Abstract
Objective
Women with preeclampsia and those who delivered a small-for-gestational-age (SGA) neonate share several mechanisms of disease, including chronic uteroplacental ischemia and failure of physiologic transformation of the spiral arteries. However, the clinical manifestation of these obstetrical syndromes is remarkably different. It has been proposed that an altered maternal metabolic state, as well as a unique circulating cytokines milieu, predispose women to develop either preeclampsia or SGA. Compelling evidence suggests that adipose tissue orchestrates both metabolic pathways and immunological responses via the production of adipokines. Visfatin is a novel adipocytokine with metabolic and immunomodulating properties. The objective of this study was to determine whether preeclampsia and SGA are associated with alterations in maternal circulating visfatin concentrations.
Methods
This cross-secitonal study included pregnant women in the following groups: (1) normal pregnancy (n = 158); (2) patients with preeclampsia (n = 43) of which 32 had an AGA and 11 had an SGA neonate; (3) patients without preeclampsia who delivered an SGA neonate (n = 55). Maternal plasma visfatin concentrations were measured by ELISA. Nonparametric tests and multiple linear regression analysis were used.
Results
(1) Women who delivered an SGA neonate had a higher median maternal plasma visfatin concentration than those with a normal pregnancy (20.0 ng/ml, interquartile range: 17.2-24.6 vs. 15.2 ng/ml, 12.1-19.2, respectively; P < 0.001) than those with preeclampsia (14.5 ng/ml, 12.5-18.7; P < 0.001); (2) the median maternal plasma visfatin concentration did not differ significantly between patients with preeclampsia and those with a normal pregnancy (P = 0.8); (3) among patients with preeclampsia, there was no significant difference in the median maternal plasma visfatin concentration between those with or without an SGA neonate (P = 0.5); (4) in a linear regression model, delivery of an SGA neonate and pregestational body mass index were independently associated with increased visfatin concentration after adjustment for confounding factors (maternal age, smoking, gestational age at blood collection and the presence of preeclampsia or SGA).
Conclusion
(1) Patients with SGA, but not those with preeclampsia, had a higher maternal plasma visfatin concentration than those with a normal pregnancy; (2) this finding suggests differential involvement of visfatin in SGA and preeclampsia; (3) we propose that changes in circulating maternal visfatin concentration may be implicated in the phenotypic definitions and distinction of preeclampsia and SGA.
Keywords: Adipokine, pregnancy, fetal growth, metabolism, obesity
INTRODUCTION
Preeclampsia and small-for-gestational-age (SGA) neonates are two of the most prevalent and important ‘great obstetrical syndromes’ [1]. Consistent with their syndromic nature, and in accordance with the related risk factors [2-11], several common mechanisms of disease have been proposed for both entities, including defective physiologic transformation of the spiral arteries [12-14], an antiangiogenic state [15-35], endothelial cell dysfunction [36-41], chronic uteroplacental ischemia [42,43] and an increased maternal intravascular inflammatory response [32,44-54].
Despite these similarities, the clinical manifestation of preeclampsia and SGA is different. Preeclampsia is characterized by hypertension, proteinuria and multiple organ involvement; in 10-25% of patients, concomitant fetal growth restriction will be diagnosed [55]. SGA is usually defined as a birthweight below the 10th percentile for gestational age at birth according to the birthweight distribution of a particular population [56]. The clinical features of hypertension, proteinuria and multiple organ involvement are not present in patients with isolated SGA. This apparent paradox can be articulated by the following question: why do some patients develop preeclampsia while others develop SGA, in the presence of similar risk factors and underlying mechanisms of disease?
Ness and SIbai [37] have proposed that underlying maternal metabolic derangements are the cause for the distinct clinical manifestations of these complications of pregnancy. Specifically, this concept holds that patients with increased adiposity, insulin resistance/hyperglycemia or dyslipidemia will develop preeclampsia, whereas in those without these metabolic complications, SGA will be the clinical manifestation.
