Abstract
Context
Volumetric studies have reported relatively decreased cortical thickness and gray matter volumes in adults with Attention-Deficit/Hyperactivity Disorder (ADHD) whose childhood status was retrospectively recalled. We present the first prospective study combining cortical thickness and voxel-based morphometry (VBM) in adults diagnosed with ADHD in childhood.
Objective
In adults who had Combined Type ADHD in childhood, to 1) test whether they exhibit cortical thinning and decreased gray matter in regions hypothesized related to ADHD, and 2) test whether anatomic differences are associated with current ADHD diagnosis, including persistence versus remission.
Design
Cross-sectional analysis embedded in a 33-year prospective follow-up at mean age 41.
Setting
Research outpatient center.
Participants
ADHD probands were from a cohort of 207 6–12 year old Caucasian boys; male comparison subjects (n=178) had been free of ADHD in childhood. We obtained MRI scans in 59 probands and 80 comparisons (28% and 45% of original samples, respectively).
Main Outcome Measure
Whole-brain VBM and vertex-wise cortical thickness analyses.
Results
Cortex was significantly thinner in ADHD probands than comparisons in the dorsal attentional network and limbic areas (FDR<0.05, corrected). Additionally, gray matter was significantly decreased in probands in right caudate, right thalamus and bilateral cerebellar hemispheres. Probands with persistent ADHD (n=17) did not differ significantly from remitters (n=26) at FDR<0.05. At uncorrected p<0.05, remitters had thicker cortex relative to those with persistent ADHD in medial occipital cortex, insula, parahippocampus, and prefrontal regions.
Conclusions
We observed anatomic gray matter reductions in adults with childhood ADHD, regardless of current diagnosis. The most affected regions underpin top-down control of attention and regulation of emotion and motivation. Exploratory analyses suggest that diagnostic remission may result from compensatory maturation of prefrontal, cerebellar, and thalamic circuitry.
CONTEXT
Volumetric studies in children with Attention-Deficit/Hyperactivity Disorder (ADHD) have consistently found global reductions of total brain volume with prefrontal cortex, anterior and posterior cingulate cortex, basal ganglia, cerebellum and parieto-temporal regions particularly affected relative to typically developing subjects.1–4 These findings are consistent with a model of ADHD as a disorder of frontal-striatal-cerebellar circuitry. The diagnosis of ADHD requires onset in childhood, but persistence of ADHD into adulthood is now well documented.4, 5 This longitudinal course together with smaller brain volumes in children with ADHD has raised questions about brain development into adulthood.
A sparse literature on brain anatomy in adults with ADHD also reports decreased volumes in orbitofrontal cortex,6 anterior cingulate cortex (ACC),7, 8 dorsolateral prefrontal cortex (DLPFC),9 superior frontal cortex and cerebellum.10 Complementary analyses of cortical thickness11 reveal overall decreased cortical thickness in children11–14 and adults with ADHD with reductions in ACC, medial frontal regions and parieto-temporo-occipital cortex.12–14 Recently, Almeida et al.15 found cortical thinning in right frontal lobe of children, adolescents and adults with ADHD.
Faute de mieux, investigations of structural brain abnormalities in adults have relied on adults’ retrospective recall of their childhood status.8, 9, 16–22 The documented inaccuracies of such reports23 highlight the advantage of assessing brain anatomy in individuals with established childhood-onset ADHD prospectively followed into adulthood. Additionally, clinical ADHD remits in a substantial proportion of individuals followed into adulthood,24, 25 but the neurobiology of remission has not been previously examined in middle adulthood.
We report cortical thickness and voxel-based morphometry (VBM) analyses on the largest sample to date of adults with childhood ADHD diagnoses (mean age 8) consistent with DSM-IV. Follow-up assessments occurred at mean ages 18, 25 and 41 (18FU, 25FU, and 41FU, respectively). At 18FU, a comparison group free of childhood ADHD, matched for age, sex, ethnicity, and childhood social class was recruited.26–30 Systematic diagnostic assessments at each follow-up were conducted by interviewers “blind” to past history and group membership. At 41FU, we conducted anatomic brain magnetic resonance imaging in probands with childhood ADHD and comparisons. We performed analyses based on childhood diagnosis as well as on current diagnostic status in adulthood. Primary aims were to: (1) test whether adults with a childhood diagnosis of Combined Type ADHD (probands), relative to comparisons, exhibit cortical thinning and decreased gray matter in regions hypothesized to be related to ADHD,12–14, 31 and (2) assess whether anatomic differences are associated with current ADHD diagnosis.
METHODS
PARTICIPANTS
The ADHD group originally comprised 207 6 to 12 year-old Caucasian boys referred to a research clinic from 1970 to 1977 (mean age 8.3 years). Briefly, they were referred by schools because of behavioral problems, had elevated parent and teacher ratings of hyperactivity, IQ≥85, and a diagnosis of Hyperkinetic Reaction of Childhood.32, 33 Children with a pattern of aggressive or antisocial behavior were excluded to rule out comorbid conduct disorder. Further details of proband characteristics appear in previous publications.30, 34 These subjects were assessed at mean ages 18.4±1.3, 25.0±1.3, and 41.2±2.7. Comparison male subjects (n=178) were recruited at 18FU. Medical center pediatric charts were reviewed for children seen for routine physical exams from 1970–1977 when they were 6 through 12 years-old, group-matched for probands’ race, childhood socioeconomic status and geographical residence. Parents of suitable children (by then adolescents) were called, informed of the study and, if interested, recruited, provided parents reported that no teacher had complained about their child’s behavior in elementary school. Refusal was low (circa 5%).
ADULT-FOLLOW UP ASSESSMENT (41FU)
On average 33 years after initial childhood diagnosis, clinical data were obtained on 135 male probands (65% of original sample, 69% of those living) and 136 male comparisons (76% of 178 recruited in adolescence, 77% of those living). Major DSM-IV disorders, as well as multiple aspects of function, were assessed for the interval between 25FU and 41FU by trained clinicians “blind” to all antecedent data. A special interview, Assessment of Adult Attention Deficit Hyperactivity Disorder, was developed for diagnosing DSM-IV ADHD in adults (see Author e-Methods and Author e-Instrument). Current ADHD was defined as meeting DSM-IV criteria during the preceding six months. Participants were invited to take part in an anatomical MRI study. Due to refusals and MRI exclusions (see Table 1), we obtained MRI scans in 59 ADHD probands and 80 comparisons. Nearly all probands (n=57; 97% of those scanned) were treated with methylphenidate in childhood between ages 6 and 12, for an average of 2.2 years.35 (See Author e-Table 1 for further details of childhood medication treatment, including thioridazine.30) All participants provided written informed consent as approved by the NYU School of Medicine Institutional Review Board.
Table 1.
Derivation of MRI Sample and Demographics
| ADHD Male Probands N (%) | Male Comparisons N (%) | |||
|---|---|---|---|---|
| INITIAL SAMPLE | 207 (100) | 178 (100) | ||
| Unable to locate | 21 (10) | 20 (11) | ||
| Deceased | 15 (7) | 5 (3) | ||
| Incarcerated | 6 (3) | 1 (1) | ||
| Refused MRI | 43 (21) | 34 (19) | ||
| Not evaluated prior to termination of funding | 29 (14) | 22 (12) | ||
| SUBTOTAL-AVAILABLE FOR SCAN | 93 (45) | 96 (54) | ||
| MRI Exclusions: | ||||
| Size (too large for scanner) | 17 (8) | 6 (3) | ||
| Claustrophobic | 7 (3) | 3 (2) | ||
| Metal contraindications | 3 (2) | 1 (1) | ||
| Failed scan quality criteria | 7 (3) | 6 (3) | ||
| TOTAL NUMBER WITH USABLE DATA | 59 (29) | 80 (45) | ||
| DEMOGRAPHICS* | Mean (SD) | Mean (SD) | t | P (2-tailed) |
|
| ||||
| Age at Follow-Up (Years) | 41.1 (2.7) | 41.3 (3.1) | 0.51 | 0.61 |
| Socioeconomic Status** at Follow-Up | 3.37 (1.1) | 2.48 (1.0) | 5.01 | 0.001 |
| Educational Attainment† | 13.5 (2.4) | 15.6 (2.3) | 5.31 | 0.001 |
| WAIS Full Scale IQ at 18FU | 104(13) | 113(13) | 3.58 | 0.001 |
| WASI Full Scale IQ at 41FU | 101 (13) | 110 (15) | 3.42 | 0.001 |
| Global Assessment Scale Rating*** | 63.4 (12.5) | 71.4 (10.5) | 4.05 | 0.001 |
All ADHD probands and comparisons: Caucasian
Hollingshead and Redlich (1958) scale, based on the participant’s education and occupation.
