Abstract
Purpose
No reliable classification exists for the therapeutic stratification of children with ependymoma, such that disease-risk might be identified and patients treated to ensure a combination of maximal cure rates and minimal adverse therapeutic effects. This study examined associations between clinicopathological and cytogenetic variables and outcome in a trial cohort of children with ependymoma, with the aim of defining a practical scheme for grading this heterogeneous tumor.
Methods
Intracranial ependymomas (n=146) from children treated on the RT1 trial at St. Jude Children’s Research Hospital were evaluated for the status of multiple pathological features. Interphase FISH (iFISH) defined the status of chromosomes 1q, 6q (LATS1), and 9p21 (CDKN2A). Data relating to these variables were compared with survival data in order to model disease-risk groups.
Results
Extent of surgical resection was a significant determinant of outcome. Tumor cell density and mitotic count were associated with outcome among children with posterior fossa ependymomas (n=119). Among pathologic factors, only brain invasion was associated with outcome in children with supratentorial ependymomas (n=27). Gain of 1q was independently associated with outcome and in combination with clinicopathological variables defined a three-tier system of disease-risk for posterior fossa tumors.
Conclusions
Among children developing posterior fossa ependymomas treated with maximal surgical resection and conformal radiotherapy, key clinicopathological variables and chromosome 1q status can be used to define tiers of disease-risk. In contrast, risk factors for pediatric supratentorial tumors are limited to subtotal resection and brain invasion.
Introduction
Of the three most common central nervous system (CNS) neoplasms presenting in childhood, astrocytoma, medulloblastoma, and ependymoma, it is ependymoma that has the poorest outcome 1,2. Progression-free survival (PFS) for ependymoma is at best 60% after 5 years 3,4, and many survivors suffer the same therapeutic adverse effects that afflict survivors of standard-risk medulloblastoma, with its better 5-year PFS of over 80% 5,6.
Attempts to improve outcome for patients with ependymoma have been frustrated by various factors. Fundamentally, this is a rare disease, which hampers the study of its biology and interpretation of clinical studies based on small patient cohorts. Radiotherapy undeniably improves outcome in ependymoma 3,7,8, but a clear survival advantage to augmenting radiotherapy with a specific chemotherapeutic regimen has yet to be conclusively demonstrated 9,10. In addition, pathological classifications of ependymoma are difficult to apply and of uncertain clinical utility 11,12; few new trials recommend a grading scheme to stratify patients. In recent years, genetic studies of ependymoma have begun to improve our understanding of its biology 13–15, but no robust diagnostic or therapeutic molecular markers are yet in clinical use.
We report a detailed histopathological and cytogenetic analysis of intracranial ependymomas from children treated on the St. Jude Children’s Research Hospital (SJCRH) RT1 trial 4. This trial utilized conformal radiotherapy following a rigorous approach to surgical resection and has the most favorable outcome data reported for pediatric ependymoma. Our aim was to define, for this optimal therapeutic paradigm, a grading scheme of disease-risk based on clinical, pathological, and cytogenetic outcome indicators.
Methods
Patient cohort
The study cohort consisted of intracranial ependymomas from children (n=146) entered onto the RT1 trial at SJCRH. Patient treatment (TEM)... Radiologic assessment of surgical resection -including numbers of GTR vs STR by site... (TEM and/or neuroradiologist). Clinical characteristics of study patients are summarized in Table 1.
Table 1.