Adipose tissue is now recognized as a highly active endocrine organ [57-60] that can exert its pleiotropic effects via the production and secretion of adipokines. Moreover, Dysregulation of adipokines has been implicated in the pathophysiology of insulin resistance [57,61-63], obesity [64], dyslipidemia and the metabolic syndrome [65]. Consistent with these findings, adipokine have been implicated in metabolic adaptations to normal gestation [57,66-73], as well as in preeclampsia [74-94], SGA [95-97] and other complications of pregnancy [69,93,98-109].
Visfatin, a newly discovered 52 kDa adipokine, has been implicated in regulation of glucose hemostasis [110,111]. Indeed, high circulating concentrations of visfatin are associated with hyperglycemia [112], Type-2 diabetes mellitus (Type-2 DM) [113,114] and gestational diabetes mellitus (GDM) [99,115-118]. Thus, the objective of this study was to determine if preeclampsia and SGA are associated with alterations in maternal circulating visfatin concentrations.
MATERIALS AND METHODS
Study population
A cross-sectional study was conducted by searching our clinical database and bank of biological samples, and included pregnant women in the following groups: (1) normal pregnant women who delivered an appropriate for gestational age (AGA) newborn (n = 158); (2) patients with preeclampsia (n = 43) of which 32 had an AGA and 11 had an SGA neonate; (3) patients without preeclampsia who delivered an SGA neonate (n = 55).
Samples and data were retrieved from our bank of biological samples and clinical databases. Many of these samples have previously been employed to study the biology of inflammation, hemostasis, angiogenesis regulation and growth factor concentrations in normal pregnant women and those with pregnancy complications.
All participating women provided written informed consent prior to enrollment and the collection of blood samples. The collection and use of blood for research purposes was approved by the Institutional Review Boards of the Sotero del Rio Hospital (Santiago, Chile) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH, DHHS, Bethesda, MD).
Definitions
The definition of normal pregnancy included all of the following: (1) no medical, obstetrical or surgical complications; (2) intact membranes; (3) delivery of a term neonate (> 37 weeks) with a birth weight above the 10th percentile [119]; (4) a normal oral 75-g oral glucose tolerance test (OGTT) between 24 and 28 weeks of gestation based on World Health Organization (WHO) criteria [120].
Clinical definitions
The inclusion criteria for normal pregnant women were as follows: singleton gestation, no prior diabetes mellitus, no maternal or fetal complications during pregnancy, normal plasma glucose concentration in the first trimester, normal oral glucose challenges test [121] and delivery at term of a healthy neonate with a birthweight above the 10th percentile for gestational age [122].
Preeclampsia was defined as the presence of hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on at least two occasions, 4 h to 1 week apart) occurring after 20 weeks of gestation in women with previously normal blood pressure, and proteinuria (≥ 300 mg in a 24-h urine collection or at least one dipstick measurement ≥ 1+) [123]. Severe preeclampsia was defined as severe hypertension (diastolic blood pressure ≥ 110 mmHg) plus mild proteinuria, or mild hypertension and sever proteinuria (a 24-h urine sample containing ≥ 3.5 g of protein or a urine specimen ≥ 3+ protein by dipstick measurement on at least two occasions) or severe hypertension plus severe proteinuria [124]. Patients with abnormal liver function test (aspartate aminotransferase > 70 IUI/1) and thrombocytopenia (platelet count < 100,000/cm3) were also classified as having severe preeclampsia [125]. The diagnosis of SGA was based on ultrasonographic estimated fetal weight and confirmed by a birth weight below the 10th percentile for gestational age [56,122].
The body mass index (BMI) was calculated according to the formula: weight (kg)/height (m2). Normal weight women were defined as those with a BMI of 18.5-24.9 kg/m2 according to the definition of the WHO [126].
Sample collection and human visfatin C-terminal immunoassay
Maternal blood samples were collected at clinical visit. Blood was centrifuged at 1300g for 10 min at 4 °C. The plasma obtained was stored at -80 °C until analysis. Concentrations of visfatin in maternal plasma were determined using specific and sensitive enzyme immunoassays (Phoenix Pharmaceuticals, Belmont, CA). An initial assay validation was performed in our laboratory prior to the conduction of this study. Detailed description of the assay has been previously published [73,99,103,107]. The calculated inter and intraassay coefficients of variation for visfatin C-terminal immunoassays in our laboratory were 5.3% and 2.4%, respectively. The sensitivity was calculated to be 0.04 ng/ml.
