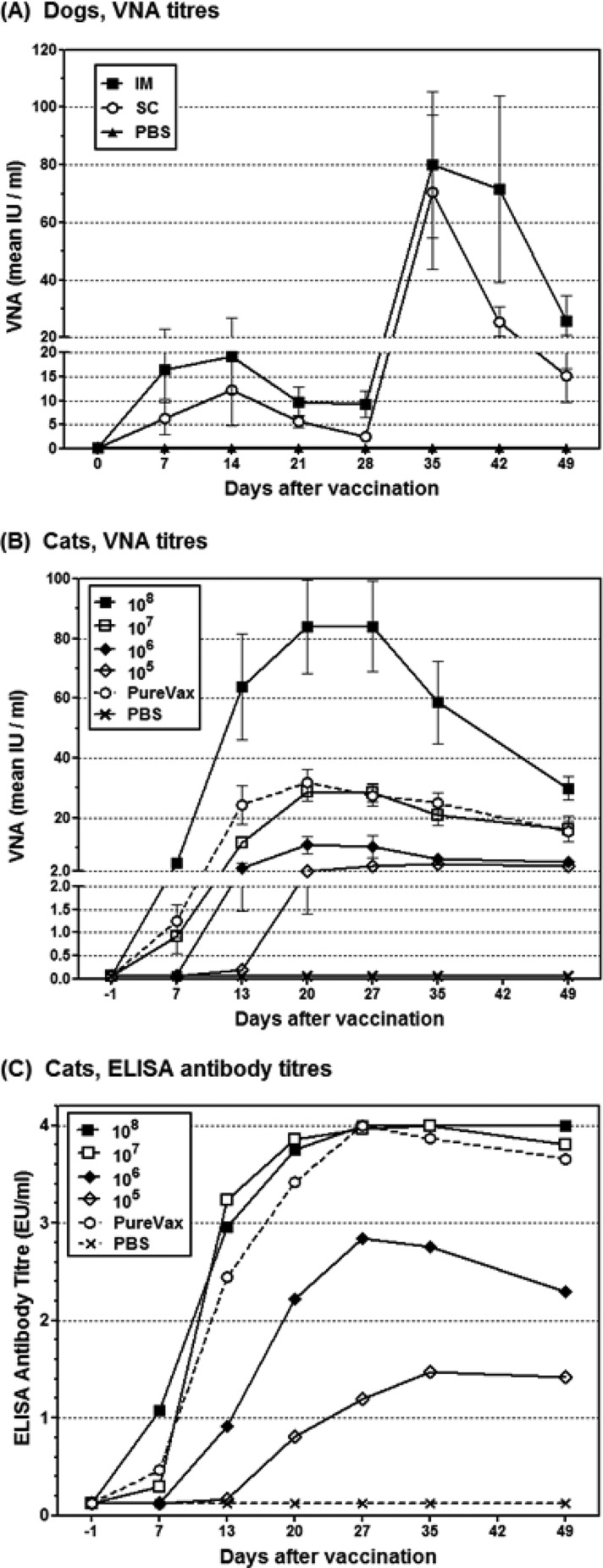
Dogs are the main reservoir for human RABV infections and have to be vaccinated for controlling rabies. Therefore, we examined the immunogenic potential of D1701-V-RabG in dogs after s.c. or i.m. immunization. Dogs (n = 5) were immunized twice with 107.57 TCID50 of the recombinant ORFV in 28-day intervals. For detection of VNA, sera were collected weekly from day 0 (prevaccination) until day 49. As controls, one dog was immunized s.c. and another one i.m. with PBS. Figure 8A shows that all animals of the s.c. immunized group seroconverted within 7 days, exhibiting VNA titers from 1.6 IU to 19.5 IU (mean, 6.3 IU). After prime immunization, the VNA response peaked at day 14, ranging from 2.8 IU to 41.6 IU (mean, 12.1 IU). Subsequently, the mean titer slightly decreased to 5.7 IU at day 21 and to 2.5 IU at day 28, whereby the minimal VNA titer of 1.6 IU of the animals still exceeded the protective titer of 0.5 IU per ml serum. Booster immunization at day 28 markedly increased the VNA titers, ranging 1 week later between 10.9 IU to 169.4 IU (mean, 70.5 IU). At day 42 and 49 postimmunization, the VNA titers slightly declined to a mean titer of 25.5 IU (6.3 to 32.3 IU) and 15.2 IU (4.8 to 32.3 IU), respectively (Fig. 8A).
Fig 8.
Serum antibody response in vaccinated cats and dogs. (A) Dogs (n = 5) were vaccinated s.c. (filled squares) or i.m. (open squares) with 107.5 TCID50 of D1701-V-RabG, and the serum VNA response was determined by RIFFT. At day 28, all animals received a booster immunization with the same dose of D1701-V-RabG. Control animals were mock immunized with PBS (stars). (B) VNA GMT titers of cats (n = 7 or 8) s.c. immunized with 108 TCID50, 107 TCID50, 106 TCID50, 105 TCID50 D1701-V-RabG, or PureVax feline rabies or control-immunized with PBS. (C) ELISA titers were obtained from Platelia II ELISA as described in Materials and Methods.
