Abstract
Tyrosine phosphorylation-dependent signaling, as mediated by members of the epidermal growth factor receptor (EGFR) family (ErbB1 to -4) of protein tyrosine kinases (PTKs), Src family PTKs (SFKs), and cytokines such as interleukin-6 (IL-6) that signal via signal transducer and activator of transcription 3 (STAT3), is critical to the development and progression of many human breast cancers. EGFR, SFKs, and STAT3 can serve as substrates for the protein tyrosine phosphatase TCPTP (PTPN2). Here we report that TCPTP protein levels are decreased in a subset of breast cancer cell lines in vitro and that TCPTP protein is absent in a large proportion of “triple-negative” primary human breast cancers. Homozygous TCPTP deficiency in murine mammary fat pads in vivo is associated with elevated SFK and STAT3 signaling, whereas TCPTP deficiency in human breast cancer cell lines enhances SFK and STAT3 signaling. On the other hand, TCPTP reconstitution in human breast cancer cell lines severely impaired cell proliferation and suppressed anchorage-independent growth in vitro and xenograft growth in vivo. These studies establish TCPTP's potential to serve as a tumor suppressor in human breast cancer.
INTRODUCTION
The transformation of the breast epithelium to malignant and metastatic disease involves an amalgam of genetic and epigenetic events and is deeply influenced by both estrogen receptor (ER) and growth factor signaling, in particular, that involving the epidermal growth factor receptor (EGFR)/ErbB family of protein tyrosine kinases (PTKs). Breast cancers can be subclassified according to the expression of ER, progesterone receptor (PR), and ErbB2, tumor grade, and transcript profiles (1). Subtypes include (i) luminal A tumors that account for up to 60% of breast cancers and express ER and/or PR but not ErbB2, (ii) luminal B tumors that account for 4% to 19% of breast tumors, express ER or PR, and are highly proliferative and/or express ErbB2, (iii) highly aggressive ErbB2-positive (ErbB2+) tumors that are negative for ER and PR (7% to 12% of breast cancers), and (iv) basal-like tumors that account for 14% to 20% of breast cancers and include the so-called “triple-negative” tumors that do not express ErbB2, ER, or PR and are resistant to endocrine- and trastuzumab-based therapies (1).
In breast cancer, ErbB2 is amplified and overexpressed in 15% to 20% of primary breast cancers and plays a causal role in mammary carcinogenesis (2). Other EGFR family PTKs implicated in the development of breast cancer include ErbB1, which is activated in many triple-negative tumors and correlates with poor prognosis (3). Although ErbB1 is less transforming than ErbB2 (4), ErbB1 cooperates with the PTK c-Src to promote breast cancer cell migration and anchorage-independent growth and aberrant human mammary epithelial cell acinar formation in three-dimensional cultures (5, 6). Elevated c-Src protein levels and/or activity occurs in a striking 70% of primary breast cancers and can coincide with ErbB1 or ErbB2 overexpression (7–9). In ductal carcinoma in situ, activated c-Src correlates with high tumor grade, high proliferation, and high risk of recurrence or progression to invasive cancer (10). Src family PTKs (SFKs) can also cooperate with ErbB2 to promote tumorigenicity and metastasis (11). Also, SFKs can play an integral role in mediating ER signaling and may also be important in basal-like tumors, promoting cellular proliferation, survival, invasion, and chemotherapeutic resistance (12–14).
Signal transducer and activator of transcription 3 (STAT3) is activated constitutively in many human breast cancers, mediating signaling by ErbB1 and ErbB2 and serving as a direct substrate for SFKs (12, 15–17). STAT3 can also be phosphorylated by Janus kinases (JAKs) downstream of the common interleukin-6 (IL-6) cytokine family receptor β subunit gp130. In breast cancer, an increase in IL-6/gp130 signaling correlates with poor prognosis (18, 19) and promotes an invasive phenotype in mammospheres in vitro (20), whereas knockdown of STAT3 attenuates xenograft growth and sensitizes tumors to chemotherapeutics (13, 21). Furthermore, STAT3 deletion in ErbB2-induced breast cancer suppresses angiogenesis and inflammation and mammary tumor metastases in mice (22).
TCPTP (encoded by PTPN2) is a ubiquitous tyrosine-specific phosphatase (23). Two splice variants of TCPTP are expressed that have identical N termini and catalytic domains but varied C termini: a 48-kDa form (TC48) that is targeted to the endoplasmic reticulum (ER) by a hydrophobic C terminus and a 45-kDa variant (TC45) that is targeted to the nucleus by a nuclear localization sequence (23). Despite an apparently exclusive nuclear localization in resting cells, TC45 can shuttle between the nucleus and cytoplasm to access substrates in both compartments (23). Substrates for TC45 include both receptor and nonreceptor PTKs such as ErbB1, JAK1/3, and SFKs (23–28) and nuclear substrates such as STAT3 (23, 29, 30) that have been implicated in the genesis and progression of many human tumors. Recent studies have shown that TCPTP is deleted in 6% of all human T-cell acute lymphoblastic leukemias (T-ALLs), contributing to JAK/STAT signaling and tumorigenesis (31, 32). However, TCPTP's role in the development and progression of solid tumors remains unknown. In this study, we explored TCPTP's role in breast cancer.
MATERIALS AND METHODS
Reagents.
Recombinant human IL-6 and prolactin were purchased from PeproTech, EGF from R&D Systems, and CMP6 (JAK inhibitor I) and SU6656 from Calbiochem. Rabbit anti-phospho-Akt-S473, anti-phospho-STAT3-Y705, anti-phospho-EGFR-Y1068, anti-phospho-EGFR-Y1173, anti-phospho-STAT5-Y694, anti-phospho-SFK-Y418, and anti-phospho-extracellular signal-regulated kinase 1/2 (anti-ERK1/2)-T202/Y204 and murine anti-STAT3 and anti-Akt were from Cell Signaling, rabbit anti-phospho-JAK1-Y1022/Y1023 was from Biosource International, mouse anti-ERK2 and antiactin were from Santa Cruz Biotechnology, antitubulin was from Sigma-Aldrich, anti-c-Src (GD11) was from BD Biosciences, antiphosphotyrosine (4G10) was from Millipore, and anti-TCPTP (6F3) was from Medimabs. Mouse anti-TCPTP (clone CF4) was provided by N. K. Tonks (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY).
Mice.
We maintained mice on a 12-h light-dark cycle in a temperature-controlled high-barrier facility (Monash University Animal Research Laboratory with free access to food and water. Ptpn2ex2−/ex2− (C57BL/6) mice were genotyped as described previously (33). Littermates from heterozygous breeding pairs were used in all experiments. All animal experiments were performed in accordance with the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care and Use of Animals and approved by the Monash University School of Biomedical Sciences Animal Ethics Committee.
Human breast cancers.
Ethics approval for human tissues used in this study was obtained from the standing subcommittee on ethics in research involving humans, Monash University (CF08/1279-2008000613). Primary human breast cancer samples used for immunoblot analysis of TCPTP protein were from the Victorian Cancer Biobank. Tissues (∼4 mm3) were minced with scalpel blades and mechanically homogenized in ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM HEPES [pH 7.4], 1% [vol/vol] Triton X-100, 1% [vol/vol] sodium deoxycholate, 0.1% [vol/vol] SDS, 150 mM NaCl, 10% [vol/vol] glycerol, 1.5 mM MgCl2, 1 mM EGTA, 50 mM sodium fluoride, leupeptin [5 μg/ml], pepstatin A [1 μg/ml], 1 mM benzamadine, 2 mM phenylmethylsulfonyl fluoride, 1 mM sodium vanadate), sonicated (four 5-min bursts at 4°C), clarified by centrifugation (16,000 × g for 30 min at 4°C), and then resolved by SDS-PAGE and immunoblotted. The use of human tissue collected as part of the Melbourne Collaborative Cohort Study was approved by the Human Research Ethics Committee of the Cancer Council of Victoria (HREC0622) and included 119 formalin-fixed and paraffin-embedded breast cancer samples. Breast carcinomas were considered positive for ER or PR if staining occurred in >5% of epithelial cells and positive for HER2 if the staining intensity was given a score of ≥2.
Cell culture and stimulations.