Highest Grade Completed WAIS: Wechsler Adult Intelligence Scale. Obtained for 39 (66%) of the 59 Probands and all Comparisons.
WASI: Wechsler Abbreviated Scale of Intelligence. Obtained on 54 (92%) of the 59 Probands and 73 (91%) of the 80 Comparisons.
Completed by the “blind” clinician that conducted the mental status and diagnostic assessments.
To test whether cortical thickness differed as a function of current ADHD, we subdivided probands into three subgroups: 1) those who met diagnostic criteria for DSM-IV ADHD at 41FU (“persistents” n=17, including seven Predominantly Inattentive, six Predominantly Hyperactive/Impulsive, and four Combined Type); 2) those who did not (“remitters” n=26); and 3) those diagnosed with ADHD Not Otherwise Specified (“ADHD-NOS” n= 16; see Author e-Methods). Comparisons were dichotomized into subjects who did not meet criteria for any type of ADHD (“non-ADHD comparisons” n=57) and those who were diagnosed with ADHD-NOS (“comparisons with ADHD” n=23). Although all probands and all comparisons were included in initial vertex-wise and VBM analyses, subgroup analyses focused on current diagnostic status. Accordingly, probands and comparisons with current ADHD-NOS, which is not well-defined and did not differ between groups (27% and 29%, respectively), were excluded from subgroup analyses.
IMAGING
Anatomic T1-weighted images were obtained on a 3T Siemens Trio with an 8-channel Siemens head coil (41 scans; 20 ADHD probands, 21 comparisons) and a 3T Siemens Allegra with a Siemens single channel head coil (98 scans; 39 ADHD probands, 59 comparisons; proportions did not differ significantly across scanners, (χ(1)2=0.96, p=0.33) with the following parameters: TR=2100ms; flip angle=12; slice thickness=1.5mm; inversion time=1100ms; matrix=192×256; FOV=172.5mm. The only parameter that differed was TE, which was 3.87ms on the Trio and 3.90ms on the Allegra.
Structural MRI scans were preprocessed through the fully automated CIVET-MNI pipeline.36–39 The initial preprocessing step was to mask MRI native images using an automated brain extraction method.40 Data were corrected for non-uniformity artifacts and registered to stereotaxic space (MNI152) using a 9-parameter linear transformation. Voxel-wise tissue type classification was performed using a neural network classifier followed by a partial volume estimation step.38, 41
For VBM, the classified tissue maps were blurred with a Gaussian kernel of 10mm full width at half-maximum. Cortical thickness measures were assessed using a fully automated algorithm which defines the distances between a set of vertices at the white matter (WM) surface and then expands outward to find the intersection with GM in order to generate surface meshes that represent WM and GM interfaces.42 A total of 40,962 linked vertices were calculated per hemisphere. Each individual cortical thickness map was blurred using a 30mm surface-based diffusion-smoothing kernel to reduce noise while preserving anatomical location, as this method produces less volumetric blurring than the equivalent Gaussian kernel.43
STATISTICAL ANALYSES
Global cortical thickness
We obtained a single global cortical thickness value for each subject by averaging across all 81,924 vertices. Linear regression models controlled for age at time of scan and scanner model (Trio vs. Allegra).
Vertex-wise and voxel-based morphometry analyses
Following the study aims, group analyses tested for regional differences in cortical thickness and GM density between (1) all adults with a childhood diagnosis of Combined Type ADHD and all comparisons; (2a) persistents versus non-ADHD comparisons; (2b) remitters versus non-ADHD comparisons; and (2c) persistents versus remitters. For each comparison, we regressed cortical thickness at each of 81,924 vertices or whole-brain GM density on group, controlling for age at time of scan and scanner model. The software package ‘mni.cortical.statistics’ (Brain Imaging Centre of the Montreal Neurological Institute) for the R environment44 was used for cortical thickness analyses and the FMRIB Software Library (FSL, www.fmrib.ox.ac.uk)tool Feat, for VBM. Results were thresholded using a false discovery rate (FDR) of 0.05.45, 46 Maps of t-statistics for group effects on cortical thickness at each vertex or GM density at each voxel were projected onto an average brain template revealing clusters that differed significantly between groups. We retained clusters comprising at least 50 contiguous vertices for cortical thickness47 and five voxels for VBM.
Region-based analyses of cortical thickness and voxel-based morphometry
To test whether childhood or current ADHD was associated with significant differences in specific regions, we performed post-hoc region-of-interest (ROI)-based analyses. For each participant, we computed mean cortical thickness or GM density within each cluster exhibiting significant (FDR<0.05) group differences in primary analyses by averaging across all vertices or voxels within each cluster. We then compared the diagnostic subgroups of probands (persistents, remitters) and the comparisons without current ADHD, Bonferroni corrected for the number of clusters. For completeness, Author e-Table 2 contains means and SD for the subgroups with current ADHD-NOS.
Table 2.
Cortical Thickness Values for Significant Clusters for Subgroups Defined by Current ADHD Diagnostic Status in Mid-Adulthood.
| Regions | MNI X, Y, Z (No. of vertices) | Non-ADHD Controls (n=57) | Probands w/Persistent ADHD (n=17) | Remitted Probands (n=26) | Non-ADHD Controls vs. Persistents | Non-ADHD Controls vs. Remitters | Remitters vs. Persistents | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | p | ES | p | ES | p | ES | ||
| L Sup Parietal (BA7) | −26, −55, 68 (4,290) | 2.97 | 0.13 | 2.85 | 0.17 | 2.86 | 0.13 | 0.004 | 0.83 | 0.000 | 0.88 | 0.978 | 0.01 |
| L Precentral G (BA6) | −35, 37, 36 (784) | 3.35 | 0.13 | 3.26 | 0.15 | 3.26 | 0.16 | 0.022 | 0.65 | 0.006 | 0.67 | 0.917 | −0.03 |
| L Sup Temp G (BA38) | −54, 10, −22 (915) | 3.80 | 0.19 | 3.60 | 0.23 | 3.66 | 0.19 | 0.001 | 0.98 | 0.002 | 0.75 | 0.401 | 0.26 |
| L Frontal Pole (BA10) | −31, 62, −6 (638) | 3.23 | 0.18 | 3.06 | 0.18 | 3.11 | 0.23 | 0.001 | 0.96 | 0.010 | 0.62 | 0.453 | 0.24 |
| L Cuneus (BA19) | −13, −91, 35 (618) | 2.78 | 0.20 | 2.65 | 0.17 | 2.73 | 0.16 | 0.025 | 0.63 | 0.325 | 0.23 | 0.134 | 0.48 |
| L Precuneus (BA31) | −6, −65, 30 (62) | 3.35 | 0.20 | 3.23 | 0.19 | 3.26 | 0.15 | 0.028 | 0.62 | 0.029 | 0.53 | 0.630 | 0.15 |
| R. Precuneus (BA7) | 10, −73, 51 (1148) | 3.23 | 0.16 | 3.12 | 0.13 | 3.15 | 0.15 | 0.010 | 0.73 | 0.040 | 0.49 | 0.430 | 0.25 |
| R Inf Parietal (BA40) | 49, −40, 50 (4836) | 3.03 | 0.14 | 2.91 | 0.18 | 2.93 | 0.14 | 0.007 | 0.77 | 0.002 | 0.74 | 0.828 | 0.07 |
| R Sup Temp G (BA38) | 30, 15, −40 (1141) | 3.87 | 0.27 | 3.62 | 0.25 | 3.75 | 0.22 | 0.001 | 0.96 | 0.044 | 0.48 | 0.080 | 0.56 |
| R Temporal G extending to Insula (BA13) | 48, −1, −3 (315) | 3.81 | 0.21 | 3.69 | 0.24 | 3.72 | 0.21 | 0.049 | 0.55 | 0.053 | 0.46 | 0.747 | 0.10 |
| R Precentral G (BA6) | 58, 0, 36 (315) | 3.41 | 0.15 | 3.27 | 0.19 | 3.35 | 0.18 | 0.003 | 0.86 | 0.109 | 0.38 | 0.207 | 0.40 |
| R Frontal Pole (BA10) | 27, 47, 32 (98) | 3.37 | 0.16 | 3.28 | 0.17 | 3.27 | 0.18 | 0.057 | 0.53 | 0.021 | 0.56 | 0.907 | −0.04 |
| R Middle Frontal G (BA9) | 25, 47, −14 (130) | 3.36 | 0.20 | 3.19 | 0.18 | 3.33 | 0.17 | 0.002 | 0.90 | 0.497 | 0.16 | 0.011 | 0.83 |
| R Occipital (BA19) | 27, −87, 26 (210) | 2.96 | 0.20 | 2.86 | 0.19 | 2.87 | 0.19 | 0.095 | 0.47 | 0.079 | 0.42 | 0.862 | 0.05 |
| R Occipital (BA18) | 10, −80, 10 (94) | 2.79 | 0.20 | 2.69 | 0.21 | 2.71 | 0.19 | 0.079 | 0.49 | 0.080 | 0.42 | 0.776 | 0.09 |
Results for regions which survived FDR<0.05 and extent > 50 vertices in analyses of the entire sample (Figure 1a).