Intramuscular injection of D1701-V-RabG elicited approximately 2- to 3-fold higher VNA GMT titers in the dogs compared to s.c. application. Seven days after immunization, all animals had seroconverted, and a VNA mean titer of 16.5 IU was found (Fig. 8A). The VNA response after i.m. application also peaked at day 14, with a mean titer of 19.1 IU, and decreased thereafter to 9.8 IU and 9.3 IU at day 21 and day 28, respectively. The second i.m. immunization boosted the VNA titers to 80.3 IU at day 35, and similar to the s.c. immunized animals, a slight reduction was observed at day 42 (mean 71.5 IU) and at day 49 (mean 25.7 IU).
Collectively, the results demonstrate that all immunized dogs seroconverted during the first week after prime vaccination and displayed VNA titers, which remained beyond the OIE recommendation for a protective response (0.5 IU/ml serum) during the complete observation period. Additionally, no signs of illness, inflammation, increased rectal temperature, or pain were observed, except of one i.m. immunized dog showing mild pain on palpation for the duration of 1 day.
Immunogenicity in cats.
Vaccination of cats is also important for controlling spread of RABV. Therefore, we were interested in testing the immunogenicity of D1701-V-RabG after a single s.c. vaccination with several amounts of D1701-V-RabG. For comparison, one group of animals received the commercial Canarypox-based PureVax feline rabies live vaccine (corresponding to ≥106.8 FAID50). After vaccination, sera were collected weekly over a period of 7 weeks, and RABV antibody responses were determined by RFFIT (VNA) and by ELISA (Platelia Rabies II). Both tests proved all animals seronegative to RABV antibodies prior to vaccination. The level of the induced serum VNA titers clearly depended on the used immunization dose of D1701-V-RabG (Fig. 8B). All cats receiving the highest dose (108 TCID50) developed RABV-specific serum antibody titers higher than 0.5 IU/ml within 7 days, ranging from 1.3 to 10.0 IU/ml. Peak VNA titers of 84 IU/ml were found in this group at days 20 to 27, which were approximately 3 to 40 times higher than those obtained after application of the lower doses of the ORFV recombinant. Immunization with 107 TCID50 D1701-V-RabG stimulated very similar VNA titers compared to the use of the commercial Canarypox-vectored vaccine. Seroconversion of all animals was found in both groups just at day 13 after vaccination, and titers ranged from 4.3 to 13.0 IU/ml (107 PFU D1701-V-RabG) and from 2.8 to 39.0 IU/ml in PureVax-vaccinated animals. VNA titers above the protective threshold of 0.5 IU/ml serum were reached in all vaccinated groups, the latest at day 27, except for one animal after injection of 105 PFU of D1701-V-RabG, and persisted during the whole observation period (Fig. 8B).
The development of the serum ELISA antibody response showed comparably high titers using 108 or 107 TCID50 D1701-V-RabG or the canarypox-vectored vaccine (Fig. 8C). Again, the vaccination with 108 TCID50 of the ORFV recombinant elicited the highest antibody titers already during the first week. Adequate ELISA titers were also induced after vaccination with the smaller amounts of the ORFV recombinant (Fig. 8C). Taken together, the most potent antibody response was achieved by the application of a single dose of 108 TCID50 D1701-V-RabG. In addition, this immunization did not lead to any adverse effect in the vaccinated cats, and excretion of the ORFV recombinant was not detectable by virus isolation. Very similar immune responses were obtained by the use of 107 TCID50 D1701-V-RabG and by vaccination with the commercial vaccine PureVax feline rabies.
DISCUSSION
Rabies is still a global public health threat caused by RABV infection from rabid canids or felids controllable also by wildlife vaccination (1). To this end, improved efficacious and safe vaccines against RABV would be desirable. Since ORFV-vectored vaccines can be regarded as a potent, safe alternative to other poxvirus-based recombinant vaccines (44, 49, 51, 57), we generated the novel recombinant D1701-V-RabG containing the RABV glycoprotein known as the principal correlate of protective immunity against rabies. The RabG gene was inserted into the vegf-e gene locus of the genome of the attenuated ORFV strain D1701-V and was correctly expressed in good amounts, which did not detectably alter the growth characteristics, the plaque phenotype, or the replication of parental D1701-V, as reported after expression of RabG in a Newcastle disease virus vector (22). Immunofluorescence experiments revealed strong expression of RABV-characteristic RabG surface projections already in the early phase of D1701-V-RabG replication (Fig. 2). As demonstrated earlier for other D1701-V recombinants, the regulation of the foreign gene by the early vegf-e promoter allows its expression without the need for a productive ORFV replication (44, 49–51). Therefore, RabG expression and induction of RABV-specific immunity was achieved in ORFV nonpermissive hosts as mice, cats, or dogs, which implies one important prerequisite for the potential usage of D1701-V-RabG as a safe vaccine.
