Abstract
Background
Young adult survivors of childhood brain tumors (BT) may have late-effects that compromise physical performance and everyday task participation.
Objective
To evaluate muscle strength, fitness, physical performance, and task participation among adult survivors of childhood BT.
Design/Method
In-home evaluations and interviews were conducted for 156 participants (54% male). Results on measures of muscle strength, fitness, physical performance, and participation were compared between survivors and population-group members with chi-squared statistics and two-sample t-tests. Associations between late effects and physical performance, and physical performance and participation, were evaluated in regression models.
Results
BT survivors were a median age of 22 (18–58), and 14.7 (6.5–45.9) years from diagnosis. Survivors had lower estimates of grip strength (Female: 24.7±9.2 vs. 31.5±5.8, Male: 39.0±12.2 vs. 53.0±10.1 kilograms), knee extension strength (Female: 246.6±95.5 vs. 331.5±5.8, Male: 304.7±116.4 vs. 466.6±92.1 Newtons) and peak oxygen uptake (Female: 25.1±8.8 vs. 31.3±5.1, Male: 24.6±9.5 vs. 33.2±3.4 milliliters/kilogram/minute) than population-group members. Physical performance was lower among survivors and associated with not living independently (OR=5.0, 95% CI=2.0–12.2) and not attending college (OR=2.3, 95% CI 1.2–4.4).
Conclusion
Muscle strength and fitness values among BT survivors are similar to those among persons 60+ years, and are associated with physical performance limitations. Physical performance limitations are associated with poor outcomes in home and school environments. These data indicate an opportunity for interventions targeted at improving long-term physical function in this survivor population.
Keywords: physical performance, disability, brain tumor, cancer survivor, pediatric
Brain tumors (BT) account for approximately 20 percent of cancers in children 0–19 years of age. Incidence in the United States is approximately 29/1,000,000 persons or 2,277 new cases per year.1 While multimodal treatment approaches are often successful, resulting in cure for nearly 70% of children with BT, patients frequently suffer significant long term deficits. Physical, sensory, cognitive, neurological, and endocrine complications are reported.2
Impairments of the central nervous system as a consequence of either the tumor or treatment may alter cognitive, emotional, and/or physical performance. Less than optimal function in these domains may influence activities of daily living and greatly affect the BT survivor’s ability to fully participate in expected roles at home, school and work. Although there is a substantial body of literature that documents the prevalence and types of cognitive and emotional problems experienced by childhood BT survivors,3–9 physical performance limitations, while recognized,5, 10–15 are poorly quantified. The etiology of these limitations and their impact on life roles among childhood BT survivors is largely unknown.
We report the results of a study designed to document the prevalence of and risk factors for specific impairments likely to be associated with physical performance limitations. In addition, we assessed the association between observed limitations in physical performance and the inability of BT survivors to fully participate in expected social roles.
Methods
Participants
BT survivors 18 years of age or older, and treated between 1970 and 2000 when younger than 21 years of age, were randomly recruited from clinical populations at St. Jude Children’s Research Hospital and the University of Minnesota Children’s Hospital. Pregnant women or individuals being treated for an active tumor were not eligible. A population-based comparison group was also enrolled and was frequency-matched to participants by age group (18–29, 30–39, 40–49, 50–59 years), sex, and zip code. Lists of randomly selected names and residential addresses were generated for same gender and age group individuals within the zip codes of eligible BT survivors using Melissa Data services (www.melissadata.com). Potentially eligible comparison group members received letters to introduce the study and return post cards to indicate interest/disinterest. Both BT survivors and comparison group members were reimbursed for participation and had home visits to eliminate the potential for healthy participation bias based on inability to travel to the hospital. Institutional approval for Human Subjects Research and consent from all study participants or legal guardians were obtained prior to completing study measures.
Outcomes of interest
The study outcomes, evaluated during the home visit, were body mass index (BMI), sensation, muscle strength, fitness, physical performance and participation. The examiners (CRH, LSG, KKN) measured weight in kilograms and standing height in to the nearest centimeter with a portable electronic scale and a tape measure. BMI was calculated as weight in kilograms divided by height in meters squared, and classified as underweight, normal weight, overweight, and obese (<18.5, 18.5–24.9, 25.0–29.9, 30+ kg/m2). Visual deficits and hearing loss were documented by patient report and verified by examination of patients’ records. Touch sensation was tested with a 5.07 Semmes Weinstein monofilament.16, 17 Hand grip and knee extension strength were assessed using hand held myometry. Muscle weakness was classified as present if values were 2.5 standard deviations or more below expected means for age and sex.18, 19 Exercise tolerance was estimated with the Duke Activity Status Index20 and classified as impaired if estimated peak oxygen uptake was 2.5 standard deviations or more below age and sex predicted values.21
Physical performance limitations were evaluated with the Physical Performance Test (PPT), the Berg Balance Test (Berg) and the Functional Status Index (FSI). The 7-item PPT includes a series of timed tasks: writing a sentence, eating, dressing, picking up a small object, putting an object on a shelf, standing and turning, and walking. The PPT is internally consistent, with excellent test-retest reliability. A score of 28 indicates no physical performance deficit.22 The Berg appraises ability to maintain an upright position during typical movements. The Berg has high internal consistency, inter-rater and intra-rater reliability. A score of ≤ 45 indicates risk for a fall. A person with no impairment will score 56 on the Berg.23 The FSI is a questionnaire that measures physical performance in three dimensions; assistance, difficulty and pain. It is internally consistent and has test-retest reliability. A score of 54 (range 54–252) indicates no disability.24
Participation status was evaluated by asking open-ended questions about employment, education and current living situation. Employment was categorized as employed or student, sheltered employment, or unemployed. Individuals classified in the sheltered employment category required direct supervision/assistance of a caregiver/coach at work. Four survivors required caregiver assistance to answer these questions. Educational attainment was classified as less than high school, high school graduate or more than high school; and living situation was classified as independent, living with family support beyond housing or shared meals, or custodial care. Young adults who were still completing their education but who were living with their parents were included in the independent category.