Non-ADHD Controls: comparisons who did not meet criteria for any type of ADHD at 41FU longitudinal assessment; ES: Effect Size; BA: Brodmann area; L.: Left; Sup.: Superior; Temp.: Temporal; G.: Gyrus; R.: Right; Inf.: Inferior. P-values surviving Bonferroni correction for multiple comparisons or ES>.50 are indicated in bold.
Exploratory analyses of cortical thickness
To further investigate primary hypotheses for which no FDR<0.05 vertices were found, we reexamined subgroup differences heuristically using an uncorrected p<0.05 threshold with a cluster threshold of 50 vertices.47 Because of significant between-group differences in IQ, we confirmed cortical thickness results by also adjusting for IQ.
RESULTS
Table 1 summarizes the derivation of the sample. A larger proportion of comparisons (45% of originally enrolled participants) than probands (29%) had analyzable MRI scans. This discrepancy reflects a significantly higher rate of unavoidable factors in probands (27%) (i.e., deaths, incarcerations and MRI exclusions) than in comparisons (12%) (χ2(1)=12.08, p<0.001). By contrast, rates of refusal, failure to schedule or to locate subjects did not differ significantly (45% of probands versus 43% of comparisons). Accordingly, results are based on anatomic images from 59 ADHD probands and 80 comparisons.
We compared diagnoses and demographic information at 18FU of subjects who were scanned and those who were not (data available for 57/59 probands and all comparisons; see Author e-Table 3). Within both proband and comparison groups, individuals scanned and those not scanned did not differ significantly on prevalence of ADHD, Antisocial Personality Disorder, mood or anxiety disorders, any DSM-III disorders, age at referral, IQ, socioeconomic status, or Teacher Conners Hyperactivity Factor score. However, scanned probands had significantly higher rates of alcohol substance use disorder (SUD), non-alcohol SUD, and any SUD than probands who were not scanned (Author e-Table 3)
Table 3.
Grey Matter Density within Clusters for Subgroups Defined by Current ADHD Diagnostic Status in Mid-Adulthood.
| Regions | Maximum Z-scores | MNI Peak (No. of voxels) | Non- ADHD Controls (n=57) | Probands Persistent ADHD (n=17) | Probands Remitters ADHD (n=26) | Non-ADHD Controls vs. P-ADHD | Non-ADHD Controls vs. Remitters | Remitters vs. P-ADHD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| mean | SD | mean | SD | mean | SD | p | ES | p | ES | P | ES | |||
| L Sup Parietal (BA7) | 5.3 | −28, −58, 66 (1747) | 0.42 | 0.04 | 0.39 | 0.03 | 0.39 | 0.05 | 0.003 | 0.86 | 0.001 | 0.78 | 0.993 | 0 |
| L Cerebellum | 4.01 | −14, −70, −34 (430) | 0.57 | 0.15 | 0.51 | 0.14 | 0.56 | 0.16 | 0.146 | 0.41 | 0.821 | 0.05 | 0.279 | 0.34 |
| L Inf Cerebellum | 3.44 | −32, −42, −58 (41) | 0.41 | 0.17 | 0.28 | 0.15 | 0.34 | 0.18 | 0.009 | 0.74 | 0.116 | 0.38 | 0.27 | 0.35 |
| L Middle Temp G (BA21) | 3.32 | −48, 10, −42 (32) | 0.39 | 0.1 | 0.33 | 0.1 | 0.34 | 0.11 | 0.048 | 0.56 | 0.088 | 0.41 | 0.682 | 0.13 |
| L Temp-occipital (BA37) | 3.5 | −50, −42, −22 (17) | 0.6 | 0.08 | 0.55 | 0.09 | 0.54 | 0.07 | 0.024 | 0.64 | 0.002 | 0.78 | 0.724 | −0.1 |
| Brainstem extending to Cerebellum | 3.37 | 0, −44, −48 (16) | 0.36 | 0.14 | 0.3 | 0.12 | 0.32 | 0.11 | 0.1 | 0.46 | 0.148 | 0.35 | 0.653 | 0.14 |
| L Temp-parahippocampal (BA35) | 3.33 | −34, −10, −22 (13) | 0.75 | 0.06 | 0.73 | 0.06 | 0.72 | 0.07 | 0.117 | 0.44 | 0.027 | 0.53 | 0.727 | −0.1 |
| L Frontal pole (BA10) | 3.31 | −16, 52, 32 (9) | 0.45 | 0.05 | 0.43 | 0.06 | 0.43 | 0.06 | 0.062 | 0.52 | 0.035 | 0.51 | 0.972 | 0.01 |
| R Parietal Postcentral (BA3) extending to BA6 | 4.7 | 48,−18, 56 (1196) | 0.41 | 0.04 | 0.37 | 0.04 | 0.38 | 0.05 | 0 | 1.04 | 0.003 | 0.72 | 0.444 | 0.24 |
| R Cerebellum | 3.84 | 32, −60, −38 (235) | 0.51 | 0.17 | 0.44 | 0.14 | 0.53 | 0.17 | 0.146 | 0.41 | 0.581 | −0.1 | 0.078 | 0.56 |
| R Thalamus | 3.94 | 4, −8, 12 (170) | 0.73 | 0.1 | 0.7 | 0.09 | 0.74 | 0.07 | 0.202 | 0.36 | 0.541 | −0.2 | 0.065 | 0.59 |
| R Occipital, Cuneus (BA18/19) | 3.88 | 2, −76, 36 (122) | 0.67 | 0.05 | 0.63 | 0.06 | 0.65 | 0.05 | 0.005 | 0.79 | 0.082 | 0.42 | 0.2 | 0.41 |
| R Sup Frontal G (BA10) | 4.25 | 12, 64, 12 (68) | 0.47 | 0.06 | 0.45 | 0.04 | 0.46 | 0.05 | 0.261 | 0.31 | 0.398 | 0.2 | 0.696 | 0.12 |
| R Frontal lobe (BA6) | 3.66 | 12, −12, 78 (32) | 0.46 | 0.11 | 0.41 | 0.11 | 0.45 | 0.14 | 0.107 | 0.45 | 0.75 | 0.08 | 0.319 | 0.31 |
| R Middle Frontal G (BA10) extending to OFC (BA11) | 3.41 | 24, 38, −18 (23) | 0.51 | 0.06 | 0.45 | 0.05 | 0.47 | 0.06 | 0.001 | 0.96 | 0.005 | 0.69 | 0.434 | 0.25 |
| R Temp Fusiform (BA36) | 3.23 | 34, −34, −26 (13) | 0.63 | 0.08 | 0.58 | 0.05 | 0.6 | 0.07 | 0.014 | 0.69 | 0.062 | 0.45 | 0.423 | 0.25 |
| R Caudate | 3.3 | 8, 20, −2 (5) | 0.51 | 0.09 | 0.49 | 0.07 | 0.48 | 0.1 | 0.41 | 0.23 | 0.18 | 0.32 | 0.715 | −0.1 |
| R Middle Temp (BA21) extending to (BA38) | 3.16 | 52, 6, −32 (5) | 0.55 | 0.06 | 0.52 | 0.05 | 0.51 | 0.08 | 0.063 | 0.52 | 0.009 | 0.63 | 0.614 | −0.2 |
| R Middle Temp (BA21) extending to (BA38) | 5.3 | 46, 8, −42 (5) | 0.52 | 0.06 | 0.51 | 0.08 | 0.51 | 0.05 | 0.557 | 0.16 | 0.561 | 0.14 | 0.894 | 0.04 |
| Anterior Cingulate/limbic (BA24) | 3.53 | 0, 44, −10 (20) | 0.82 | 0.04 | 0.79 | 0.04 | 0.81 | 0.04 | 0.016 | 0.68 | 0.248 | 0.28 | 0.246 | 0.37 |
Results for regions which survived FDR<0.05 and extent > 5 voxels in analyses of the entire sample (Figures 3 and 4). Non-ADHD Controls: comparisons who did not meet criteria for any type of ADHD at 41FU longitudinal assessment; ES: Effect Size; BA: Brodmann area; L: Left; Sup: Superior; Temp: Temporal; G: Gyrus; R: Right; Inf: Inferior. OFC: Orbitofrontal cortex; P-values surviving Bonferroni correction for multiple comparisons or ES>.50 are indicated in bold.