| All patients (n=146) | ST tumors (n=27) | PF tumors (n=119) | |
|---|---|---|---|
| Median Age @ Diagnosis (yrs) | 2.5 | 5.6 | 2.3 |
| Sex (M:F) | 1.28:1 | 1.08:1 | 1.33:1 |
| Recurrent tumor: | |||
| none | 100 (68%) | 20 (74%) | 80 (67%) |
| local only | 22 | 4 | 18 |
| metastatic only | 17 | 2 | 15 |
| local & metastatic | 7 | 1 | 6 |
Pathological evaluation
Standard histological preparations from each tumor (formalin-fixed paraffin wax-embedded (FFPE) 5μm sections stained with hematoxylin and eosin) were initially reviewed by one neuropathologist (DWE). The following features from the WHO classification of ependymomas were assessed: cell density, microvascular proliferation (MVP), necrosis, and mitotic count. High cell density was recorded when tumor cells exhibited a high nuclear:cytoplasmic ratio across more than 25% of the sectioned tumor’s area (Supp. Figure 1). Invasion was also assessed where an interface between ependymoma and brain was evident in submitted tissue and was defined as infiltration of adjacent CNS parenchyma by individual tumor cells (Supp. Figure 1). Mitotic count was recorded per 10 high-powered fields (hpfs), preferentially in areas of tumor with high cell density, and a mean value calculated for three series of 10hpfs. When mitotic count was studied as a categorical variable, the integer above the median value for the cohort (mitotic count = 4) was used as a cut-off value. Additionally, the presence of two morphological phenotypes was recorded (Supp. Figure 1), one combining nodules of high cell density with a focal papillary or cerebriform architecture, the ‘biphasic-cerebriform phenotype’, and the other combining a network of fine capillaries with focal clear cell change, the ‘vascular phenotype’ 16. Two other microscopists (CG and JMK) independently evaluated the same pathological features, after the three reviewers had conferred in detail and arrived at a consensus about how each feature was to be evaluated.
Interphase fluorescence in situ hybridization (iFISH)
Multi-color iFISH was performed on 5μm FFPE sections as previously described 17. Probes were derived from BAC clones (BACPAC Resources, Oakland, CA) and labeled with either AlexaFluor-488 or Rhodamine fluorochromes. Probes were co-denatured with target cells on a hotplate at 90°C for 12 minutes. Slides were incubated overnight at 37°C and then washed in 4M Urea/2xSSC at 25°C for 1 minute. Nuclei were counterstained with DAPI (200ng/ml). The following BACs were used to assess copy number abnormalities (CNAs) at genetic loci of interest: EXO1 at 1q43, RP11-610O24 (1p control, CTD-3241G19); LATS1 at 6q25, CTD-2221P18 (6p control, RP11-945O22/RP11-186N7); CDKN2A at 9p21, RP11-149I2/RP11-145E5 (9q control, RP11-235C23).
Statistical analysis
Progression-free survival (PFS) was defined as the interval between end of radiotherapy and date of progression or death, and overall survival (OS) was defined as the interval between end of radiotherapy and death. Patients without an event (PFS or OS) were censored at the time of last follow-up. Survival distributions were estimated using the Kaplan-Meier method and compared between two or more groups of patients using the log-rank test. To investigate associations between multiple covariates and PFS or OS, Cox Proportional Hazards regression models were employed. For pathology review data by three microscopists, descriptive statistics and graphical tools were used to present the level of consensus among reviewers on various pathological features. Logistic regression models were used to investigate the association between a factor of interest and the likelihood of a 3-in-3 consensus on any pathological feature. P-values were not adjusted for multiplicity.
Results
Clinicopathological variables and outcome
PFS and OS at 5 years post-diagnosis for the entire cohort were 68.9% ± 4.1% and 82.6% ± 3.4% respectively. PFS (and OS) for children with supratentorial tumors was slightly better than that for children with posterior fossa tumors (Supp. Figure 2), though this difference was not significant (PFS, P=0.11; OS P=0.44). In contrast, there was a clear association between a favorable outcome and gross total resection; PFS and OS were significantly better for completely resected tumors across all patients (both variables, P<0.0001) and among children with posterior fossa ependymomas alone (both variables, P<0.0001; Supp. Figure 3, Table 2).
Table 2.