Demographic and treatment information
Demographic information was obtained from study participants and/or caregivers. Treatment information was obtained from medical records using trained abstractors. Tumor type and location, surgical interventions, and chemotherapy agents and doses were recorded. Cranial and spinal radiation doses were abstracted using written descriptions from medical records, treatment diagrams, and photographs taken in treatment positions. Using methods described by Packer et al,25 four different anatomic segments were identified (frontal cortex, temporal lobe, posterior fossa, and parietal or occipital cortex). Maximum dose was estimated for each segment.
Cognitive performance
Cognitive performance was evaluated with the Kaufman Brief Intelligence Test (K-BIT, Version 2).26 The K-BIT2 results in an Intelligence Quotient Composite with a population mean of 100 and a standard deviation of 15. Internal consistency and test-retest reliability are documented.
Emotional health
Emotional health was evaluated with the 18-item Brief Symptom Inventory (BSI-18). The BSI-18 evaluates mental health globally and across three subscales (depression, somatization, and anxiety).27 It has been validated in cancer survivors and population controls. 27–29
Data analyses
Descriptive statistics were calculated to describe study participants. Percentages for impaired BMI, sensation, muscle strength and fitness were compared between groups (BT survivors and population members) with Fisher’s exact tests.30 Means were calculated for physical, cognitive and emotional performance scales, and compared between groups with two-sample t-tests. Results are presented with effect sizes (mean differences between groups divided by pooled standard deviations).31 Percentages for categories of employment, education and living situation were compared between groups with Fisher’s exact tests.30 In analyses limited to survivors, associations between diagnosis and treatment variables and impairments or physical performance outcomes were evaluated in multiple variable logistic regression models.32 The associations between impairments and scores on the PPT, Berg and FSI were evaluated in multiple variable linear regression models.33 The association between performance limitation and participation were evaluated in multiple variable logistic regression models.32 SAS version 9.2 (Cary N.C.) was used for all analyses.
Results
Participants
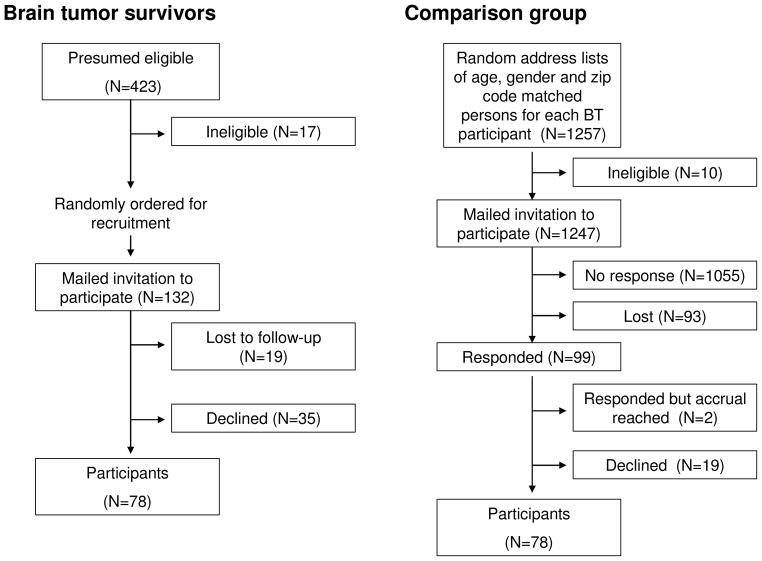
Participants included 78 of the first 132 eligible BT survivors randomly selected for contact. Non-participants included 19 (14.4%) individuals lost to follow-up and 35 (26.5%) individuals who actively or passively declined participation (Figure 1). Comparison group members included 78 of 99 individuals who responded to an invitation mailed to randomly selected names/addresses from a public use database based on BT participants’ ages, sexes and zip codes (Figure 1). BT participants did not differ from non-participants by sex, current age, age at diagnosis, survival time or tumor type (p-values > 0.50). Fifty-four percent (54%) of survivors were male, and 85.9% reported their race/ethnicity as white. Sex and race distributions of comparison group members were identical to those of BT survivors. Survivors current ages ranged from 18.4–58.3 years (median 22 years). Comparison group members were slightly older (median 25 (range 18.0–54.0) years). Additional characteristics of survivors are shown in Table 1. More than half were younger than age 10 years when diagnosed and 84.6% had survived ≥ ten years since diagnosis. The most common tumor type was astrocytic. The most common tumor location was the cerebellum. Surgical resection was included in treatment for 77.0%, cranial radiation for 66.7%, and chemotherapy for 30.8% of survivors.
Figure 1.
Recruitment and participation
Table 1.
