Abstract
Purpose
The presence of multidrug resistance-associated protein (MRP) in cancer cells is known to be responsible for many therapeutic failures in current oncological treatments. Here, we show that the combination of different effectors like hyperthermia, iron oxide nanoparticles, and chemotherapeutics influences expression of MRP 1 and 3 in an adenocarcinoma cell line.
Methods
BT-474 cells were treated with magnetic nanoparticles (MNP; 1.5 to 150 μg Fe/cm2) or mitomycin C (up to 1.5 μg/cm2, 24 hours) in the presence or absence of hyperthermia (43°C, 15 to 120 minutes). Moreover, cells were also sequentially exposed to these effectors (MNP, hyperthermia, and mitomycin C). After cell harvesting, mRNA was extracted and analyzed via reverse transcription polymerase chain reaction. Additionally, membrane protein was isolated and analyzed via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
Results
When cells were exposed to the effectors alone or to combinations thereof, no effects on MRP 1 and 3 mRNA expression were observed. In contrast, membrane protein expression was influenced in a selective manner. The effects on MRP 3 expression were less pronounced compared with MRP 1. Treatment with mitomycin C decreased MRP expression at high concentrations and hyperthermia intensified these effects. In contrast, the presence of MNP only increased MRP 1 and 3 expression, and hyperthermia reversed these effects. When combining hyperthermia, magnetic nanoparticles, and mitomycin C, no further suppression of MRP expression was observed in comparison with the respective dual treatment modalities.
Discussion
The different MRP 1 and 3 expression levels are not associated with de novo mRNA expression, but rather with an altered translocation of MRP 1 and 3 to the cell membrane as a result of reactive oxygen species production, and with shifting of intracellular MRP storage pools, changes in membrane fluidity, etc, at the protein level. Our results could be used to develop new treatment strategies by repressing mechanisms that actively export drugs from the target cell, thereby improving the therapeutic outcome in oncology.
Keywords: magnetic nanoparticles, hyperthermia, chemotherapy, drugs, MDR, MRP, cancer, nanotechnology, iron oxide
Introduction
Even though the therapeutic outcome of chemotherapeutic treatments has considerably improved over recent years, one of the main reasons for failure is associated with the presence of multidrug resistance-associated protein (MRP) in cancer cells. These circumstances call for the development of new and innovative therapeutic strategies to effectively circumvent export of drugs from the target cells.
From the cell biological point of view, MRP is allocated to the adenosine triphosphate (ATP)-binding cassette transporters (ABC transporters), which are currently subdivided into seven subfamilies (ABC transporters A to G).1 By hydrolysis of ATP, this protein family carries proteins and endo- and xenobiotica out of the cells, thereby protecting them from harmful effects.2 Their substrate specificity is rather low and varies in terms of structure, functionality, and size of the substrate.3 Among the members of this family, the MDR 1 protein was discovered first and is known to be prominently expressed in proliferating tissues, as well as in cells with a specific barrier function.4 The protein is considered to be one of the main ubiquitary transporters for endo- and xenobiotics.5 Another multidrug resistance protein, MRP 3, is also being discussed as being distinctly associated with multiple resistance to drugs.6 Its expression is highly upregulated in drug-resistant breast cancer cells, even though the knowledge of its particular physiological function is currently limited to upregulation of cholestasis.7
Among the currently proposed and available therapeutic modalities, hyperthermia has been considered with increasing interest in the last few years. In this modality, heating is used to inactivate tumor cells. Some researchers have found that hyperthermic temperatures between 42°C to 45°C induced necrosis and apoptosis in cells,8,9 destabilized cell membrane integrity, induced protein denaturation, and inactivated DNA repair systems, etc.10–12 In numerous cases synergistic effects have been observed when combined with chemotherapeutic drugs.13 In contrast to the increased knowledge of the effects of hyperthermia at the molecular level, little is known about the impact on the expression of MRP.
A similar situation is encountered in relation to the exposure of cancer cells to magnetic nanoparticles and multidrug resistance expression. Different nanoparticle formulations (metallic iron oxides, liposomes, polymers, and others), sizes and configurations (eg, surface coatings) have been proposed for cancer treatments, by coupling chemotherapeutic drugs or by inducing magnetic heating.14,15 Commonly, they have been shown to be internalized by defined endocytotic processes and to be accumulated in the endolysosomal compartment without considering their effects on multidrug resistance.
To provide new insights on the mechanistic effects of multidrug resistance and how they could be used to prevent future therapeutic failures due to active export of drugs from the cell, we sought to shed some light on the effects of MRP 1 and MRP 3 expression in a human breast adenocarcinoma cell line when exposing it to the effectors of hyperthermia, magnetic nanoparticles, and a chemotherapeutic drug (mitomycin C) as a single modality and selected combinations thereof. We will show that their impact on MRP 1 and 3 expression occurs in a very selective manner.
Materials and methods
Materials
The human breast adenocarcinoma cell line BT-474 (Cell Lines Service, Eppelheim, Germany) was used for the experiments. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) cell culture medium (Invitrogen, Karlsruhe, Germany) containing fetal calf serum (10% [v/v]; GIBCO BRL, Paisley, Scotland) at 37°C in a 5% CO2 atmosphere. Determination of cell numbers was carried out using a cell counter (CASY TT; Innovatis AG, Reutlingen, Germany). Iron oxide nanoparticles with dextran coating (FluidMAG-DX) were obtained from Chemicell GmbH (Berlin, Germany). Mitomycin C was purchased from Applichem GmbH (Darmstadt, Germany).
Characterization of magnetic nanoparticles
The morphologic and magnetic features of the nanoparticles were assessed by utilization of vibration magnetometry and photon correlation spectroscopy in order to determine core diameter, hydrodynamic diameter, and zeta-potential. Samples were measured in DMEM with and without serum proteins (10% [v/v]) to elucidate potential effects from opsonisation.
Exposure of cells to hyperthermia, magnetic nanoparticles, and mitomycin C
BT474 cells were seeded onto culture dishes and allowed to grow until reaching a confluency of 80% to 90% of the available grow matrix surface. To assess the effects of hyperthermia, the old culture medium was replaced by fresh medium and the cells were incubated at 43°C (incubator, 5% CO2, 95% humidity) for 15 to 120 minutes. After replacing the culture medium, the cells were allowed to recover up to 48 hours postincubation time. The effects of mitomycin C were elucidated by incubating the cells with 0.15 and 1.5 μg/cm2 mitomycin C for 24 hours, and to examine the combinatorial effect of hyperthermia, the cells were firstly treated at temperatures of 43°C for 90 minutes, followed by incubation with mitomycin C. The impact of magnetic nanoparticles on MRP expression was elucidated by treatment of cells with nanoparticles (1.5 to 150 μg Fe/cm2) for 24 hours in the presence or absence of hyperthermia (43°C for 90 minutes after nanoparticle exposure). Finally, the combined treatment of cells (triple modality) was studied by treating the cells first with magnetic nanoparticles (up to 150 μg Fe/cm2, 24 hours), then with hyperthermia (43 °C, 90 minutes), and finally with mitomycin C (1.5 m/cm2, 24 hours). Nontreated cell populations were used as controls for normal MRP expression in BT474 cells. After finalization of each treatment protocol, cells were detached from the growing substrate, counted and processed as described below.