Hazard Ratios for PFS & OS in a Cox Proportional Hazards Regression Model Posterior fossa ependymomas (n=119)
| Variable | PFS | OS | ||
|---|---|---|---|---|
| HR | P-value | HR | P-value | |
| Age at diagnosis | 0.92 | 0.13 | 0.95 | 0.42 |
| Sex (male : female) | 1.76 | 0.062 | 2.02 | 0.073 |
| Extent of surgical resection (STR : GTR) | 3.13 | <0.0001 | 3.85 | <0.0001 |
| Cell density (high : low) | 2.95 | 0.004 | 3.74 | 0.009 |
| MVP (present : absent) | 1.42 | 0.29 | 1.86 | 0.17 |
| Necrosis (present : absent) | 1.51 | 0.22 | 1.37 | 0.45 |
| Mitotic count (≥4% : <4%) | 2.13 | 0.009 | 2.46 | 0.013 |
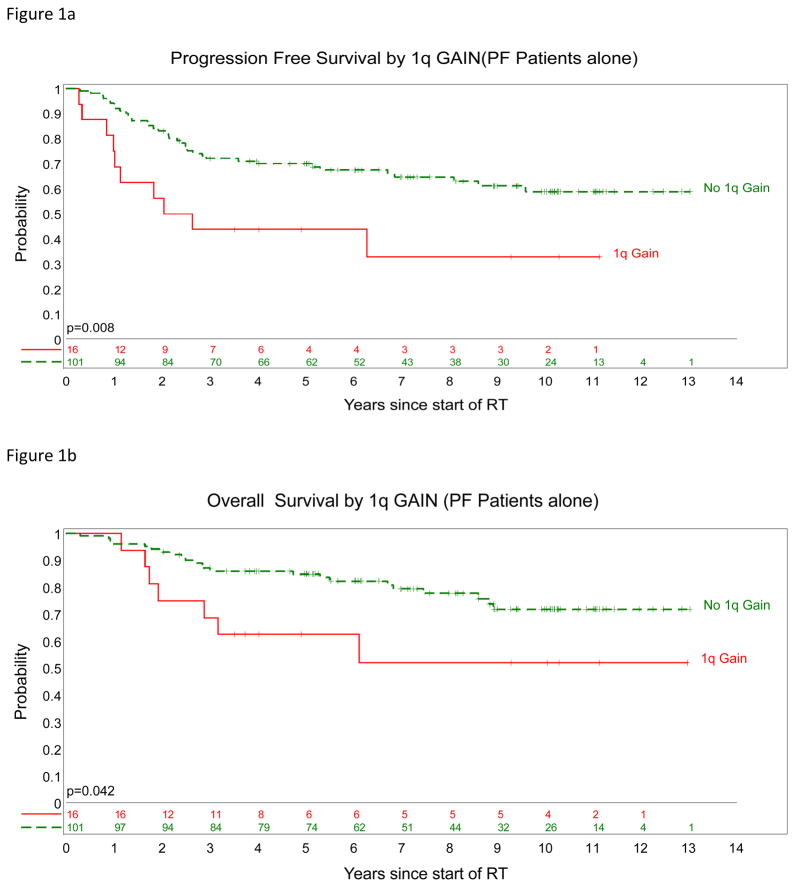
| Chromosome 1q (gain : balance) | 2.51 | 0.008 | 2.35 | 0.042 |
PFS = progression-free survival
OS = overall survival
HR = hazard ratio
STR = sub-total resection
GTR = gross-total resection
MVP = microvascular proliferation
Among children with supratentorial ependymomas (n=27), a gross total resection had been achieved for all those alive and disease-free (n=20; Table 1). Total resection could not be achieved in 3 of 27 cases, and all suffered post-radiotherapy relapse. Of 7 children with recurrent supratentorial disease, 5 had tumors characterized by infiltration of adjacent parenchyma. Only one other supratentorial ependymoma, which had been totally excised from a child now disease-free, showed brain invasion. Brain infiltration was the only histological variable significantly associated with outcome in supratentorial disease (PFS, P=0.008; OS, P=0.003).