DEMOGRAPHICS
Probands and comparisons did not differ significantly in age at scan, or in lifetime prevalence of substance abuse or dependence (see Table 1). As expected, probands and comparisons differed significantly in IQ in childhood and 41FU assessments. See Author e-Table 5 for demographics of subgroups based on current diagnosis. Current substance use and comorbid diagnoses are presented in Author e-Table 5.
GLOBAL CORTICAL THICKNESS
Surface-wide, mean cortical thickness was significantly lower in probands (n=59) than comparisons (n=80) (mean ± SD 3.18±0.11mm and 3.24±0.11mm, respectively; p<0.001 in regression controlling for age and scanner; Cohen’s d=0.54). At 41FU, probands with persistent ADHD differed significantly from non-ADHD comparisons (3.14±0.13mm and 3.25±0.10mm, respectively; p=0.0005; d=1.02). The remitters (3.20±0.11mm) also differed from non-ADHD comparisons in overall cortical thickness (p=0.04, d=0.48). However, persistents and remitters did not differ significantly (p=0.10, d=0.51).
VERTEX-WISE ANALYSES OF CORTICAL THICKNESS
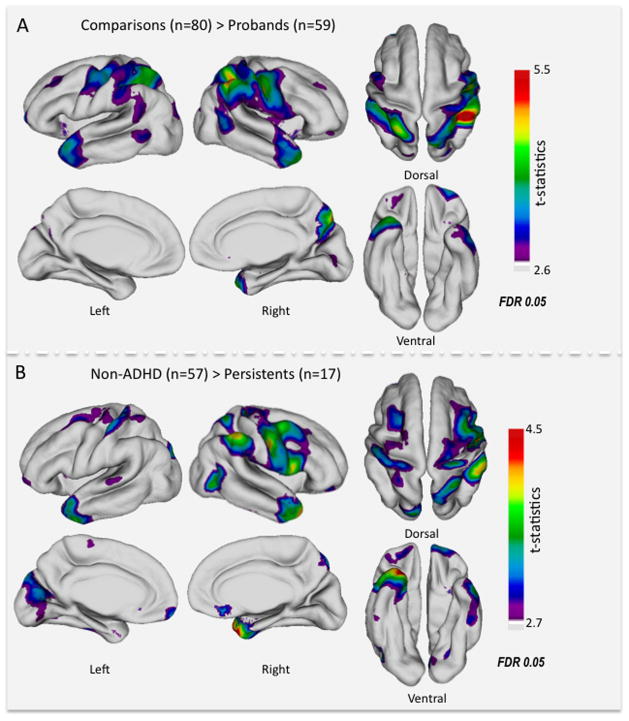
Figure 1A displays the multiple clusters of vertices (detailed in Table 2) for which the cortex was significantly thinner (surface-wide FDR<0.05) in ADHD probands; the largest cluster extended from right precuneus to precentral gyrus. Other right hemisphere clusters were located in inferior parietal lobe, temporal pole, and insula. Left hemisphere clusters were located in superior frontal gyrus/frontal pole, precentral gyrus, insula, temporal pole, and cuneus. There was no instance in which cortical thickness was significantly increased in probands. As shown in eFigure 1 and Author e-Table 6, after covarying for IQ (in addition to scanner and age), significant cluster centers remained largely unchanged in location, but the clusters were less extensive.
Figure 1.
(A) t-map of the significant cortical thinning in probands with ADHD (n=59) compared to comparisons (n=80). (B) t-map of the significant cortical thinning in probands with persistent ADHD (n=17) compared to non-ADHD comparisons (n=57). False Discovery Rate (FDR) threshold depends on the data and is different for the right and left hemispheres. Here the t-statistics at the lowest FDR threshold are projected across each hemisphere for each comparison.
In order to assess associations with current ADHD diagnosis, we performed vertex-wise comparisons among the different diagnostic subgroups. The 17 individuals with persistent ADHD differed significantly from the 57 non-ADHD comparisons in most but not all the regions identified in the initial inclusive analyses (see Table 2 and Figure 1B). Additionally, this analysis revealed thinner cortex related to persistent ADHD in the left medial occipital cortex and right subgenual ACC. Using FDR<0.05, remitters (n=26) did not differ significantly from non-ADHD comparisons; persistents and remitters also did not differ in any region at this threshold. There were no vertices at which cortical thickness was significantly associated with lifetime or current substance abuse diagnoses, dimensional measures of substance abuse, lifetime smoking history, or thioridazine treatment, nor were there any significant interactions between group and scanner for any cortical or VBM measures.
REGION-BASED ANALYSES OF CORTICAL THICKNESS
To examine potential differences associated with remission from childhood ADHD, we focused on the clusters in which ADHD probands exhibited significantly thinner cortex than comparisons (FDR<0.05). Both remitters and persistents had thinner cortex than non-ADHD comparisons, with medium to large effect sizes. Average effect sizes between persistents and non-ADHD comparisons (d=0.73) were larger than for remitters (d=0.52), although all confidence intervals overlapped (not shown); persistents and remitters did not differ significantly from each other in any cluster at FDR<0.05 (see Table 2).
EXPLORATORY VERTEX-WISE ANALYSES
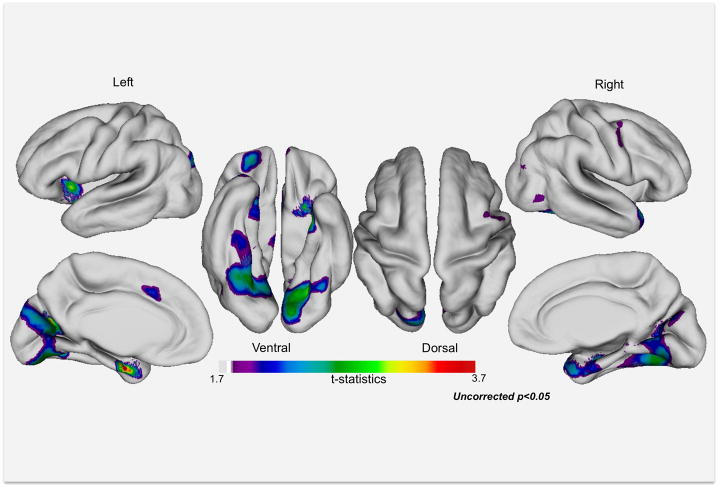
When vertex-wise results were thresholded at p<0.05 (uncorrected), we observed thinner cortex for persistents versus remitters in insula, bilateral temporal cortex including right temporal pole and in left occipital Brodmann area (BA) 19, orbitofrontal cortex and medial ACC (see Figure 2, Author e-Table 7). There were no regions exceeding our cluster size threshold of 50 vertices in which remitters exhibited thinner cortex than those with persistent ADHD.
Figure 2.
Exploratory uncorrected analyses (p<0.05) reveal regions in which remitted probands (n=27) exhibit thicker cortex than probands with persistent ADHD (n=17). See Author e-Table 7 for peaks and coordinates of clusters.
EXPLORATORY REGION-BASED ANALYSES
In the clusters that differentiated persistents from remitters in exploratory vertex-wise analyses, persistents differed markedly from non-ADHD comparisons (average d=0.75), whereas remitters did not (average d=0.03; t(9)=8.26, p<0.0001). Relative to comparisons, remitters had (non-significantly) greater cortical thickness in left superior temporal gyrus extending to insula and orbitofrontal cortex, left parahippocampus, left ACC, and left medial occipital cortex (see Author e-Table 7).