MRP 1 and MRP 3 mRNA expression
To determine MRP 1 and MRP 3 mRNA expression, the cells were lysed mRNA was isolated and transcribed to cDNA by the use of a high purity RNA isolation kit from Roche Diagnostics GmbH (Mannheim, Germany) and the Omniscript RT Kit (Qiagen, Hilden, Germany), respectively, according to the instructions of the manufacturer. Then, reverse-transcription polymerase chain reaction (RT-PCR) was performed using the following primers: MRP 1 forward: 5′-ACCAAGACGTATCAGGTGGCC-3′, reverse: 5′-CTGTCTGGGCATCCAGGAT-3′ (286 bp); MRP 3 forward: 5′-ACACGTTTGTGAGCTCCCAG-3′, reverse: 5′-GCAATGAGGTTGGCTGGAGAAT-3′ (322 bp). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as internal standard the negative control consisted of water instead of cDNA. Transcripts were identified by agarose gel electrophoresis (1.5% [w/v] agarose gel in Trisacetate-EDTA buffer containing ethidium bromide). PCR products were detected using the Imagemaster VDS (Pharmacia Biotech, Freiburg, Germany). The program Gene Tools (Syngene, Cambridge, UK) was used for semi-quantitative analysis of the intensity of the bands, which were proportional to the number of molecules. Hereto, regions of interest were placed on the respective bands corresponding to MRP 1 and 3 (corresponding to 286 and 322 bp, respectively) and GAPDH and intensities were normalized to nontreated controls.
MRP 1 and MRP 3 protein expression
To elucidate the role of MRP 1 and MRP 3 on the protein level, a modified protocol from Vellonen et al16 was used. Briefly, 7 × 106 BT474 cells were suspended in a protein lysis buffer (1% [v/v] Triton X-100, 20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA) for 30 minutes on ice and centrifuged for 30 minutes at 10,000 g (4°C) to decant cell detritus and nuclei. Proteins in the supernatants were used for protein quantification using the QuantiPro BCA Assay kit (Sigma-Aldrich GmbH, Munich, Germany) according to the instructions of the manufacturer. Afterwards, 20 μg protein per sample were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 4.3% (w/v) stacking and a 10% (w/v) separating SDS-gel. GAPDH was used as an internal loading control. Electrophoresis was carried out at 120 V. MRP 1 and 3 protein bands were identified via immunoblotting using an MRP 1 antibody (1:2,000, clone QCRL-; Sigma-Aldrich GmbH), an MRP 3 antibody (1:2,000, M3II-21; Abcam, Cambridge, UK), or an GAPDH antibody (1:1,000, sc-25778; Santa Cruz Biotechnology, Heidelberg, Germany) and a secondary antibody conjugated to horse radish peroxidase (1:10,000; Jackson ImmunoResearch, West Grove, PA, USA). Semiquantitative analysis of the band intensity (corresponding to 190 kDa for MRP 1 and 3 according to Kruh et al)17 was performed as described above.
Statistics
All investigations related to the determination of the MRP 1 and 3 expression profile on mRNA or protein level were performed in duplicate. Values were depicted as bars indicating the mean value and symbols to show the variability. To determine whether the findings from the first experiment could be corroborated by the second one, a Spearman correlation coefficient between both experiments was determined. Correlation coefficients with values higher than 0.5 were considered to indicate a good reproducibility.
Results
Our data show, for the first time, that the exposure of human BT-474 adenocarcinoma cells to hyperthermia, mitomycin C, and magnetic nanoparticles has an influence on the membrane MRP expression in a very selective manner. The effects were shown to be particularly prominent for MRP 1 compared with MRP 3. In contrast, mRNA expression was much less sensitive.
Effects of hyperthermia on MRP expression profile
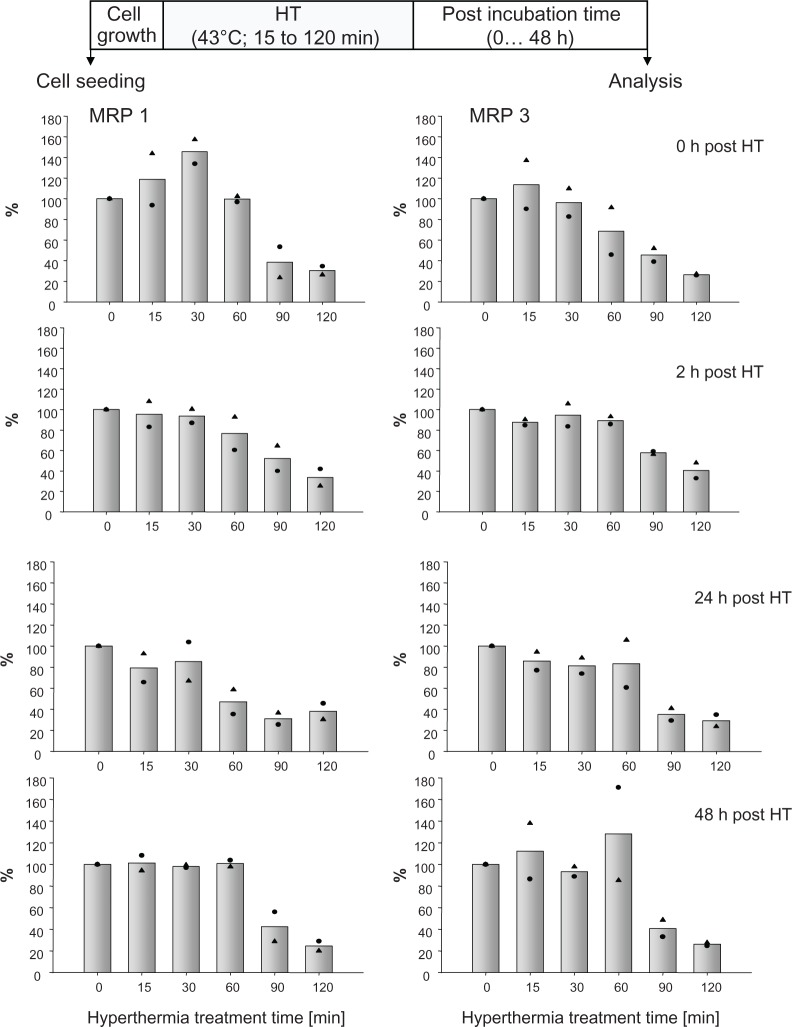
Hyperthermia alone (43°C, 15 to 120 minutes exposure time) induced no changes in MRP 1 and 3 mRNA expression profiles compared with nontreated controls. Also long-term effects on MRP mRNA expression (up to 48 hours after hyperthermic treatment) were absent (data not shown). On the protein expression level, short hyperthermia treatments (15 to 30 minutes) led immediately to an increased membrane MRP 1 expression, which disappeared with increasing post-treatment incubation times. Longer hyperthermic treatments (90 to 120 minutes) induced a marked decrease in membrane MRP 1 expression, which turned out to be lower than 50% compared with nontreated controls. Low MRP 1 expression profiles persisted with ongoing time after treatments (up to 48 hours posthyperthermia). Similar but comparatively lower effects were encountered for MRP 3 (Figure 1).
