Abstract
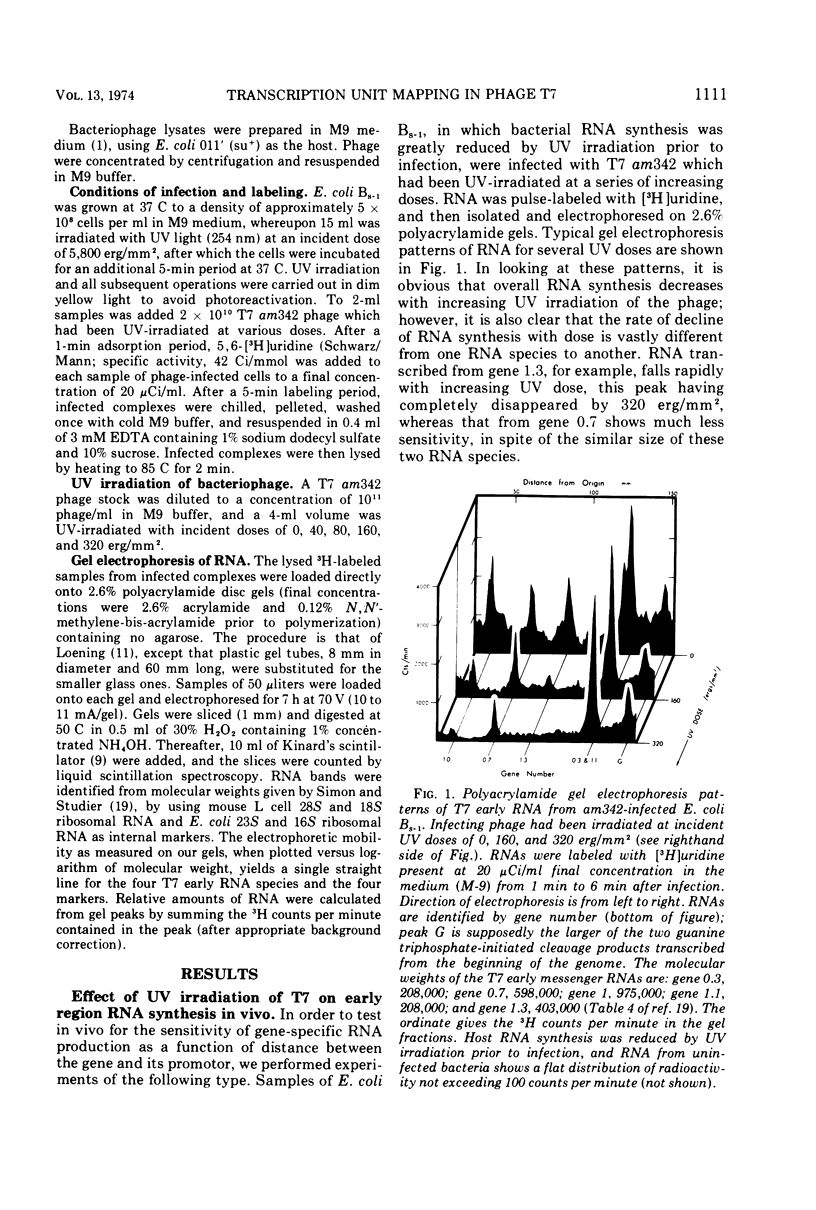
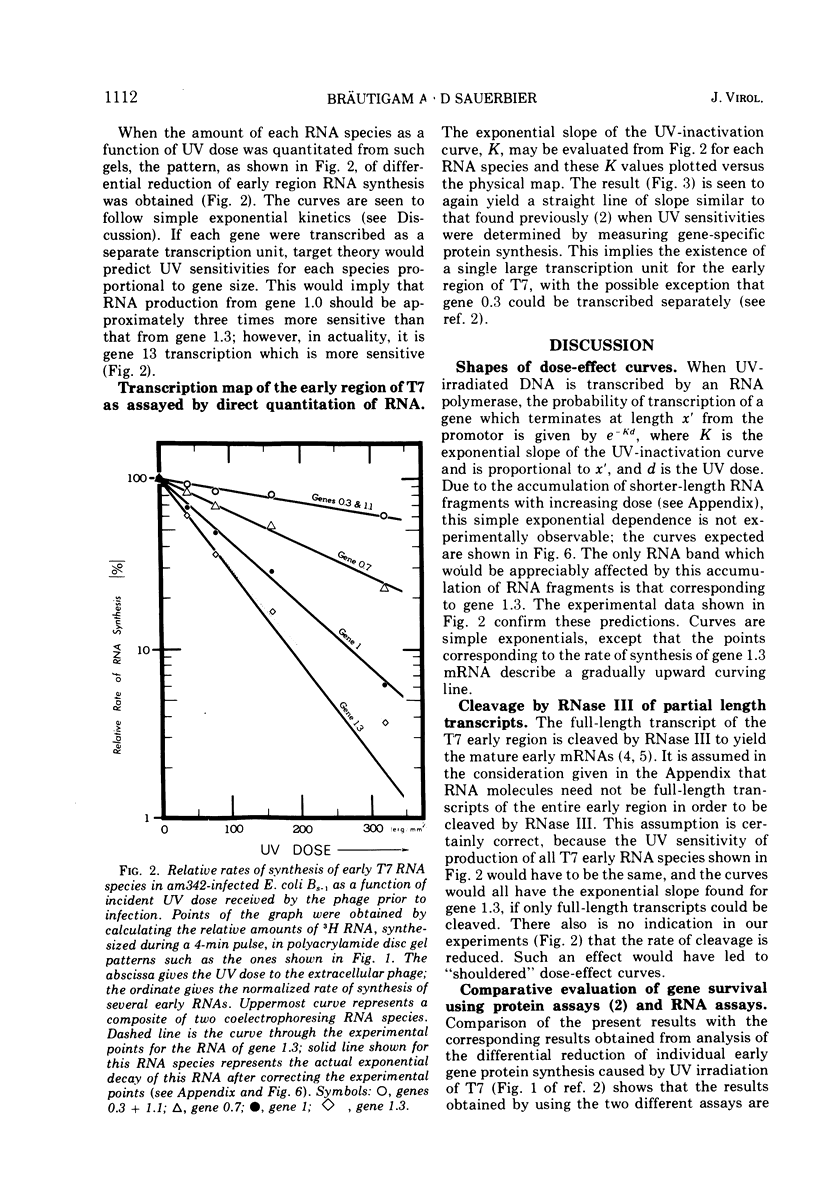
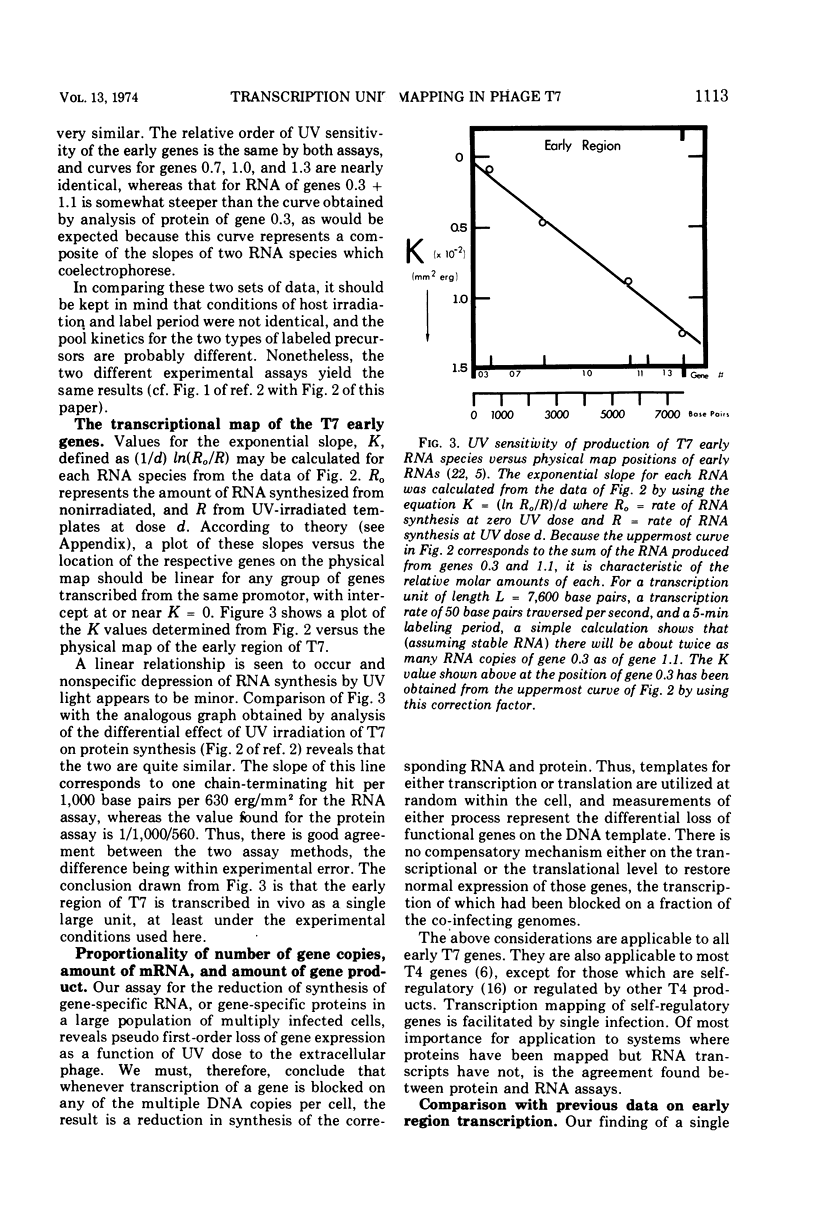
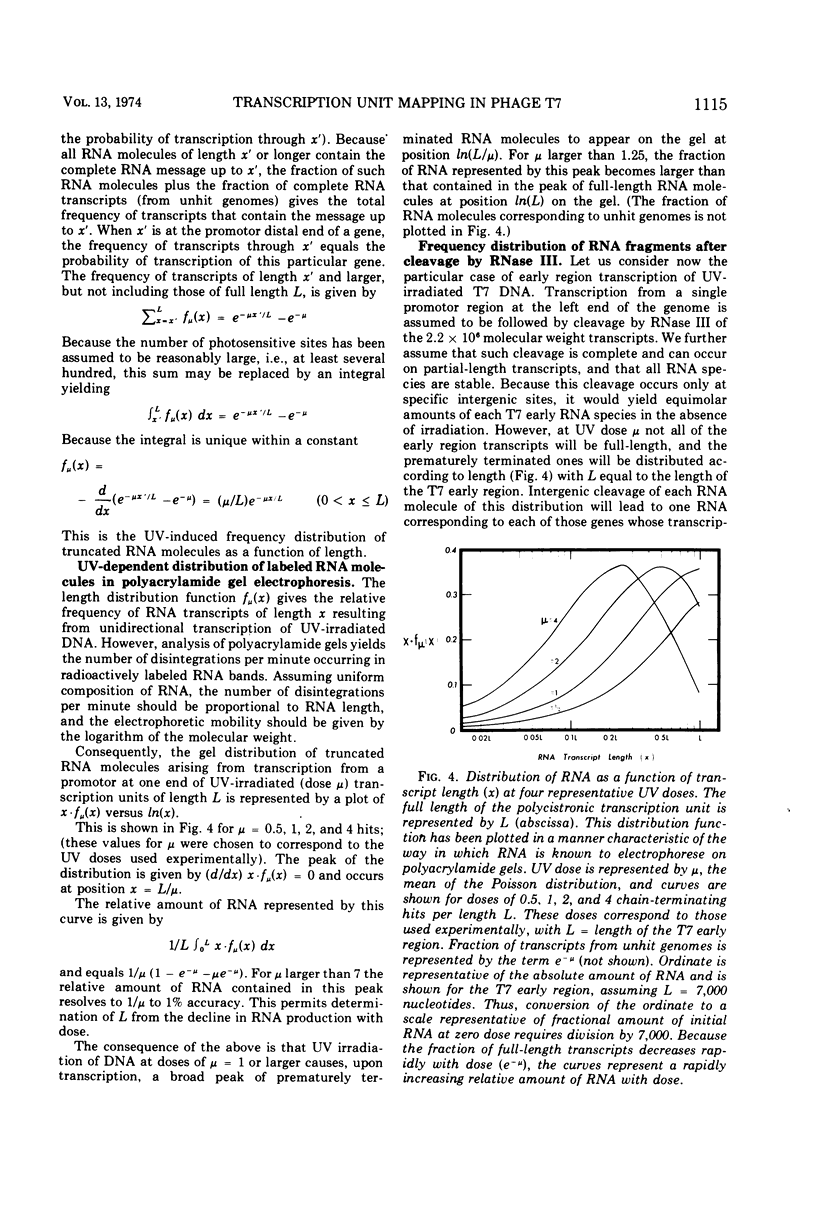
The effect of UV irradiation of bacteriophage T7 on in vivo early RNA synthesis has been studied by direct quantitation of the gene-specific RNA transcripts. The results show that the early region of phage T7 is transcribed from left to right as a single unit. Furthermore, gene inactivation, the UV sensitivity of synthesis of gene-specific RNA, and the UV sensitivity of synthesis of the corresponding proteins all follow pseudo first-order kinetics in multiply infected cells, demonstrating a random statistical correlation between both transcriptional sampling of gene copies and translational sampling of the resultant RNA transcripts. In addition, these simple kinetics imply an absence of positive feedback mechanisms compensating for the differential decline of individual early gene products in cells multiply infected with phage T7.