HeLa, MDA-MB-231, MDA-MB-175, and HCC-1954 cells (ATCC) were cultured at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) containing 10% (vol/vol) fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. BT-483 and T-47D cells (ATCC) were cultured under the same conditions in RPMI 1640 medium supplemented with 10% to 20% (vol/vol) FBS and 10 μg/ml insulin plus antibiotics. All other cell lines were cultured as described previously (14). Cells were seeded at 5 × 105 cells/well/6-well plate and cultured for 24 to 48 h, serum starved in medium containing 0.1% (vol/vol) FBS for 6 to 24 h, and stimulated as indicated. For IL-6 stimulation assays, cells were either stimulated with 1 ng/ml IL-6 for the indicated times or pulsed with 1 to 10 ng/ml IL-6 for 10 min, medium was replenished, and incubations were continued as indicated. For integrin ligation assays, cells were detached with 10 mM EDTA–phosphate-buffered saline (PBS), resuspended in phenol red-free DMEM (Sigma-Aldrich) containing 0.1% (wt/vol) bovine serum albumin (BSA), and kept in suspension at 37°C for 30 min before replating onto rat tail collagen type I-coated plates (Roche) and incubations were continued for the indicated times. Cells were lysed in ice-cold RIPA lysis buffer and clarified by centrifugation (16,000 × g, 5 min, 4°C), and equal amounts of proteins were resolved by SDS-PAGE and immunoblotted as described previously (27).
TCPTP knockdown and overexpression.
For the stable knockdown of TCPTP, control (Mission PLKO.1-Puro control particles) and either TCPTP (TC45 and TC48; TRCN0000002783)- or TC48 (TRCN0000002783)-specific short hairpin RNA (shRNA) lentiviral particles (Sigma-Aldrich) were used to transduce HeLa, SK-BR-3, HCC-1954, or T47D breast cancer cells as indicated according to the manufacturer's instructions and cells were selected in 1 to 2 μg/ml puromycin for 6 days. For the transient knockdown of TC45, HeLa cells were transfected with a 15 nM enhanced green fluorescent protein (eGFP) control or TC45-specific (5′AAGAUUGACAGACACCUAAUAUU3′) small interfering RNAs (siRNAs) (Dharmacon) using Lipofectamine 2000 as described previously (34) and cells were stimulated after 48 h.
For transient overexpression studies, MDA-MB-231 cells were electroporated with pCG or TC45-pCG plasmid constructs described previously (24) using Amaxa Nucleofector technology (program X-013) according to the manufacturer's recommendations (Lonza). For clonogenic assays, the indicated cell lines were transduced with pWZL-hygro or TC45-pWZL-hygro retroviruses for 24 h as described previously (24) and then subjected to passage into 6-well plates and selected with 100 μg/ml hygromycin (Invitrogen) and cultured for 1 to 5 weeks for the formation of colonies visualized by staining with Coomassie brilliant blue.
For the generation of MDA-MB-231 cells that express TC45 in a doxycycline (DOX)-inducible manner (MDA-MB-231-TC45), TC45-pMT2 (27) was digested with SmaI and XbaI and cDNA corresponding to the coding region of TC45 was subcloned into pBluescript II KS (Stratagene); the EcoRI-XbaI fragment was then subcloned into pTRE (Clontech, Mountain View, CA). MDA-MB-231 cells were cotransfected with TC45-pTRE and pEFpurop-Tet-on and selected in 2 μg/ml puromycin as described previously (35). TC45 expression was induced by the addition of 2 μg/ml DOX (Sigma-Aldrich) to the culture medium for 3 days.
Soft-agar assays and tumor xenografts.
HeLa cells expressing control and TCPTP- or TC48-specific shRNAs were processed for soft-agar assays as described previously (24). MDA-MB-231-TC45 cells were cultured in the presence or absence of 2 μg/ml DOX for 3 days and then either processed for soft-agar assays as described previously (24) in the continued presence or absence of DOX or processed for xenograft studies. For the latter experiment, cells were detached with 10 mM EDTA–PBS, resuspended in PBS, and mixed with growth factor-reduced BD Matrigel (BD Biosciences) at a 1:1 ratio and injected (1 × 106 cells, 100 μl) subcutaneously into the right flanks of 6-week-old female BALB/c nu/nu mice; for DOX-treated MDA-MB-231-TC45 cells, DOX (1 mg/ml) was added to the drinking water of mice. Tumor volumes (height × width2) were measured with calipers every 2 days.
Immunohistochemistry.
Tissue sections (4 μm thick) were deparaffinized with xylene and rehydrated with three successive changes in ethanol. Antigen retrieval was performed in a pressure cooker at 120°C for 3 min in Tris-EDTA (pH 8). Nonspecific antibody binding was blocked with 1% (vol/vol) BSA and TCPTP staining performed with 5 to 10 μg/ml affinity-purified anti-TCPTP CF4. Sections were counterstained with hematoxylin. Endogenous peroxidase activity was quenched with 0.3% (vol/vol) hydrogen peroxide and TCPTP detected using horseradish peroxidase (HRP)-conjugated antibodies with diaminobenzidine (DAB) detection (Dako).
Statistical analyses.
Statistical analyzes were performed using the nonparametric, unpaired Mann-Whitney U test, the two-tailed Student t test, or Fisher's exact test and GraphPad Prism software. P values of <0.05 were considered significant.
RESULTS
TCPTP levels are reduced in breast cancer cells.
To determine whether TCPTP levels may be altered in human breast cancer, we assessed TCPTP protein expression in a panel of 26 breast cancer cell lines versus 3 immortalized human mammary epithelial cell lines. The breast cancer cell lines included luminal breast cancer cells such as ER+ T-47D, MCF-7, BT-483, MDA-MB-175, and MDA-MB-134 cells, HER2+ SK-BR-3, MDA-MB-453, MDA-MB-361, and HCC1954 cells, and ER−, PR−, and ErbB2− MDA-MB-468, MDA-MB-231, and MDA-MB-157 cells. Proteins from asynchronous cells were resolved to monitor the expression of TC48 and TC45. We found that TCPTP was variably expressed in breast cancer cell lines and that TC48 and TC45 protein levels were reduced in several cell lines, including BT-483, MDA-MB-134, MDA-MB-157, and MDA-MB-175 cells, compared, for example, to MDA-MB-468, SK-BR-3, and T-47D cells or immortalized and nontumorigenic human mammary epithelial cells (Fig. 1A). In addition, although TC48 and TC45 levels in the nontumorigenic human mammary epithelial cells and in many breast cancer cell lines were roughly similar, we found that TC45 levels were reduced in several cell lines, including MCF-7, MDA-MB-231, and HCC1569 cells, such that TC48/TC45 ratios were ≥2 (Fig. 1A). The decrease in TC48 and TC45 or in TC45 alone did not correlate with a specific tumor subtype and occurred in 27% (7/26) of the breast cancer cell lines tested. The alterations in TCPTP expression did not coincide with overt changes in the levels of other protein tyrosine phosphatases (PTPs), including SHP-2 or PTP1B (Fig. 1B), previously implicated in breast cancer development (36–38).
Fig 1.
TCPTP expression in human breast cancer cell lines. Cell lysates from the indicated asynchronous human breast cancer cell lines and immortalized normal breast epithelial cell lines were resolved by SDS-PAGE and immunoblotted for TCPTP (CF4) and tubulin (A) or PTP1B, SHP-2, and actin (B). The TCPTP variants, TC48 and TC45, are indicated, and the TC48/TC45 ratios shown. Cells with a low TC45 (TC48/TC45 ratios ≥ 2) or TCPTP (TC48 and TC45) level are indicated by one star or two stars, respectively.
TC48 and TC45 differentially contribute to PTK signaling.