Figure 1.
Hyperthermia treatment times influence MRP protein expression and corresponding effects remain unchanged up to 48 hours thereafter.
Notes: After exposure to 43°C (0 to 120 minutes), BT474 cells were allowed to recover for defined periods in time (0 to 48 hours). Then, cell membrane protein was isolated, SDS-PAGE and immunoblotting was performed. Semiquantitative analysis of MRP 1 and 3 protein bands on immunoblots (190 kDa). For details see Methods. Bars indicate mean of two independent experiments, both obtained experimental values are indicated by symbols.
Abbreviations: HT, hyperthermia; MRP, multidrug resistance protein; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Effects of mitomycin C on MRP expression profile
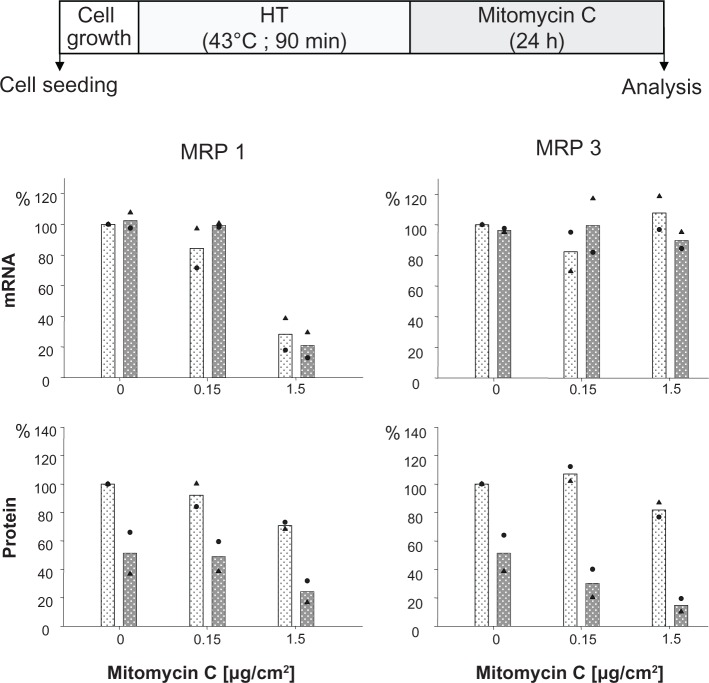
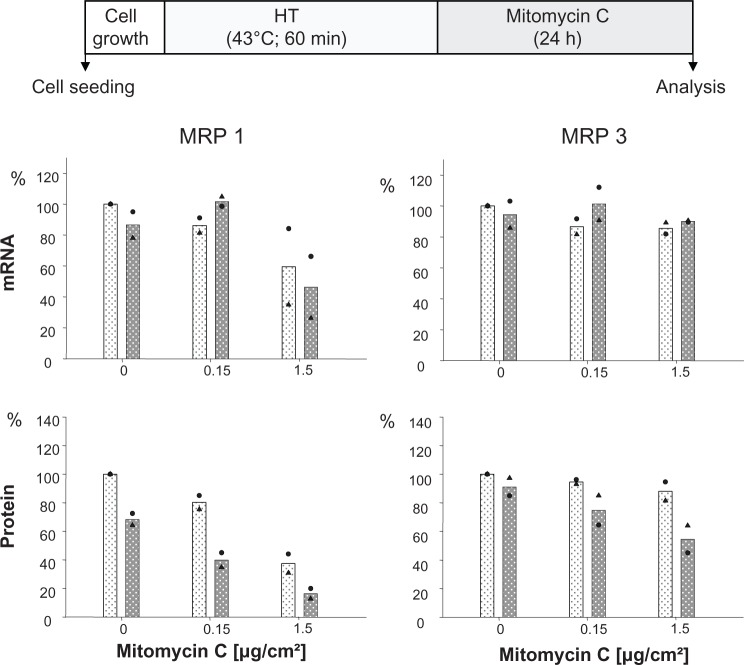
The presence of mitomycin C alone induced a decrease of MRP 1 mRNA expression at higher drug concentrations (1.5 μg/cm² for 24 hours), while MRP 3 remained unaffected. The additional treatment of cells via hyperthermia (43°C, 90 minutes) prior to mitomycin C exposure did not markedly influence this mRNA expression pattern. At the protein expression level, presence of membrane MRP 1 and 3 was diminished in a drug concentration dependent manner (0.15 and 1.5 μg/cm2 mito-mycin C for 24 hours) under the same experimental conditions. Interestingly, the observed reduction of membrane MRP 1 expression was distinctly boosted by combination with a hyperthermic treatment of the cells (43°C, 90 minutes) (Figure 2). On the other hand, only a minor reversal of MRP expression was visible if one shortens the hyperthermia treatment time window from 90 to 60 minutes (Figure 1S).
Figure 2.
Mitomycin C concentration influences MRP 1 and MRP 3 expression pattern and hyperthermia intensifies these effects.
Notes: After treating with hyperthermia (43°C, 90 minutes), BT474 cells were exposed to mitomycin C (up to 1.5 μg/cm2 for 24 hours). Immediately afterwards, mRNA and protein were isolated and finally RT-PCR and immunoblotting were performed. Semiquantitative analysis of MRP 1 and 3 specific PCR products (286 and 322 bp, respectively) separated via agarose gel electrophoresis (top panel) as well as of corresponding MRP-specific protein bands on immunoblots (190 kDa) (bottom panel). For details see Methods. Expression was given in per cent of nontreated controls. All data were additionally normalized to GAPDH. Bars indicate mean of two independent experiments, both experimental values are indicated by symbols. Light and dark bars: cells without and with hyperthermic treatment, respectively.
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HT, hyperthermia; MRP, multidrug resistance protein; RT-PCR, reverse transcription polymerase chain reaction.
Figure 1S.
Mitomycin C concentration influences MRP 1 and MRP 3 expression pattern and hyperthermia treatment (43°C) for 60 instead of 90 minutes also intensifies these effects, but to a lesser extent compared to 90 min (see Figure 4).
Notes: After treatment with hyperthermia (43°C, 60 min), BT474 cells were exposed to mitomycin C (up to 1.5 μg Fe for 24 hours) and incubated for another 24 hours. mRNA and protein were isolated, and finally RT-PCR or SDS-PAGE/immunoblotting were performed. Semiquantitative analysis of MRP 1 and 3 specific PCR products (286 and 322 bp, respectively) separated via agarose gel electrophoresis (top panel) as well as of corresponding MRP-specific protein bands on immunoblots (190 kDa) (bottom panel). For details see Methods. Expression was given in per cent of nontreated controls. All data were additionally normalized to GAPDH. Bars indicate mean of two independent experiments, corresponding values are indicated by symbols. Light and dark bars: cells without and with hyperthermic treatment, respectively.
