Summary
Background
Childhood cancer survival remains dismal in low-income [A4] countries, but initiatives for treating paediatric cancer have substantially improved care in some of these countries. The My Child Matters programme was launched to fund projects for controlling paediatric cancer in low-income and mid-income countries. We aimed to assess the baseline status of paediatric cancer in ten countries that were receiving support (Bangladesh, Egypt, Honduras, Morocco, Philippines, Senegal, Tanzania, Ukraine, Venezuela, and Vietnam). [A5]
Methods
Qualitative face-to-face interviews with clinicians, hospital managers, health officials, and others were done by a multidisciplinary public-health research company. Estimates of paediatric cancer from population-based data were used to project the number of current and future patients for comparison with survey-based data. 5-year survival was postulated on the basis of interviews with health-care professionals. Field survey data were statistically compared with demographic, health, and socioeconomic data from global health organisations. The main outcome was to assess baseline status of paediatric cancer in the countries. [A6]
Findings
The baseline status of paediatric oncology varied substantially between the countries. The number of patients reportedly receiving medical care (obtained from survey data [A7]) differed markedly from the number predicted by population-based incidence data. Postulated 5-year survival was directly proportional to several demographic, economic, and health indicators, and most substantially, annual government healthcare expenditure per capita [ADD DATA] [A8].
Interpretation
Management of paediatric cancer and access to care are poor or deficient (ie, nonexistent, unavailable, or inconsistent access for most children with cancer [A48]) in seven of the ten countries studied, and accurate baseline data on incidence and outcome are very sparse [A10]. Alliances between public, private, and international agencies can rapidly improve the outcome of children with cancer in these countries.
Introduction
Until recently, paediatric-cancer care has been largely neglected in low-income and mid-income countries. An estimated 160 000 new cases of cancer are diagnosed annually in children younger than 15 years of age [A11].1 Only about 20–30% of patients (mostly in high-income countries) are thought to be adequately diagnosed and treated. A child's probability [A12] of surviving cancer is dismal in less developed countries, and extreme discomfort is likely in the absence of palliative care [A13]. Paradoxically, most cases of childhood cancers, if diagnosed at an early stage, are highly curable if treatment is available. Furthermore, today's effective treatment regimens are relatively simple, inexpensive, and well established.
Paediatric oncology has improved substantially in some comparatively low-income countries, and therefore, might be improved in other countries as well. Successful initiatives have improved access to treatment in countries in central and south America, Africa, and Asia.2–9 Collectively, these initiatives are twinning partnerships that pair medical institutions in high-income countries with those in low-income and mid-income countries. These programmes can rapidly improve survival when the collaborating institutions have a long-term commitment and when their efforts are supported locally by alliances between public and private sectors.7
On the basis of these successes, the My Child Matters programme was launched by the Sanofi-Aventis Humanitarian Sponsorship Department (Paris, France) and International Union Against Cancer (UICC; Geneva, Switzerland) in collaboration with a consortium comprising the US National Cancer Institute (Bethesda, MD, USA), St Jude Children's Research Hospital (Memphis, TN, USA), the International Network for Cancer Treatment and Research (Brussels, Belgium), the International Society of Pediatric Oncology (Amsterdam, Netherlands [A15]), the French-African Pediatric Oncology Group (Villejuif, France), the International Agency for Research on Cancer (IARC; Lyon, France), Epidaure Center Val D'Aurelle-Paul Lamarque (Montepellier, France), and the International Confederation of Childhood Cancer Parent Organizations (ICCCPO; Nieuwegein, Netherlands). The programme's purpose is to fund promising projects in paediatric-cancer control in selected low-income and mid-income countries. 14 proposed projects in ten countries10,11 were selected for funding. Project selection was based on five main points: feasibility, potential benefits for the community, sustainability, possibility for serving as a model for other countries, and accountability.10 Substantial weight was given to the accountability criterion to avoid potential mismanagement of funds. The current status of paediatric oncology was surveyed in the ten countries to provide baseline data. Here we describe the status of paediatric cancer care and the correlates of survival in these ten countries.
Methods
Procedures
In the absence of more reliable data sources, the status of paediatric oncology in Bangladesh, Egypt, Honduras, Morocco, Philippines, Senegal, Tanzania, Ukraine, Venezuela, and Vietnam was assessed by field survey. Population-based incidence estimates were used for comparison with survey data; otherwise, no information from the application or selection process was used.
Field survey
Interviews and data analysis were done by seven employees of Sanisphere (Neuillysur-Seine, France [A16]), a research company who specialise in public health, international affairs, project and health-services management, business, international health-care development, and public management (webtable 1). The data were extensively reviewed by the My Child Matters steering committee,10 comprising mainly medical and paediatric oncologists with extensive international experience. Survey data are shown in tables 1 and 2.
Table 1.