Tumor cell invasion could be assessed in few posterior fossa ependymomas, insufficient for meaningful survival analysis. However, cell density and mitotic count both showed (Supp. Figure 4, Table 2) a significant association with PFS (both variables, P=0.01) and OS (P=0.013 and P=0.006, respectively), while necrosis, MVP, and ‘biphasic-cerebriform’ morphology were not associated with outcome (Table 2). A few children (n=5) had posterior fossa ependymomas with very high (>40/10hpfs) mitotic counts, and four of these quickly suffered progressive disease.
Concordance on histopathological interpretation among the three microscopists differed according to variable, being greater for cell density than microvascular proliferation or necrosis (Supp. Table 1). In a regression analysis of mitotic counts, correlation among reviewers was strongly positive and highly significant (all combinations, P<0.0001; Supp. Figure 5). This analysis and a comparison of calls on other variables between combinations of two observers suggested that concordance was associated to only a minor degree with experience of microscopy or familiarity with ependymomas (Supp. Table 1).
Cytogenetic abnormalities
Gain of 1q was observed at diagnosis in sixteen (13%) posterior fossa ependymomas, but in no supratentorial tumor. Hemizygous deletion at the LATS1 locus (6q25) was found in three posterior fossa ependymomas, but in no supratentorial tumor. In contrast, homozygous deletion at the CDKN2A locus (9p21) was detected in two supratentorial ependymomas, but in no posterior fossa tumor. Two of three tumors with LATS1 deletion also had gain of 1q. Monosomy 6 was detected in another eleven ependymomas, nine of which (82%) presented in the posterior fossa.
There was a clear relationship between 1q gain at diagnosis and poor outcome, with P values of 0.003 for PFS and 0.028 for OS (Figure 1, Table 2). Of three children with posterior fossa ependymomas incorporating a hemizygous deletion of LATS1, two have died of disease, and one is alive with disease. Loss at the LATS1 6q25 locus, whether by focal hemizygous deletion or monosomy 6, was not significantly associated with an adverse outcome among posterior fossa tumors (PFS, P=0.11; OS, P=0.35). Of the children with supratentorial tumors characterized by CDKN2A deletion, one has died of disease and the other is alive and disease-free.
Figure 1.
Posterior fossa ependymomas. PFS (a) and OS (b) survival curves split by 1q status; gain (
 ) and no gain (green).
) and no gain (green).
Stratification models of disease risk
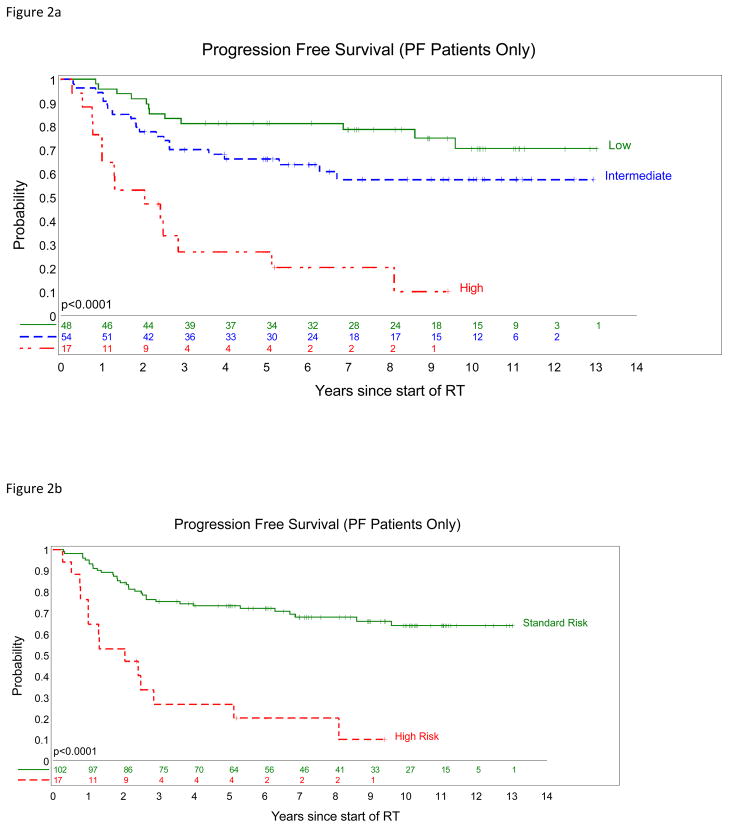
Multivariate survival analysis focused upon posterior fossa ependymomas identifying extent of surgical resection, tumor cell density, and gain of 1q as independent outcome indicators (Table 3). The significance of mitotic count as an outcome indicator was lost in this analysis, because of the close association between (high) cell density and (increased) mitotic count (all reviewers, P<0.0001). If cell density is dropped from the multivariate analysis, then mitotic count is an independent outcome indicator (PFS, HR=1.93, P=0.028). The above variables were used in stratification models of disease risk. In one (Figure 2a), low-risk disease was defined as a totally resected ependymoma with low cell density or mitotic count <4/10hpfs and no 1q gain, and high-risk disease as tumors not totally resected and having 1q gain, high cell density, or a mitotic count ≥4/10hpfs. Remaining tumors were classified as intermediate-risk disease. In another (Figure 2b), high-risk disease was defined as above, and the two other categories were combined as standard-risk.