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HT, hyperthermia; MRP, multidrug resistance protein; RT-PCR, reverse transcription polymerase chain reaction; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
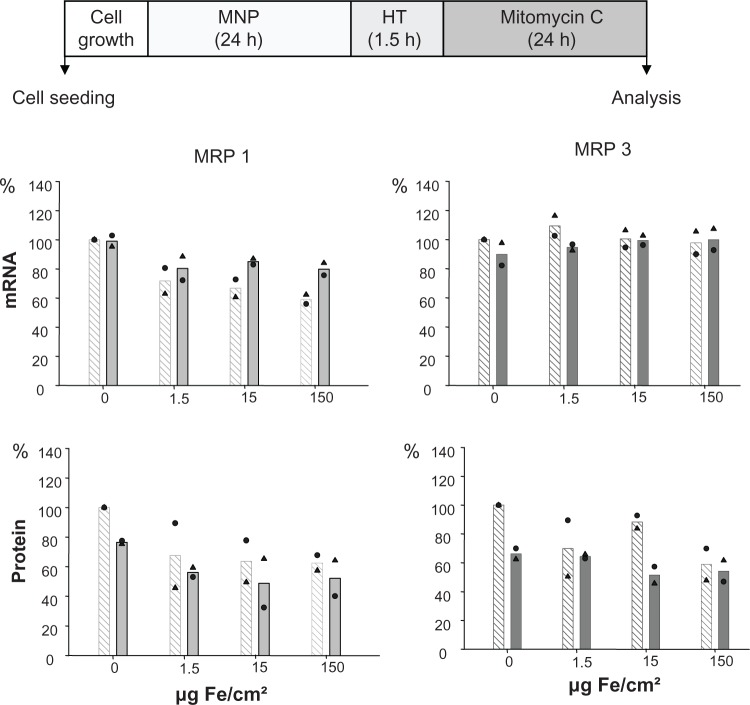
Combination of nanoparticle exposure with hyperthermia
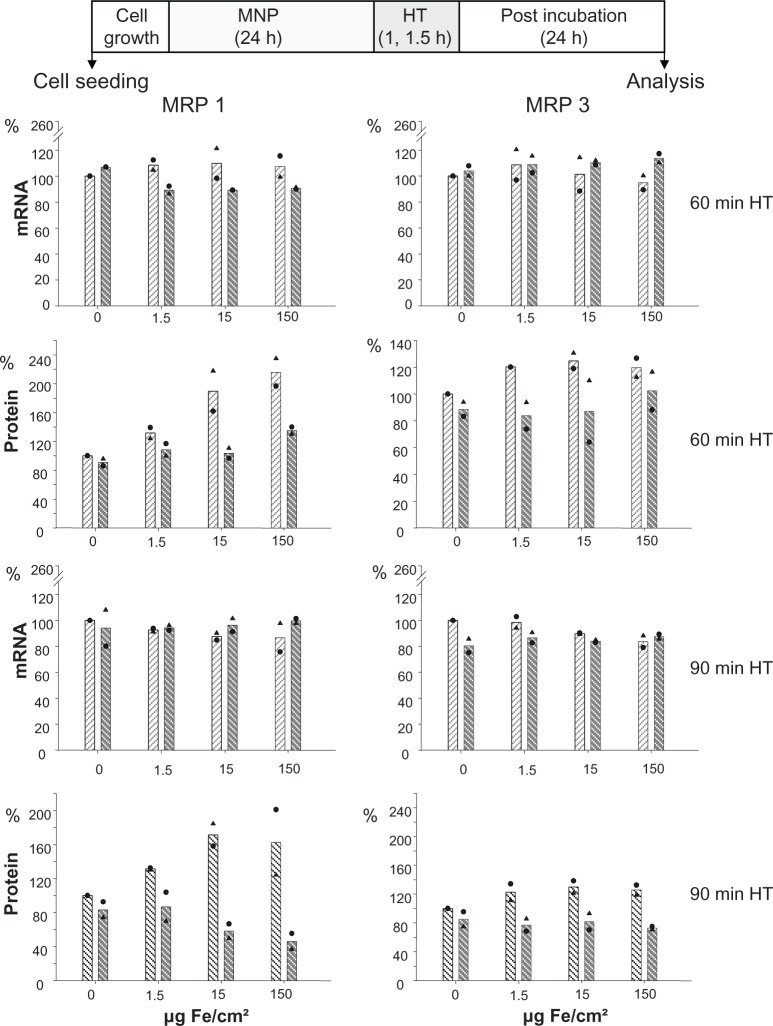
The nanoparticles used in the present study exhibit a core and hydrodynamic diameter of 10 and 150 nm, respectively. Moreover, interactions with serum proteins were rather low as indicated by corresponding measurements (Table 1). The exposure of cells to both magnetic nanoparticles and hyperthermia did not affect MRP mRNA expression in BT-474 cells, independently of the amount of nanoparticles the cells were exposed to (1.5 to 150 Fe/cm² for 24 hours; Figure 3). As far as protein levels are concerned, again, a distinctly increased membrane MRP 1 and 3 protein expression with a marked nanoparticle concentration dependency, particularly for MRP 1, was observed. An additional hyperthermic treatment reversed these effects and, interestingly, the previously observed nanoparticle concentration dependency was abolished (1.5 to 150 Fe/cm² for 24 hours). In contrast, increase of MRP 3 in presence of nanoparticles was only moderate, and combination with hyperthermia induced a reversal to the control level. All these observations apply for 24 hours postincubation time after finalization of treatments (Figure 3).
Table 1.
Morphological features of the magnetic nanoparticles used in the present study. Culture medium: Dulbecco’s modified Eagle’s medium with 10% (v/v) fetal calf serum
| Suspension conditions | Core diameter (nm) | Hydrodynamic diameter (nm) | Zeta-potential (mV) |
|---|---|---|---|
| Aqua destillata | 10 ± 0.4 | 163 ± 0.7 | 2.8 ± 3.5 |
| Culture medium | – | 143 ± 1.2 | -2.2 ± 0.3 |
Figure 3.
Treatment of adenocarcinoma cells with magnetic nanoparticles increases and hyperthermia decreases MRP 1 to a higher extent than MRP 3 protein expression while mRNA expression remains unaltered.
Notes: There is no distinct benefit of a 90-minute over a 60-minute hyperthermia treatment time. After exposure to magnetic nanoparticles (up to 1.5 μg Fe/cm2 for 24 hours), BT-474 cells were treated with hyperthermia (43°C, 60 or 90 minutes), and allowed to grow for further 24 hours. Then, mRNA and protein were isolated, and finally RT-PCR and immunoblotting were performed. Semi-quantitative analysis of MRP 1 and 3 specific PCR products (286 and 322 bp, respectively) separated in agarose gel electrophoresis (top panel) and of corresponding MRP-specific protein bands in immunoblots (190 kDa) (bottom panel). Expression was given in per cent of nontreated controls. Further on, data were additionally normalized to GAPDH. Bars indicate mean of 2 independent experiments, both experimental values are indicated by symbols. Light and dark bars: cells without and with hyperthermic treatment, respectively.
Abbreviations: HT, hyperthermia; MRP, multidrug resistance protein; RT-PCR, reverse transcription polymerase chain reaction.