Number of centres visited and individuals interviewed by Sanisphere in the ten surveyed countries
| Centres visited, n | Total individuals interviewed | Physicians* | Cancer foundation representatives | Ministry of Health officials | Other† | |||
|---|---|---|---|---|---|---|---|---|
| Paediatric haematologists or oncologists | Hospital or programme directors | Other | ||||||
| Bangladesh | 11 | 17 | 2 | 6 | 3 | 4 | 0 | 2 |
| Egypt | 12 | 37 | 18 | 12 | 2 | 0 | 1 | 4 |
| Honduras | 5 | 23 | 4 | 5 | 2 | 4 | 1 | 7 |
| Morocco | 10 | 35 | 5 | 8 | 7 | 3 | 2 | 10 |
| Philippines | 12 | 29 | 9 | 5 | 2 | 7 | 3 | 3 |
| Senegal | 9 | 32 | 1 | 2 | 11 | 2 | 4 | 12 |
| Tanzania | 8 | 19 | 0 | 6 | 9 | 0 | 1 | 3 |
| Ukraine | 14 | 21 | 11 | 13 | 0 | 0 | 0 | 1 |
| Venezuela | 7 | 32 | 14 | 6 | 0 | 5 | 1 | 7 |
| Vietnam | 13 | 37 | 7 | 21 | 0 | 1 | 5 | 3 |
Physicians might be listed in more than one category.
Social workers, pharmacists, and psychologists.
Table 2.
Field survey of paediatric oncology status in the ten surveyed countries
| Paediatric cancer units, n | Dedicated paediatric oncology beds, n | Paediatric oncology or haematology specialists, n | Availability of diagnostic services | Availability of medication, radiotherapy, blood products | Uniform treatment guidelines | Patients seen by health-care providers annually, n | Postulated 5-year survival(%)* | |
|---|---|---|---|---|---|---|---|---|
| Bangladesh | 2 in the capital | 50 | 2 | Poor | Poor | Poor | 1000–1500 | 5 |
| Egypt | 20 across the country | 350 | 100 | Limited [A1] to adequate | Adequate | Limited [A1] | 2300 | 40 |
| Honduras | 2 in two large cities | 35 | 5 | Limited [A1] | Limited [A1] | Limited [A1] | 250 | 40 |
| Morocco | 3 in three large cities | 35 | 15 | Limited [A1] | Limited [A1] | Limited [A1] | 750–800 | 30 |
| Philippines | 0 | 75 | 100 | Poor to limited [A1] | Limited [A1] | Poor | 1000 | 10 |
| Senegal | In development | NA | 1 | Poor | Poor | Poor | 100 | 5 |
| Tanzania | 1 | 20 | None | Poor | Poor | Poor | 400–450 | 10 |
| Ukraine | 30 (in all administrative regions) | NA | 250 | Adequate | Adequate | Adequate | 1000 | 50 |
| Venezuela | 20 in large cities | 100 | 35 | Adequate | Adequate | Limited [A1] | 1000 | 60 |
| Vietnam | 2 in two large cities | 200–300 | 12 | Poor to limited [A1] | Poor to limited [A1] | Poor | 1000 | 5 |
NA=not available.
All data were based on direct interviews done by Sanisphere with local health-care providers.
First, each country was visited for 3 weeks between September, 2005, and May, 2006, by three Sanisphere employees (webtable 1). The survey comprised qualitative face-to-face interviews with oncologists of paediatric and adult cancers, family doctors[A17], nurses, pharmacists, hospital managers, cancer-registry employees, government health officials, embassy employees, and representatives of international and local non-governmental and religious agencies (table 1). No standard forms were used, but a set of basic questions were asked in all interviews, including the estimated number of children at each step of the paediatric cancer care chain, the number and description of paediatric oncology units, the number of beds available for paediatric oncology, and the number of paediatric oncologists and nurses. The main purpose of the survey was to ascertain the availability of a national paediatric-cancer programmes, dedicated paediatric-cancer hospital units, diagnostic resources, regular supplies of antineoplastic and antibiotic drugs, radiotherapy facilities, treatment guidelines or protocols, palliative-care programmes, parent support or advocacy organisations, paediatric oncology or haematology society [A18], and international partners.
Diagnostic resources were classified as poor, moderate, or adequate on the basis of timely access to the minimum necessary diagnostic procedures. Poor access was defined as nonexistent or unavailable access for most children with cancer; moderate [A19] access was defined as inconsistent access or a long wait for results; and adequate access was defined as basic, timely diagnostic procedures available for most children. Availability of medications, blood products, and radiotherapy was deemed poor if they were unavailable to all or most children with cancer, moderate [A19] if their availability was irregular; and adequate if they were available for most children in a timely manner.
The minimum requirement for diagnosis of solid tumour was histological assessment of haematoxylin and eosin-stained tumour sections. For diagnosis of leukaemia, assessment of a bone-marrow smear with Wright-Giemsa and myeloperoxidase staining was needed. Ultrasonography and CT were needed to stage solid tumours. Minimum diagnostic resources for all cancers included the necessary medical expertise to interpret the diagnostic studies. The consistent availability (ie, accessibility to all or most patients) of pain control, psychosocial support, chemotherapy drugs, blood products, and antimicrobials overall was classified as adequate or inadequate.