Our studies indicated that protein levels of TC45 alone or of TC48 and TC45 are decreased in a subset of breast cancer cell lines. To determine the potential relative contributions of TC48 and TC45 to oncogenic tyrosine phosphorylation-dependent signaling, we used as a model system a HeLa adenocarcinoma cell line that we generated previously (25) in which TC48 and TC45 had been knocked down stably and compared signaling in those cells to signaling in those in which TC48 alone had been knocked down stably using shRNAs; TC45 and TC48 (here referred to as TCPTP) or TC48 alone was undetectable in the respective TCPTP and TC48 shRNA-expressing cell lines (Fig. 2A). We assessed the impact of TCPTP versus TC48 knockdown on EGF, IL-6, and integrin signaling and anchorage-independent growth in soft agar. We found that TCPTP knockdown had modest effects on EGF-induced ErbB1 tyrosine phosphorylation as assessed with antibodies to phosphotyrosine or antibodies to the ErbB1 Y1068 and Y1173 phosphorylation sites (data not shown). In contrast, TCPTP deficiency enhanced SFK activation (as monitored with antibodies to the c-Src Y418 autophorylation site) and STAT3 Y705 phosphorylation (Fig. 2B). TCPTP deficiency did not alter phosphatidylinositol 3-kinase (PI3K)/Akt signaling as monitored with antibodies to the Akt Ser-473 phosphorylation site and did not affect Ras/mitogen-activated protein kinase (MAPK) signaling as assessed by ERK1/2 phosphorylation (Fig. 2B), in line with TCPTP selectively regulating components of EGF signaling. Consistent with the regulation of EGF-induced STAT3 signaling, we found that IL-6-induced JAK1 Y1022/Y1023 phosphorylation and STAT3 Y705 phosphorylation were significantly enhanced by TCPTP deficiency (Fig. 2C). Similarly, we noted dramatic increases in JAK1 and SFK phosphorylation in detached TCPTP-deficient HeLa cells kept in suspension for 30 min and after replating onto collagen-coated dishes (Fig. 2D). Replating TCPTP-deficient cells onto collagen also resulted in a significant increase in STAT3 phosphorylation (Fig. 2D). In contrast, the effects of TC48 knockdown on EGF, IL-6, and integrin signaling were modest (Fig. 2B to D); TC48 knockdown promoted basal SFK signaling, albeit modestly, but did not promote EGF or IL-6 signaling and did not promote basal JAK1 Y1022/Y1023 phosphorylation or integrin-induced JAK1 and STAT3 signaling (Fig. 2B to D). To assess the effects of TCPTP versus TC48 knockdown on tumorigenicity, we assessed growth in soft agar. TCPTP, but not TC48, knockdown in HeLa cells resulted in a 3-fold increase in anchorage-independent growth (Fig. 2E); the increase in anchorage-independent growth was associated with significantly increased xenograft growth in nude mice (data not shown). Taken together, these results indicate that decreased protein levels of TC45 and TC48, but not TC48 alone, are associated with increased PTK/STAT3 signaling and tumorigenicity. These results imply that TC45 deficiency is necessary to promote PTK signaling and tumorigenicity. To determine whether TC45 deficiency alone was sufficient to enhance signaling, we took advantage of a previously characterized siRNA (34) to transiently suppress TC45 expression. TC45 knockdown enhanced SFK Y418 phosphorylation in response to EGF, and collagen-induced JAK1 Y1022/Y1023 phosphorylation, in a manner similar to that seen after stable TCPTP knockdown (Fig. 2F and G). Although an overt difference in STAT3 Y705 phosphorylation was not evident after TC45 knockdown, this might have been due to the incomplete repression of TC45 expression (Fig. 2F and G). In any event, these results are consistent with TC45 deficiency alone being sufficient to enhance PTK signaling and tumorigenicity.
Fig 2.
TC48 and TC45 differentially contribute to PTK signaling and anchorage-independent growth. (A) HeLa cells stably expressing control, TCPTP (TC48 and TC45), or TC48 shRNAs were processed for immunoblot analysis with antibodies to TCPTP and actin. (B to G) HeLa cells stably expressing control, TCPTP, or TC48 shRNAs (B to G) or transiently transfected with control (GFP) or TC45-specific siRNAs (F and G) were serum starved and left unstimulated or stimulated with 10 ng/ml EGF, pulsed with 10 ng/ml IL-6 for 10 min and then chased, or detached for 30 min and stimulated with plate-bound collagen. Lysates were then collected at the indicated times and resolved by SDS-PAGE and immunoblotted with antibodies to phospho-(Y705)-STAT3 (p-STAT3), phospho-(Y418)-SFK (p-SFK), phospho-(Y1022/Y1023)-JAK1 (p-JAK1), phospho-(S473)-Akt (p-Akt), or phospho-ERK1/2 (p-ERK1/2) and the corresponding indicated proteins and loading controls. (E) HeLa cells stably expressing control, TCPTP, or TC48 shRNAs were grown in soft agar for 4 to 5 weeks, and colonies were stained with crystal violet and counted. Representative images and data (means ± standard errors of the means [SEM]) from triplicate determinations from three independent experiments are shown; significance was calculated using a two-tailed Student's t test (*, P < 0.05).
TC45 reconstitution suppresses breast cancer cell growth.
To determine whether TCPTP deficiency affords breast cancer cells a proliferative and/or survival advantage, we attempted to stably reconstitute TC45 into a panel of breast cancer cell lines (Fig. 3). MDA-MB-231 and MCF-7 cells that are deficient in TC45 and BT-483 and MDA-MB-175 and MDA-MB-157 cells that are deficient in both TC48 and TC45 (Fig. 1A) were transduced with pWZL-hygro versus TC45-pWZL-hygro retroviruses and 24 h later incubated in the presence of antibiotic (hygromycin), and colony formation was assessed (Fig. 3); as a control for viral titer, we also transduced HeLa cells that express both TC48 and TC45 (Fig. 2A; HeLa cell proliferation is not affected by TCPTP knockdown [39] or TC45 overexpression [data not shown]). We found that transduction with TC45-expressing retroviruses alone was sufficient to almost completely prevent the growth of MCF-7 cells and to suppress the growth of MDA-MB-231, MDA-MB-175, and BT-483 cells (Fig. 3A); similar numbers of colonies were seen in pWZL control and TC45-pWZL-transduced HeLa cells, consistent with comparable viral titers (Fig. 3A). In contrast, TC45 reconstitution alone did not affect the growth of MDA-MB-157 cells (Fig. 3A) that are deficient for both TC48 and TC45 (Fig. 1), indicating that TC45 expression per se is not cytostatic and that both TCPTP variants may be required for the suppression of cellular proliferation and/or survival in at least some TCPTP-deficient breast cancer cell lines. To further assess the effect of TC45 reconstitution on breast cancer cell growth, we monitored MDA-MB-231 cell proliferation and survival (Fig. 3B to D). MDA-MB-231 cells were transduced with retroviral constructs for the expression of TC45 and then selected with hygromycin for 7 days before we assessed cellular proliferation using a hemocytometer or the distribution of cells in different phases of the cell cycle and survival by flow cytometry. TC45 reconstitution significantly suppressed overall cellular proliferation as assessed by monitoring growth curve rates (Fig. 3B) and increased cell death/apoptosis (as assessed by monitoring for sub-G1 cells [Fig. 3C] and annexin V staining [Fig. 3D]) without significantly affecting the distribution of cells in the G1, S, and G2/M phases (Fig. 3C), consistent with TCPTP attenuating progression throughout the cell cycle. These results indicate that TC45 reconstitution can suppress the growth of TCPTP/TC45-deficient breast cancer cell lines, consistent with TCPTP deficiency in human breast cancer cells otherwise providing a significant proliferative/survival advantage.
Fig 3.
TC45 reconstitution suppresses breast cancer cell growth. (A) The indicated breast cancer cell lines and HeLa cells were transduced with pWZL-hygro or TC45-pWZL-hygro retroviruses and selected with 100 μg/ml hygromycin for 1 to 5 weeks for the formation of foci/colonies. Representative images from three independent experiments are shown. MCF-7, MDA-MB-175, and MDA-MB-231 colonies/well from three independent experiments were counted; significance was calculated using a two-tailed Student's t test (*, P < 0.05; **, P < 0.01). (B to D) MDA-MB-231 cells were transduced with pWZL-hygro and TC45-pWZL-hygro retroviruses for 24 h, selected with 100 μg/ml hygromycin for 7 days, and then subjected to passage into 24-well plates (2 × 104 cells/well) followed by the counting of cells at the indicated times using a hemocytometer (B), fixed in 95% (vol/vol) ethanol, stained with propidium iodide, and processed for flow cytometry (2c and 4c cells are indicated) (C), or stained with annexin V-fluorescein isothiocyanate and propidium iodide and analyzed by flow cytometry (annexin V-positive, propidium iodide [PI]-negative cells were defined as apoptotic) (D). Data shown represent means ± standard deviations (SD) of the results of triplicate determinations and are representative of results from two independent retroviral transductions. In panel C, values correspond to the percentages of sub-G1, G0/G1, S, and G2/M cells.