Effects of nanoparticle exposure in combination with hyperthermia and mitomycin C treatment
Figure 4 depicts the presence of MRP mRNA and protein immediately after finalization of the sequential exposure of cells first to nanoparticles, then to hyperthermia, and finally to mitomycin C. Hyperthermia did not further foster these effects. Interestingly, a decrease of membrane MRP 1 and 3 protein expression in the presence of nanoparticles was detectable, but without a concentration dependency (see controls without magnetic nanoparticles in Figure 4).
Figure 4.
MRP 1 and 3 expression pattern after combining treatment of cells with MNP, hyperthermia, and mitomycin C: a decrease in MRP 1 mRNA as well as MRP1 and MRP 3 protein expression takes place.
Notes: After exposure to magnetic nanoparticles (up to 150 μg Fe/cm2 for 22.5 hours), BT-474 cells were treated with hyperthermia (43°C, 90 minutes), followed by mitomycin C (1.5 μg/cm2, 24 hours). Afterwards, cells were immediately harvested (0 hours posthyperthermia), mRNA and protein were isolated, and finally RT-PCR or SDS-PAGE/immunoblotting were performed. Semiquantitative analysis of MRP 1 and 3 specific PCR products (286 and 322 bp, respectively) separated in agarose gel electrophoresis (top panel) and of corresponding MRP-specific protein bands in immunoblots (190 kDa) (bottom panel). Expression was given in percent of nontreated controls. Data was additionally normalized to GAPDH. Bars indicate mean of two independent experiments, both experimental values are indicated by symbols. Light and dark bars: cells without and with hyperthermic treatment, respectively.
Abbreviations: MRP, multidrug resistance protein; RT-PCR, reverse transcription polymerase chain reaction; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Discussion
Our data clearly show that hyperthermia with temperatures of 43°C and treatment times longer than 60 minutes induce a distinct decrease in the membrane MRP 1 and 3 protein expression pattern. The underlying molecular mechanisms for these observations are poorly understood. Currently, modifications of the protein expression level correlating with mRNA expression and transcription in relation to the p-glycoprotein are being discussed.18–22 In view of our data, this would imply that, in fact, a downregulation of mRNA expression would have anteceded modifications on the protein level. Nevertheless, the fact that none of the postincubation times showed any changes on the mRNA expression levels can be attributed either to short mRNA lifetimes or to an explicit absence of mRNA expression activity.
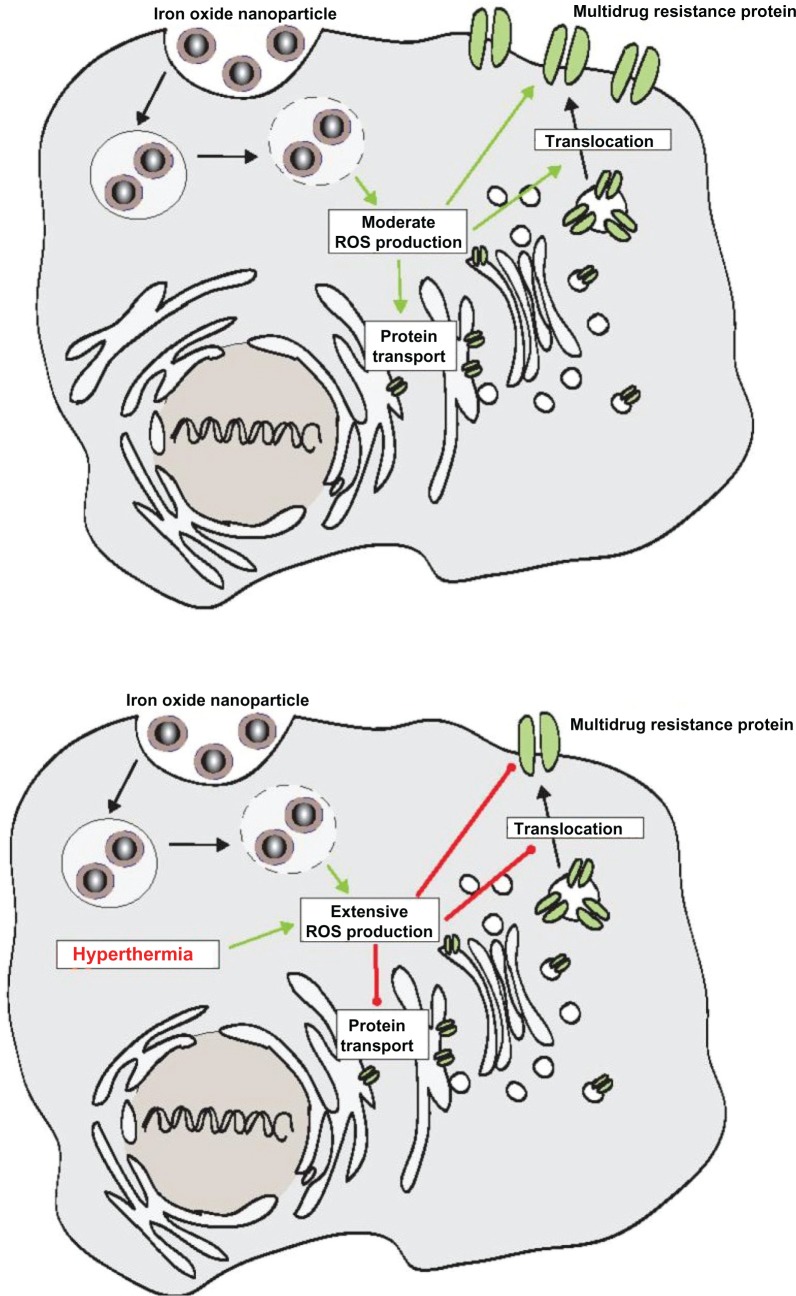
Three different mechanisms are possible, which could be responsible for the effects of hyperthermia on the membrane MRP protein expression profile in adenocarcinoma cells. It is known that ABC transporters contain redox-sensitive amino acids like cysteine which stabilize the whole protein molecule.23 Moreover, ROS-linked mechanisms have been associated with the MDR transporter p-glycoprotein.20,23–31 Low reactive oxygen species (ROS) levels upregulate,20,24 whereas high levels downregulate23,25,27–31 p-glycoprotein expression. Since an oxidative responsive element has also been detected in the proximity of its promoter, MRP 1 protein has also been associated with redox mechanisms.32 In relation to MRP 3, less is known in this context. In view of the present investigations, we hypothesize the occurrence of three mechanisms: first the initially observed increase of MRP 1 and 3 following up to 30 minutes of hyperthermia could be associated with low intracellular ROS levels. In the presence of high temperatures, intracellular ROS levels increase23,26 which accounts for the observed downregulation of MRP 1 and 3 during longer hyperthermic treatment times (from 60 minutes upwards). The second mechanism responsible for the observed effects on membrane MRP protein expression could be associated with shifting of subcellular MRP protein storage pools, an aspect which is presently discussed in relation to rapid changes in MRP protein expression profiles.33–36 The hypothesis is further reinforced by the fact that hyperthermic temperatures are responsible for a reduced incorporation of p-glycoprotein in membranes.37 These observations also imply that hyperthermic temperatures influence directly, possibly ROS-mediated, the incorporation of MRP 1 and 3 in the cell membrane. The third mechanism could be related to a modified stability of these proteins through increased cell membrane fluidity as a consequence of the administered heating stimulus. Interestingly, intracellular ROS production has been observed when cells were incubated with MNP.38–41 Taking these observations into account, we postulate that the presence of MNP produces only moderate intracellular ROS levels promoting the translocation of MDR proteins to the cellular surface. When ROS production is extensive, eg, as a consequence of an additional treatment with hyperthermia, MDR translocation to the cellular membrane will be decreased (Figure 5). Furthermore, inactivation of MRP activity in presence of hyperthermia is conceivable as well, since it has been observed in relation to the inactivation of DNA repair enzymes.42 Further detailed investigations are necessary to elucidate the hypothesis derived from our data.