Statistical analysis
Normality of the data was tested using the Kolmogorov-Smirnov test. Because maternal plasma visfatin concentrations were not normally distributed, Kruskal-Wallis tests with post-hoc analysis by Mann-Whitney U-tests were used for comparisons of continuous variables between the different groups. Comparison of proportions was performed by Chi-square or Fisher’s exact tests. Multiple linear regression analysis was performed to determine which factors were significantly and independently associated with maternal plasma visfatin concentrations; because of skewed distribution, logarithmic (log) transformation was employed in the latter analysis. The following parameters were included in the model: maternal age, maternal pregestational BMI (as continuous variable), gestational age at blood sampling, smoking status and the presence of preeclampsia of SGA. A P-value of < 0.05 was considered statistically significant. Analysis was performed with SPSS< version 14 (SPSS, Chicago, IL).
RESULTS
Demographic and clinical characteristics of the study groups are presented in Table I. The median gestational age at blood sampling was significantly higher in patients with an SGA neonate than in those with either normal pregnancy or preeclampsia. The median gestational age at delivery of patients with preeclampsia was significantly lower than those with either normal pregnancy or SGA (Table I).
Table I.
Clinical and demographic characteristics of the study population.
| Normal pregnancy (n = 158) | P * | Preeclampsia (n = 43) | P χ | SGA (n = 55) | P ‡ | |
|---|---|---|---|---|---|---|
| Maternal age (years) | 26 (22-31) | 0.8 | 26 (21-30) | 0.1 | 22 (19-29) | 0.35 |
| Parity | 1 (0-2) | 0.1 | 1 (0-1) | 0.1 | 1 (0-1) | 0.5 |
| Pre-gestational BMI (kg/m2) | 23.5 (21.9-29.9) | 0.62 | 24.5 (21.1-28.5) | 0.2 | 23.2 (21.1-25.0) | 0.24 |
| GA at sampling (weeks) | 33.0 (28.0-38.7) | 0.22 | 31.7 (29.7-33.8) | < 0.001 | 39.0 (38.0-39.7) | < 0.001 |
| GA at delivery (weeks) | 39.7 (38.7-40.4) | < 0.001 | 32.4 (30.4-34.1) | 0.003 | 38.5 (38.0-39.8) | < 0.001 |
| Birth weight (g) | 3430 (3190-3690) | < 0.001 | 1460 (1130-2010) | < 0.001 | 2600 (2330-2777) | < 0.001 |
Values expressed as median (interquartile range).
GA, gestational age; BMI, body mass index.
P: comparison between normal pregnancy and preeclampsia groups.
P: comparison between normal pregnancy and SGA groups.
P: comparison between preeclampsia and SGA groups.
Visfatin concentrations in patients with preeclampsia and those with an SGA neonate
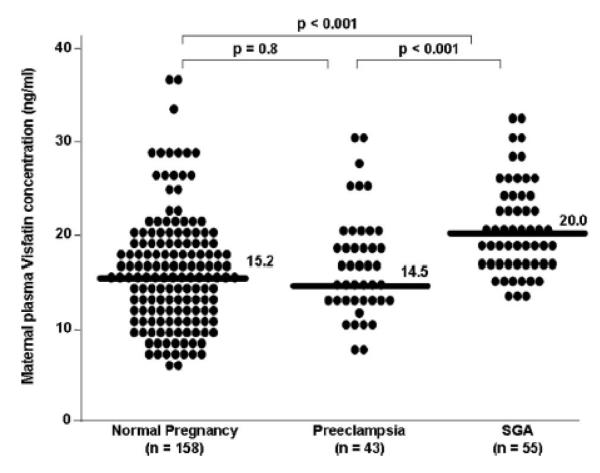
The median maternal plasma concentration of visfatin was significantly higher in patients with an SGA neonate than in those with either normal pregnancy (20.0 ng/ml, interquartile range [IQR]: 17.2-24.6 vs. 15.2 ng/ml, IQR: 12.1-19.2; P < 0.001, Figure 1) or those with preeclampsia (14.5 ng/ml, IQR: 12.5-18.7; P < 0.001, Figure 1). The median maternal plasma visfatin concentration did not differ significantly between patients with preeclampsia and those with a normal pregnancy (P = 0.8, Figure 1).