Paediatric-cancer burden
The interviewers estimated the number of patients seen by each country's medical services on the basis of interview data from health-care providers and hospital-based registries. To assess the survey data, we used incidence data for each country obtained from existing, internationally reviewed, population-based cancer registries within that country or region for various time periods between 1982 and 2002.1,12 –15 Incidence data for Egypt,14 Philippines,14 and Vietnam12,13,15 were obtained from regional population-based cancer registries. Incidence data for the remaining countries were obtained from cancer registries in neighbouring countries. The assumptions underlying the choice of surrogate population-based registries have been described elsewhere.13 Briefly, surrogate countries were chosen on the basis of location adjacent to the country of interest, availability of population-based cancer data, and similarity to the reference population.
Postulated survival of paediatric cancer
Postulated survival in each country was derived from all available interview data. It was not possible to meet the criteria for standard statistical methodology because of great variation in the data sources and settings, and the scarcity of population-based or hospital-based registries. When consistent survival estimates were obtained from different sources in a country, they were averaged. When the estimates were inconsistent, more weight was given to sources closest to the clinical management of childhood cancer, unless their estimates differed substantially from all others. For example, in Morocco, 750–800 patients were seen annually (table 2). All but 20 were treated in hospitals with cancer registries. We therefore based the postulated survival on the cancer registry survival information (about 300–350 survivors) and the estimated 1000 new cancers [A20] per year from incidence data (table 3), arriving at about 30% survival. Because of the absence of systematic follow-up, postulated survival represents [A21] only short-term survival and might be overestimated.
Table 3.
Estimated current and future paediatric cancer burden in the ten surveyed countries
| Current estimated population <15 years of age16 | Current estimated annual incidence of paediatric cancer (age <15 years) | Projected population <15 years of age in 202516 | Projected annual number of new cancer cases in paediatric population (age <15 years) in 2025 | |||||
|---|---|---|---|---|---|---|---|---|
| Data collection period for reference rate [A3] | Reference (data sources, method of estimation) | Incidence (per million) | Cases, n | At current estimated population-based [Al] incidence [A2] | At European incidence (140 per million)17 | |||
| Bangladesh | 47 759 | 1982–92 | 12,13 | 82 | 3916 | 61314 | 5028 | 8584 |
| Egypt | 25 589 | 1999–2002 | 14 | 125 | 3199 | 26 062 | 3258 | 3649 |
| Honduras | 2699 | 1982–92 | 12,13 | 130 | 351 | 2906 | 378 | 407 |
| Morocco | 10 499 | 1993–99 | 1 | 97 | 1018 | 10 413 | 1010 | 1458 |
| Philippines | 31 125 | 1998–2002 | 14 | 115 | 3579 | 32 698 | 3760 | 4578 |
| Senegal | 5016 | 1995–99 | 1 | 59 | 296 | 6717 | 396 | 940 |
| Tanzania | 16 174 | 1992–95 | 12,13 | 134 | 2167 | 19 946 | 2673 | 2792 |
| Ukraine | 6863 | 1990–98 | 13,24 | 135 | 927 | 5677 | 766 | 795 |
| Venezuela | 7578 | 1982–92 | 12,13 | 134 | 1015 | 7206 | 966 | 1009 |
| Vietnam | 23 278 | 1991–97 | 12, 13, 15 | 117 | 2724 | 20 833 | 2852 | 3412 |
Demographic, health, and socioeconomic data
For comparison and analysis purposes, we obtained available data for each country from various sources. Government annual health-care expenditure per capita and number of physicians and nurses per thousand in 2006 were obtained from WHO,18 and mortality data in patients aged under 5 years and per capita gross national income (GNI) in 2006 [A22] were obtained from the United Nations Children's Fund (UNICEF).19 2005 human development and human poverty indices were obtained from the UN Development Programme (UNDP).20 The human development index, a composite, normalised measure of life expectancy, literacy, education, standard of living, and gross domestic product (GDP) per capita, is a standard measure of well being, especially child welfare, for countries worldwide. The human poverty index is a composite index measuring deprivation in three basic dimensions: a long and healthy life, knowledge, and standard of living. The statistical procedures used to derive these indices have been described elsewhere.21 2005 per capita GDP was obtained from the International Monetary Fund;22 the 2006 total population and population under 15 years of age were obtained from the US Census Bureau;16 and reports on childhood cancer survival were obtained from European23 and US24 cancer registry data. We studied the correlation of these parameters, including incidence of paediatric cancer, survival, and access to care, with data obtained from the field surveys.