Figure 5.
Scheme showing our hypothesis that the presence of MNP promotes translocation of MDR proteins to the cellular surface through a moderate ROS production (upper panel), whereas extensive ROS production (lower panel) resulting from the combination with hyperthermia decreases translocation of MDR proteins to the cellular membrane.
Abbreviations: MNP, magnetic nanoparticles; MDR, multidrug resistance; ROS, reactive oxygen species.
In future, these processes could be exploited to distinctly modulate intracellular retention of drug-loaded nanoparticles in tumor cells, leading to an increase in therapeutic efficacy. Obviously, the above-mentioned observations occur in viable cells, corroborating the fact that hyperthermia as a single tumor treatment modality (43°C up to 120 minutes) has a limited impact on tumor cell viability,43,44 which makes it necessary for heating cycles with other oncologic modalities. Furthermore, it was shown that repeated heating cycles could further strengthen the damages to already weakened cells. In this context, strong cell destruction was found via the use of repeated heating and cooling rates rather than heating at continuous temperatures.45,46
Interestingly, we noted only slight changes of hydrodynamic diameter and the zeta potential in presence of culture medium. These effects are associated with absorbtion of serum proteins to the nanoparticle surface.47 In connection with cellular uptake the same nanoparticle formulations were shown to be internalized in endolysosomes.48
We were also able to show, for the first time, that mitomycin C affects mRNA as well as the membrane MRP 1 and 3 protein expression. Such mechanisms are currently known for the p-glycoprotein only. For example, Ihnat et al49 and Maitra et al50 reported that mitomycin C induces decreased p-glycoprotein expression. The effects vary among different cell lines and are detectable between 24 and 96 hours after treatment with drugs, a fact which is in agreement with the observations made in the present study. Mitomycin C is known to induce alkylation, monoadducts, as well as DNA cross-linking via guanidine residues, and all these effects are lethal. Moreover, mitomycin C induces cross-linking on promoters of inducible genes.50,51 Comparable relationships could be associated with the inducible genes for MRP 1 and 3. The differential effects of MRP 1 and MRP 3 are possibly related to different basal expression levels of MRP 1 (ubiquitary) and MRP 3 (selective, predominantly in the liver) in adenocarcinoma cells as a consequence of different functionality. Also different sensitivity of MRP 1 and 3 in response to stress stimuli and/or different thresholds is conceivable.
The underlying reasons for mitomycin C-induced effects of MRP 1 and 3 could also be derived from findings on maturation and trafficking of MDR proteins.50 For example, Maitra et al observed a distinct increase of membrane p-glycoprotein directly after a 4-hour incubation time with mitomycin C.50 At the same time, the total p-glycoprotein level remained constant, and a decrease was not observed until 12 to 24 hours thereafter. In a similar way, mitomycin C could also have induced a redistribution of intracellular MDR protein storage pools which were not detectable in our study, since the incubation times employed were too long (24 hours) and MRP expression analyses were started immediately after finalization of treatments. In this context, our findings complement the present knowledge on the combined effects of hyperthermia and chemotherapeutic drugs.11,52
To the best of our knowledge, we are the first group to observe that magnetic nanoparticles have an impact on membrane MRP 1 and 3 protein expression. A few years ago, some groups reported that p-glycoprotein expression is not influenced in the presence of magnetic nanoparticles.53–55 At the same time, a free iron-induced expression of MDR proteins similar to p-glycoprotein56 could be excluded, since this would implicate modifications at the transcription level. Therefore, potential explanations for the observed upregulation of membrane MRP 1 and 3 expression can be attributed primarily to a protein translocation to the cell membrane as was observed for the p-glycoprotein in the presence of mitomycin C.50 Moreover, magnetic nanoparticles could have had an additional impact on membrane fluidity as a result of transient attachment to the glycocalyx, which could lead to conformational modification and stabilization of MRP 1 and 3 at normothermic conditions. Interestingly, as far as the p-glycoprotein is concerned, conformational modifications leading to a decreased affinity to ATP after modification of membrane fluidity is being investigated.54,57 This would mean that drugs coupled to nanoparticles are increasingly exocytosed, which limits their intracellular retention and therapeutic efficacy.
The combination of nanoparticle exposure with hyperthermia abolished the aforementioned increase of membrane MRP 1 and 3 protein expression, which apparently was not driven by changes at the mRNA level. These findings could not be associated with ROS-linked effects according to the corresponding regulative mechanisms associated with the p-glycoprotein described above. The underlying mechanisms remain to be elucidated. In view of potential nanoparticle-related therapeutic applications, hyperthermia will have beneficial effects on intracellular nanoparticle retention by reducing the MRP expression on the cellular membrane.
The sequential application of all three treatment modalities (nanoparticle exposure followed by hyperthermia and mitomycin C) led to a reduced expression of membrane MRP, but in this case hyperthermia did not further foster this effect as it was observed in relation to the dual combination modalities (hyperthermia vs mitomycin C or hyperthermia vs nanoparticle exposure). With respect to our hypothesis on ROS-mediated translocation of MRP to the cell surface, yet unknown, but highly specific interrelations driven by the different modalities take place that results in a nonlinear MRP transport. Further investigations are necessary to completely elucidate these aspects. Additionally, it remains to be shown to what extent the attachment of mitomycin C molecules to nanoparticles could further reduce membrane MRP expression and kill tumor cells as a result of an increased intracellular accumulation of drugs after inhibition of MRP-driven efflux mechanisms. The elucidated effects apply to the exposure of cells to heating from “outside”. Further studies should enlighten potential effects when heating is produced from nanoparticles accumulated inside the cells (intracellular hyperthermia).
In summary, we were able to show that all effectors alone or in combination had an influence on MRP 1 and 3 membrane protein expression to varying extents. The effects encountered are not found to be associated with de novo MRP expression. Rather, they seem to be related to altered translocation of MRP to the cell membrane as a result of ROS production at the protein level. The intracellular levels of ROS, which, for example, result from additional treatment with hyperthermia seem to be significant. Other mechanisms can be attributed to shifting of intracellular MRP storage pools, changes in membrane fluidity, or inactivation of MRP activity (particularly via hyperthermia). Moreover, hyperthermia seems to efficiently reverse MNP-induced over-expression in cellular membranes. These results could open up new strategies in the future to reduce therapeutic failures in oncology by repressing mechanisms which actively export drugs from the cancer cell.