VOXEL-BASED MORPHOMETRY
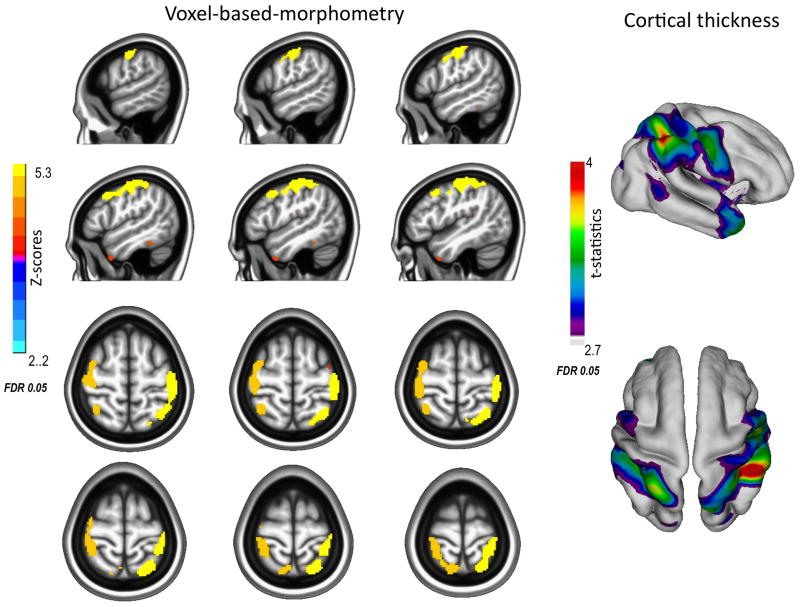
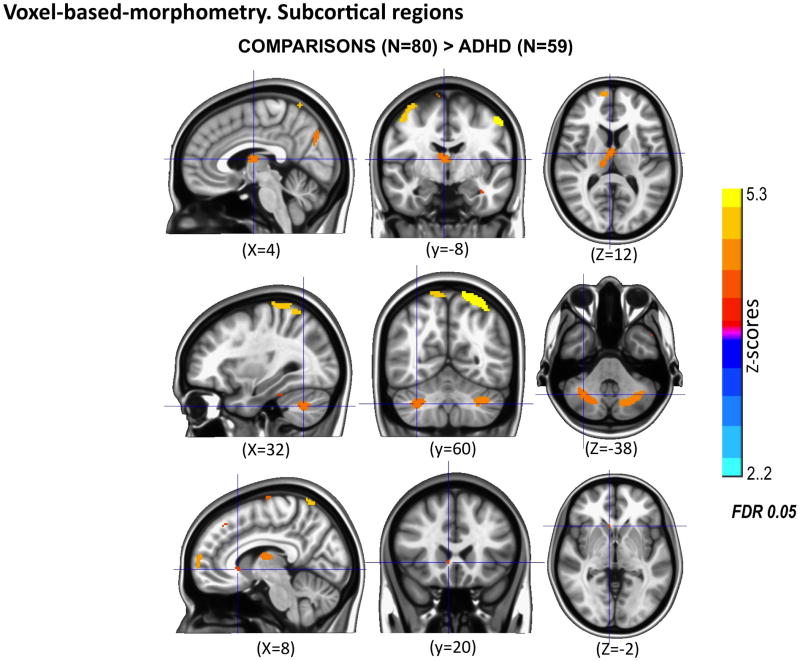
As shown in Table 3 and Figure 3, GM density was significantly greater (FDR<0.05) for comparisons than for probands in many of the same regions identified through cortical thickness analyses as well as in subcortical regions inaccessible to cortex-based measures. Figure 4 displays decreased GM in probands in right caudate, right thalamus and bilateral cerebellar hemispheres. VBM analyses of diagnostic subgroups or of medication treatment in childhood with methylphenidate or thioridazine did not yield significant results even with more lenient thresholds (FDR≤0.2).
Figure 3.
Comparisons (N=80) exhibit greater gray matter density (left) and cortical thickness (right) in the bilateral dorsal attentional network than probands (n=59) with childhood combined type ADHD. Images are in radiological convention, right is left and left is right.
Figure 4.
Voxel-based morphometry reveals that comparisons (N=80) exhibit significantly greater gray matter density (FDR <0.05) in right ventral caudate, right thalamus, bilateral cerebellum than probands (n=59) with childhood combined type ADHD. Images are in radiological convention, right is left and left is right.
COMMENT
In a prospective 33-year longitudinal follow-up of 59 probands (mean age 41 years) with established ADHD in childhood and 80 prospectively enrolled non-ADHD comparisons, we found an overall significant reduction in mean cortical thickness in probands. Beyond this global difference, the greatest cortical thinning associated with childhood ADHD was located in bilateral parietal lobes, temporal poles, insula, precentral gyri, frontal poles, and right precuneus. No cortical region was significantly thicker in probands than comparisons. Although less sensitive,48 VBM also revealed significantly decreased GM in probands versus comparisons in right precentral, bilateral parietal, left temporal, and right cuneus. Additionally, VBM detected decreased GM in probands in caudate, thalamus and cerebellar hemispheres.
With respect to current adult diagnosis, probands with persistent ADHD differed most from non-ADHD comparisons in the same cortical regions identified in our primary analyses, as well as in additional clusters in left medial occipital cortex and subgenual ACC. Probands with remitted ADHD did not differ significantly from persistents when analyses were corrected for full-brain comparisons. In exploratory uncorrected analyses, probands with persistent ADHD exhibited reduced cortical thickness relative to remitters in bilateral medial occipital lobes, temporal lobes extending to insula, and left parahippocampus.
Our results extend prior volumetric and cortical thickness findings in ADHD. First, consistent with decreased total cerebral volume in ADHD,2–4 our observation of reduced global cortical thickness in probands with ADHD confirms prior reports.13, 14, 20 Furthermore, although we found less frontal and prefrontal cortical thinning in ADHD than others,12–15, 20, 49 we confirmed thinner cortical mantle in occipito-parietal,12, 13, 20 temporal cortex and precentral regions13, 14 in ADHD. In subcortical analyses, we also confirmed anatomic abnormalities in caudate,3, 50, 51 thalamus52, 53 and cerebellum3 in ADHD.
Studies of cortical thickness in adults with ADHD have focused on specific regions associated with executive function and attentional control.54, 55 Makris et al.9 selected nine parcellation units (from 48) per hemisphere and found thinner cortex related to ADHD in prefrontal and cingulate cortex and inferior parietal lobe, albeit without correcting for multiple comparisons.9 A cross-sectional study of children, adolescents and adults found that individuals with ADHD, regardless of age, had significantly thinner right superior frontal cortex than controls.15 In the adults with ADHD, the specific reduction, with correction for multiple comparisons limited to the frontal lobe, was localized to BA9. In contrast, we did not find group differences in much of prefrontal cortex but found widespread cortical thinning in bilateral parietal-temporal cortex. We found similar results in analyses that included all participants as well as in those limited to probands with persistent ADHD versus non-ADHD comparisons. The latter contrasts are comparable to studies in adults that define group membership by current diagnostic status.15, 20
Studies of cortical thickness in children with ADHD are more numerous than those in adults,12–14, 33, 47, 56, 57 and typically have examined the entire cerebrum, although nearly all (except14) report results uncorrected for multiple comparisons. Thinner cortex has been reported in children with ADHD in prefrontal and precentral regions12, 14 parietal and temporal lobes12, 13 and inferior frontal gyrus bilaterally.58 In our main analyses, we applied FDR full-brain correction for multiple comparisons, and observed significant differences whether groups were defined by initial childhood history or by current adult diagnoses. We speculate that the robustness of our results reflects having established the diagnosis of ADHD in childhood as well as our medium to large sample sizes.
Broadly, our results implicate disruptions in large-scale neural systems involved in the regulation of both attention and emotion in adults with childhood ADHD. We found convincing converging anatomic evidence implicating the dorsal attentional network55 and distributed regions within limbic circuits that were thinner in ADHD probands than in comparisons. Similar findings were obtained when we contrasted probands with persistent ADHD versus comparisons without ADHD. However, we failed to observe hypothesized group differences in prefrontal regions.1, 3 Below we discuss our main findings and non-findings in turn.