TC45 suppresses breast cancer cell PTK signaling and tumorigenicity.
Given the overt effects of TC45 expression on breast cancer cell growth, we next assessed TCPTP's role in PTK signaling and tumorigenicity. First, we transiently overexpressed TC45 (TC45 levels increased by approximately 20-fold) in MDA-MB-231 cells (Fig. 4A) and assessed IL-6 and EGF signaling. TC45 expression suppressed IL-6-induced STAT3 Y705 phosphorylation (Fig. 4B). TC45 expression did not suppress IL-6-induced JAK1 Y1022/Y1023 phosphorylation (data not shown), consistent with TC45 acting at the level of STAT3. In addition, TC45 expression attenuated EGF-induced ErbB1 tyrosine phosphorylation, as assessed with pan-tyrosine phosphorylation-specific antibodies or those specific to the Y1068-phosphorylated EGFR, and suppressed downstream tyrosine phosphorylation-dependent signaling (Fig. 4C). TC45 expression also suppressed basal SFK signaling as assessed with antibodies to the Y418 activation loop autophosphorylation site on c-Src (Fig. 4C). However, TC45 expression did not attenuate JAK1 Y1022/Y1023 or STAT3 Y705 phosphorylation induced by plating detached cells onto collagen (Fig. 4D), indicating that TCPTP expression per se does not attenuate signaling in MDA-MB-231 cells and that TC45 regulates STAT3 signaling in a stimulus-dependent manner. To complement these overexpression studies, we knocked down TCPTP expression in SK-BR-3 and HCC-1954 breast cancer cells that expressed ErbB1 and ErbB2 (representing ErbB2+ ER− breast tumor subtypes) and in T-47D breast cancer cells that were ER positive (representing luminal A/B tumors) and assessed signaling in response to EGF and IL-6 as appropriate (Fig. 5); for T-47D cells, we also assessed STAT3 and STAT5 signaling in response to prolactin, which is normally associated with mammary gland development and lactation (40). We found that TCPTP knockdown in SK-BR-3 cells did not have any overt effect on EGF-induced ErbB1 tyrosine phosphorylation or downstream Ras/MAPK and PI3K/Akt signaling but enhanced EGF-induced STAT3 Y705 phosphorylation and basal SFK Y418 phosphorylation (Fig. 5A and data not shown). Moreover, IL-6-induced STAT3 Y705 phosphorylation was significantly enhanced after TCPTP knockdown in SK-BR-3 cells; JAK1 Y1022/Y1023 phosphorylation and Ras/MAPK or PI3K/Akt signaling were not altered (Fig. 5B). In HCC-1954 cells, TCPTP knockdown did not overtly alter EGF- or IL-6-induced signaling but resulted in enhanced basal STAT3 Y705 phosphorylation and SFK Y418 phosphorylation (Fig. 5C and data not shown). In T-47D cells, TCPTP knockdown significantly enhanced IL-6-induced STAT3 Y705 phosphorylation, but not JAK1 phosphorylation (Fig. 5D); for reasons that remain unclear, TCPTP knockdown in T-47D cells resulted in a reproducible suppression of IL-6-induced PI3K/Akt signaling and attenuated Ras/MAPK signaling (Fig. 5D and E). Nonetheless, TCPTP knockdown in T-47D cells also resulted in an enhancement in prolactin-induced STAT3 Y705 phosphorylation, but not JAK1 Y1022/Y1023, SFK Y418, or STAT5 Y694 phosphorylation (Fig. 5E), consistent with TCPTP acting directly on STAT3. These results are consistent with TCPTP deficiency promoting SFK and STAT3 signaling in breast cancer cells.
Fig 4.
TC45 overexpression attenuates MDA-MB-231 cell PTK signaling. MDA-MB-231 cells were transfected transiently with pCG control or pCG-TC45 and then left untreated (A) or serum starved and stimulated with either 1 ng/ml IL-6 or 10 ng/ml EGF or detached for 30 min and stimulated with plate-bound collagen for the indicated times (B to D), and lysates were processed for immunoblot analysis with antibodies to phosphotyrosine (pTyr), p-STAT3, p-SFK, or p-EGFR 1068, TCPTP, and the indicated loading controls. Results shown in panels A to C are representative of three independent experiments, and those in panel D are representative of two independent experiments. In panels B and C, IL-6-induced pSTAT3 and EGF-induced p-EGFR Y1068 and the p-SFK species indicated by arrows were quantified from 3 experiments and normalized as indicated. AU, arbitrary units. Statistical significance was calculated using a two-tailed Student's t test (*, P < 0.05; **, P < 0.01).
Fig 5.
TCPTP knockdown enhances PTK signaling in SK-BR-3 and T47-D breast cancer cells. SK-BR-3 (A and B), HCC-1954 (C), and T47-D (D and E) cells stably expressing control or TCPTP shRNAs were serum starved and left unstimulated or stimulated with 10 ng/ml EGF, pulsed with the indicated concentrations of IL-6, and chased, or stimulated with 100 ng/ml prolactin, and lysates were collected at the times indicated, resolved by SDS-PAGE, and processed for immunoblot analysis with antibodies to p-EGFR Y1173, p-EGFR Y1068, p-STAT3, p-SFK, p-ERK1/2, p-Akt, p-STAT5, and TCPTP and the indicated loading controls. In panels B and D, IL-6-induced pSTAT3 from 3 experiments was quantified and normalized to STAT3. AU, arbitrary units. Statistical significance was calculated using a two-tailed Student's t test (*, P < 0.05).
Next we assessed the effects of TC45 overexpression on tumorigenicity. For these studies, we first assessed MDA-MB-231 anchorage-independent growth in soft agar (Fig. 6A). To this end, we generated a MDA-MB-231 cell line that expressed TC45 (TC45 levels increased 5-fold) in response to doxycycline (MDA-MB-231-TC45). Induction of TC45 with doxycycline suppressed MDA-MB-231-TC45 anchorage-independent growth by approximately 4-fold (Fig. 6A). Next we assessed the impact of TCPTP reconstitution on tumor growth in vivo. The growth of MDA-MB-231-TC45 tumor cells was assessed in immunocompromised BALB/c (nu/nu) mice with and without TC45 induction (Fig. 6B). For these studies, MDA-MB-231-TC45 cells were left untreated or treated with doxycycline for 3 days to induce TC45 prior to inoculation and TC45 expression was maintained by administering doxycycline in drinking water for the duration of the experiment. We found that TC45 induction suppressed the growth of MDA-MB-231-TC45 xenografts for up to 2 weeks, after which increases in TC45 expression were also evident in untreated MDA-MB-231-TC45 xenografts and differences in growth diminished (Fig. 6B and data not shown). Thus, TC45 reconstitution in TC45-deficient breast cancer cell lines suppresses the transformed phenotype in vitro and in vivo. Taken together, these results are consistent with TCPTP deficiency promoting breast cancer cell tumorigenicity.
Fig 6.

TC45 attenuates MDA-MB-231 cell tumorigenicity. MDA-MB-231-TC45 cells were cultured for 3 days in the absence or presence of 2 μg/ml doxycycline (DOX) for the induction of TC45 expression and then either grown in soft agar with or without DOX (A) or injected into the flanks of BALB/c nu/nu mice and tumor volumes were measured every 2 days using calipers (B). Tumors were extracted after 16 days and homogenates were processed for immunoblot analysis to assess TC45 protein levels. Results in panel A represent the means ± SEM of the results from triplicate determinations from three independent experiments; significance was calculated using a two-tailed Student's t test (*, P < 0.05). Results in panel B represent the means ± SEM of the tumor volumes of 10 mice per group and are representative of two independent experiments; significance was determined using a two-tailed Mann-Whitney U test (*, P < 0.05).