The protective potential of the novel ORFV recombinant D1701-V-RabG was first evaluated in mice, as a proof of concept. The capacity of the mediated immunity was tested on severe infectious conditions by the use of high lethal doses (100 to 3,000 LD50) for the i.c. challenge infections performed with the virulent RABV strain CVS-11. One important demand for improved rabies vaccines is the induction of a solid protective immunity after a single vaccine inoculation, which can be achieved with 107 PFU of D1701-V-RabG. Dose-dependent reduction in protection rates was accompanied by a reduction of VNA titers as reported for other recombinant rabies vaccines (22, 33, 35, 58). The presented data, however, do not allow an unambiguous correlation between protection and magnitude of VNA titers in mice. The absence of a relationship between VNA titers and mortality rates was reported earlier (59), albeit the presented results are difficult to compare with other reported mouse studies because of the use of different RABV strains, dosages, or routes for challenge infection. Moreover, RABV-specific antibodies determined by the in vitro virus neutralization assay did not necessarily correlate with RABV neutralization or protection in vivo, and low titers of VNA with high avidity can be more effective in protection than high titers of VNA with low avidity, as reported for influenza virus (60).
Immunization with smaller amounts of D1701-V-RabG (106 PFU) also mediated complete protection, albeit booster immunizations were necessary. In contrast to other poxvirus or adenovirus vectors (26, 32, 61–64), the ORFV vector allows repeated immunizations to enhance protective immunity against the transgene, which can be explained by the lack of serum antibodies efficiently neutralizing ORFV as well as by the generally short-living ORFV immunity (38, 40). Preexisting ORFV immunity in vaccine candidates is also highly unlikely due to the very restricted host range. Furthermore, in immunized hosts that are nonpermissive for ORFV, only early ORFV genes are expressed, and thus, immunogenic ORFV late proteins are not produced and consequently cannot induce efficient ORFV-specific immunity.
Although no substantial increase of RABV-specific VNA titers was obtained after a third immunization with 106 or more PFU of the recombinant, the protection rate against challenge infection was improved. Mice challenged 43 weeks after prime immunization with 106 PFU were completely protected after a third immunization, whereas a single boost protected only 67% of the mice. The longevity of the protective immune response might need booster immunizations with the ORFV recombinant, at least in mice or as earlier reported in rats (51). The findings that groups of animals showed different survival rates but comparable VNA titers indicate that apart from humoral immunity, additional cellular immune mechanisms contribute to protection, particularly the induction and activation of T lymphocytes, including their cytokine production (8, 65). The presented in vivo depletion experiments supported the importance of CD4-positive T cells for protection after application of D1701-V-RabG. Removal of CD4-positive T-helper cells impedes stimulation and enhancement of B cells and consequently reduces antibody production (65, 66) and therefore resulted in decreased survival of mice missing CD4-positive T cells. This is corroborated by the result that D1701-V-RabG immunization did not protect B-cell-knockout mice. The presented data confirm earlier reports on the importance of CD4-positive T cells for developing immunity and protection against rabies (59, 67), which were also induced by the application of the ORFV recombinant. Removal of the CD4- and the CD8-positive T cell subsets just before the time point of challenge only marginally influenced the survival rate of the previously immunized animals. The presented results show that CD4-positive T cells are needed to prime protective immunity, but deleting this T cell subset later on, as for instance shortly before challenge infection, does not substantially reduce protection. These results confirm earlier studies that CD4-positive T cell help is no more required for protection against RABV approximately 10 days after infection or immunization (67). Our results also support findings by Perry and Lodmell that CD8-positive T cells play a rather minor role in conferring resistance against rabies (67). When CD8- and CD4-positive T cell subsets were missing before and during vaccination, the survival rate was mostly reduced, which indicates some protective role of CD8-positive T cells. The protective role of CD8-positive cytolytic T cells was not investigated in the present study, but their importance to eliminate RABV from the brain by providing antiviral cytokines and collaborating in a concerted action with VNA was reported (68). Recently, it was shown that an induction of the recruitment of dendritic cells significantly enhanced the protective immune response against rabies (17). The general property of ORFV to attract dendritic cells to the virus inoculation site (40) was also demonstrated recently for the ORFV strain D1701 (46). This might be one explanation for the excellent immune-stimulating properties of the ORFV recombinants without the need of adjuvant.