First, we found widespread thinner cortex and decreased GM density in bilateral parietal and precentral regions, overlapping areas of the dorsal attentional network. The bilateral dorsal network, which mediates goal-directed, top-down executive control processes, interacts with a right-sided ventral system (stimulus-driven, bottom-up) during attentional functioning,1, 55 particularly in redirecting attention. The core areas constituting the dorsal attentional network include the intraparietal sulcus and the conjunction of the precentral and superior frontal sulcus (frontal eye fields)55 which were particularly affected in the ADHD probands. Strikingly, we also observed significantly thinner cortex in precuneus and superior parietal lobe, which along with the dorsal network core regions are implicated in top-down processing of shifting of attention.59 These findings are consistent with studies of ADHD that report abnormal patterns of activation in parietal regions52 during working memory,60–62 attentional63–65 or response inhibition tasks.66, 67
We also found occipital cortical thinning in probands with persistent ADHD versus non-ADHD comparisons. Occipital cortex has been recently found to interact with the dorsal network in maintaining attention59 and in suppressing responses to irrelevant stimuli.68, 69 Individuals with ADHD are easily distracted when required to ignore extraneous signals.70, 71 Top-down control deficits when responding to irrelevant stimuli are associated with impaired working memory.72, 73 Abnormal activation of occipital cortex has been found in youth74 and adults75–77 with ADHD during working memory tasks. Similarly, in a meta-analysis of functional imaging studies, children and adolescents with ADHD showed activation decreases in left middle occipital gyrus (BA19) compared to controls.52 Additionally, a recent VBM study in adults with ADHD found significant bilateral reduction of GM volume only in early visual cortex.78
Our VBM analysis revealed cerebellar, thalamic and striatal GM deficits in ADHD. Cerebellar involvement in ADHD is well-established, with findings in children reported mostly in the vermis,1–4, 79 and in the hemispheres in adults, as in this sample.60, 80, 81 Early anatomical studies of ADHD did not specifically examine thalamic nuclei, although thalamic hypoactivation emerged in an unbiased meta-analysis.52 Recently, several studies have identified thalamic abnormalities in children/adolescents53, 82 and adults with ADHD.83, 84
Second, our analyses revealed thinner cortex in probands, and particularly those with persistent ADHD, across multiple limbic regions such as temporal poles (BA38), insula (BA13) and subgenual ACC (BA25). The insula and ACC play important roles in sensorimotor, emotional and cognitive function.85, 86 Specifically, subgenual ACC is implicated in emotional processing and pain perception.87 In humans, subgenual ACC is functionally connected with multiple limbic regions including temporal poles88 and insula.89 In turn, the insula, along with participating in performance of demanding tasks,90 is clearly also related to affective processing.91 Abnormal activations in insula and subgenual ACC were reported in a meta-analysis of ADHD functional imaging.52
Cortical thickness studies in ADHD have downplayed findings in the temporal pole, which have been reported but not discussed.12–14 The temporal pole (BA38) is classified as a paralimbic region, based on its interconnections with both amygdala and orbitofrontal cortex, and is implicated in social and emotional processes.92 Altered activation in temporal pole is associated with deficits in face recognition93–100 and mentalizing, i.e., theory of mind.101–104 The temporal poles have been proposed as a channel for the integration of emotion and perception, playing an important role in both emotional and social functions.92
Our findings are consistent with pathophysiological models of ADHD highlighting not only cognitive executive functions (“cool” processes) but also emotion/motivational deficits (“hot” processes).105 Anatomic “spiraling” circuits begin with emotion/motivation pathways which influence “cool” cognitive processes, which in turn control motor responses.106 We observed thinner cortex in regions subserving both emotional regulation (temporal pole, insula, parahippocampus and subgenual ACC) and top-down attentional regulation (dorsal attentional network and medial occipital cortex). Further, our exploratory analyses suggest that thinner cortex and diminished gray matter in the dorsal attentional network and limbic relay regions is related to the trait of having had ADHD in childhood, regardless of current diagnostic status.
Third, the lack of proband-comparison differences in prefrontal cortex or ACC was unexpected.8, 9, 17, 20, 21 To better understand possible differences between persistents and remitters, we performed uncorrected exploratory analyses. In regions in which we found suggestive differences, we observed remarkable congruence between remitters and controls in left superior temporal gyrus, ACC, parahippocampus, and occipital cortical thickness as well as in thalamus and cerebellum gray matter density. We cannot rule out that remitters may have differed from persistents in these regions since childhood, but the most parsimonious explanation is offered by the hypothesis that remission entails compensatory processes12, 107 underpinned by prefrontal cortical maturation. While we found supporting evidence for ACC and orbitofrontal involvement in diagnostic remission of ADHD, our data also suggest superior temporal, medial occipital and thalamo-cerebellar involvement in remission.
Our findings must be interpreted in light of several limitations. First, despite our prospective longitudinal design, we examined brain imaging data only cross-sectionally in middle adulthood. Nevertheless, this is the largest sample of children with ADHD followed into adulthood, obviating the unreliability of retrospective recall of childhood symptoms. Additionally, we report on the largest sample to date of adults with confirmed childhood ADHD who had remitted. We were able to analyze imaging data from only 28% of initially diagnosed probands with ADHD and 45% of comparison subjects. However, these probands and comparisons did not differ from the original sample, and the probands studied did not differ significantly from those excluded on nearly all clinical and demographic variables, except for significantly higher rates of substance use disorders at 18FU in scanned probands. Nevertheless, we did not observe significant relationships between brain anatomic measures and substance use disorders. Finally, as is generally the case, our probands had significantly lower IQ than comparisons both in childhood/adolescence and adulthood. The issue of whether to covary for IQ in disorders such as ADHD is not settled.108 As shown in eFigure 1 and Author e-Table 7, our principal findings of persistent differences in brain anatomy survived covarying for IQ even with conservative full-brain correction.
We were surprised by the rate of ADHD-NOS diagnosed in comparisons, which was comparable to the rate in probands. We speculate that secular changes in the general public’s awareness of ADHD may have contributed. While we cannot rule out instrument-related error (see Author e-Instrument), using similar approaches did not yield high rates of ADHD symptoms in comparisons in two previous “blind” assessments.24, 26 Nevertheless, analyses excluding ADHD-NOS did not alter results appreciably.
Subjects were limited to Caucasian males, since the number of originally diagnosed females with ADHD was too small for meaningful statistical comparisons. Thus our results may not generalize to ADHD in women or to other racial or ethnic groups. However, this constraint avoided potential confounds from possible sex, ethnic, or socioeconomic differences. Exclusion of conduct disorder comorbidity (see Author e-Text) in childhood also averted confusion as to the origin of the deficits found in cortical thickness or GM density.
We cannot comment on cortical thickness or GM density in ADHD in the absence of medication treatment, as all but four of the scanned probands were treated with methylphenidate as children. We also did not detect significant effects of childhood treatment with stimulants or thioridazine in cortical thickness or VBM analyses. Medication treatment has been reported to affect cortical thickness47 although the durability of such effects is unknown, and treatment had been discontinued for all subjects for several decades.
For logistical reasons, we used two scanners. Fortunately, scans were approximately counterbalanced across probands and comparisons, and there were no significant main effects or interactions related to scanner type. Secondary analyses (see eFigure 2) also showed that we obtained comparable results when we examined only the 98 scans obtained on the Allegra scanner. Finally, the analyses presented here were limited to cortical thickness and VBM; ongoing analyses will examine white matter structure using diffusion tensor imaging.
In conclusion, in this first study of childhood ADHD prospectively examined in adulthood, we found thinner overall cortex in probands with childhood ADHD that was even more pronounced in those with persistent ADHD. Beyond this global effect, we also detected significant reductions in cortex thickness in parietal, temporal and posterior frontal regions corresponding to the dorsal attentional network and limbic areas. These findings were largely echoed by VBM, which additionally highlighted decreased GM in caudate. These regions underpin top-down control of attention and the regulation of emotion and motivation and were comparably diminished in probands with remitted ADHD or persistent ADHD. Thus these differences seem to primarily reflect the childhood diagnosis of ADHD. By contrast, remitters tended to differ from persistents in medial occipital cortex, temporal pole, insula, orbitofrontal cortex, parahippocampus, frontal pole, and subcortically in cerebellum and thalamus. This supports the suggestion that symptom amelioration and diagnostic remission may result in part from compensatory maturation of frontal thalamic cerebellar circuits.107, 109
Supplementary Material
Acknowledgments
We thank the participants and their families who have contributed to this research over the decades. We also thank Christine Cox, Ph.D., for careful reading of an earlier version of the manuscript and Pierre Bellec, Ph.D., Yasser Aleman, Ph.D., and Joost Janssen, Ph.D. for helpful discussions regarding methods.
Footnotes
Presented at the 16th Meeting of the Organization for Human Brain Mapping, June 6–10, 2010 in Barcelona, Spain and at the Third International Congress on ADHD, May 26–29, 2011 in Berlin, Germany.