Finally, we assessed whether the attenuation of SFK and STAT3 signaling by TC45 overexpression contributes to the suppression of MDA-MB-231 cellular growth/proliferation and tumorigenicity. To do this, we determined whether the JAK PTK-selective inhibitor CMP6 (41) or the SFK inhibitor SU6656 (42) could attenuate MDA-MB-231 colony formation or anchorage-independent growth in soft agar. CMP6 and SU6656 suppressed MDA-MB-231 proliferation as assessed by colony formation and almost completely prevented anchorage-independent growth (Fig. 7). As expected, CMP6 attenuated STAT3 signaling and SU6656 attenuated SFK Y418 phosphorylation and the phosphorylation of the SFK substrate STAT3 (Fig. 7B). Taken together, these results are consistent with TC45 deficiency in MDA-MB-231 cells contributing to tumorigenicity through the promotion of SFK and STAT3 signaling.
Fig 7.

CMP6 and SU6656 suppress MDA-MB-231 focus formation and anchorage-independent growth. (A) MDA-MB-231 cells were seeded at a density of 2 × 103 cells/well in a 6-well dish and cultured in the absence or presence of 2 μM CMP6 or 5 μM SU6656 for 7 days and then fixed with 3.2% paraformaldehyde and stained with 0.05% crystal violet. (B) MDA-MB-231 cells were seeded at a density of 2 × 103 cells/well in a 6-well dish and cultured in the absence or presence of 10 μM CMP6 or 10 μM SU6656 for 60 min and processed for immunoblotting as indicated. (C) MDA-MB-231 cells were cultured in 0.3% (wt/vol) soft agar in the absence or presence of 10 μM CMP6 or 10 μM SU6656 for 10 to 14 days and colonies stained with 0.05% crystal violet and counted using a dissecting microscope. Results in panels A and B are representative of two independent experiments performed in triplicate. Results in panel C represent the means ± SEM of triplicate determinations from three independent experiments; significance was calculated using a two-tailed Student's t test (**, P < 0.01).
Activation of SFK and STAT3 in mouse mammary tissue.
Previous studies have established that STAT3 can serve as a bona fide substrate for TC45 (23, 25, 29, 30). In particular, our studies have shown that TCPTP attenuates STAT3 signaling in the liver and hypothalamus (29, 30). To further assess TCPTP's role in STAT3 signaling and to examine TCPTP's potential to regulate STAT3 in mouse mammary tissue, we took advantage of mice with a global deficiency in TCPTP. Ptpn2ex2−/ex2− (C57BL/6) mice exhibit growth retardation and a median life expectancy of 32 days (33), precluding a detailed analysis of mammary gland development and tumorigenesis. Nonetheless, tissues were extracted from 5- to-6-week-old Ptpn2ex2−/ex2− (C57BL/6) mice and their corresponding wild-type littermates and processed for immunoblot analysis. We found that TCPTP deficiency resulted in pronounced increases in STAT3 Y705 phosphorylation in all tissues examined, including mammary fat pads that were isolated from virgin mice (Fig. 8 and data not shown). Furthermore, we found that TCPTP deficiency resulted in pronounced increases in SFK Y418 phosphorylation in select tissues, including mammary fat pads (Fig. 8), but unaltered STAT5 Y694 and JAK1 Y1022/Y1023 phosphorylation (data not shown). Therefore, these results highlight the potential for TCPTP deficiency to contribute to breast cancer tumorigenicity through the promotion of oncogenic SFK and STAT3 signaling.
Fig 8.

TCPTP deficiency in mice enhances STAT3 Y705 and SFK Y418 phosphorylation in mammary fat pads. The indicated tissues were extracted from 5- to 6-week-old Ptpn2ex2−/ex2− (C57BL/6) TCPTP-deficient mice and wild-type littermates and homogenates processed for immunoblot analysis with the indicated antibodies.
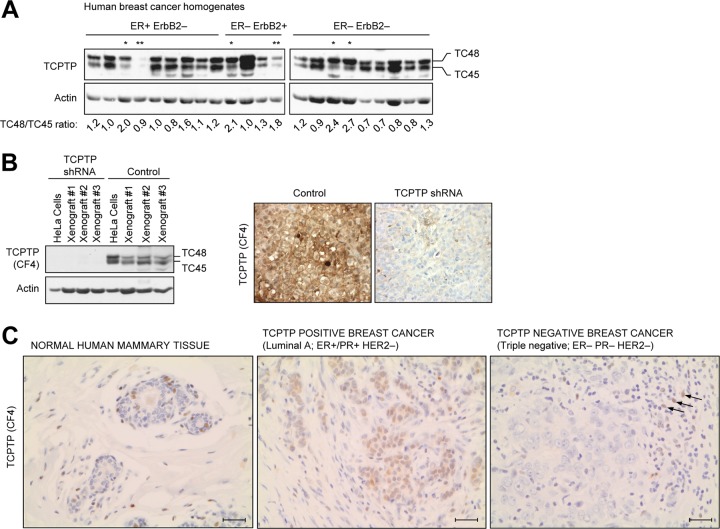
TCPTP protein levels are decreased in human breast cancer.
Our studies indicated that TCPTP protein levels are diminished in a subset of human breast cancer cell lines and that TCPTP deficiency is associated with increased PTK/STAT3 signaling and tumorigenicity. Moreover, our studies indicated that TCPTP attenuates STAT3 signaling in a variety of murine tissues, including the mammary gland, consistent with the possibility that TCPTP deficiency in mammary tissue promotes oncogenic STAT3 signaling and tumorigenicity. To explore this possibility, we monitored TCPTP expression in human breast cancer. First, we assessed TCPTP expression in primary human breast tissue homogenates from patients with ER+ ErbB2−, ER− ErbB2+, and ER− ErbB2− breast carcinomas (Fig. 9A). Consistent with our findings in breast cancer cell lines, we found that protein levels of TC45 and TC48 or of TC45 alone were diminished in 6/22 of the human breast tumor samples and that this occurred irrespective of subtype (Fig. 9A). To determine whether this occurred as a consequence of TCPTP deficiency in breast epithelial cells, as opposed to differences in cellular composition, we also assessed TCPTP deficiency in formalin-fixed and paraffin-embedded human breast cancer samples by immunohistochemistry. For these studies, we used a human TCPTP monoclonal antibody (CF4) that detected TCPTP in formalin-fixed control but not in TCPTP knockdown HeLa xenografts (Fig. 9B) and detected TCPTP in human lymph nodes where TCPTP is expressed abundantly in residing T and B cells (data not shown). In normal human mammary tissue (normal tumor-adjacent tissue), we found that TCPTP was expressed in some but not all lobular and ductal cells and was evident in the nucleus and to a lesser extent in the cytoplasm (Fig. 9C and data not shown). To determine whether TCPTP levels may be altered in human breast cancer cells, we screened a panel of breast cancer samples by immunohistochemistry. We screened a total of 119 primary human breast cancer samples, including 79 luminal A, 9 luminal B, 7 HER2+-only, and 24 triple-negative tumor samples (Table 1). In TCPTP-positive tumors, TCPTP was both nuclear and cytoplasmic, cytoplasmic alone, or focally nuclear (data not shown). Since TC45 can exit the nucleus and subcellular localization as assessed by immunohistochemistry cannot discriminate between TC45 and TC48, we screened for tumors that were TCPTP positive irrespective of localization and those that were TCPTP negative (Fig. 9C and Table 1). In TCPTP-negative tumors, care was taken to ensure that TCPTP was detected in at least some infiltrating lymphocytes (Fig. 9C). We found that TCPTP could not be detected in 34 of the 119 tumors examined. Of the 45 ER− tumors screened, we found that 20 (44%) were negative for TCPTP expression. Furthermore, 16 (66%) of the 24 ER− PR− HER2− triple-negative tumors lacked TCPTP. TCPTP expression was significantly different in ER− versus ER+ tumors and in triple-negative tumors versus all other subtypes (Table 1). No significant association was found with histological grade (Bloom Richardson; data not shown). Taken together, these results indicate that TCPTP protein levels are altered in human breast cancer and significantly reduced in ER− and triple-negative breast cancers, compared to other breast cancer subtypes.
Fig 9.