The comparison of mice vaccinated with D1701-V-RabG to mice vaccinated with Nobivac or Rabipur, two commercial inactivated RABV vaccines, may indicate some superiority of D1701-V-RabG. Although Nobivac i.m. prime immunization induced the highest VNA titers, they also rapidly decreased and remained low even after another booster injection, which indicates the development of only short-lived VNA by that inactivated rabies vaccine. Also, the vaccinated mice were less protected than those immunized with the ORFV recombinant. The failure of Nobivac booster immunizations to increase the VNA response has not been reported and cannot be explained. In contrast to Nobivac, Rabipur immunization resulted in relatively low VNA titers after prime, but one booster immunization increased the immune response to serum VNA titers comparable to the i.m. administration of 105 PFU of D1701-V-RabG. However, the protective potential of Rabipur was low (14% survivors), a finding that is in contrast to other studies that reported efficacy even of a single immunization (23). We suppose that the different challenge virus and the high i.c. challenge dose can explain the poor protection efficacy of both inactivated vaccines in our experiments, which also argue for the excellent potency of D1701-V-RabG.
Vaccination of wildlife is considered the most effective strategy for rabies control, but application of effective vaccines requires more practicable inoculation routes than i.m. injection. Therefore, we examined the efficacy of D1701-V-RabG immunization after alternate inoculation routes. In summary, the results demonstrated that in all cases, adequate immunity against RABV could be stimulated at least after 2 vaccinations. The i.n. and i.m. immunized mice developed the highest VNA titers among the different inoculation ways and were protected against RABV challenge (Table 2). Only weak VNA responses and partial protection were found after the i.d. or the s.c. administration of the recombinant, which, however, can be explained by technical drawbacks of the mouse model. The oral application, a favorite route of vaccination for wildlife animals, showed promising results for the use of the ORFV recombinant with superior potential compared with other RabG-expressing recombinant vaccines. For example, a canine adenovirus 2-RabG recombinant was not able to protect mice against RABV challenge (58). VACV vector strain MVA expressing RabG failed to elicit detectable VNA even after booster with high doses of this recombinant and consequently did not protect against RABV challenge infection. Similarly, V-RG or a recombinant derived from the VACV strain Western Reserve mediated only partial protection against mild RABV challenge infection (33). Nevertheless, those vaccines were found effective in other animals by oral application, indicating some limitations of the murine model for evaluating the efficacy of the oral vaccination and, therefore, encourages testing the oral vaccination efficacy of D1701-V-RabG in wildlife reservoirs. In addition, the complete protection of i.n. immunized animals indicates the potential of D1701-V-RabG to trigger RABV-specific immunity by targeting mucosa-associated lymphoid tissue as described for other poxviruses (for review, see reference 69). The presented results now demonstrate that ORFV recombinants can be successfully used to elicit protective immunity by different routes of inoculation, which will be an important practical benefit for field application if shown for target animals.
Important prerequisites for controlling rabies are effective vaccination of dogs and cats, which both represent important reservoirs for RABV. As in mice (44), there was no evidence for replication of D1701-V in these animals (unpublished data). The success of rabies vaccination is inferred from the induced VNA response, as VNA titers equal to or higher than 0.5 IU per ml serum are regarded protective against rabies. In this study, cats were s.c. vaccinated with different dosages of D1701-V-RabG and compared to cats vaccinated with the commercial feline rabies vaccine PureVax, a licensed canarypox virus-vectored rabies live vaccine. The results showed that cats receiving comparable amounts of D1701-V-RabG or of the canarypox virus recombinant also develop similar, adequate serum VNA titers, and all animals had seroconverted during 13 days after immunization. By increasing the dose of the ORFV recombinant, very high VNA titers can be elicited during the first 10 days, which could be of interest for vaccinations needed for rapid induction of high VNA response. The amount of the applied dosage of D1701-V-RabG determines the magnitude of the VNA titer, but even after the inoculation of the lowest dose (105 TCID50), all animals seroconverted (i.e., VNA higher than 0.5 IU/ml), albeit lasting 35 days. The potential of D1701-V-RabG was also tested in dogs using i.m. and s.c. vaccination. Both application routes elicit VNA titers higher than 0.5 IU/ml within 7 days, peaking around day 14 after immunization, similar to results after application of inactivated rabies vaccines (70). Booster s.c. or i.m. immunization strongly increased the VNA titers, indicating low vector immunity, which, for instance, was not possible using a recombinant canine adenovirus expressing RabG (71). Intramuscular application appeared to lead to a better and more durable VNA response at later times after vaccination compared to the s.c. vaccinated animals, which was reported for other rabies vaccines, too (9).
In conclusion, the presented results are promising for the use of D1701-V-RabG as a potent, nonadjuvanted vaccine candidate, e.g., for companion animals against rabies. The VNA titers remaining after the deemed normal decline of serum antibodies were still above the protective threshold, which has to be shown by forthcoming challenge studies. This report adds another example of an ORFV recombinant mediating an effective and sustained protective immune response in hosts nonpermissive for ORFV, as replication of the recombinant virus is not required and underlines the excellent potential of the Parapoxvirus ORFV strain D1701-V as an attractive virus vector platform for the development of live ORFV-vectored vaccines.