Characteristics of brain tumor survivors
| N | % | |
|---|---|---|
| Age at diagnosis | ||
| < 5 years | 22 | (28.2) |
| 5–9 years | 26 | (33.3) |
| 10–14 years | 21 | (26.9) |
| 15–20 years | 9 | (11.5) |
| Time since diagnosis | ||
| 5–9 years | 12 | (15.4) |
| 10–14 years | 30 | (38.5) |
| 15–19 years | 22 | (28.2) |
| 20+ years | 14 | (17.9) |
| Tumor type | ||
| Astrocytic | 40 | (51.3) |
| Medulloblastoma/Ependymoma | 22 | (28.2) |
| Other | 16 | (20.5) |
| Primary tumor location | ||
| Cerebrum | 11 | (14.1) |
| Thalamus | 5 | (6.4) |
| Sellar/parasellar/hypothalamic | 18 | (23.1) |
| Pineal | 4 | (5.1) |
| Brainstem | 8 | (10.3) |
| Brainstem and spine | 2 | (2.6) |
| Cerebellum | 25 | (32.1) |
| Optic nerve | 5 | (6.4) |
| Extent of surgery | ||
| None | 10 | (12.8) |
| Biopsy | 8 | (10.3) |
| Partial resection | 11 | (14.1) |
| Near total resection | 13 | (16.7) |
| Gross total resection | 36 | (46.2) |
| Chemotherapy | ||
| Any | 24 | (30.8) |
| Specific chemotherapy agents | ||
| Vincristine | 19 | (79.2) |
| Cisplatin | 15 | (62.5) |
| Carboplatin | 7 | (29.2) |
| Radiation | ||
| None | 26 | (33.3) |
| Cranial | 52 | (66.7) |
| Cranial and spinal | 23 | (29.5) |
| Median | Range | |
|---|---|---|
| Cranial doses (cGy) | 5400 | 3600–7020 |
| Site specific radiation doses | ||
| Spine | 3600 | 2430–6050 |
| Posterior fossa | 5070 | 0–7020 |
| Temporal lobe | 5040 | 0–7200 |
| Frontal cortex | 3520 | 0–6000 |
| Occipital/parietal lobe | 3520 | 0–7020 |
Impairments
BMI, sensory, muscle strength and fitness impairments are shown in Table 2. Obesity was prevalent in 35.9% of survivors and 26.9% of comparison group members. Less than 4% of comparison group members experienced sensory loss, while 20.5% of survivors had loss of touch sensation, 26.9% had a visual deficit, and 23.1% had hearing loss. Leg muscle weakness was prevalent in 55.1% of survivors and 11.5% of comparison group members. Knee extension strength values among BT survivors were comparable to norms reported for 60–69 year olds in the general population (246.6±95.5 vs. 269.8±81.6 Newtons (N) females; 304.7±116.4 vs. 381.7±80.8 N males).18 Hand grip strength was 2.5 standard deviations or more below expected in 20.5% of survivors and no comparison group members. Poor exercise tolerance was also more common in BT survivors than in the comparison group. Average peak oxygen uptake estimates among (female: 25.1±8.8 milliliters per kilogram per minute (ml/kg/min); male 24.6±9.5 ml/kg/min) BT survivors were within ranges typically seen among 60–69 year olds in the general population (22–27 ml/kg/min females; 26–31 ml/kg/min males).21
Table 2.
Specific impairments among brain tumor survivors and a population frequency matched on sex, age and zip code.
| Survivors | Comparison group | p-value | |||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Body mass index (kg/m2) | |||||
| Underweight (<18.5) | 1 | (1.3) | 4 | (5.1) | 0.37 |
| Normal weight (18.5–24.9) | 26 | (33.3) | 23 | (29.5) | 0.12 |
| Overweight (25–29.9) | 23 | (29.5) | 30 | (38.5) | 0.31 |
| Obese (30+) | 28 | (35.9) | 21 | (26.9) | 0.30 |
| Sensory loss | |||||
| Protective sensation | 16 | (20.5) | 3 | (3.8) | 0.002 |
| Visual deficit | 21 | (26.9) | 1 | (1.3) | <0.001 |
| Hearing loss | 18 | (23.1) | 2 | (2.6) | <0.001 |
| Muscle weakness┼ | |||||
| Hand grip | 16 | (20.5) | 0 | (0.0) | <0.001 |
| Knee extension | 43 | (55.1) | 9 | (11.5) | <0.001 |
| Poor exercise tolerance┼ | 26 | (33.3) | 5 | (6.4) | <0.001 |
| Mean | SD | Mean | SD | p-value | |
|---|---|---|---|---|---|
| Muscle weakness | |||||
| Hand grip (kg) | |||||
| Female | 24.7 | (9.2) | 31.5 | (5.8) | <0.001 |
| Male | 39.0 | (12.2) | 53.0 | (10.1) | <0.001 |
| Knee extension (N) | |||||
| Female | 246.6 | (95.5) | 339.1 | (92.4) | <0.001 |
| Male | 304.7 | (116.4) | 466.6 | (92.1) | <0.001 |
| Exercise tolerance (VO2) ml/kg/min | |||||
| Female | 25.1 | (8.8) | 31.1 | (5.1) | <0.001 |
| Male | 24.6 | (9.5) | 33.2 | (3.4) | <0.001 |
Age and sex matched z-score ≤ −2.5
N=Newtons; kg=kilograms; ml=milliliters; min=minute; VO2=estimated peak oxygen consumption
Performance limitations
Mean scores on physical, cognitive and emotional performance measures are shown in Table 3. Physical performance deficits were evident among BT survivors when compared to the general population with effect sizes of 0.75 or higher for the FSI, Berg, and PPT. On average, cognitive performance was impaired among BT survivors, and not in the comparison group. Emotional health did not differ between the two groups.
Table 3.