Table 3.
Hazard Ratios for PFS & OS in a Cox Proportional Hazards Multiple Regression Model Posterior fossa ependymomas (n=119)
| Variable | PFS | OS | ||
|---|---|---|---|---|
| HR | P-value | HR | P-value | |
| Extent of surgical resection (STR : GTR) | 4.76 | <0.0001 | 5.00 | <0.0001 |
| Cell density (high : low) | 3.04 | 0.008 | 3.82 | 0.021 |
| Mitotic count (≥4% : <4%) | 1.28 | 0.45 | 1.17 | 0.71 |
| Chromosome 1q (gain : balance) | 3.04 | 0.006 | 2.33 | 0.086 |
PFS = progression-free survival
OS = overall survival
HR = hazard ratio
STR = sub-total resection
GTR = gross-total resection
Figure 2.
Models of disease risk for posterior fossa ependymomas. PFS (a) low-risk (green) = totally resected tumor with low cell density or mitotic count <4/10hpfs and no 1q gain; high-risk (
 ) = tumors not totally resected and having 1q gain, high cell density, or a mitotic count ≥4/10hpfs. Remaining tumors (
) = tumors not totally resected and having 1q gain, high cell density, or a mitotic count ≥4/10hpfs. Remaining tumors (
 ) were classified as intermediate-risk. PFS (b), high-risk (
) were classified as intermediate-risk. PFS (b), high-risk (
 ) = tumors not totally resected and having 1q gain, high cell density, or a mitotic count ≥4/10hpfs. Remaining tumors (
) = tumors not totally resected and having 1q gain, high cell density, or a mitotic count ≥4/10hpfs. Remaining tumors (
 ) were classified as standard-risk.
) were classified as standard-risk.
Another model derived from cytogenetic studies across all tumors generated three groups: (i) tumors with polysomy 9 and/or loss of chromosome 6 (monosomy 6 or hemizygous deletion of LATS1), (ii) tumors with a balanced profile on chromosomes 1, 6, and 9, and (iii) tumors with gain of 1q and/or homozygous deletion of CDKN2A, and produced survival curves that were not quite statistically different (P=0.08; Supp. Figure 6).
Discussion
Ependymoma is a heterogeneous disease, and this is reflected in histopathological and histogenetic diversity 13. However, our understanding of the biology behind this heterogeneity remains poor and has yet to be translated into refined therapeutic strategies. The WHO classification includes distinct pathological variants of ependymoma, but established clinical associations are limited to the grade I myxopapillary variant and subependymoma; the classic (grade II) tumor and its variants (clear cell, tanycytic, and papillary) or the anaplastic (grade III) tumor have not been linked unequivocally to clinical phenotype or outcome 12.