TCPTP expression in human breast cancer. (A) ER+ ErbB2−, ER− ErbB2+, and ER− ErbB2− primary human breast cancers were homogenized, and clarified extracts were processed for immunoblot analysis with antibodies to TCPTP (CF4) and actin. The TCPTP variants, TC48 and TC45, are indicated, and the TC48/TC45 ratios are shown. Cells with a low TC45 (TC48/TC45 ratios ≥ 2) or TCPTP (TC48 and TC45) level are indicated by one star or two stars, respectively. (B) HeLa cells expressing control or TCPTP-specific shRNAs were injected into the flanks of BALB/c nu/nu mice, and tumors were extracted after 7 weeks and processed for immunoblot analysis or fixed in formalin and processed for immunohistochemistry; TCPTP was detected with the CF4 monoclonal, HRP-conjugated secondary antibodies and DAB (staining TCPTP-positive cells brown). (C) Formalin-fixed and paraffin-embedded breast cancers were deparaffinized and rehydrated, and sections were processed for immunohistochemistry, stained for TCPTP (CF4), and counterstained with hematoxylin. Representative images (×400) from TCPTP-positive and -negative tumors (arrows indicate TCPTP-positive lymphocytes) and surrounding normal tissue (showing TCPTP expression in a normal terminal duct lobular unit) are shown. Scale bar, 50 μm.
Table 1.
Analysis of TCPTP loss in human breast cancer subtypesa
| Tumor sample category | Total no. of tumor samples (n = 119) | No. (%) of tumor samples with TCPTP loss (n = 34) | P value |
|---|---|---|---|
| ER status | |||
| ER positive | 74 | 14 (19) | 0.0036 |
| ER negative | 45 | 20 (44) | |
| PR status | |||
| PR positive | 64 | 13 (20) | 0.0418 |
| PR negative | 55 | 21 (38) | |
| HER2 status | |||
| HER2 positive | 16 | 3 (19) | 0.5526 |
| HER2 negative | 103 | 31 (30) | |
| Receptor status | |||
| Luminal A | 79 | 15 (19) | <0.0001 |
| Triple negative | 24 | 16 (67) | |
| HER2 positive | 16 | 3 (19) |
Luminal A, ER and PR or ER/PR positive and HER2 negative; triple negative, ER negative, PR negative, and HER2 negative. Statistical significance was determined using Fisher's exact test.
DISCUSSION
Although previous studies have established TCPTP as a tumor suppressor in T-ALL (31, 32), until now there has been little evidence for TCPTP being altered in solid tumors. In this report, we have shown that TCPTP deficiency promotes PTK and STAT3 signaling and tumorigenicity in breast cancer cells in vitro and in vivo and that TCPTP protein is lost in a subset of primary human breast cancers, consistent with TCPTP serving a tumor suppressor.
TCPTP deficiency promoted STAT3 Y705 phosphorylation in response to various stimuli in breast cancer cells. The elevated STAT3 signaling was important for the tumorigenicity of MDA-MB-231 cells, since JAK or SFK inhibitors that suppressed STAT3 signaling attenuated cellular proliferation and anchorage-independent growth. Heightened STAT3 signaling has been linked with the progression of many human malignancies, including breast cancer (12, 15). Whereas STAT3 promotes cell death during normal mammary gland involution (43), in breast cancer, elevated STAT3 signaling enhances tumor cell proliferation and survival, angiogenesis, chemotherapeutic resistance, and metastatic spread (12, 13, 21, 22). During mammary gland development, STAT3 is activated by IL-6 family cytokines, including leukemia inhibitory factor (LIF) (44). In breast cancer, multiple factors contribute to the promotion of STAT3 signaling, including increased levels of cytokines such as IL-6, overexpression and activation of oncogenic PTKs such as ErbB1 and c-Src, and the downregulation of negative regulators such as SOCS3 (8, 9, 12, 16, 18, 19, 45). The results of our studies performed using mice with a global deficiency in TCPTP are consistent with TCPTP being a key negative regulator of STAT3 in the breast; further studies should focus on delineating TCPTP's role in mammary gland development. Nonetheless, consistent with TCPTP's capacity to negatively regulate STAT3 in vivo, we found that TCPTP knockdown promoted the IL-6–STAT3 pathway in SK-BR-3 and T-47D breast cancer cells, whereas TCPTP overexpression in MDA-MB-231 breast cancer cells suppressed IL-6–STAT3 signaling. Although TCPTP has the capacity to regulate JAK1 phosphorylation, this was not overtly altered by TCPTP knockdown in the breast cancer cells examined, consistent with TCPTP acting on STAT3 directly or otherwise mediating its effects via the regulation of SFKs. In breast cancer, increased IL-6 signaling correlates with poor prognosis (18, 19), and IL-6 levels and STAT3 activation may be frequently upregulated in triple-negative breast cancers (46, 47). Accordingly, we predict that decreased TCPTP levels in triple-negative breast cancers may exacerbate IL-6–STAT3 signaling to promote tumorigenesis and/or tumor invasion-metastasis. Recent studies have shown that sustained IL-6–STAT3 signaling in breast cancer cells can promote tumorigenesis by promoting the expression of microRNA 21 (miR-21) and miR-181b-1 to repress the expression of tumor suppressors such as PTEN (48). Yet other studies have shown that STAT3 can promote the expression of miRs to enhance IL-6 transcription as part of a positive-feedback loop (49). Although STAT3 deletion does not repress ErbB2-induced tumor initiation in mice (22), it would be of interest to determine if TCPTP deficiency in mammary epithelial cells, in the context of Erbb2 or other oncogenes, promotes tumorigenesis by exacerbating the STAT3 pathway.
Our studies indicated that TC45 deficiency alone may be sufficient to promote PTK signaling and tumorigenicity. In contrast to TC48, which is restricted to the endoplasmic reticulum, TC45 has access to substrates such as ErbB1, JAK1/3, and SFKs in the cytoplasm and substrates such as STAT3 in the nucleus (23–25, 28, 30). We showed previously that overexpressing TC45 in U87MG glioblastoma cells that have a low level of TC45 inhibits cellular proliferation in vitro and suppresses the growth of intracerebral xenografts in vivo (24). Furthermore, TC45 expression in Ptpn2−/− mouse embryonic fibroblasts (MEFs) attenuates STAT3 signaling and G1/S progression and prevents the checkpoint bypass otherwise associated with sustained STAT3 signaling and cyclin D1 expression in the context of DNA replication stress (25). In this study, we found that the stable reconstitution of TC45 in TCPTP- or TC45-deficient MCF-7, MDA-MB-231, BT-483, MDA-MB-231, and MDA-MB-175 cells overtly suppressed cellular proliferation and growth in vitro. In MDA-MB-231 cells, inducible TC45 expression was also associated with a significant repression of anchorage-independent growth in vitro and xenograft growth in vivo. Paradoxically, our preliminary studies indicated that TCPTP knockdown in breast cancer cell lines expressing both TC48 and TC45 does not significantly enhance anchorage-independent growth (B. J. Shields and T. Tiganis, unpublished observations). Accordingly, we suggest that cells with low TC45 levels may be reliant on the growth-promoting effects of TCPTP deficiency. It would be of interest to determine if this “addiction” is linked to STAT3 signaling and whether triple-negative breast cancers lacking TCPTP are similarly reliant on the potential growth-promoting effects.
Other PTPs have been previously implicated in the development of human breast cancer. For example, ErbB2-overexpressing murine mammary tumors are reliant on PTP1B for growth (38), whereas PTP1B overexpression activates c-Src (50, 51) to promote breast cancer cell tumorigenicity. On the other hand, PTP deletion or decreased expression, as noted for PTPRJ (52) and PTPN12 (53), can promote tyrosine phosphorylation-dependent signaling and contribute to breast tumorigenesis. In particular, recent studies have indicated that PTPN12/PTP-PEST may serve as a tumor suppressor in triple-negative breast cancers (53). ErbB1 is a direct substrate of PTP-PEST, and immortalized human mammary epithelial cells lacking PTP-PEST exhibit enhanced ErbB1 phosphorylation and downstream PI3K/Akt and Ras/MAPK signaling (53). In triple-negative breast cancers, PTPN12 deletion, single nucleotide polymorphisms (SNPs) associated with a partial loss of function, or the miR-124-mediated repression of PTPN12 expression results in undetectable PTP-PEST levels in 60% of triple-negative breast cancers (53). Although PTPN2 SNPs have been linked with autoimmune diseases (54), no associations have been reported for cancer. Furthermore, beyond T-cell leukemias (31, 32), no evidence has emerged for PTPN2 deletion in solid tumors. Also, an evaluation of PTPN2 expression in publically available breast cancer data sets points toward PTPN2 mRNA levels being normally distributed (T. J. Molloy, S. A. O'Toole, R. L. Sutherland, and T. Tiganis, unpublished observations), but these analyses do not exclude the contribution of stroma and, in particular, immune cells, where TCPTP is abundant, to the overall PTPN2 mRNA levels. Additional studies are needed to define the molecular basis for the decreased TCPTP levels in triple-negative breast cancers and to determine whether alterations are associated with changes in, for example, DNA methylation, as reported for PTPRO and SOCS3 (45, 55), or miR-mediated PTPN2 repression, as reported for PTPN12 (53). In any event, it would of interest to determine if PTP-PEST deficiencies coincide with low TCPTP levels for the concerted promotion of oncogenic ErbB1 or STAT3 signaling and whether defining PTP-PEST and TCPTP status would stratify triple-negative breast cancer patients and be of prognostic and diagnostic value.