Figure 1.
Maternal plasma visfatin concentration in normal pregnant women, patients with preeclampsia and patients who delivered an SGA neonate. The median maternal plasma concentration of visfatin was significantly higher in patients with an SGA neonate than in those with either a normal pregnancy (20.0 ng/ml, interquartile range [IQR]: 17.2-24.6 vs. 15.2 ng/ml, IQR: 12.1-19.2, respectively; P < 0.001). The median maternal plasma visfatin concentration did not differ significantly between patients with preeclampsia and those with a normal pregnancy (P = 0.8)
Visfatin concentrations in patients with preeclampsia with and without SGA
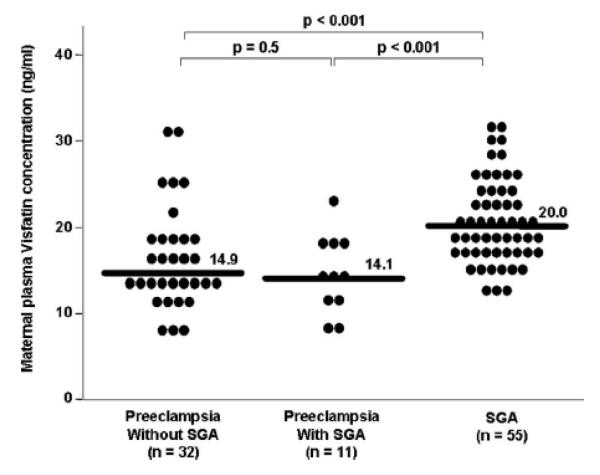
Among patients with preeclampsia, there was no significant difference in the median maternal plasma visfatin concentration between those with and without an SGA neonate (14.1 ng/ml, IQR: 11.8-17.5 vs. 14.9 ng/ml, IQR 12.8-19.0, respectively; P = 0.5, Figure 2). The median maternal plasma concentration of visfatin was significantly higher in patients with an isolated SGA neonate than in those with preeclampsia either with or without an SGA neonate (P < 0.001 for both comparisons, Figure 2).
Figure 2.
Maternal plasma visfatin concentration in patients with preeclampsia who delivered an SGA neonate, patients with preeclampsia who delivered and AGA neonate and patients without preeclampsia who delivered an SGA neonate. Among patients with preeclampsia, there was no significant difference in the median maternal plasma visfatin concentration between those with and without an SGA neonate (14.1 ng/ml, IQR: 11.8-17.5 vs. 14.9 ng/ml, IQR: 12.8-19.0, respectively; P = 0.5). The median maternal plasma concentrations of visfatin were significantly higher in patients with an isolated SGA neonate than in those with preeclampsia either with or without an SGA neonate (P , 0.001 for both comparisons).
Visfatin concentrations in patients with mild vs. severe preeclampsia
Among patients with preeclampsia, there was no significant difference in the median maternal plasma visfatin concentration between those with mild and severe preeclampsia (14.0 ng/ml, IQR: 9.9-21.7 vs. 14.5 ng/ml, IQR: 12.6-18.3, respectively; P = 0.8). The median maternal plasma concentrations of visfatin were significantly higher in patients with an isolated SGA neonate than in those with either mild or severe preeclampsia (P < 0.001 for both comparisons).
Linear regression analysis was used to examine the association between the presence of SGA or preeclampsia and maternal plasma visfatin concentration while adjusting for maternal age, pregestational BMI (as a continuous variable), gestational age at blood sampling and smoking status. The final regression model suggested that the presence of SGA and pregestational BMI were independently associated with maternal plasma concentrations visfatin (P < 0.001 and P = 0.04, respectively).
DISCUSSION
Principal findings of the study
(1) Women who delivered an SGA neonate had a higher median maternal plasma visfatin concentration than those either with normal pregnancy or with preeclampsia; (2) the median maternal plasma visfatin concentration did not differ significantly between patients with preeclampsia and those with normal pregnancy; (3) among patients with preeclampsia, there was no significant difference in the median maternal plasma visfatin concentration between those with or without an SGA neonate.