Statistical analysis
The correlation between postulated childhood-cancer survival in the ten countries overall and demographic, health, and socioeconomic data was calculated as the Pearson's correlation coefficient by use of SAS (version 9.1). Logistic regression models were used to ascertain the correlation between combinations of predictive variables and postulated cancer survival. A corresponding probability value of 0·05 or less was deemed to show a significant correlation. Because the incidence of paediatric cancer is expected to increase as mortality from diseases of poverty decreases in most of the ten surveyed countries, we also estimated the future annual incidence of paediatric cancer in these countries by assuming that it will equal current European incidence (140 cases per million)17 by 2025.
Role of the funding source
The sponsors of the My Child Matters programme (Sanofi-Aventis and International Union Against Cancer) had no role in the study concept, design, or in the collection, analysis, or interpretation of the data. The sponsors contracted Sanisphere to undertake a field study to ascertain baseline data on the countries chosen to receive My Child Matters grants. After Sanisphere employees presented the data to the My Child Matters steering committee, the authors analysed the data further and prepared the report. The steering committee are volunteers and did not receive honoraria from UICC or Sanofi-Aventis for this activity. The authors were responsible for the concept, design, and the data analysis and preparation of the report. All authors had access to all the data in the study. PCR had the final decision to submit for publication. [A23]
Results
Table 2 summarises the findings of the field survey and table 3 shows the number of current and future cases of paediatric cancer estimated from population-based data. A comparison of these two tables shows a marked discrepancy between the number of patients seen by health-care providers and the number of cases expected in 2025 [A24]. In Bangladesh, Philippines, Tanzania, and Vietnam only about 15–35% of expected cases would have been seen by health-care providers [A25], suggesting insufficient access to appropriate care. Only Ukraine had a national paediatric-oncology programme.
The number of paediatric-cancer units (PCUs) varied substantially between the countries. This essential component of contemporary paediatric oncology25 was unavailable in the Philippines and Senegal. In Senegal, a unit with beds used for the treatment of Burkitt's lymphoma, acute lymphoblastic leukaemia, Wilms' tumour, Hodgkin's lymphoma, and retinoblastoma has since been established in partnership with the French–African Paediatric Oncology Group. Six countries had too few PCUs or beds (or both) to accommodate all paediatric-cancer referrals. Only Egypt, Ukraine, and Venezuela seemed to have an adequate number of PCUs and beds.
Tanzania had no formally trained paediatric haematologists or oncologists. In the public sector, paediatric cancer was managed in the single Tanzanian PCU by a clinician-assisted radiation oncologist or by radiation oncologists [A26]; in the private sector, the PCU was managed by paediatricians and medical oncologists. By contrast, Ukraine had 250 paediatric haematologists or oncologists (one specialist for every four incident cases). These ratios were 1:10 in the Philippines, 1:23 in Egypt, and 1:28 in Venezuela, which are considered adequate.25 The remaining countries had ratios of 1:50 to 1:750, which are clearly inadequate for proper cancer care. Data from WHO suggested a median of 0·55 physicians of any type per 1000 population in the surveyed countries (ranged 0·06 in Senegal to 2·95 in Ukraine) and a median of 0·56 nurses (range 0·14 [A27] in Bangladesh to 7·62 in Ukraine; table 4).
Table 4.
Demographic, economic, and health indicators in the surveyed countries
| Total population (×1000)16 | Physicians per 1000 population*18 | Nurses per 1000 population18 | Under-5 mortality (per 1000)19 | Per capita GDP (US$)22 | Total per capita health-care expenditure (US$)18 | Per capita public health-care expenditure (US$)18 | Human development index20 | Human poverty index20 | |
|---|---|---|---|---|---|---|---|---|---|
| Bangladesh | 144 320 | [A1] | [A1] | 73 | 400 | 13 | 4 | 0.53 | 44.2 |
| Egypt | 77 506 | 0.54 | 2 | 33 | 1265 | 55 | 24 | 0.70 | 20 |
| Honduras | 7168 | 0.57 | 1.29 | 40 | 1148 | 72 | 41 | 0.68 | 17.2 |
| Morocco | 32 760 | 0.51 | 0.78 | 40 | 1713 | 72 | 24 | 0.64 | 33.4 |
| Philippines | 87 857 | 0.58 | 1.69 | 33 | 1168 | 31 | 14 | 0.76 | 15.3 |
| Senegal | 11 860 | 0.06 | 0.32 | 136 | 738 | 29 | 12 | 0.46 | 44 |
| Tanzania | 36 766 | 0.02 | 0.37 | 122 | 336 | 12 | 7 | 0.43 | 36 |
| Ukraine | 46 959 | 2.95 | 7.62 | 17 | 1766 | 60 | 40 | 0.77 | NA |
| Venezuela | 25 375 | 1.94 | NA | 21 | 5026 | 146 | 65 | 0.78 | 8.8 |
| Vietnam | 85 536 | 0.53 | 0.56 | 19 | 618 | 26 | 7 | 0.71 | 15.7 |
GDP=gross domestic product. NA=not available.
Refers to all physicians.