Performance and participation outcomes among brain tumor survivors and a population frequency matched on sex, age, and zip code.
| Survivors | Comparison group | Effect size | p-value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Performance | ||||||
| Physical Function | ||||||
| Functional Status Index┼ | 77.3 | 42.3 | 54.8 | 3.1 | 0.75 | <0.001 |
| Berg Balance Test* | 48.6 | 12.4 | 56.0 | 1.0 | 0.85 | <0.001 |
| Physical Performance Test* | 21.5 | 7.9 | 26.8 | 1.4 | 1.06 | <0.001 |
| Kaufmann Brief Intelligence Test 2 | ||||||
| (Composite) | 83.1 | 23.1 | 102.7 | 14.3 | 1.01 | <0.001 |
| Brief Symptom Inventory-18 | ||||||
| Somatization | 50.3 | 9.5 | 48.0 | 6.8 | 0.28 | 0.08 |
| Depression | 49.2 | 10.2 | 47.0 | 8.3 | 0.24 | 0.13 |
| Anxiety | 48.8 | 10.6 | 46.6 | 7.7 | 0.23 | 0.15 |
| Global status index | 49.1 | 11.0 | 46.8 | 8.3 | 0.23 | 0.16 |
| N | % | N | % | ||
|---|---|---|---|---|---|
| Participation | |||||
| Employment | |||||
| Employed/student | 51 | (65.4) | 73 | (93.6) | <0.001 |
| Sheltered employment | 4 | (5.1) | 0 | (0.0) | |
| Unemployed | 23 | (29.5) | 5 | (6.4) | |
| Education | |||||
| < High school | 4 | (5.1) | 2 | (2.6) | <0.001 |
| High school graduate | 27 | (34.6) | 8 | (10.3) | |
| More than high school | 47 | (60.3) | 68 | (87.2) | |
| Living situation | |||||
| Independent | 58 | (74.4) | 78 | (100.0) | <0.001 |
| Family support | 18 | (23.1) | 0 | (0.0) | |
| Custodial care | 2 | (2.6) | 0 | (0.0) | |
a higher score indicates a greater degree of limitation
A lower score indicates a greater degree of limitation
Participation restrictions
The percentage of participants in each category of employment, educational attainment and living situation are shown in Table 3. BT survivors were more likely to be in sheltered employment or unemployed than comparison group members. Survivors were also less likely to have educational achievement post high school or to live independently than comparison group members.
Effects of treatment on BMI, sensation, muscle strength, fitness and overall performance
The results of the final multiple variable logistic regression models designed to evaluate associations between treatment and BMI, sensory, muscle strength and fitness impairments among BT survivors are shown in Table 4. Sex, current age, extent of surgery, and tumor type were included in original models, but did not demonstrate independent associations with the outcomes, nor appreciably alter the strength of associations for other variables, so were not included in final models. After adjusting for tumor location, segment specific doses, and treatment with either vincristine or platinum, age at diagnosis was the only predictor of hand weakness and poor exercise tolerance.
Table 4.
Treatment related predictors of impaired body composition, sensation, strength and exercise tolerance among brain tumor survivors
| Obesity | Loss of touch sensation | Leg weakness | Hand weakness | Reduced exercise tolerance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Tumor location | ||||||||||
| Posterior fossa | 0.7 | 0.3–2.1 | 0.3 | 0.1–1.2 | 1.2 | 0.4–3.3 | 0.4 | 0.1–1.4 | 0.9 | 0.3–3.1 |
| Supratentorial | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Segmental radiation | ||||||||||
| Posterior fossa/spine | ||||||||||
| Yes | 0.2 | 0.1–1.1 | 2.3 | 0.3–20.1 | 1.2 | 0.2–6.9 | 2.3 | 0.2–23.0 | 1.1 | 0.1–8.4 |
| None/scatter | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| Temporal lobe | ||||||||||
| Yes | 1.7 | 0.4–8.1 | 0.6 | 0.1–4.1 | 0.9 | 0.2–3.8 | 1.0 | 0.1–8.0 | 1.5 | 0.2–10.8 |
| None/scatter | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Frontal cortex | ||||||||||
| Yes | 1.7 | 0.3–10.5 | 1.9 | 0.2–15.3 | 3.0 | 0.5–17.0 | 2.3 | 0.3–17.4 | 2.4 | 0.4–14.8 |
| None/scatter | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Occipital/parietal lobe | ||||||||||
| Yes | 3.5 | 0.5–22.8 | 0.5 | 0.1–3.7 | 0.8 | 0.2–4.1 | 0.8 | 0.1–6.0 | 3.1 | 0.5–20.1 |
| None/scatter | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Received platinum or vincristine | ||||||||||
| Yes | 0.2 | 0.1–1.1 | 1.6 | 0.3–7.1 | 3.8 | 0.8–17.8 | 1.2 | 0.3–5.6 | 0.8 | 0.2–3.3 |
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Age at diagnosis | ||||||||||
| < 5 years | 4.2 | 0.9–18.4 | 2.3 | 0.7–7.9 | 0.8 | 0.3–2.6 | 4.7* | 1.4–16.2 | 5.8* | 1.7–20.7 |
| 5–20 years | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
Estimates are statistically significant at p < 0.01
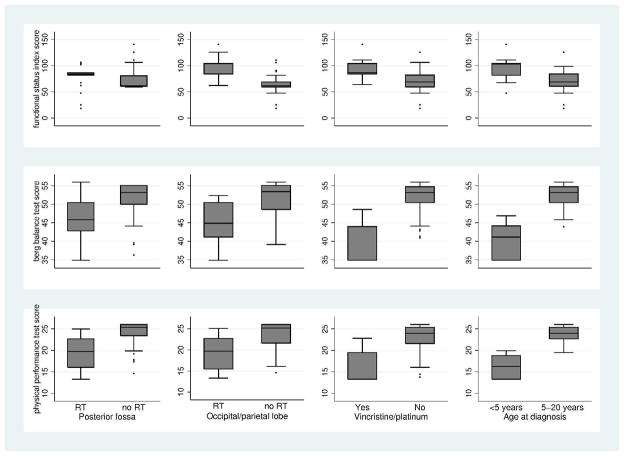
Figure 2 shows the multivariate associations between treatment variables and scores on the PPT, Berg and FSI for BT survivors. After adjusting for tumor location and radiation to the temporal lobe and frontal cortex, radiation to the posterior fossa or occipital/parietal lobe, treatment with platinum or vincristine, and being younger than age 5 at diagnosis were associated with lower scores on the PPT and Berg and higher scores on the FSI. These four treatment variables explained 25% of the variance on the FSI, 26% on the Berg and 34% on the PPT.