Our studies have established for the first time TCPTP's potential to suppress oncogenic PTK and STAT3 signaling and tumorigenicity in human breast cancer. Importantly, we have shown that the reconstitution of TCPTP suppresses tumor cell proliferation and xenograft growth. Defining the molecular basis for TCPTP regulation in normal mammary tissue and breast cancer may ultimately provide a means for increasing TCPTP levels in triple-negative breast cancers. Such an approach, in combination with the use of PTK inhibitors, may provide a therapeutic strategy for what is otherwise an aggressive and recalcitrant disease.
ACKNOWLEDGMENTS
We thank Maria Sardellis for immunohistochemistry and Teresa Tiganis for assistance with animal studies.
Grant support was provided by the National Health and Medical Research Council (NHMRC) of Australia (T.T., C.A.M., R.J.D., and R.L.S.), Cancer Council New South Wales (R.J.D.), Cancer Institute NSW (R.L.S. and S.A.O.), Sydney Breast Cancer Foundation Australia (R.L.S.), Australian Cancer Research Foundation, RT Hall Trust, Petre Foundation (R.L.S.), and the Cancer Council of Victoria (T.T.). T.T., R.J.D., and R.L.S. (in memoriam) are NHMRC Research Fellows, and T.J.M. and S.A.O. are recipients of Cancer Institute NSW Fellowships.
Footnotes
Published ahead of print 19 November 2012
REFERENCES
- 1. Reddy KB. 2011. Triple-negative breast cancers: an updated review on treatment options. Curr. Oncol. 18:e173–e179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yarden Y. 2001. Biology of HER2 and its importance in breast cancer. Oncology 61(Suppl 2):1–13 [DOI] [PubMed] [Google Scholar]
- 3. Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. 2004. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 10:5367–5374 [DOI] [PubMed] [Google Scholar]
- 4. Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. 2001. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 3:785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biscardi JS, Belsches AP, Parsons SJ. 1998. Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol. Carcinog. 21:261–272 [DOI] [PubMed] [Google Scholar]
- 6. Dimri M, Naramura M, Duan L, Chen J, Ortega-Cava C, Chen G, Goswami R, Fernandes N, Gao Q, Dimri GP, Band V, Band H. 2007. Modeling breast cancer-associated c-Src and EGFR overexpression in human MECs: c-Src and EGFR cooperatively promote aberrant three-dimensional acinar structure and invasive behavior. Cancer Res. 67:4164–4172 [DOI] [PubMed] [Google Scholar]
- 7. Muthuswamy SK, Siegel PM, Dankort DL, Webster MA, Muller WJ. 1994. Mammary tumors expressing the neu proto-oncogene possess elevated c-Src tyrosine kinase activity. Mol. Cell. Biol. 14:735–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosen N, Bolen JB, Schwartz AM, Cohen P, DeSeau V, Israel MA. 1986. Analysis of pp60c-src protein kinase activity in human tumor cell lines and tissues. J. Biol. Chem. 261:13754–13759 [PubMed] [Google Scholar]
- 9. Verbeek BS, Vroom TM, Adriaansen-Slot SS, Ottenhoff-Kalff AE, Geertzema JG, Hennipman A, Rijksen G. 1996. c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J. Pathol. 180:383–388 [DOI] [PubMed] [Google Scholar]
- 10. Wilson GR, Cramer A, Welman A, Knox F, Swindell R, Kawakatsu H, Clarke RB, Dive C, Bundred NJ. 2006. Activated c-SRC in ductal carcinoma in situ correlates with high tumour grade, high proliferation and HER2 positivity. Br. J. Cancer 95:1410–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muthuswamy SK, Muller WJ. 1995. Direct and specific interaction of c-Src with Neu is involved in signaling by the epidermal growth factor receptor. Oncogene 11:271–279 [PubMed] [Google Scholar]
- 12. Diaz N, Minton S, Cox C, Bowman T, Gritsko T, Garcia R, Eweis I, Wloch M, Livingston S, Seijo E, Cantor A, Lee JH, Beam CA, Sullivan D, Jove R, Muro-Cacho CA. 2006. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin. Cancer Res. 12:20–28 [DOI] [PubMed] [Google Scholar]
- 13. Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, Yoder S, Enkemann S, Eschrich S, Lee JH, Beam CA, Cheng J, Minton S, Muro-Cacho CA, Jove R. 2006. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin. Cancer Res. 12:11–19 [DOI] [PubMed] [Google Scholar]
- 14. Hochgräfe F, Zhang L, O'Toole SA, Browne BC, Pinese M, Porta Cubas A, Lehrbach GM, Croucher DR, Rickwood D, Boulghourjian A, Shearer R, Nair R, Swarbrick A, Faratian D, Mullen P, Harrison DJ, Biankin AV, Sutherland RL, Raftery MJ, Daly RJ. 2010. Tyrosine phosphorylation profiling reveals the signaling network characteristics of basal breast cancer cells. Cancer Res. 70:9391–9401 [DOI] [PubMed] [Google Scholar]
- 15. Alvarez JV, Febbo PG, Ramaswamy S, Loda M, Richardson A, Frank DA. 2005. Identification of a genetic signature of activated signal transducer and activator of transcription 3 in human tumors. Cancer Res. 65:5054–5062 [DOI] [PubMed] [Google Scholar]
- 16. Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R. 2001. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 20:2499–2513 [DOI] [PubMed] [Google Scholar]
- 17. Yarden Y. 2001. The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 37(Suppl 4):S3–S8 [DOI] [PubMed] [Google Scholar]
- 18. Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. 2003. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br. J. Cancer 88:1721–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Selander KS, Li L, Watson L, Merrell M, Dahmen H, Heinrich PC, Muller-Newen G, Harris KW. 2004. Inhibition of gp130 signaling in breast cancer blocks constitutive activation of Stat3 and inhibits in vivo malignancy. Cancer Res. 64:6924–6933 [DOI] [PubMed] [Google Scholar]
- 20. Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafe M. 2007. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J. Clin. Invest. 117:3988–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ling X, Arlinghaus RB. 2005. Knockdown of STAT3 expression by RNA interference inhibits the induction of breast tumors in immunocompetent mice. Cancer Res. 65:2532–2536 [DOI] [PubMed] [Google Scholar]
- 22. Barbieri I, Quaglino E, Maritano D, Pannellini T, Riera L, Cavallo F, Forni G, Musiani P, Chiarle R, Poli V. 2010. Stat3 is required for anchorage-independent growth and metastasis but not for mammary tumor development downstream of the ErbB-2 oncogene. Mol. Carcinog. 49:114–120 [DOI] [PubMed] [Google Scholar]
- 23. Tiganis T, Bennett AM. 2007. Protein tyrosine phosphatase function: the substrate perspective. Biochem. J. 402:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klingler-Hoffmann M, Fodero-Tavoletti MT, Mishima K, Narita Y, Cavenee WK, Furnari FB, Huang HJ, Tiganis T. 2001. The protein tyrosine phosphatase TCPTP suppresses the tumorigenicity of glioblastoma cells expressing a mutant epidermal growth factor receptor. J. Biol. Chem. 276:46313–46318 [DOI] [PubMed] [Google Scholar]
- 25. Shields BJ, Hauser C, Bukczynska PE, Court NW, Tiganis T. 2008. DNA replication stalling attenuates tyrosine kinase signaling to suppress S phase progression. Cancer Cell 14:166–179 [DOI] [PubMed] [Google Scholar]
- 26. Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. 2002. The T cell protein tyrosine phosphatase is a negative regulator of Janus family kinases 1 and 3. Curr. Biol. 12:446–453 [DOI] [PubMed] [Google Scholar]