In particular, the pathologic grading of pediatric intracranial ependymomas has always provoked debate 18–20. From the pathologist’s perspective, there is considerable histological variability among and within tumors, making grading difficult. Such difficulty is reflected by studies that report ratios of grade II to grade III tumors that range between 17:1 and 1:7, a striking discordance that likely represents intratumoral heterogeneity, the uneven application of criteria for anaplasia by review pathologists, and idiosyncratic patient cohorts. Whether children with grade II and those with grade III ependymomas have significantly different outcomes remains unclear; among articles with a focus on prognostic factors, those that do not show histopathological grade as an independent prognostic or predictive factor outnumber those that do 1,8,11,21,22.
In the present study, we chose to study associations between outcome and specific histological variables, rather than tumor grade, in the setting of a large cohort of children with intracranial ependymoma treated uncompromisingly with surgery to remove as much tumor as possible prior to conformal radiotherapy, but without producing significant neurological deficit. This approach has produced the best survival data so far reported in the literature and thus represents the optimal setting in which to determine the role of histopathological evaluation.
Ependymomas uncommonly infiltrate adjacent brain; so a rigorous approach to surgical resection possibly involving several operations to remove residual tumor will probably be more successful with a supratentorial than posterior fossa tumor, as the latter is more likely to be apposed to vital structures. This perspective is supported by lower frequencies of subtotal resection and relapse among supratentorial tumors across our cohort, and it is significant that the only histopathological feature linked to the outcome of children with supratentorial ependymomas was microscopic infiltration of adjacent brain.
With posterior fossa tumors and less chance of a macroscopic or microscopic removal of tumor, other histopathological variables become relevant. In particular, our data suggest that high cell density and mitotic count are significant outcome indicators. Microvascular proliferation and necrosis, which are included among WHO criteria for high-grade gliomas, were not significantly associated with outcome. In ependymomas, regions and particularly nodules characterized by high cell density are often where mitotic activity can be found, and a significant correlation between these two variables was demonstrated across data from each of the three reviewers.
Previous studies have used measures of cell proliferation, including mitotic count, to separate ependymomas into groups with distinct survivals, although widely different cut-off values have prevented consensus on how to integrate such measures into diagnostic practice 21,23–26. Some of these studies also explored the utility of evaluating microvascular proliferation and necrosis to grade ependymomas. Agreement on the presence of these two histopathological features was less frequent among three observers in the present study, suggesting that aspects of their evaluation might be more subjective than for cell density or mitotic count, and this conclusion was similarly reported in a separate study of assessment of histological variables in ependymoma by five neuropathologists 11. In contrast, good concordance on tumor cell density and mitotic count was achieved in this study by three observers with contrasting experience of ependymomas, which varied from (i) pediatric neuropathologist with track record as reviewer for multiple ependymoma trials, through (ii) experienced neuropathologist, to (iii) medical student with four hours of intensive training on the pathologic features of ependymoma and its variants.
Cytogenetic abnormalities in ependymoma have also been the focus of survival studies 14,15,27–30. Gain of 1q is one of the commonest cytogenetic abnormalities in ependymoma (26% in one study) and was detected in 13% of posterior fossa tumors in the present study. It has been shown to associate independently with poor outcome in several cohorts and was a strong predictor of adverse outcome in our study 14,27,30,31. Furthermore, the frequency of 1q gain appears to increase among recurrent ependymomas 30. CNAs at 6q25 (LATS1) and 9p21 (CDKN2A) occurred too infrequently for meaningful outcome analysis in this study, but have been linked to favorable or poor outcome in other studies 14,32. A recent study utilizing iFISH data in a “molecular staging” model proposed three tiers of risk defined by different combinations of cytogenetic abnormalities 14. Our data could be fitted to this model and provided modest support for it, our cytogenetic subgroups just failing to show significant separation on survival analysis.