Figure 2. The association between cranial radiation, treatment with vincristine or platinum, age at diagnosis and scores on physical performance scales from a linear regression model.
Higher scores on the FSI represent worse performance. Lower scores on the Berg and PPT represent worse performance. Models are adjusted for the other variables in the figure and for radiation to the spine, frontal cortex and temporal lobes.
Effects of BMI, sensation, muscle strength, and fitness on overall performance
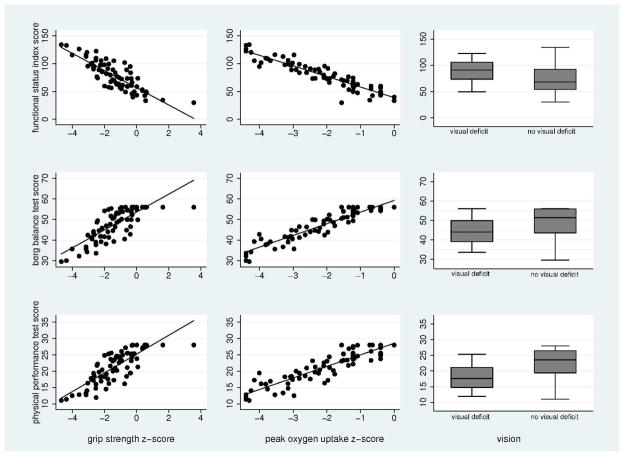
Figure 3 shows the multivariate associations between sensory, muscle strength and fitness impairments and scores on the three physical performance tests for BT survivors. After adjusting for obesity, grip strength, peak oxygen uptake and normal/corrected to normal vision were associated with higher scores on the PPT and Berg and lower scores on the FSI. These three variables explained 36% of the variance on the FSI, 47% on the Berg and 55% on the PPT.
Figure 3. The association between grip strength, peak oxygen uptake and visual deficits and scores on physical performance scales from a linear regression model.
Higher scores on the FSI represent worse performance. Lower scores on the Berg and PPT represent worse performance. Grip strength and peak oxygen uptake z-scores were calculated based on published age and gender matched reference values from “healthy populations.”19, 21
Effects of physical performance on participation
Associations between performance and participation were evaluated in multiple variable models with dependent living status, unemployment and not attending college as separate outcomes. After adjusting for emotional health and cognitive performance, a one standard deviation (SD) decrease (5.87 points) on the PPT increased the odds of a dependent living situation by 5.0 (95% CI 2.0–12.2), and of not attending college by 2.3 (95% CI 1.2–4.4). In the same models, a one SD decrease (15 points) in IQ increased the odds of a dependent living situation by 2.0 (95% CI 1.1–3.7), of unemployment by 1.8 (95% CI 1.1–2.8) and of not attending college by 2.4 (95% CI 1.4–4.0).
Discussion
To our knowledge, this study is one of the first to document specific deficits in muscle force production and exercise tolerance among adult survivors of childhood BT. Young age at diagnosis is the strongest predictor of weakness and poor fitness. In this study, we also describe associations between poor exercise tolerance, muscle weakness and overall physical performance, and between segment specific radiation, treatment with vincristine or platinum, young age at diagnosis and overall physical performance. BT survivors with poor physical performance outcomes face several life challenges. Even after taking into account cognitive status and emotional health, we document that physical performance limitations are associated with restricted participation in both home and educational environments.
Our results are striking, because our measures of muscle strength, peak oxygen uptake, and overall physical performance scores are slightly lower than published reference norms for individuals in the seventh decade of their lives.18, 21 The survivors tested had a median age of 22. This information indicates that childhood BT survivors may be particularly vulnerable to declines in physical performance that typically accompany aging, perhaps increasing their risks for other health problems typically associated inactivity, like osteoporosis, cardiovascular disease and obesity.34–38 Muscle weakness and reduced exercise capacity are known independent predictors of heart disease, osteoporosis, and mortality in the general population,39–44 and may also predict increased risk for chronic disease and early death in childhood BT survivors.45, 46
Our findings of an association between young age at diagnosis and muscle weakness, poor fitness or diminished overall motor performance are consistent with one investigation that included survivors of other types of childhood cancer, and diverge from two other studies that evaluated predictors of motor performance among childhood BT survivors. Talvensaari et al47 reported that age at diagnosis explained 44% of the variation in isokinetic trunk strength among 46 survivors of leukemia or solid tumors a median of 9.4 years off treatment. Conversely, Helseth et al,48 found no association between diagnosis age and score on the locomotion section of the Karnofsky performance index among 28 medulloblastoma survivors. In addition, another study12 reported that boys treated for BT when older than age nine, and who received local irradiation, had the worst motor outcomes when examined by a neurologist.