Availability of diagnostic testing was poor or limited [A28] in eight of the ten countries, in which there was typically no expertise in the histological diagnosis of paediatric cancers, no consistent supply of immunohistochemical reagents, long delays for pathology reports (as long as 1 month in Tanzania), or limited [A28] access to modern imaging or to other diagnostic technologies (or both) [A29]. Modern diagnostic equipment [A30] and access to them were adequate only in Ukraine and Venezuela. In Egypt, Honduras, Morocco, and Philippines, diagnostic resources were available but were inaccessible to most patients living outside of the countries' largest cities.
Overall management of paediatric cancer was deemed poor or limited [A28] in seven countries. Only in Egypt, Ukraine, and Venezuela did most children diagnosed with cancer seem to have access to anticancer drugs, antibiotics, blood products, and radiotherapy. Uniform treatment guidelines were absent in all countries except Ukraine, which uses national guidelines based on international protocols.
Postulated 5-year survival (table 2) was 5% to 10% in Bangladesh, Philippines, Senegal, Tanzania, and Vietnam; 30% in Morocco; and 40% to 60% in Egypt, Honduras, Ukraine, and Venezuela. Demographic, economic, and health-care indicators relevant to population health also varied widely between all the countries (table 4). Several of these indicators were significantly correlated with postulated survival in all the countries overall: per capita annual government health-care expenditure, per capita annual total health-care expenditure, per capita GDP, per capita GNI, and number of physicians and nurses per 1000 population (table 5). Annual government health-care expenditure per capita was most highly correlated with postulated survival in the countries overall (r2=0·882; p<0·0001). This indicator was a better predictor of postulated survival than any other demographic, economic, or health indicator, and a better predictor than any combination of these variables (data not shown). Mortality in patients aged under 5 years, deemed a standard measure of children's health, was not correlated [A31] with postulated paediatric cancer survival (r2=−0·333; p=0·081). Surprisingly, the human development index (r2=0·398; p=0·050) and human poverty index (r2=0·351; p=0·093), which are commonly used to rank countries' economic performance, were also not correlated [A31] with postulated survival [A32]. We also studied the correlation between the findings of the field survey data (table 1) and postulated 5-year survival. As expected, indicators suggestive of [A33] the availability of paediatric cancer services were correlated with postulated survival (webtable 2).
Table 5.
Correlation of health and economic indicators with paediatric cancer postulated 5-year survival in the surveyed countries
| Pearson's correlation coefficient (r) | Pearson r2 | p | |
|---|---|---|---|
| Government annual health-care expenditure per capita | 0.939 | 0.882 | <0.0001 |
| Total annual health-care expenditure per capita | 0.872 | 0.760 | 0.001 |
| Per capita GDP | 0.777 | 0.603 | 0.008 |
| Per capita GNI | 0.756 | 0.572 | 0.011 |
| Physicians per thousand population | 0.749 | 0.560 | 0.013 |
| Nurses per thousand population | 0.712 | 0.506 | 0.032 |
| Human development index | 0.631 | 0.398 | 0.050 |
| Human poverty index | −0.593 | 0.351 | 0.093 |
| Under-5 mortality | −0.577 | 0.333 | 0.081 |
GDP=gross domestic product. GNI=gross national income.
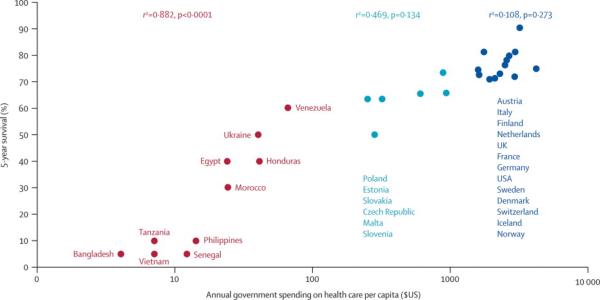
We then compared the correlation of annual government health-care expenditure per capita with the estimated survival rates of children with cancer in all ten countries, 18 European countries,23 and the USA24 (figure). The correlation was strongest at the low end of the expenditure range (p<0.0001 for expenditures <US$100) and weakest at the high end (p=0.27 for expenditures >US$1000). [Emma: I thought about rewording this para to give directions of the correlations, but I think it's obvious as it is]
Discussion
The overall survival of children with cancer as postulated from interviews with local health-care professionals is dismal in Bangladesh, Philippines, Senegal, Tanzania, and Vietnam, but is much better in Ukraine and Venezuela. Egypt, Honduras, and Morocco rank between these two groups. Postulated survival in the ten countries was significantly correlated with several socioeconomic and health-related indices established [A34] by international agencies, including total annual health-care expenditure, per capita GDP, per capita GNI, and the number of physicians and nurses per 1000 population, but only annual government health-care spending per capita was independently correlated. Future research will focus on specific characteristics of public-health infrastructure represented by these expenditures and how they are related to childhood-cancer survival.