Studies focused on the biology of ependymoma indicate that the disease is distinct at sites across the neuraxis 13. This anatomic heterogeneity also manifests as distinct histological phenotypes and cytogenetic abnormalities in supratentorial and posterior fossa tumors and argues for a different therapeutic approach to ependymomas above and below the tentorium 16,27. Our data support this proposal; outcome indicators are different for tumors at these two sites. The recommendation for supratentorial ependymomas would be to optimize surgical resection and to regard brain invasion, alone among histopathological features, as a risk factor for recurrence. In contrast, pediatric posterior fossa ependymomas could be stratified into two or three risk groups, using cytogenetic data on 1q status and clinicopathological variables; extent of surgical resection, tumor cell density, and mitotic count.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge support from the CERN foundation and American Lebanese Syrian Associated Charities. This study was conducted with institutional ethics committee approval - St. Jude Children’s Research Hospital XPD07-107/IRB.
References
- 1.McGuire CS, Sainani KL, Fisher PG. Both location and age predict survival in ependymoma: a SEER study. Pediatr Blood Cancer. 2009;52:65–9. doi: 10.1002/pbc.21806. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez D, Cheung MC, Housri N, et al. Outcomes of malignant CNS ependymomas: an examination of 2408 cases through the Surveillance, Epidemiology, and End Results (SEER) database (1973–2005) J Surg Res. 2009;156:340–51. doi: 10.1016/j.jss.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merchant TE, Fouladi M. Ependymoma: new therapeutic approaches including radiation and chemotherapy. J Neurooncol. 2005;75:287–99. doi: 10.1007/s11060-005-6753-9. [DOI] [PubMed] [Google Scholar]
- 4.Merchant TE, Li C, Xiong X, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258–66. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. The lancetoncology. 2006;7:813–20. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 6.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–8. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 7.Merchant TE. Three-dimensional conformal radiation therapy for ependymoma. Childs Nerv Syst. 2009;25:1261–8. doi: 10.1007/s00381-009-0892-9. [DOI] [PubMed] [Google Scholar]
- 8.Shu HK, Sall WF, Maity A, et al. Childhood intracranial ependymoma: twenty-year experience from a single institution. Cancer. 2007;110:432–41. doi: 10.1002/cncr.22782. [DOI] [PubMed] [Google Scholar]
- 9.Grundy RG, Wilne SA, Weston CL, et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol. 2007;8:696–705. doi: 10.1016/S1470-2045(07)70208-5. [DOI] [PubMed] [Google Scholar]
- 10.Massimino M, Gandola L, Barra S, et al. Infant Ependymoma in a 10-year AIEOP (Associazione Italiana Ematologia Oncologia Pediatrica) Experience with Omitted or Deferred Radiotherapy. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 11.Ellison DW, Kocak M, Figarella-Branger D, et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. doi: 10.1186/1477-5751-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfraind C. Classification and controversies in pathology of ependymomas. Childs Nerv Syst. 2009;25:1185–93. doi: 10.1007/s00381-008-0804-4. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RA, Wright KD, Poppleton H, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466:632–6. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korshunov A, Witt H, Hielscher T, et al. Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol. 2010;28:3182–90. doi: 10.1200/JCO.2009.27.3359. [DOI] [PubMed] [Google Scholar]
- 15.Puget S, Grill J, Valent A, et al. Candidate genes on chromosome 9q33-34 involved in the progression of childhood ependymomas. J Clin Oncol. 2009;27:1884–92. doi: 10.1200/JCO.2007.15.4195. [DOI] [PubMed] [Google Scholar]