- 27. Tiganis T, Bennett AM, Ravichandran KS, Tonks NK. 1998. Epidermal growth factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase. Mol. Cell. Biol. 18:1622–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Vliet C, Bukczynska PE, Puryer MA, Sadek CM, Shields BJ, Tremblay ML, Tiganis T. 2005. Selective regulation of tumor necrosis factor-induced Erk signaling by Src family kinases and the T cell protein tyrosine phosphatase. Nat. Immunol. 6:253–260 [DOI] [PubMed] [Google Scholar]
- 29. Fukushima A, Loh K, Galic S, Fam B, Shields B, Wiede F, Tremblay ML, Watt MJ, Andrikopoulos S, Tiganis T. 2010. T-cell protein tyrosine phosphatase attenuates STAT3 and insulin signaling in the liver to regulate gluconeogenesis. Diabetes 59:1906–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loh K, Fukushima A, Zhang X, Galic S, Briggs D, Enriori PJ, Simonds S, Wiede F, Reichenbach A, Hauser C, Sims NA, Bence KK, Zhang S, Zhang ZY, Kahn BB, Neel BG, Andrews ZB, Cowley MA, Tiganis T. 2011. Elevated hypothalamic TCPTP in obesity contributes to cellular leptin resistance. Cell Metab. 14:684–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kleppe M, Lahortiga I, El Chaar T, De Keersmaecker K, Mentens N, Graux C, Van Roosbroeck K, Ferrando AA, Langerak AW, Meijerink JP, Sigaux F, Haferlach T, Wlodarska I, Vandenberghe P, Soulier J, Cools J. 2010. Deletion of the protein tyrosine phosphatase gene PTPN2 in T-cell acute lymphoblastic leukemia. Nat. Genet. 42:530–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kleppe M, Soulier J, Asnafi V, Mentens N, Hornakova T, Knoops L, Constantinescu S, Sigaux F, Meijerink JP, Vandenberghe P, Tartaglia M, Foa R, Macintyre E, Haferlach T, Cools J. 2011. PTPN2 negatively regulates oncogenic JAK1 in T-cell acute lymphoblastic leukemia. Blood 117:7090–7098 [DOI] [PubMed] [Google Scholar]
- 33. Wiede F, Hui Chew S, van Vliet C, Poulton IJ, Kyparissoudis K, Sasmono T, Loh K, Tremblay ML, Godfrey DI, Sims NA, Tiganis T. 2012. Strain-dependent differences in bone development, myeloid hyperplasia, morbidity and mortality in Ptpn2-deficient mice. PlosOne 7:e36703 doi:10.1371/journal.pone.0036703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galic S, Hauser C, Kahn BB, Haj FG, Neel BG, Tonks NK, Tiganis T. 2005. Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Mol. Cell. Biol. 25:819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu HJ, Iaria J, Sizeland AM. 1999. Smad7 differentially regulates transforming growth factor beta-mediated signaling pathways. J. Biol. Chem. 274:32258–32264 [DOI] [PubMed] [Google Scholar]
- 36. Aceto N, Sausgruber N, Brinkhaus H, Gaidatzis D, Martiny-Baron G, Mazzarol G, Confalonieri S, Quarto M, Hu G, Balwierz PJ, Pachkov M, Elledge SJ, van Nimwegen E, Stadler MB, Bentires-Alj M. 2012. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat. Med. 18:529–537 [DOI] [PubMed] [Google Scholar]
- 37. Bentires-Alj M, Neel BG. 2007. Protein-tyrosine phosphatase 1B is required for HER2/Neu-induced breast cancer. Cancer Res. 67:2420–2424 [DOI] [PubMed] [Google Scholar]
- 38. Julien SG, Dube N, Read M, Penney J, Paquet M, Han Y, Kennedy BP, Muller WJ, Tremblay ML. 2007. Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nat. Genet. 39:338–346 [DOI] [PubMed] [Google Scholar]
- 39. Shields BJ, Court NW, Hauser C, Bukczynska PE, Tiganis T. 2008. Cell cycle-dependent regulation of SFK, JAK1 and STAT3 signaling by the protein tyrosine phosphatase TCPTP. Cell Cycle 7:3405–3416 [DOI] [PubMed] [Google Scholar]
- 40. Brisken C, Kaur S, Chavarria TE, Binart N, Sutherland RL, Weinberg RA, Kelly PA, Ormandy CJ. 1999. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev. Biol. 210:96–106 [DOI] [PubMed] [Google Scholar]
- 41. Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR, Cameron PM, Meinke PT, Liverton N, Weng Y, DeMartino JA. 2002. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg. Med. Chem. Lett. 12:1219–1223 [DOI] [PubMed] [Google Scholar]
- 42. Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. 2000. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol. Cell. Biol. 20:9018–9027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chapman RS, Lourenco PC, Tonner E, Flint DJ, Selbert S, Takeda K, Akira S, Clarke AR, Watson CJ. 1999. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 13:2604–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kritikou EA, Sharkey A, Abell K, Came PJ, Anderson E, Clarkson RW, Watson CJ. 2003. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development 130:3459–3468 [DOI] [PubMed] [Google Scholar]
- 45. Sutherland KD, Lindeman GJ, Choong DY, Wittlin S, Brentzell L, Phillips W, Campbell IG, Visvader JE. 2004. Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene 23:7726–7733 [DOI] [PubMed] [Google Scholar]
- 46. Bertucci F, Finetti P, Cervera N, Charafe-Jauffret E, Buttarelli M, Jacquemier J, Chaffanet M, Maraninchi D, Viens P, Birnbaum D. 2009. How different are luminal A and basal breast cancers? Int. J. Cancer 124:1338–1348 [DOI] [PubMed] [Google Scholar]
- 47. D'Anello L, Sansone P, Storci G, Mitrugno V, D'Uva G, Chieco P, Bonafe M. 2010. Epigenetic control of the basal-like gene expression profile via Interleukin-6 in breast cancer cells. Mol. Cancer 9:300 doi:10.1186/1476-4598-9-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. 2010. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol. Cell 39:493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rokavec M, Wu W, Luo JL. 2012. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol. Cell 45:777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bjorge JD, Pang A, Fujita DJ. 2000. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. J. Biol. Chem. 275:41439–41446 [DOI] [PubMed] [Google Scholar]
- 51. Wiener Jr, Kerns BJ, Harvey EL, Conaway MR, Iglehart JD, Berchuck A, Bast RC., Jr 1994. Overexpression of the protein tyrosine phosphatase PTP1B in human breast cancer: association with p185c-erbB-2 protein expression. J. Natl. Cancer Inst. 86:372–378 [DOI] [PubMed] [Google Scholar]
- 52. Ruivenkamp C, Hermsen M, Postma C, Klous A, Baak J, Meijer G, Demant P. 2003. LOH of PTPRJ occurs early in colorectal cancer and is associated with chromosomal loss of 18q12-21. Oncogene 22:3472–3474 [DOI] [PubMed] [Google Scholar]
- 53. Sun T, Aceto N, Meerbrey KL, Kessler JD, Zhou C, Migliaccio I, Nguyen DX, Pavlova NN, Botero M, Huang J, Bernardi RJ, Schmitt E, Hu G, Li MZ, Dephoure N, Gygi SP, Rao M, Creighton CJ, Hilsenbeck SG, Shaw CA, Muzny D, Gibbs RA, Wheeler DA, Osborne CK, Schiff R, Bentires-Alj M, Elledge SJ, Westbrook TF. 2011. Activation of multiple proto-oncogenic tyrosine kinases in breast cancer via loss of the PTPN12 phosphatase. Cell 144:703–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wellcome Trust Case Control Consortium 2007. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447:661–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Motiwala T, Kutay H, Ghoshal K, Bai S, Seimiya H, Tsuruo T, Suster S, Morrison C, Jacob ST. 2004. Protein tyrosine phosphatase receptor-type O (PTPRO) exhibits characteristics of a candidate tumor suppressor in human lung cancer. Proc. Natl. Acad. Sci. U. S. A. 101:13844–13849 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]