ACKNOWLEDGMENTS
The excellent technical assistance of Karin Kegreiss and Berthilde Bauer is greatly acknowledged. We thank Lothar Stitz for assistance with mice experiments, fruitful discussions, and critical commentary on the manuscript. The most valuable comments of Stefan Finke and Gregor Meyers after critically reading the manuscript are highly appreciated. The dog experiments were carried out by Charles River Laboratory Pre-Clinical Services, Ireland.
This study was financially supported by Pfizer Animal Health.
Footnotes
Published ahead of print 21 November 2012
REFERENCES
- 1. Lembo T, Hampson K, Kaare MT, Ernest E, Knobel D, Kazwala RR, Haydon DT, Cleaveland S. 2010. The feasibility of canine rabies elimination in Africa: dispelling doubts with data. PLoS Negl. Trop. Dis. 4:e626 doi:10.1371/journal.pntd.0000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dietzschold B, Schnell M, Koprowski H. 2005. Pathogenesis of rabies. Curr. Top. Microbiol. Immunol. 292:45–56 [DOI] [PubMed] [Google Scholar]
- 3. Schnell MJ, McGettigan JP, Wirblich C, Papaneri A. 2010. The cell biology of rabies virus: using stealth to reach the brain. Nat. Rev. Microbiol. 8:51–61 [DOI] [PubMed] [Google Scholar]
- 4. Cox JH, Dietzschold B, Schneider LG. 1977. Rabies virus glycoprotein. II. Biological and serological characterization. Infect. Immun. 16:754–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foley HD, McGettigan JP, Siler CA, Dietzschold B, Schnell MJ. 2000. A recombinant rabies virus expressing vesicular stomatitis virus glycoprotein fails to protect against rabies virus infection. Proc. Natl. Acad. Sci. U. S. A. 97:14680–14685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macfarlan RI, Dietzschold B, Koprowski H. 1986. Stimulation of cytotoxic T-lymphocyte responses by rabies virus glycoprotein and identification of an immunodominant domain. Mol. Immunol. 23:733–741 [DOI] [PubMed] [Google Scholar]
- 7. Wiktor TJ, Gyorgy E, Schlumberger D, Sokol F, Koprowski H. 1973. Antigenic properties of rabies virus components. J. Immunol. 110:269–276 [PubMed] [Google Scholar]
- 8. Moore SM, Wilkerson MJ, Davis RD, Wyatt CR, Briggs DJ. 2006. Detection of cellular immunity to rabies antigens in human vaccinees. J. Clin. Immunol. 26:533–545 [DOI] [PubMed] [Google Scholar]
- 9. Aubert MF, Masson E, Artois M, Barrat J. 1994. Oral wildlife rabies vaccination field trials in Europe, with recent emphasis on France. Curr. Top. Microbiol. Immunol. 187:219–243 [DOI] [PubMed] [Google Scholar]
- 10. Rupprecht CE, Wiktor TJ, Johnston DH, Hamir AN, Dietzschold B, Wunner WH, Glickman LT, Koprowski H. 1986. Oral immunization and protection of raccoons (Procyon lotor) with a vaccinia-rabies glycoprotein recombinant virus vaccine. Proc. Natl. Acad. Sci. U. S. A. 83:7947–7950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rupprecht CE, Hanlon CA, Blanton J, Manangan J, Morrill P, Murphy S, Niezgoda M, Orciari LA, Schumacher CL, Dietzschold B. 2005. Oral vaccination of dogs with recombinant rabies virus vaccines. Virus Res. 111:101–105 [DOI] [PubMed] [Google Scholar]
- 12. Conzelmann KK, Schnell M. 1994. Rescue of synthetic genomic RNA analogs of rabies virus by plasmid-encoded proteins. J. Virol. 68:713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomme EA, Wanjalla CN, Wirblich C, Schnell MJ. 2011. Rabies virus as a research tool and viral vaccine vector. Adv. Virus Res. 79:139–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ertl HC. 2009. Novel vaccines to human rabies. PLoS Negl Trop. Dis. 3:e515 doi:10.1371/journal.pntd.0000515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cenna J, Hunter M, Tan GS, Papaneri AB, Ribka EP, Schnell MJ, Marx PA, McGettigan JP. 2009. Replication-deficient rabies virus-based vaccines are safe and immunogenic in mice and nonhuman primates. J. Infect. Dis. 200:1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Faber M, Li J, Kean RB, Hooper DC, Alugupalli KR, Dietzschold B. 2009. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc. Natl. Acad. Sci. U. S. A. 106:11300–11305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wen Y, Wang H, Wu H, Yang F, Tripp RA, Hogan RJ, Fu ZF. 2011. Rabies virus expressing dendritic cell-activating molecules enhances the innate and adaptive immune response to vaccination. J. Virol. 85:1634–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao L, Toriumi H, Wang H, Kuang Y, Guo X, Morimoto K, Fu ZF. 2010. Expression of MIP-1alpha (CCL3) by a recombinant rabies virus enhances its immunogenicity by inducing innate immunity and recruiting dendritic cells and B cells. J. Virol. 84:9642–9648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ullas PT. 2012. Rabies DNA vaccines: current status and future. World J. Vaccines 2:36–45 [Google Scholar]