Again, in contrast to published findings, we found no association between segment specific radiation and impaired fitness. Jakacki et al49 reported maximal cardiac index values below the 5th percentile in 19/26 BT survivors treated with craniospinal radiation, and Jenney et al50 reported an association between craniospinal radiation and reduced values of transfer for carbon monoxide among 70 survivors of childhood leukemia. We did, however, find an association between segment specific radiation and both muscle strength and overall physical performance. These findings are consistent with data from a study that included 75 long term survivors of acute lymphoblastic leukemia51 where cranial radiation was associated with leg weakness, and with data from the Childhood Cancer Survivor Study (CCSS), where self-reported physical performance limitations were 1.4 times more likely among survivors who received radiation compared to those who did not receive radiation.52
The association between grip strength and overall motor performance is supported by literature describing performance outcomes in children with other cancer types. Marchese et al53 reported that knee extension strength explained 63% of the variation in performance on the timed up and go among 16 children with acute lymphoblastic leukemia (ALL). Hartman et al54 described an association between grip strength and performance on the movement ABC among 64 survivors of ALL, Wilms tumor, B-non-Hodgkin lymphoma, or malignant mesenchymal tumors, and Gerber et al55 reported an association between muscle weakness and functional loss among 30 pediatric sarcoma survivors.
The impact of poor overall motor performance on participation in life roles is consistent with other research among childhood BT survivors. Odame et al56 reported an association between self-reported physical performance and participation in physical activity among 25 adolescent survivors of childhood BT, and Sutton et al57 reported an association between self-reported physical performance and score on a BT specific scale of the Functional Assessment of Cancer Therapy Questionnaire (FACT-Br) among survivors of germinoma treated with craniospinal radiation. The FACT-Br includes items related to everyday tasks like driving and attending work. Physical performance was also a strong indicator of participation in the CCSS cohort, where survivors with limitations in physical performance were less likely than those without physical performance limitations to be employed, graduate from high school, or be married.58
We acknowledge that our study has potential limitations. First, our participation rate among survivors was only 59%. It is possible that those who chose to participate in our study had either more or fewer limitations than those who did not participate, which would bias our results. However, participants did not differ from non-participants on demographic and treatment factors, and we made every attempt to allow eligible participants to enroll, performing evaluations in their homes, and accommodating evening and weekend visits. Non-response to the mailed invitation among potential comparison group members was also high. Our comparison group members were not more or less healthy than the general population, except that they had a higher than expected prevalence of leg weakness. This would result in an underestimate of the degree of this impairment among BT survivors. Finally, although we selected validated instruments and measurement techniques for exercise tolerance and muscle strength, used limited numbers of examiners, and conducted extensive training to assure reliability, we did perform testing in the home. The use of self-report data and field testing are subject to measurement error, and may have reduced the precision of our estimates.59, 60
In summary, adult survivors of childhood BT, on average, have significant muscle weakness and poor exercise tolerance. While not unexpected, this research makes clear the strong association between muscle weakness, poor exercise tolerance and overall physical performance, which in turn interfere with abilities to participate fully in life roles. Recognition of this association provides ready targets for interventions to address these important problems. Rehabilitation strategies to restore, teach compensatory strategies for losses in, or recommend environmental adaptations to optimize function should be designed and tested for both adult survivors and for children currently undergoing therapy. Aerobic and resistance training interventions may be particularly beneficial for this population, and will need tailored adaptations to take into account comorbid cognitive and other neurological deficits.
Acknowledgments
Support: Funded by the American Cancer Society - RSGPB-06-210-01-CPPB. Additional funding at St. Jude Children’s Research Hospital provided by ALSAC, and at the University of Minnesota by the Masonic Cancer Center.