- 16.Rousseau E, Palm T, Scaravilli F, et al. Trisomy 19 ependymoma, a newly recognized genetico-histological association, including clear cell ependymoma. Mol Cancer. 2007;6:47. doi: 10.1186/1476-4598-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellison DW, Dalton J, Kocak M, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121:381–96. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouffet E, Perilongo G, Canete A, et al. Intracranial ependymomas in children: a critical review of prognostic factors and a plea for cooperation. Med Pediatr Oncol. 1998;30:319–29. doi: 10.1002/(sici)1096-911x(199806)30:6<319::aid-mpo1>3.0.co;2-h. discussion 329–31. [DOI] [PubMed] [Google Scholar]
- 19.Bouffet E, Tabori U, Huang A, et al. Ependymoma: lessons from the past, prospects for the future. Childs Nerv Syst. 2009;25:1383–4. doi: 10.1007/s00381-009-0915-6. author reply 1385. [DOI] [PubMed] [Google Scholar]
- 20.Tihan T, Zhou T, Holmes E, et al. The prognostic value of histological grading of posterior fossa ependymomas in children: a Children’s Oncology Group study and a review of prognostic factors. Mod Pathol. 2008;21:165–77. doi: 10.1038/modpathol.3800999. [DOI] [PubMed] [Google Scholar]
- 21.Figarella-Branger D, Civatte M, Bouvier-Labit C, et al. Prognostic factors in intracranial ependymomas in children. J Neurosurg. 2000;93:605–13. doi: 10.3171/jns.2000.93.4.0605. [DOI] [PubMed] [Google Scholar]
- 22.Gerszten PC, Pollack IF, Martinez AJ, et al. Intracranial ependymomas of childhood. Lack of correlation of histopathology and clinical outcome. Pathol Res Pract. 1996;192:515–22. doi: 10.1016/s0344-0338(96)80100-2. [DOI] [PubMed] [Google Scholar]
- 23.Bennetto L, Foreman N, Harding B, et al. Ki-67 immunolabelling index is a prognostic indicator in childhood posterior fossa ependymomas. Neuropathol Appl Neurobiol. 1998;24:434–40. doi: 10.1046/j.1365-2990.1998.00143.x. [DOI] [PubMed] [Google Scholar]
- 24.Prayson RA. Clinicopathologic study of 61 patients with ependymoma including MIB-1 immunohistochemistry. Ann Diagn Pathol. 1999;3:11–8. doi: 10.1016/s1092-9134(99)80004-5. [DOI] [PubMed] [Google Scholar]
- 25.Preusser M, Heinzl H, Gelpi E, et al. Ki67 index in intracranial ependymoma: a promising histopathological candidate biomarker. Histopathology. 2008;53:39–47. doi: 10.1111/j.1365-2559.2008.03065.x. [DOI] [PubMed] [Google Scholar]
- 26.Zamecnik J, Snuderl M, Eckschlager T, et al. Pediatric intracranial ependymomas: prognostic relevance of histological, immunohistochemical, and flow cytometric factors. Mod Pathol. 2003;16:980–91. doi: 10.1097/01.MP.0000087420.34166.B6. [DOI] [PubMed] [Google Scholar]
- 27.Carter M, Nicholson J, Ross F, et al. Genetic abnormalities detected in ependymomas by comparative genomic hybridisation. Br J Cancer. 2002;86:929–39. doi: 10.1038/sj.bjc.6600180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyer S, Prebble E, Davison V, et al. Genomic imbalances in pediatric intracranial ependymomas define clinically relevant groups. Am J Pathol. 2002;161:2133–41. doi: 10.1016/S0002-9440(10)64491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirose Y, Aldape K, Bollen A, et al. Chromosomal abnormalities subdivide ependymal tumors into clinically relevant groups. Am J Pathol. 2001;158:1137–43. doi: 10.1016/S0002-9440(10)64061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendrzyk F, Korshunov A, Benner A, et al. Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin Cancer Res. 2006;12:2070–9. doi: 10.1158/1078-0432.CCR-05-2363. [DOI] [PubMed] [Google Scholar]
- 31.Rousseau A, Idbaih A, Ducray F, et al. Specific chromosomal imbalances as detected by array CGH in ependymomas in association with tumor location, histological subtype and grade. J Neurooncol. 2010;97:353–64. doi: 10.1007/s11060-009-0039-6. [DOI] [PubMed] [Google Scholar]
- 32.Monoranu CM, Huang B, Zangen IL, et al. Correlation between 6q25.3 deletion status and survival in pediatric intracranial ependymomas. Cancer Genet Cytogenet. 2008;182:18–26. doi: 10.1016/j.cancergencyto.2007.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.