- 20. Kaur M, Saxena A, Rai A, Bhatnagar R. 2010. Rabies DNA vaccine encoding lysosome-targeted glycoprotein supplemented with Emulsigen-D confers complete protection in preexposure and postexposure studies in BALB/c mice. FASEB J. 24:173–183 [DOI] [PubMed] [Google Scholar]
- 21. Liu MA. 2011. DNA vaccines: an historical perspective and view to the future. Immunol. Rev. 239:62–84 [DOI] [PubMed] [Google Scholar]
- 22. Ge J, Wang X, Tao L, Wen Z, Feng N, Yang S, Xia X, Yang C, Chen H, Bu Z. 2011. Newcastle disease virus-vectored rabies vaccine is safe, highly immunogenic, and provides long-lasting protection in dogs and cats. J. Virol. 85:8241–8252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saxena S, Dahiya SS, Sonwane AA, Patel CL, Saini M, Rai A, Gupta PK. 2008. A sindbis virus replicon-based DNA vaccine encoding the rabies virus glycoprotein elicits immune responses and complete protection in mice from lethal challenge. Vaccine 26:6592–6601 [DOI] [PubMed] [Google Scholar]
- 24. Yuan Z, Zhang S, Liu Y, Zhang F, Fooks AR, Li Q, Hu R. 2008. A recombinant pseudorabies virus expressing rabies virus glycoprotein: safety and immunogenicity in dogs. Vaccine 26:1314–1321 [DOI] [PubMed] [Google Scholar]
- 25. Prehaud C, Takehara K, Flamand A, Bishop DH. 1989. Immunogenic and protective properties of rabies virus glycoprotein expressed by baculovirus vectors. Virology 173:390–399 [DOI] [PubMed] [Google Scholar]
- 26. Weyer J, Rupprecht CE, Nel LH. 2009. Poxvirus-vectored vaccines for rabies—a review. Vaccine 27:7198–7201 [DOI] [PubMed] [Google Scholar]
- 27. Kieny MP, Lathe R, Drillien R, Spehner D, Skory S, Schmitt D, Wiktor T, Koprowski H, Lecocq JP. 1984. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature 312:163–166 [DOI] [PubMed] [Google Scholar]
- 28. Wiktor TJ, Macfarlan RI, Reagan KJ, Dietzschold B, Curtis PJ, Wunner WH, Kieny MP, Lathe R, Lecocq JP, Mackett M, et al. 1984. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc. Natl. Acad. Sci. U. S. A. 81:7194–7198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blancou J, Kieny MP, Lathe R, Lecocq JP, Pastoret PP, Soulebot JP, Desmettre P. 1986. Oral vaccination of the fox against rabies using a live recombinant vaccinia virus. Nature 322:373–375 [DOI] [PubMed] [Google Scholar]
- 30. Rupprecht CE, Charlton KM, Artois M, Casey GA, Webster WA, Campbell JB, Lawson KF, Schneider LG. 1990. Ineffectiveness and comparative pathogenicity of attenuated rabies virus vaccines for the striped skunk (Mephitis mephitis). J. Wildl. Dis. 26:99–102 [DOI] [PubMed] [Google Scholar]
- 31. Rupprecht CE, Blass L, Smith K, Orciari LA, Niezgoda M, Whitfield SG, Gibbons RV, Guerra M, Hanlon CA. 2001. Human infection due to recombinant vaccinia-rabies glycoprotein virus. N. Engl. J. Med. 345:582–586 [DOI] [PubMed] [Google Scholar]
- 32. Lodmell DL, Ewalt LC. 2000. Rabies vaccination: comparison of neutralizing antibody responses after priming and boosting with different combinations of DNA, inactivated virus, or recombinant vaccinia virus vaccines. Vaccine 18:2394–2398 [DOI] [PubMed] [Google Scholar]
- 33. Weyer J, Rupprecht CE, Mans J, Viljoen GJ, Nel LH. 2007. Generation and evaluation of a recombinant modified vaccinia virus Ankara vaccine for rabies. Vaccine 25:4213–4222 [DOI] [PubMed] [Google Scholar]
- 34. Esposito JJ, Knight JC, Shaddock JH, Novembre FJ, Baer GM. 1988. Successful oral rabies vaccination of raccoons with raccoon poxvirus recombinants expressing rabies virus glycoprotein. Virology 165:313–316 [DOI] [PubMed] [Google Scholar]
- 35. Taylor J, Trimarchi C, Weinberg R, Languet B, Guillemin F, Desmettre P, Paoletti E. 1991. Efficacy studies on a canarypox-rabies recombinant virus. Vaccine 9:190–193 [DOI] [PubMed] [Google Scholar]
- 36. Taylor J, Weinberg R, Languet B, Desmettre P, Paoletti E. 1988. Recombinant fowlpox virus inducing protective immunity in non-avian species. Vaccine 6:497–503 [DOI] [PubMed] [Google Scholar]