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A. R., Sauerbier W. Transcription unit mapping in bacteriophage T7. I. In vivo transcription by Escherichia coli RNA polymerase. J Virol. 1973 Oct;12(4):882–886. doi: 10.1128/jvi.12.4.882-886.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Sauerbier W. Transcription units in bacteriophage T4. J Virol. 1973 Oct;12(4):872–881. doi: 10.1128/jvi.12.4.872-881.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Keil T. U., Hofschneider P. H. Secondary structure of RNA phage M12 replicative intermediates in vivo. Biochim Biophys Acta. 1973 Jun 23;312(2):297–310. doi: 10.1016/0005-2787(73)90375-4. [DOI] [PubMed] [Google Scholar]
- Le Talaer J. Y., Kermici M., Jeanteur P. Isolation of Escherichia coli RNA polymerase binding sites on T5 and T7 DNA: further evidence for sigma-dependent recognition of A-T-rich DNA sequences. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2911–2915. doi: 10.1073/pnas.70.10.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. L., Yanofsky C. Host and bacteriophage specific messenger RNA degradation in T7-infected Escherichia coli. Nat New Biol. 1971 Dec 8;234(49):168–170. doi: 10.1038/newbio234168a0. [DOI] [PubMed] [Google Scholar]
- Michalke H., Bremer H. RNA synthesis in Escherichia coli after irradiation with ultraviolet light. J Mol Biol. 1969 Apr 14;41(1):1–23. doi: 10.1016/0022-2836(69)90122-3. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Russel M. Control of bacteriophage T4 DNA polymerase synthesis. J Mol Biol. 1973 Sep 5;79(1):83–94. doi: 10.1016/0022-2836(73)90271-4. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Millette R. L., Hackett P. B., Jr The effects of ultraviolet irradiation on the transcription of T4 DNA. Biochim Biophys Acta. 1970;209(2):368–386. doi: 10.1016/0005-2787(70)90735-5. [DOI] [PubMed] [Google Scholar]
- Schweiger M., Herrlich P., Millette R. L. Gene expression in vitro from deoxyribonucleic acid of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6707–6712. [PubMed] [Google Scholar]
- Simon M. N., Studier F. W. Physical mapping of the early region of bacteriophage T7 DNA. J Mol Biol. 1973 Sep 15;79(2):249–265. doi: 10.1016/0022-2836(73)90004-1. [DOI] [PubMed] [Google Scholar]
- Stevens A. Studies of DNA-dependent RNA polymerase. J Cell Physiol. 1969 Oct;74(2 Suppl):205+–205+. doi: 10.1002/jcp.1040740421. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Brunovskis I., Hyman R. W. The process of infection with coliphage T7. VII. Characterization and mapping of the major in vivo transcription products of the early region. J Mol Biol. 1973 Mar 5;74(3):291–300. doi: 10.1016/0022-2836(73)90374-4. [DOI] [PubMed] [Google Scholar]
- Young R. J., Smith G. The end groups of T7 mRNA. Biochem Biophys Res Commun. 1973 Aug 6;53(3):952–959. doi: 10.1016/0006-291x(73)90184-8. [DOI] [PubMed] [Google Scholar]