Importantly, we noted that per capita annual health-care expenditure was significantly associated with childhood-cancer survival only in the lowest expenditure range. Not surprisingly, survival data were most favourable in countries where children are promptly referred to well-equipped tertiary-care centres. However, about 25–30% of patients are not cured, even with optimum treatment. Therefore, once access to early diagnosis and adequate care (with the requisite hospital infrastructure) is available, additional investment of public-health resources has less effect on the survival of children with cancer. Because childhood cancer has a low overall incidence and most patients can be managed without complex infrastructure or procedures, a relatively small [A35] investment by governments or private sectors in conjunction with local [A36] organisations might make a large difference in survival in low-income and mid-income countries.
The absence of correlation between mortality in patients aged under 5 years and postulated survival was not surprising; this disparity has been seen in many low-income and mid-income countries.26–28 We made this comparison because global health agencies deem mortality in patients aged under 5 years an important indicator of children's health. However, paediatric cancer is not a factor in mortality in this age group because of its relative rarity and its underdiagnosis in many countries. For example, even if all childhood cancer in Senegal were cured, Senegal's mortality in patients aged under 5 years would diminish only negligibly. Therefore, assistance or advocacy, or both, for treatment of paediatric cancer is unlikely to come from agencies that focus on child health in general. The relatively low mortality in this age group in some surveyed countries with poor postulated cancer survival suggests they have adequate basic public health measures, but the economic, professional, technological, and infrastructure resources needed for effective management of childhood cancer remain unavailable; abandonment of therapy is also likely to be a factor.29,30
Our study was substantially affected by a scarcity of population-based or even hospital-based cancer registries in most of the countries surveyed. Egypt, Philippines, and Vietnam have regional population-based cancer registries that provide data for international comparative studies.12,14 Ukraine has an established national population-based cancer registry, although its data has not been reviewed internationally. The estimated incidence of childhood cancer in Ukraine (based on reliable data from surrounding countries) is 135 per million population [A37],13 and our survey data yielded a postulated overall survival of about 50%. These estimates differ slightly from those reported by the Ukrainian national population-based registry31 (incidence 120 per million population [A38], and mortality 46 per million population [A38] in children aged under 15 years [A39]). However, under the crude assumption that mortality=incidencex(1–survival), the Ukraine national population-based registry would predict overall survival as 62%. Because there is an estimated 20% rate of under-reporting to the Ukraine national population-based registry, the survey data are not inconsistent with this survival prediction [A40].
Our study had some limitations [A41]. We used incidence data estimated from reliable (although not necessarily representative) sources in or outside of the ten countries. Use of incidence data from surrogate countries is not an ideal method of estimation but yielded the best available approximation (equally likely to deviate from the true incidence in either direction). The probability of 5-year survival was estimated by interviewing clinicians who directly cared for children with cancer but who do not usually provide long-term follow-up. We should also acknowledge that low-income and mid-income countries can undergo rapid changes in health, demographic, and economic measures, especially during war or natural disaster, although to our knowledge there have been no substantial changes in the surveyed countries. Therefore, despite the possibility that our data are incomplete and biased, they provide the only currently available means of defining a baseline for use in assessing future progress.
Improvement of paediatric-cancer survival in low-income and mid-income countries might need alliances that combine government, public and private sectors, and medical societies.7 Chile provides a remarkable example of what can be accomplished. Paediatric-oncology care in Chile has improved substantially over the past two decades through a strong alliance between the public and private sectors, and the oversight of the Chilean Minister of Health.32,33 Honduras and Morocco, which have relatively high mortality in children aged under 5 years, have also made substantial progress in the past few years, including expansion of access to care, improvement of supportive care and diagnostic capabilities, decrease of abandonment of therapy and late diagnosis, and establishment of uniform treatment guidelines adapted to local resources. This progress has been helped by use of twinning programmes with St Jude Children's Research Hospital and by Morocco's participation in the French–African Paediatric Oncology Group.5,29,34,35 The Honduran and Moroccan institutions have also created local non-governmental organisations that provide psychosocial and financial support to patients' families. Such organisations also work to increase awareness that paediatric cancer is curable, enlist community leaders, and campaign for national paediatric-cancer programmes. Most importantly, their fundraising activities sustain these programmes.
Twinning programmes are a mechanism that allows rapid and relatively inexpensive improvement of survival of childhood cancer even in countries without optimum medical infrastructure and public-health funds. In countries where mortality in children aged under 5 years is relatively low, but where overall childhood-cancer survival is extremely poor—such as the Philippines, Vietnam, and many others—effective twinning programmes might prompt rapid progress. Paediatric-oncology units implemented and maintained through such programmes also promote national, regional, and international alliances, as exemplified in Honduras, Morocco, and other countries. As countries develop economically and can increase their investment in health care, the beneficial [A42] effect of twinning programmes is likely to decrease. A major challenge for the My Child Matters programme is the long-term sustainability of funded projects in paediatric oncology. Sustainability is an especially crucial consideration in countries such as Tanzania, Senegal, Vietnam, and Philippines, which have many competing needs and few resources. One or more twinning sites in these countries, once established, might rapidly incorporate contemporary paediatric-cancer care. These centres could then serve as training sites for additional health-care providers and as community education resources. Eventually, regional collaboration and the participation of government and private agencies could expand access to a national level.