- 37. Aspden K, van Dijk AA, Bingham J, Cox D, Passmore JA, Williamson AL. 2002. Immunogenicity of a recombinant lumpy skin disease virus (neethling vaccine strain) expressing the rabies virus glycoprotein in cattle. Vaccine 20:2693–2701 [DOI] [PubMed] [Google Scholar]
- 38. Büttner M, Rziha H-J. 2002. Parapoxviruses: from the lesion to the viral genome. J. Vet. Med. B Infect. Dis. Vet. Public Health 49:7–16 [DOI] [PubMed] [Google Scholar]
- 39. Hussain KA, Burger D. 1989. In vivo and in vitro characteristics of contagious ecthyma virus isolates: host response mechanism. Vet. Microbiol. 19:23–36 [DOI] [PubMed] [Google Scholar]
- 40. Haig DM. 2006. Orf virus infection and host immunity. Curr. Opin. Infect. Dis. 19:127–131 [DOI] [PubMed] [Google Scholar]
- 41. Fachinger V, Schlapp T, Strube W, Schmeer N, Saalmuller A. 2000. Poxvirus-induced immunostimulating effects on porcine leukocytes. J. Virol. 74:7943–7951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Friebe A, Siegling A, Friederichs S, Volk HD, Weber O. 2004. Immunomodulatory effects of inactivated parapoxvirus ovis (ORF virus) on human peripheral immune cells: induction of cytokine secretion in monocytes and Th1-like cells. J. Virol. 78:9400–9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siegemund S, Hartl A, von Buttlar Dautel FH, Raue R, Freudenberg MA, Fejer G, Buttner M, Kohler G, Kirschning CJ, Sparwasser T, Alber G. 2009. Conventional bone marrow-derived dendritic cells contribute to Toll-like receptor-independent production of alpha/beta interferon in response to inactivated parapoxvirus ovis. J. Virol. 83:9411–9422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fischer T, Planz O, Stitz L, Rziha H-J. 2003. Novel recombinant parapoxvirus vectors induce protective humoral and cellular immunity against lethal herpesvirus challenge infection in mice. J. Virol. 77:9312–9323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Friebe A, Friederichs S, Scholz K, Janssen U, Scholz C, Schlapp T, Mercer A, Siegling A, Volk HD, Weber O. 2011. Characterization of immunostimulatory components of Orf virus (parapoxvirus ovis). J. Gen. Virol. 92:1571–1584 [DOI] [PubMed] [Google Scholar]
- 46. McGuire MJ, Johnston SA, Sykes KF. 2012. Novel immune-modulator identified by a rapid, functional screen of the parapoxvirus ovis (Orf virus) genome. Proteome Sci. 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weber O, Siegling A, Friebe A, Limmer A, Schlapp T, Knolle P, Mercer A, Schaller H, Volk HD. 2003. Inactivated parapoxvirus ovis (Orf virus) has antiviral activity against hepatitis B virus and herpes simplex virus. J. Gen. Virol. 84:1843–1852 [DOI] [PubMed] [Google Scholar]
- 48. Rziha H-J, Henkel M, Cottone R, Bauer B, Auge U, Gotz F, Pfaff E, Rottgen M, Dehio C, Büttner M. 2000. Generation of recombinant parapoxviruses: nonessential genes suitable for insertion and expression of foreign genes. J. Biotechnol. 83:137–145 [DOI] [PubMed] [Google Scholar]
- 49. Rohde J, Schirrmeier H, Granzow H, Rziha H-J. 2011. A new recombinant Orf virus (ORFV, parapoxvirus) protects rabbits against lethal infection with rabbit hemorrhagic disease virus (RHDV). Vaccine 29:9256–9264 [DOI] [PubMed] [Google Scholar]
- 50. Voigt H, Merant C, Wienhold D, Braun A, Hutet E, Le Potier MF, Saalmuller A, Pfaff E, Büttner M. 2007. Efficient priming against classical swine fever with a safe glycoprotein E2 expressing Orf virus recombinant (ORFV VrV-E2). Vaccine 25:5915–5926 [DOI] [PubMed] [Google Scholar]
- 51. Henkel M, Planz O, Fischer T, Stitz L, Rziha H-J. 2005. Prevention of virus persistence and protection against immunopathology after Borna disease virus infection of the brain by a novel Orf virus recombinant. J. Virol. 79:314–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dory D, Fischer T, Beven V, Cariolet R, Rziha H-J, Jestin A. 2006. Prime-boost immunization using DNA vaccine and recombinant Orf virus protects pigs against pseudorabies virus (herpes suid 1). Vaccine 24:6256–6263 [DOI] [PubMed] [Google Scholar]
- 53. van Rooij EM, Rijsewijk FA, Moonen-Leusen HW, Bianchi AT, Rziha H-J. 2010. Comparison of different prime-boost regimes with DNA and recombinant Orf virus based vaccines expressing glycoprotein D of pseudorabies virus in pigs. Vaccine 28:1808–1813 [DOI] [PubMed] [Google Scholar]