References
- 1.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(12):1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. J Am Med Assoc. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 3.Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26(4):433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Durston S, Hulshoff Pol HE, Schnack HG, Buitelaar JK, Steenhuis MP, Minderaa RB, Kahn RS, van Engeland H. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2004;43(3):332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the national comorbidity survey replication. Am J Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hesslinger B, Tebartz van Elst L, Thiel T, Haegele K, Hennig J, Ebert D. Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neurosci Lett. 2002;328(3):319–321. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- 7.Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60(10):1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Makris N, Seidman LJ, Valera EM, Biederman J, Monuteaux MC, Kennedy DN, Caviness VS, Jr, Bush G, Crum K, Brown AB, Faraone SV. Anterior cingulate volumetric alterations in treatment-naive adults with ADHD: a pilot study. J Atten Disord. 2010;13(4):407–413. doi: 10.1177/1087054709351671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60(10):1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Monuteaux MC, Seidman LJ, Faraone SV, Makris N, Spencer T, Valera E, Brown A, Bush G, Doyle AE, Hughes S, Helliesen M, Mick E, Biederman J. A preliminary study of dopamine D4 receptor genotype and structural brain alterations in adults with ADHD. Am J Med GenetB NeuropsychiatrGenet. 2008;147B(8):1436–1441. doi: 10.1002/ajmg.b.30870. [DOI] [PubMed] [Google Scholar]
- 11.Castellanos FX, Proal E. Location, location, and thickness: volumetric neuroimaging of attention-deficit/hyperactivity disorder comes of age. J Am Acad Child Adolesc Psychiatry. 2009;48(10):979–981. doi: 10.1097/CHI.0b013e3181b45084. [DOI] [PubMed] [Google Scholar]
- 12.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del’homme M, Caplan R, Toga AW, McCracken JT, Levitt JG. Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(10):1014–1022. doi: 10.1097/CHI.0b013e3181b395c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 15.Almeida LG, Ricardo-Garcell J, Prado H, Barajas L, Fernandez-Bouzas A, Avila D, Martinez RB. Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: A cross-sectional study. J Psychiatr Res. 2010;44(16):1214–1212. doi: 10.1016/j.jpsychires.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Faraone SV, Biederman J, Feighner JA, Monuteaux MC. Assessing symptoms of attention deficit hyperactivity disorder in children and adults: Which is more valid? J Consult Clin Psychol. 2000;68(5):830–842. [PubMed] [Google Scholar]
- 17.Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, Brown AB, Bush G, Monuteaux MC, Caviness VS, Kennedy DN, Seidman LJ. Attention and executive systems abnormalities in adults with childhood ADHD: a DT-MRI study of connections. Cereb Cortex. 2008;18(5):1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- 18.Monuteaux MC, Seidman LJ, Faraone SV, Makris N, Spencer T, Valera E, Brown A, Bush G, Doyle AE, Hughes S, Helliesen M, Mick E, Biederman J. A preliminary study of dopamine D4 receptor genotype and structural brain alterations in adults with ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1436–1441. doi: 10.1002/ajmg.b.30870. [DOI] [PubMed] [Google Scholar]
- 19.Hesslinger B, Tebartz van Elst L, Thiel T, Haegele K, Hennig J, Ebert D. Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neuroscience Letters. 2002;328(3):319–321. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- 20.Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17(6):1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 21.Biederman J, Makris N, Valera EM, Monuteaux MC, Goldstein JM, Buka S, Boriel DL, Bandyopadhyay S, Kennedy DN, Caviness VS, Bush G, Aleardi M, Hammerness P, Faraone SV, Seidman LJ. Towards further understanding of the co-morbidity between attention deficit hyperactivity disorder and bipolar disorder: a MRI study of brain volumes. Psychol Med. 2008;38(7):1045–1056. doi: 10.1017/S0033291707001791. [DOI] [PubMed] [Google Scholar]
- 22.Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 23.Mannuzza S, Klein RG, Klein DF, Bessler A, Shrout P. Accuracy of adult recall of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 2002;159(11):1882–1888. doi: 10.1176/appi.ajp.159.11.1882. [DOI] [PubMed] [Google Scholar]
- 24.Mannuzza S, Klein RG, Bonagura N, Malloy P, Giampino TL, Addalli KA. Hyperactive boys almost grown up. V. Replication of psychiatric status. Arch Gen Psychiatry. 1991;48(1):77–83. doi: 10.1001/archpsyc.1991.01810250079012. [DOI] [PubMed] [Google Scholar]
- 25.Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157(5):816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- 26.Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up: I. Psychiatric status. Arch Gen Psychiatry. 1985;42(10):937–947. doi: 10.1001/archpsyc.1985.01790330017002. [DOI] [PubMed] [Google Scholar]
- 27.Mannuzza S, Klein RG, Bonagura N, Malloy P, Giampino TL, Addalli KA. Hyperactive boys almost grown up. V. Replication of psychiatric status. Arch Gen Psychiatry. 1991;48(1):77–83. doi: 10.1001/archpsyc.1991.01810250079012. [DOI] [PubMed] [Google Scholar]
- 28.Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50(7):565–576. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- 29.Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155(4):493–498. doi: 10.1176/ajp.155.4.493. [DOI] [PubMed] [Google Scholar]
- 30.Gittelman-Klein R, Klein DF, Katz S, Saraf K, Pollack E. Comparative effects of methylphenidate and thioridazine in hyperkinetic children. I. Clinical results. Arch Gen Psychiatry. 1976;33(10):1217–1231. doi: 10.1001/archpsyc.1976.01770100079008. [DOI] [PubMed] [Google Scholar]
- 31.Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17(6):1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association. DSM-II: Diagnostic and Statistical Manual of Mental Disorders. 1968. [Google Scholar]
- 33.Shaw P, Gornick M, Lerch J, Addington A, Seal J, Greenstein D, Sharp W, Evans A, Giedd JN, Castellanos FX, Rapoport JL. Polymorphisms of the dopamine D4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64(8):921–931. doi: 10.1001/archpsyc.64.8.921. [DOI] [PubMed] [Google Scholar]
- 34.Gittelman R, Abikoff H, Pollack E, Klein DF, Katz S, Mattes JA. Controlled trial of behavior modification and methylphenidate in hyperactive children. In: Whalen CHB, editor. Hyperactive Children: The Ecology of Identification and Treatment. New York: Academic Press; 1980. pp. 221–243. [Google Scholar]
- 35.Mannuzza S, Klein RG, Truong NL, Moulton JL, III, Roizen ER, Howell KH, Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiatry. 2008;165(5):604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robbins S, Evans AC, Collins DL, Whitesides S. Tuning and comparing spatial normalization methods. Med Image Anal. 2004;8(3):311–323. doi: 10.1016/j.media.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 38.Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21(10):1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- 39.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34(4):1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23(1):84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 42.MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12(3):340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 43.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24(1):163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 44.R Development Core Team. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 45.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 46.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 47.Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, Evans AC, Rapoport JL. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166(1):58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48(2):371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. The Lancet. 2003;362(9397):1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 50.Carmona S, Proal E, Hoekzema EA, Gispert JD, Picado M, Moreno I, Soliva JC, Bielsa A, Rovira M, Hilferty J, Bulbena A, Casas M, Tobena A, Vilarroya O. Ventro-striatal reductions underpin symptoms of hyperactivity and impulsivity in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;66(10):972–977. doi: 10.1016/j.biopsych.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Carmona S, Vilarroya O, Bielsa A, Tremols V, Soliva JC, Rovira M, Tomas J, Raheb C, Gispert JD, Batlle S, Bulbena A. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci Lett. 2005;389(2):88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 52.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 53.Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, Sanchez-Pena J, Miller AM, Chakravarty MM, Klahr K, Durkin K, Greenhill LL, Peterson BS. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167(4):397–408. doi: 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 55.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 56.Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, Greenstein D, Evans A, Giedd JN, Rapoport J. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2009;66(8):888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH. Abnormal cerebral cortex structure in children with ADHD. Hum Brain Mapp. 2009;30(1):175–184. doi: 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ, Scerif G, Liotti M, Liddle PF, Paus T, Hollis C. Cortical gray matter in attention-deficit/hyperactivity disorder: a structural magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 49(3):229–238. doi: 10.1016/j.jaac.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, McAvoy MP, Corbetta M. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29(14):4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf RC, Plichta MM, Sambataro F, Fallgatter AJ, Jacob C, Lesch KP, Herrmann MJ, Schonfeldt-Lecuona C, Connemann BJ, Gron G, Vasic N. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2009;30(7):2252–2266. doi: 10.1002/hbm.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayerl M, Dielentheis TF, Vucurevic G, Gesierich T, Vogel F, Fehr C, Stoeter P, Huss M, Konrad A. Disturbed brain activation during a working memory task in drug-naive adult patients with ADHD. Neuroreport. 2010;21(6):442–446. doi: 10.1097/WNR.0b013e328338b9be. [DOI] [PubMed] [Google Scholar]