Acknowledgments
This study was supported by the German Research Foundation (DFG) under contract number HI-689/6-1 and HI 698/8-2 (the latter in parts). We gratefully acknowledge Susann Burgold, Yvonne Ozegowski, Doreen May, and Beate Ziegenhardt for their valuable technical assistance.
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Stavrovskaya AA, Stromskaya TP. Transport proteins of the ABC family and multidrug resistance of tumor cells. Biochemistry (Mosc) 2008;73:592–604. doi: 10.1134/s0006297908050118. [DOI] [PubMed] [Google Scholar]
- 2.Seeger MA, van Veen HW. Molecular basis of multidrug transport by ABC transporters. Biochim Biophys Acta. 2009;1794:725–737. doi: 10.1016/j.bbapap.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Gutmann DA, Ward A, Urbatsch IL, Chang G, van Veen HW. Understanding polyspecificity of multidrug ABC transporters: closing in on the gaps in ABCB1. Trends Biochem Sci. 2009;35:36–42. doi: 10.1016/j.tibs.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Bakos E, Homolya L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1) Pflugers Arch. 2007;453:621–641. doi: 10.1007/s00424-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Peng H, Zhang JT. Expression profiling of ABC transporters in a drug-resistant breast cancer cell line using AmpArray. Mol Pharmacol. 2005;68:430–438. doi: 10.1124/mol.105.011015. [DOI] [PubMed] [Google Scholar]
- 7.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 8.Hildebrandt B, Wust P. Interactions between hyperthermia and cytotoxic drugs. Cancer Treat Res. 2007;134:185–193. doi: 10.1007/978-0-387-48993-3_11. [DOI] [PubMed] [Google Scholar]
- 9.Bergs JW, Haveman J, Ten Cate R, Medema JP, Franken NA, Van Bree C. Effect of 41 degrees C and 43 degrees C on cisplatin radiosensitization in two human carcinoma cell lines with different sensitivities for cisplatin. Oncol Rep. 2007;18:219–226. [PubMed] [Google Scholar]
- 10.Katschinski DM. On heat and cells and proteins. News Physiol Sci. 2004;19:11–15. doi: 10.1152/nips.01403.2002. [DOI] [PubMed] [Google Scholar]
- 11.Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44:2546–2554. doi: 10.1016/j.ejca.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 13.van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13:1173–1184. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- 14.Jordan A, Maier-Hauff K. Magnetic nanoparticles for intracranial thermotherapy. J Nanosci Nanotechnol. 2007;7:4604–4606. [PubMed] [Google Scholar]
- 15.Hilger I, Dietmar E, Linß W, Streck S, Kaiser WA. Developments for the minimally invasive treatment of tumours by targeted magnetic heating. J Phys Condens Matter. 2006;18:S2951–2958. [Google Scholar]
- 16.Vellonen KS, Mannermaa E, Turner H, et al. Effluxing ABC transporters in human corneal epithelium. J Pharm Sci. 2010;99:1087–1098. doi: 10.1002/jps.21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruh GD, Zeng H, Rea PA, et al. MRP subfamily transporters and resistance to anticancer agents. J Bioenerg Biomembr. 2001;33:493–501. doi: 10.1023/a:1012827221844. [DOI] [PubMed] [Google Scholar]
- 18.Chin KV, Tanaka S, Darlington G, Pastan I, Gottesman MM. Heat shock and arsenite increase expression of the multidrug resistance (MDR1) gene in human renal carcinoma cells. J Biol Chem. 1990;265:221–226. [PubMed] [Google Scholar]
- 19.Vilaboa NE, Galan A, Troyano A, de Blas E, Aller P. Regulation of multidrug resistance 1 (MDR1)/P-glycoprotein gene expression and activity by heat-shock transcription factor 1 (HSF1) J Biol Chem. 2000;275:24970–24976. doi: 10.1074/jbc.M909136199. [DOI] [PubMed] [Google Scholar]
- 20.Wartenberg M, Gronczynska S, Bekhite MM, et al. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular prostate tumor spheroids by hyperthermia and reactive oxygen species. Int J Cancer. 2005;113:229–240. doi: 10.1002/ijc.20596. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki M, Kohno K, Uchiumi T, et al. Activation of human multidrug resistance-1 gene promoter in response to heat shock stress. Biochem Biophys Res Commun. 1992;187:677–684. doi: 10.1016/0006-291x(92)91248-o. [DOI] [PubMed] [Google Scholar]
- 22.Pallis M, Zhu YM, Russell NH. Bcl-x(L) is heterogenously expressed by acute myeloblastic leukaemia cells and is associated with autonomous growth in vitro and with P-glycoprotein expression. Leukemia. 1997;11:945–949. doi: 10.1038/sj.leu.2400705. [DOI] [PubMed] [Google Scholar]
- 23.Kuo MT. Redox regulation of multidrug resistance in cancer chemotherapy: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2009;11:99–133. doi: 10.1089/ars.2008.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziemann C, Burkle A, Kahl GF, Hirsch-Ernst KI. Reactive oxygen species participate in mdr1b mRNA and P-glycoprotein overexpression in primary rat hepatocyte cultures. Carcinogenesis. 1999;20:407–414. doi: 10.1093/carcin/20.3.407. [DOI] [PubMed] [Google Scholar]
- 25.Wartenberg M, Ling FC, Schallenberg M, et al. Down-regulation of intrinsic P-glycoprotein expression in multicellular prostate tumor spheroids by reactive oxygen species. J Biol Chem. 2001;276:17420–17428. doi: 10.1074/jbc.M100141200. [DOI] [PubMed] [Google Scholar]
- 26.Wartenberg M, Hoffmann E, Schwindt H, et al. Reactive oxygen species-linked regulation of the multidrug resistance transporter P-glycoprotein in Nox-1 overexpressing prostate tumor spheroids. FEBS Lett. 2005;579:4541–4549. doi: 10.1016/j.febslet.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Xu J, Min T, Huang W. Up-regulation of P-glycoprotein expression by catalase via JNK activation in HepG2 cells. Redox Rep. 2006;11:173–178. doi: 10.1179/135100006X116682. [DOI] [PubMed] [Google Scholar]
- 28.Cai Y, Lu J, Miao Z, Lin L, Ding J. Reactive oxygen species contribute to cell killing and P-glycoprotein downregulation by salvicine in multidrug resistant K562/A02 cells. Cancer Biol Ther. 2007;6:1794–1799. doi: 10.4161/cbt.6.11.4860. [DOI] [PubMed] [Google Scholar]
- 29.Huang XZ, Wang J, Huang C, et al. Emodin enhances cytotoxicity of chemotherapeutic drugs in prostate cancer cells: the mechanisms involve ROS-mediated suppression of multidrug resistance and hypoxia inducible factor-1. Cancer Biol Ther. 2008;7:468–475. doi: 10.4161/cbt.7.3.5457. [DOI] [PubMed] [Google Scholar]