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2005 Incidence and Mortality Web-based Report. Vol. 2009. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2009. [Google Scholar]
- 2.Packer RJ. Childhood brain tumors: accomplishments and ongoing challenges. J Child Neurol. 2008;23:1122–7. doi: 10.1177/0883073808320758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DM, Rennie KM, Ziegler RS, Neglia JP, Robison LR, Gurney JG. Medical and neurocognitive late effects among survivors of childhood central nervous system tumors. Cancer. 2001;92:2709–19. doi: 10.1002/1097-0142(20011115)92:10<2709::aid-cncr1625>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Houtzager BA, Grootenhuis MA, Hoekstra-Weebers JE, Caron HN, Last BF. Psychosocial functioning in siblings of paediatric cancer patients one to six months after diagnosis. Eur J Cancer. 2003;39:1423–32. doi: 10.1016/s0959-8049(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 5.Ilveskoski I, Pihko H, Wiklund T, Lamminranta S, Perkkio M, Makipernaa A, et al. Neuropsychologic late effects in children with malignant brain tumors treated with surgery, radiotherapy and “8 in 1” chemotherapy. Neuropediatrics. 1996;27:124–9. doi: 10.1055/s-2007-973762. [DOI] [PubMed] [Google Scholar]
- 6.Mulhern RK, Palmer SL. Neurocognitive late effects in pediatric cancer. Curr Probl Cancer. 2003;27:177–97. doi: 10.1016/s0147-0272(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 7.Reddick WE, White HA, Glass JO, Wheeler GC, Thompson SJ, Gajjar A, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97:2512–9. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- 8.Glauser TA, Packer RJ. Cognitive deficits in long-term survivors of childhood brain tumors. Childs Nerv Syst. 1991;7:2–12. doi: 10.1007/BF00263824. [DOI] [PubMed] [Google Scholar]
- 9.Fuemmeler BF, Elkin TD, Mullins LL. Survivors of childhood brain tumors: behavioral, emotional, and social adjustment. Clin Psychol Rev. 2002;22:547–85. doi: 10.1016/s0272-7358(01)00120-9. [DOI] [PubMed] [Google Scholar]
- 10.Foreman NK, Faestel PM, Pearson J, Disabato J, Poole M, Wilkening G, et al. Health status in 52 long-term survivors of pediatric brain tumors. J Neurooncol. 1999;41:47–53. doi: 10.1023/a:1006145724500. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy CR, Leyland K. Comparison of screening instruments for disability and emotional/behavioral disorders with a generic measure of health-related quality of life in survivors of childhood brain tumors. Int J Cancer Suppl. 1999;12:106–11. doi: 10.1002/(sici)1097-0215(1999)83:12+<106::aid-ijc19>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Macedoni-Luksic M, Jereb B, Todorovski L. Long-term sequelae in children treated for brain tumors: impairments, disability, and handicap. Pediatr Hematol Oncol. 2003;20:89–101. doi: 10.1080/0880010390158595. [DOI] [PubMed] [Google Scholar]
- 13.Jenkin D, Danjoux C, Greenberg M. Subsequent quality of life for children irradiated for a brain tumor before age four years. Med Pediatr Oncol. 1998;31:506–11. doi: 10.1002/(sici)1096-911x(199812)31:6<506::aid-mpo7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald TJ, Rood BR, Santi MR, Vezina G, Bingaman K, Cogen PH, et al. Advances in the diagnosis, molecular genetics, and treatment of pediatric embryonal CNS tumors. Oncologist. 2003;8:174–86. doi: 10.1634/theoncologist.8-2-174. [DOI] [PubMed] [Google Scholar]
- 15.Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Jama. 2003;290:1583–92. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 16.Jeng C, Michelson J, Mizel M. Sensory thresholds of normal human feet. Foot Ankle Int. 2000;21:501–4. doi: 10.1177/107110070002100609. [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Bassini L. A comparison of five tests for determining hand sensibility. J Reconstr Microsurg. 1999;15:523–6. doi: 10.1055/s-2007-1000132. [DOI] [PubMed] [Google Scholar]
- 18.Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78:26–32. doi: 10.1016/s0003-9993(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 19.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 20.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–4. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 21.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 6. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 22.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990;38:1105–12. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 23.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83 (Suppl 2):S7–11. [PubMed] [Google Scholar]
- 24.Jette AM. The Functional Status Index: reliability and validity of a self-report functional disability measure. J Rheumatol. 1987;14 (Suppl 15):15–21. [PubMed] [Google Scholar]
- 25.Packer RJ, Gurney JG, Punyko JA, Donaldson SS, Inskip PD, Stovall M, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21:3255–61. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman A, Kaufman N. Kaufman Brief Intelligence Test, Second Edition (KBIT-2) Vol. 2004. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- 27.Derogotis L. Brief Symptom Inventory (BSI) administration, scoring, and procedures manual. 3. Minneapolis: NCS Pearson, Inc; 2000. [Google Scholar]
- 28.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 29.Recklitis CJ, Parsons SK, Shih MC, Mertens A, Robison LL, Zeltzer L. Factor structure of the brief symptom inventory--18 in adult survivors of childhood cancer: results from the childhood cancer survivor study. Psychol Assess. 2006;18:22–32. doi: 10.1037/1040-3590.18.1.22. [DOI] [PubMed] [Google Scholar]
- 30.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85:87–94. [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 32.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York: Wiley; 2000. [Google Scholar]
- 33.Cohen JPC, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 34.Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;27:2650–6. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leon AS, Connett J, Jacobs DR, Jr, Rauramaa R. Leisure-time physical activity levels and risk of coronary heart disease and death. The Multiple Risk Factor Intervention Trial. Jama. 1987;258:2388–95. [PubMed] [Google Scholar]