- 62.Burgess GC, Depue BE, Ruzic L, Willcutt EG, Du YP, Banich MT. Attentional control activation relates to working memory in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;67(7):632–640. doi: 10.1016/j.biopsych.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banich MT, Burgess GC, Depue BE, Ruzic L, Bidwell LC, Hitt-Laustsen S, Du YP, Willcutt EG. The neural basis of sustained and transient attentional control in young adults with ADHD. Neuropsychologia. 2009;47(14):3095–3104. doi: 10.1016/j.neuropsychologia.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J Psychiatr Res. 2010;44(10):629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 65.Schneider MF, Krick CM, Retz W, Hengesch G, Retz-Junginger P, Reith W, Rosler M. Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults - a functional magnetic resonance imaging (fMRI) study. Psychiatry Res. 2010;183(1):75–84. doi: 10.1016/j.pscychresns.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Karch S, Thalmeier T, Lutz J, Cerovecki A, Opgen-Rhein M, Hock B, Leicht G, Hennig-Fast K, Meindl T, Riedel M, Mulert C, Pogarell O. Neural correlates (ERP/fMRI) of voluntary selection in adult ADHD patients. Eur Arch Psychiatry Clin Neurosci. 2010;260(5):427–440. doi: 10.1007/s00406-009-0089-y. [DOI] [PubMed] [Google Scholar]
- 67.Dillo W, Goke A, Prox-Vagedes V, Szycik GR, Roy M, Donnerstag F, Emrich HM, Ohlmeier MD. Neuronal correlates of ADHD in adults with evidence for compensation strategies--a functional MRI study with a Go/No-Go paradigm. Ger Med Sci. 2010;8:Doc09. doi: 10.3205/000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29(18):5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mevorach C, Hodsoll J, Allen H, Shalev L, Humphreys G. Ignoring the elephant in the room: a neural circuit to downregulate salience. J Neurosci. 2010;30(17):6072–6079. doi: 10.1523/JNEUROSCI.0241-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol. 2005;114(2):216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- 71.Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, Sahakian BJ. Association between response inhibition and working memory in adult ADHD: a link to right frontal cortex pathology? Biol Psychiatry. 2007;61(12):1395–1401. doi: 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 72.Zanto TP, Rubens MT, Bollinger J, Gazzaley A. Top-down modulation of visual feature processing: The role of the inferior frontal junction. Neuroimage. 2010;53(2):736–745. doi: 10.1016/j.neuroimage.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gazzaley A, D’Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- 74.Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53(10):871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 75.Schweitzer JB, Faber TL, Grafton ST, Tune LE, Hoffman JM, Kilts CD. Alterations in the functional anatomy of working memory in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2000;157(2):278–280. doi: 10.1176/appi.ajp.157.2.278. [DOI] [PubMed] [Google Scholar]
- 76.Schweitzer JB, Lee DO, Hanford RB, Tagamets MA, Hoffman JM, Grafton ST, Kilts CD. A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003;28(5):967–973. doi: 10.1038/sj.npp.1300110. [DOI] [PubMed] [Google Scholar]
- 77.Schweitzer JB, Hanford RB, Medoff DR. Working memory deficits in adults with ADHD: is there evidence for subtype differences? Behav Brain Funct. 2006;2:43. doi: 10.1186/1744-9081-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahrendts J, Rusch N, Wilke M, Philipsen A, Eickhoff SB, Glauche V, Perlov E, Ebert D, Hennig J, Tebartz van Elst L. Visual cortex abnormalities in adults with ADHD: A structural MRI study. World J Biol Psychiatry. doi: 10.3109/15622975.2010.518624. ePub Sep 29 2010. [DOI] [PubMed] [Google Scholar]
- 79.Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, Vaituzis AC, Blumenthal JD, Nelson J, Bastain TM, Zijdenbos A, Evans AC, Rapoport JL. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58(3):289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- 80.Perlov E, Tebarzt van Elst L, Buechert M, Maier S, Matthies S, Ebert D, Hesslinger B, Philipsen A. H(1)-MR-spectroscopy of cerebellum in adult attention deficit/hyperactivity disorder. J Psychiatr Res. 2010;44(14):938–943. doi: 10.1016/j.jpsychires.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 81.Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, Biederman J, Seidman LJ. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68(4):359–367. doi: 10.1016/j.biopsych.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qiu MG, Ye Z, Li QY, Liu GJ, Xie B, Wang J. Changes of brain structure and function in ADHD children. Brain Topogr. 2010 doi: 10.1007/s10548-010-0168-4. ePub Dec 30 2010. [DOI] [PubMed] [Google Scholar]
- 83.Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J Psychiatr Res. 2010;44(10):629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 84.Dibbets P, Evers EA, Hurks PP, Bakker K, Jolles J. Differential brain activation patterns in adult attention-deficit hyperactivity disorder (ADHD) associated with task switching. Neuropsychology. 2010;24(4):413–423. doi: 10.1037/a0018997. [DOI] [PubMed] [Google Scholar]
- 85.Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Hum Brain Mapp. 2007;28(1):9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 87.Vogt BA, Vogt L, Farber NB, Bush G. Architecture and neurocytology of monkey cingulate gyrus. J Comp Neurol. 2005;485(3):218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37(2):579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 89.Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30(9):2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30(8):2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quartz SR. Reason, emotion and decision-making: risk and reward computation with feeling. Trends Cogn Sci. 2009;13(5):209–215. doi: 10.1016/j.tics.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 92.Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(Pt 7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 93.Mackay CE, Roberts N, Mayes AR, Downes JJ, Foster JK, Mann D. An exploratory study of the relationship between face recognition memory and the volume of medial temporal lobe structures in healthy young males. Behav Neurol. 1998;11(1):3–20. doi: 10.1155/1998/285061. [DOI] [PubMed] [Google Scholar]
- 94.Nakamura K, Kawashima R, Sato N, Nakamura A, Sugiura M, Kato T, Hatano K, Ito K, Fukuda H, Schormann T, Zilles K. Functional delineation of the human occipito-temporal areas related to face and scene processing. A PET study. Brain. 2000;123 (Pt 9):1903–1912. doi: 10.1093/brain/123.9.1903. [DOI] [PubMed] [Google Scholar]
- 95.Grabowski TJ, Damasio H, Tranel D, Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001;13(4):199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dupont S. Investigating temporal pole function by functional imaging. Epileptic Disord. 2002;4 (Suppl 1):S17–22. [PubMed] [Google Scholar]
- 97.Nelson EE, McClure EB, Monk CS, Zarahn E, Leibenluft E, Pine DS, Ernst M. Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. J Child Psychol Psychiatry. 2003;44(7):1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- 98.Tsukiura T, Namiki M, Fujii T, Iijima T. Time-dependent neural activations related to recognition of people’s names in emotional and neutral face-name associative learning: an fMRI study. Neuroimage. 2003;20(2):784–794. doi: 10.1016/S1053-8119(03)00378-1. [DOI] [PubMed] [Google Scholar]
- 99.Griffith HR, Richardson E, Pyzalski RW, Bell B, Dow C, Hermann BP, Seidenberg M. Memory for famous faces and the temporal pole: functional imaging findings in temporal lobe epilepsy. Epilepsy Behav. 2006;9(1):173–180. doi: 10.1016/j.yebeh.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 100.Kim JW, Kim JJ, Jeong BS, Ki SW, Im DM, Lee SJ, Lee HS. Neural mechanism for judging the appropriateness of facial affect. Brain Res Cogn Brain Res. 2005;25(3):659–667. doi: 10.1016/j.cogbrainres.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 101.Jimura K, Konishi S, Asari T, Miyashita Y. Temporal pole activity during understanding other persons’ mental states correlates with neuroticism trait. Brain Res. 2010;1328:104–112. doi: 10.1016/j.brainres.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 102.Mier D, Lis S, Neuthe K, Sauer C, Esslinger C, Gallhofer B, Kirsch P. The involvement of emotion recognition in affective theory of mind. Psychophysiology. 2010;47(6):1028–1039. doi: 10.1111/j.1469-8986.2010.01031.x. [DOI] [PubMed] [Google Scholar]
- 103.Vollm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, Deakin JF, Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29(1):90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 104.Moriguchi Y, Ohnishi T, Mori T, Matsuda H, Komaki G. Changes of brain activity in the neural substrates for theory of mind during childhood and adolescence. Psychiatry Clin Neurosci. 2007;61(4):355–363. doi: 10.1111/j.1440-1819.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- 105.Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 106.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 107.Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- 108.Meehl P. Nuisance variables and the ex post facto design. In: Radner MWS, editor. Analyses of Theories and Methods of Physics and Psychology. Minneapolis: University of Minnesota Press; 1970. pp. 373–402. [Google Scholar]
- 109.Fassbender C, Schweitzer JB. Is there evidence for neural compensation in attention deficit hyperactivity disorder? A review of the functional neuroimaging literature. Clin Psychol Rev. 2006;26(4):445–465. doi: 10.1016/j.cpr.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.