- 30.Alakhova DY, Rapoport N Y, Batrakova EV, et al. Differential metabolic responses to pluronic in MDR and non-MDR cells: a novel pathway for chemosensitization of drug resistant cancers. J Control Release. 2009;142:89–100. doi: 10.1016/j.jconrel.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu FS. Mechanisms of chemotherapeutic drug resistance in cancer therapy – a quick review. Taiwan J Obstet Gynecol. 2009;48:239–244. doi: 10.1016/S1028-4559(09)60296-5. [DOI] [PubMed] [Google Scholar]
- 32.Kuo MT, Bao J, Furuichi M, et al. Frequent coexpression of MRP/GS-X pump and gamma-glutamylcysteine synthetase mRNA in drug-resistant cells, untreated tumor cells, and normal mouse tissues. Biochem Pharmacol. 1998;55:605–615. doi: 10.1016/s0006-2952(97)00494-2. [DOI] [PubMed] [Google Scholar]
- 33.Almquist KC, Loe DW, Hipfner DR, Mackie JE, Cole S P, Deeley RG. Characterization of the M(r) 190,000 multidrug resistance protein (MRP) in drug-selected and transfected human tumor cell. Cancer Res. 1995;55:102–110. [PubMed] [Google Scholar]
- 34.Rajagopal A, Simon SM. Subcellular localization and activity of multidrug resistance proteins. Mol Biol Cell. 2003;14:3389–3399. doi: 10.1091/mbc.E02-11-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gennuso F, Fernetti C, Tirolo C, et al. Bilirubin protects astrocytes from its own toxicity by inducing up-regulation and translocation of multidrug resistance-associated protein 1 (Mrp1) Proc Natl Acad Sci U S A. 2003;101:2470–2475. doi: 10.1073/pnas.0308452100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Luyn MJ, Muller M, Renes J, et al. Transport of glutathione conjugates into secretory vesicles is mediated by the multidrug-resistance protein 1. Int J Cancer. 1998;76:55–62. doi: 10.1002/(sici)1097-0215(19980330)76:1<55::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 37.Zhang JT, Chong CH. Co-translational effects of temperature on membrane insertion and orientation of P-glycoprotein sequences. Mol Cell Biochem. 1996;159:25–31. doi: 10.1007/BF00226059. [DOI] [PubMed] [Google Scholar]
- 38.Ahamed M, Akhtar MJ, Siddiqui MA, et al. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology. 2011;283:101–108. doi: 10.1016/j.tox.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Bae JE, Huh MI, Ryu BK, et al. The effect of static magnetic felds on the aggregation and cytotoxicity of magnetic nanoparticles. Biomaterials. 2011;32:9401–9414. doi: 10.1016/j.biomaterials.2011.08.075. [DOI] [PubMed] [Google Scholar]
- 40.Buyukhatipoglu K, Clyne AM. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J Biomed Mater Res A. 2011;96:186–195. doi: 10.1002/jbm.a.32972. [DOI] [PubMed] [Google Scholar]
- 41.Naqvi S, Samim M, Abdin M, et al. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomedicine. 2011;5:983–989. doi: 10.2147/IJN.S13244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Roti Roti JL. Heat-induced alterations of nuclear protein associations and their effects on DNA repair and replication. Int J Hyperthermia. 2007;23:3–15. doi: 10.1080/02656730601091759. [DOI] [PubMed] [Google Scholar]
- 43.van der Heijden AG, Verhaegh G, Jansen CF, Schalken JA, Witjes JA. Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: an in vitro study. J Urol. 2005;173:1375–1380. doi: 10.1097/01.ju.0000146274.85012.e1. [DOI] [PubMed] [Google Scholar]
- 44.Hildebrandt B, Wust P. The biologic rationale of hyperthermia. Cancer Treat Res. 2007;134:171–184. doi: 10.1007/978-0-387-48993-3_10. [DOI] [PubMed] [Google Scholar]
- 45.Kawai N, Ito A, Nakahara Y, et al. Complete regression of experimental prostate cancer in nude mice by repeated hyperthermia using magnetite cationic liposomes and a newly developed solenoid containing a ferrite core. Prostate. 2006;66:718–727. doi: 10.1002/pros.20394. [DOI] [PubMed] [Google Scholar]
- 46.Wilhelm C, Fortin JP, Gazeau F. Tumour cell toxicity of intracellular hyperthermia mediated by magnetic nanoparticles. J Nanosci Nanotechnol. 2007;7:2933–2937. doi: 10.1166/jnn.2007.668. [DOI] [PubMed] [Google Scholar]
- 47.Eberbeck D, Kettering M, Bergemann C, Zirpel P, Hilger I, Trahms L. Quantification of the aggregation of magnetic nanoparticles with different polymeric coatings in cell culture medium. J Phys D Appl Phys. 2010;43:405002. [Google Scholar]
- 48.Kettering M, Winter J, Zeisberger M, et al. Magnetic nanoparticles as bimodal tools in magnetically induced labelling and magnetic heating of tumour cells: an in vitro study. Nanotechnology. 2007;18:175101. [Google Scholar]
- 49.Ihnat MA, Lariviere JP, Warren AJ, et al. Suppression of P-glycoprotein expression and multidrug resistance by DNA cross-linking agents. Clin Cancer Res. 1997;3:1339–1346. [PubMed] [Google Scholar]
- 50.Maitra R, Shaw CM, Stanton BA, Hamilton JW. Increased functional cell surface expression of CFTR and DeltaF508-CFTR by the anthracycline doxorubicin. Am J Physiol Cell Physiol. 2001;280:C1031–1037. doi: 10.1152/ajpcell.2001.280.5.C1031. [DOI] [PubMed] [Google Scholar]
- 51.Caron RM, Hamilton JW. Developmentally specific effects of the DNA cross-linking agent mitomycin C on phosphoenolpyruvate carboxykinase gene expression in vivo: correlation with changes in chromatin structure within the promoter region of the gene. J Biochem Mol Toxicol. 1998;12:325–337. doi: 10.1002/(sici)1099-0461(1998)12:6<325::aid-jbt2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 52.Bhuyan BK. Kinetics of cell kill by hyperthermia. Cancer Res. 1979;39:2277–2284. [PubMed] [Google Scholar]
- 53.Chen B, Sun Q, Wang X, et al. Reversal in multidrug resistance by magnetic nanoparticle of Fe3O4 loaded with adriamycin and tetrandrine in K562/A02 leukemic cells. Int J Nanomedicine. 2008;3:277–286. doi: 10.2147/ijn.s2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen B, Cheng J, Wu Y, et al. Reversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and 5- bromotetrandrine in xenograft nude mice. Int J Nanomedicine. 2009;4:73–78. doi: 10.2147/ijn.s5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang Z, Chen BA, Xia GH, et al. The reversal effect of magnetic Fe3O4 nanoparticles loaded with cisplatin on SKOV3/DDP ovarian carcinoma cells. Int J Nanomedicine. 2009;4:107–114. doi: 10.2147/ijn.s5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fang D, Bao Y, Li X, et al. Effects of iron deprivation on multidrug resistance of leukemic K562 cells. Chemotherapy. 2010;56:9–16. doi: 10.1159/000287352. [DOI] [PubMed] [Google Scholar]
- 57.Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299:483–493. [PubMed] [Google Scholar]