In summary [A43], detailed surveys can provide useful data for baseline assessment of the status of paediatric oncology, but cannot substitute for national cancer registration. We suggest that paediatric oncology registration in low-income and mid-income countries begin with hospital-based registry,36 although population-based registry is the ultimate aim. [A44] Development of strategies to sustain and expand the successful funded projects remains a daunting challenge.
Supplementary Material
Figure. Correlation between annual government healthcare expenditure ($US) per capita and childhood-cancer survival.
5-year survival data were postulated for the ten low-income and mid-income countries surveyed in this study; the remainder were obtained from the EUROCARE study.20
Acknowledgments
This study was partly funded by the National Institutes of Health (grant CA21765), the American Lebanese Syrian Associated Chaities (ALSAC), and RETICS (grant R&D06/0020/1056). Data collection and assembly were partly funded by Sanofi-Aventis in its contract with Sanisphere [A47]. We thank Jean-Luc Faillie, Adeline Janer, Julie Lyonnard, Gregory Mercier, Luize Scherer Navarro, and Florent Tomatis for designing, undertaking, and analysing the surveys, and Sharon Naron for expert editorial review.
Funding: National Institutes of Health (grant CA21765), American Lebanese Syrian Associated Chaities (ALSAC), RETICS (grant R&D06/0020/1056), and Sanofi-Aventis [A49].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors RCR and CF were responsible for the study concept and design. TM collected and assembled the data. RCR, TM, ES-F, and SH analysed and interpreted the data. RCR, TM, ES-F wrote the report. IM, TE, CF, IT-F, and JJD revised the report. All authors approved the final report.[A45]
Conflicts of interest IT-F is employed by Sanofi-Aventis. The other authors declared no conflicts of interest [A46].
References
- 1.Ferlay J, Bray F, Pisani P, Parkin DM. International Agency for Research on Cancer (IARC). 2.0 (CancerBase No 5) IAPCPress; Lyon: 2004. GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide. [Google Scholar]
- 2.Howard SC, Pedrosa M, Lins M, et al. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource-poor area. JAMA. 2004;291:2471–75. doi: 10.1001/jama.291.20.2471. [DOI] [PubMed] [Google Scholar]
- 3.Antillon F, Baez FL, Barr R, et al. AMOR: a proposed cooperative effort to improve outcomes of childhood cancer in Central America. Pediatr Blood Cancer. 2005;45:107–10. doi: 10.1002/pbc.20280. [DOI] [PubMed] [Google Scholar]
- 4.Hesseling P, Broadhead R, Mansvelt E, et al. The 2000 Burkitt lymphoma trial in Malawi. PediatrBlood Cancer. 2005;44:245–50. doi: 10.1002/pbc.20254. [DOI] [PubMed] [Google Scholar]
- 5.Harif M, Barsaoui S, Benchekroun S, et al. Treatment of childhood cancer in Africa. Preliminary results of the French-African Paediatric Oncology Group. Arch Pediatr. 2005;12:851–53. doi: 10.1016/j.arcped.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 6.Magrath I, Shanta V, Advani S, et al. Treatment of acute lymphoblastic leukaemia in countries with limited resources; lessons from use of a single protocol in India over a twenty year period [corrected] Eur J Cancer. 2005;41:157–83. doi: 10.1016/j.ejca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Howard SC, Ribeiro RC, Pui CH. Strategies to improve outcomes of children with cancer in low-income countries. Eur J Cancer. 2005;41:1584–87. doi: 10.1016/j.ejca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Bonilla M, Moreno N, Marina N, et al. Acute lymphoblastic leukemia in a developing country: preliminary results of a nonrandomized clinical trial in El Salvador. J Pediatr Hematol Oncol. 2000;22:495–501. doi: 10.1097/00043426-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Masera G, Baez F, Biondi A, et al. North-South twinning in paediatric haematooncology: the La Mascota programme, Nicaragua. Lancet. 1998;352:1923–26. doi: 10.1016/s0140-6736(98)07077-9. [DOI] [PubMed] [Google Scholar]
- 10.Burton A. The UICC My Child Matters initiative awards: combating cancer in children in the developing world. Lancet Oncol. 2006;7:13–14. doi: 10.1016/s1470-2045(05)70433-2. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro RC, Eden T, Hartford J, et al. My child matters program: a UICC-sanofi-aventis partnership to improve pediatric cancer care in developing countries. Proc Am Soc Clin Oncol. 2007;25:532s. abstr 9526. [Google Scholar]
- 12.International Agency for Research on Cancer . In: International Incidence of Childhood Cancer. Parkin DM, Kramárová E, Draper GJ, et al., editors. volume 2. International Agency for Research on Cancer; Lyon: 1998. IARC Scientific Publications No 144. [Google Scholar]
- 13.Steliarova-Foucher E, Hery C, Pisani P. Childhood cancer: rising to the challenge. UICC; Geneva: 2006. The 10 My Child Matters countries; pp. 15–30. [Google Scholar]
- 14.International Agency for Research on Cancer . In: Cancer Incidence in Five Continents. Curado MP, Edwards B, Shin HR, et al., editors. Vol. IX. IARC; Lyon: 2007. [(accessed Jan 23, 2008)]. IARC Scientific Publications No 160. http://www-dep.iarc.fr/ [Google Scholar]
- 15.Nguyen MQ, Nguyen CH, Kramárová E, Parkin DM. Incidence of childhood cancer in Ho Chi Minh City, Vietnam, 1995-97. Paediatr Perinat Epidemiol. 2000;14:240–47. doi: 10.1046/j.1365-3016.2000.00272.x. [DOI] [PubMed] [Google Scholar]
- 16.US Census Bureau [(accessed March 2, 2008)];International Data Base. http://www.census.gov/ipc/www/idb/summaries.html.