- 36.Lissner L, Bengtsson C, Bjorkelund C, Wedel H. Physical activity levels and changes in relation to longevity. A prospective study of Swedish women. Am J Epidemiol. 1996;143:54–62. doi: 10.1093/oxfordjournals.aje.a008657. [DOI] [PubMed] [Google Scholar]
- 37.Yu S, Yarnell JW, Sweetnam PM, Murray L. What level of physical activity protects against premature cardiovascular death? The Caerphilly study. Heart. 2003;89:502–6. doi: 10.1136/heart.89.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int. 1999;9:1–12. doi: 10.1007/s001980050109. [DOI] [PubMed] [Google Scholar]
- 39.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women: A Meta-analysis. JAMA. 2009;301:2024–35. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz JR, Sui X, Lobelo F, Morrow JR, Jr, Jackson AW, Sjostrom M, et al. Association between muscular strength and mortality in men: prospective cohort study. Bmj. 2008;337:a439. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki H, Kasagi F, Yamada M, Fujita S. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med. 2007;120:337–42. doi: 10.1016/j.amjmed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Sirola J, Rikkonen T, Tuppurainen M, Jurvelin JS, Alhava E, Kroger H. Grip strength may facilitate fracture prediction in perimenopausal women with normal BMD: a 15-year population-based study. Calcif Tissue Int. 2008;83:93–100. doi: 10.1007/s00223-008-9155-0. [DOI] [PubMed] [Google Scholar]
- 43.Karkkainen M, Rikkonen T, Kroger H, Sirola J, Tuppurainen M, Salovaara K, et al. Association between functional capacity tests and fractures: an eight-year prospective population-based cohort study. Osteoporos Int. 2008;19:1203–10. doi: 10.1007/s00198-008-0561-y. [DOI] [PubMed] [Google Scholar]
- 44.Gulati M, Arnsdorf MF, Shaw LJ, Pandey DK, Thisted RA, Lauderdale DS, et al. Prognostic value of the duke treadmill score in asymptomatic women. Am J Cardiol. 2005;96:369–75. doi: 10.1016/j.amjcard.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 45.Heikens J, Ubbink MC, van der Pal HP, Bakker PJ, Fliers E, Smilde TJ, et al. Long term survivors of childhood brain cancer have an increased risk for cardiovascular disease. Cancer. 2000;88:2116–21. [PubMed] [Google Scholar]
- 46.Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–79. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talvensaari KK, Jamsen A, Vanharanta H, Lanning M. Decreased isokinetic trunk muscle strength and performance in long-term survivors of childhood malignancies: correlation with hormonal defects. Arch Phys Med Rehabil. 1995;76:983–8. doi: 10.1016/s0003-9993(95)81033-1. [DOI] [PubMed] [Google Scholar]
- 48.Helseth E, Due-Tonnessen B, Wesenberg F, Lote K, Lundar T. Posterior fossa medulloblastoma in children and young adults (0–19 years): survival and performance. Childs Nerv Syst. 1999;15:451–5. doi: 10.1007/s003810050437. discussion 56. [DOI] [PubMed] [Google Scholar]
- 49.Jakacki RI, Goldwein JW, Larsen RL, Barber G, Silber JH. Cardiac dysfunction following spinal irradiation during childhood. J Clin Oncol. 1993;11:1033–8. doi: 10.1200/JCO.1993.11.6.1033. [DOI] [PubMed] [Google Scholar]
- 50.Jenney ME, Faragher EB, Jones PH, Woodcock A. Lung function and exercise capacity in survivors of childhood leukaemia. Med Pediatr Oncol. 1995;24:222–30. doi: 10.1002/mpo.2950240403. [DOI] [PubMed] [Google Scholar]
- 51.Ness KK, Baker KS, Dengel DR, Youngren N, Sibley S, Mertens AC, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:975–81. doi: 10.1002/pbc.21091. [DOI] [PubMed] [Google Scholar]
- 52.Ness KK, Bhatia S, Baker KS, Francisco L, Carter A, Forman SJ, et al. Performance limitations and participation restrictions among childhood cancer survivors treated with hematopoietic stem cell transplantation: the bone marrow transplant survivor study. Arch Pediatr Adolesc Med. 2005;159:706–13. doi: 10.1001/archpedi.159.8.706. [DOI] [PubMed] [Google Scholar]
- 53.Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;42:127–33. doi: 10.1002/pbc.10481. [DOI] [PubMed] [Google Scholar]
- 54.Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in peripheral muscle strength and ankle dorsiflexion as long-term side effects of treatment for childhood cancer. Pediatr Blood Cancer. 2008;50:833–7. doi: 10.1002/pbc.21325. [DOI] [PubMed] [Google Scholar]
- 55.Gerber LH, Hoffman K, Chaudhry U, Augustine E, Parks R, Bernad M, et al. Functional outcomes and life satisfaction in long-term survivors of pediatric sarcomas. Arch Phys Med Rehabil. 2006;87:1611–7. doi: 10.1016/j.apmr.2006.08.341. [DOI] [PubMed] [Google Scholar]
- 56.Odame I, Duckworth J, Talsma D, Beaumont L, Furlong W, Webber C, et al. Osteopenia, physical activity and health-related quality of life in survivors of brain tumors treated in childhood. Pediatr Blood Cancer. 2006;46:357–62. doi: 10.1002/pbc.20512. [DOI] [PubMed] [Google Scholar]
- 57.Sutton LN, Radcliffe J, Goldwein JW, Phillips P, Janss AJ, Packer RJ, et al. Quality of life of adult survivors of germinomas treated with craniospinal irradiation. Neurosurgery. 1999;45:1292–7. doi: 10.1097/00006123-199912000-00002. discussion 97-8. [DOI] [PubMed] [Google Scholar]
- 58.Ness KK, Gurney JG, Zeltzer LK, Leisenring W, Mulrooney DA, Nathan PC, et al. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Arch Phys Med Rehabil. 2008;89:128–36. doi: 10.1016/j.apmr.2007.08.123. [DOI] [PubMed] [Google Scholar]
- 59.Knols RH, Aufdemkampe G, de Bruin ED, Uebelhart D, Aaronson NK. Hand-held dynamometry in patients with haematological malignancies: measurement error in the clinical assessment of knee extension strength. BMC Musculoskelet Disord. 2009;10:1–11. doi: 10.1186/1471-2474-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alonso J, Permanyer-Miralda G, Cascant P, Brotons C, Prieto L, Soler-Soler J. Measuring functional status of chronic coronary patients: Reliability, validity and responsiveness to clinical change of the reduced version of the Duke Activity Status Index (DASI) Eur Heart J. 1997;18:414–19. doi: 10.1093/oxfordjournals.eurheartj.a015260. [DOI] [PubMed] [Google Scholar]