- 54. Klingen Y, Conzelmann KK, Finke S. 2008. Double-labeled rabies virus: live tracking of enveloped virus transport. J. Virol. 82:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cobbold SP, Jayasuriya A, Nash A, Prospero TD, Waldmann H. 1984. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature 312:548–551 [DOI] [PubMed] [Google Scholar]
- 56. Le Gros GS, Prestidge RL, Watson JD. 1983. In-vivo modulation of thymus-derived lymphocytes with monoclonal antibodies in mice. I. Effect of anti-Thy-1 antibody on the tissue distribution of lymphocytes. Immunology 50:537–546 [PMC free article] [PubMed] [Google Scholar]
- 57. Brun A, Albina E, Barret T, Chapman DA, Czub M, Dixon LK, Keil GM, Klonjkowski B, Le Potier MF, Libeau G, Ortego J, Richardson J, Takamatsu HH. 2008. Antigen delivery systems for veterinary vaccine development. Viral-vector based delivery systems. Vaccine 26:6508–6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li J, Faber M, Papaneri A, Faber ML, McGettigan JP, Schnell MJ, Dietzschold B. 2006. A single immunization with a recombinant canine adenovirus expressing the rabies virus G protein confers protective immunity against rabies in mice. Virology 356:147–154 [DOI] [PubMed] [Google Scholar]
- 59. Dietzschold B, Tollis M, Rupprecht CE, Celis E, Koprowski H. 1987. Antigenic variation in rabies and rabies-related viruses: cross-protection independent of glycoprotein-mediated virus-neutralizing antibody. J. Infect. Dis. 156:815–822 [DOI] [PubMed] [Google Scholar]
- 60. Gulati U, Kumari K, Wu W, Keitel WA, Air GM. 2005. Amount and avidity of serum antibodies against native glycoproteins and denatured virus after repeated influenza whole-virus vaccination. Vaccine 23:1414–1425 [DOI] [PubMed] [Google Scholar]
- 61. Kanesa-thasan N, Smucny JJ, Hoke CH, Marks DH, Konishi E, Kurane I, Tang DB, Vaughn DW, Mason PW, Shope RE. 2000. Safety and immunogenicity of NYVAC-JEV and ALVAC-JEV attenuated recombinant Japanese encephalitis virus—poxvirus vaccines in vaccinia-nonimmune and vaccinia-immune humans. Vaccine 19:483–491 [DOI] [PubMed] [Google Scholar]
- 62. Kundig TM, Kalberer CP, Hengartner H, Zinkernagel RM. 1993. Vaccination with two different vaccinia recombinant viruses: long-term inhibition of secondary vaccination. Vaccine 11:1154–1158 [DOI] [PubMed] [Google Scholar]
- 63. McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, Li Y, Giles-Davis W, Cun A, Zhou D, Xiang Z, Letvin NL, Ertl HC. 2007. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 81:6594–6604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sharpe S, Polyanskaya N, Dennis M, Sutter G, Hanke T, Erfle V, Hirsch V, Cranage M. 2001. Induction of simian immunodeficiency virus (SIV)-specific CTL in rhesus macaques by vaccination with modified vaccinia virus Ankara expressing SIV transgenes: influence of pre-existing anti-vector immunity. J. Gen. Virol. 82:2215–2223 [DOI] [PubMed] [Google Scholar]
- 65. Whitmire JK. 2011. Induction and function of virus-specific CD4+ T cell responses. Virology 411:216–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Swain SL, McKinstry KK, Strutt TM. 2012. Expanding roles for CD4(+) T cells in immunity to viruses. Nat. Rev. Immunol. 12:136–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Perry LL, Lodmell DL. 1991. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J. Virol. 65:3429–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. 1998. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J. Virol. 72:3711–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gherardi MM, Esteban M. 2005. Recombinant poxviruses as mucosal vaccine vectors. J. Gen. Virol. 86:2925–2936 [DOI] [PubMed] [Google Scholar]
- 70. Minke JM, Bouvet J, Cliquet F, Wasniewski M, Guiot AL, Lemaitre L, Cariou C, Cozette V, Vergne L, Guigal PM. 2009. Comparison of antibody responses after vaccination with two inactivated rabies vaccines. Vet. Microbiol. 133:283–286 [DOI] [PubMed] [Google Scholar]
- 71. Hu R, Zhang S, Fooks AR, Yuan H, Liu Y, Li H, Tu C, Xia X, Xiao Y. 2006. Prevention of rabies virus infection in dogs by a recombinant canine adenovirus type-2 encoding the rabies virus glycoprotein. Microbes Infect. 8:1090–1097 [DOI] [PubMed] [Google Scholar]