- 17.Steliarova-Foucher E, Stiller C, Kaatsch P, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): an epidemiological study. Lancet. 2004;364:2097–105. doi: 10.1016/S0140-6736(04)17550-8. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization [(accessed March 2, 2008)];National Health Accounts. http://www.who.int/nha/en/
- 19.UNICEF [(accessed March 2, 2008)];The state of the world's children 2007: women and children—the double dividend of gender equality. http://www.unicef.org/publications/index_36587.html.
- 20.United Nations development programme [(accessed March 2, 2008)]; http://www.undp.org/
- 21.UNDP [(accessed May 9, 2008)];Human development reports. http://hdr.undp.org/en/statistics/indices/
- 22.International Monetary Fund [(accessed March 2, 2008)]; http://www.imf.org/external/index.htm.
- 23.Gatta G, Corazziari I, Magnani C, Peris-Bonet R, Roazzi P, Stiller C. Childhood cancer survival in Europe. Ann Oncol. 2003;14(suppl 5):v119–v127. doi: 10.1093/annonc/mdg755. [DOI] [PubMed] [Google Scholar]
- 24.NIH . In: Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. Ries LAG, Smith MA, Gurney JG, et al., editors. National Cancer Institute, SEER Program; Bethesda: 1999. pp. 17–34. NIH publication no 99-4649. [Google Scholar]
- 25.Corrigan JJ, Feig SA. Guidelines for pediatric cancer centers. Pediatrics. 2004;113:1833–35. doi: 10.1542/peds.113.6.1833. [DOI] [PubMed] [Google Scholar]
- 26.Pui CH, Ribeiro RC. International collaboration on childhood leukemia. Int J Hematol. 2003;78:383–89. doi: 10.1007/BF02983810. [DOI] [PubMed] [Google Scholar]
- 27.Usmani GN. Pediatric oncology in the third world. Curr Opin Pediatr. 2001;13:1–9. doi: 10.1097/00008480-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro RC, Pui CH. Saving the children—improving childhood cancer treatment in developing countries. N Engl J Med. 2005;352:2158–60. doi: 10.1056/NEJMp048313. [DOI] [PubMed] [Google Scholar]
- 29.Metzger ML, Howard SC, Fu LC, et al. Outcome of childhood acute lymphoblastic leukaemia in resource-poor countries. Lancet. 2003;362:706–08. doi: 10.1016/S0140-6736(03)14228-6. [DOI] [PubMed] [Google Scholar]
- 30.Arora RS, Eden T, Pizer B. The problem of treatment abandonment in children from developing countries with cancer. Pediatr Blood Cancer. 2007;49:941–46. doi: 10.1002/pbc.21127. [DOI] [PubMed] [Google Scholar]
- 31.Ukrainian Cancer Registry [(accessed March 2, 2008)]; http://users.i.com.ua/~ucr/
- 32.Ministry of Health. Government of Chile [(accessed March 2, 2008)]; http://www.minsal.cl.
- 33.Palma J, Mosso C, Paris C, et al. Establishment of a pediatric HSCT program in a public hospital in Chile. Pediatr Blood Cancer. 2006;46:803–10. doi: 10.1002/pbc.20678. [DOI] [PubMed] [Google Scholar]
- 34.Howard SC, Campana D, Coustan-Smith E, et al. Development of a regional flow cytometry center for diagnosis of childhood leukemia in Central America. Leukemia. 2005;19:323–25. doi: 10.1038/sj.leu.2403624. [DOI] [PubMed] [Google Scholar]
- 35.Leander C, Fu LC, Pena A, et al. Impact of an education program on late diagnosis of retinoblastoma in Honduras. Pediatr Blood Cancer. 2007;49:817–19. doi: 10.1002/pbc.21052. [DOI] [PubMed] [Google Scholar]
- 36.Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer. 2007;112:461–64. doi: 10.1002/cncr.23205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.