The physiological role of visfatin
Visfatin, a newly discovered 52 kDa adipokine, was originally identified as a growth factor for early B cell, and thus was termed Pre-B cell colony-enhancing factor (PBEF) [127]. Visfatin/PBEF is preferentially produced by visceral fat depot [111]. However, expression of visfatin is not limited to adipose tissue and it can be expressed in placenta, fetal membranes [128-135], myometrium [136], bone marrow, liver, muscle [127], heart, lung, kidney [127], macrophages [137] and neutrophils [127,138].
Visfatin has been implicated in regulation of glucose homeostasis, as well as in inflammatory response. Several lines of evidence support the role of this adipokine in metabolic regulation: (1) adipocytes secrete visfatin in response to treatment with glucose [112]; (2) visfatin can exert insulin-mimicking effects [110,111] through the activation of an insulin receptor; (3) visfatin deficient mice have an impaired glucose tolerance [139]; (4) a visfatin promoter polymorphism is associated with a susceptibility to Type-2 DM [140].
A compelling body of evidence suggests a role for visfatin as a mediator of the inflammatory response. The following findings characterized visfatin as an immunomodulator: (1) it synergizes with IL-7 and stem cell factors to promote the growth of B-cell precursors [67]; (2) treatment of human monocytes with visfatin results in an increased secretion of IL-6, TNF-α and IL-1β in a dose dependent manner [141]; (3) the expression of visfatin is increased following exposure to TNF-α (in monocytes [137], macrophages [142] and neutrophils [138]), IL-6 (in synovial [143] and amniotic epithelial cells), IL-8 and granulocyte/macrophage colony stimulating factor (in neutrophils [138]); (4) chronic inflammatory disorders such as inflammatory bowel disease [141] and rheumatoid arthritis [144] are associated with higher circulating visfatin concentrations than normal subjects.
Visfatin in normal gestation and in complications of pregnancy
Alterations in circulating adipokines have been associated with adaptations to gestation, as well as in complications of pregnancy [66-72,93,95,98,100-104,106,107]. Consistent with this view, normal pregnancy is associated with high maternal circulating visfatin concentrations [73,145-149]. In addition, gestational diabetes mellitus is associated with altered maternal concentrations of this adipokine than nondiabetic pregnant women [99,115,117,118,150]. Recently, we have reported that intra-amniotic infection/inflammation is associated with higher amniotic fluid concentrations of visfatin than the absence of infection [107]. This finding is in agreement with previous reports in which visfatin expression in fetal membranes increased after exposure to inflammatory stimuli [135,151].
Dysregulation of circulating maternal visfatin concentrations is a feature of patients with an SGA neonate but not of preeclampsia
Reports regarding maternal circulating visfatin concentrations in patients with preeclampsia are scarce and inconsistent. Fasshauer et al. [152] and subsequently Adali et al. [153] reported higher maternal concentrations of circulating visfatin in patients with preeclampsia than in normotensive pregnant women during eh third trimester, while Hu et al. [149] found significantly lower maternal concentrations of this adipokine in preeclampsia than in the control group. Moreover, in the latter study, patients with severe preeclampsia had a significantly lower serum visfatin concentration than those with mild preeclampsia [149]. In contrast to the aforementioned studies, we found no significant differences in maternal circulating visfatin concentrations in pregnant women with and without preeclampsia. Differences in study population and study design may account for these apparent discrepancies. Specifically, the sample size, ethnic origin, gestational age at enrollment, differences in BMI and neonatal birth weights differ among the studies.
Only two studies by Fasshauer et al. [154] and Malamitsi-Puchner et al. [155] reported the results of comparison of maternal visfatin concentrations between the patients with an SGA neonate and those with a normal pregnancy [154,155]. The results reported herein are in agreement with these studies; however, our findings extend the aforementioned reports by demonstrating that patients with an SGA neonate have higher circulating maternal visfatin concentrations than those with preeclampsia. Of note, this novel finding did not change after adjusting for maternal age, maternal pregestational BMI, gestational age at sampling and smoking. In addition, we were able to report that the presence of an SGA neonate in patients with preeclampsia was not accompanied by significant alterations in maternal circulating visfatin concentrations suggesting that the effect of the presence of an SGA neonate on maternal plasma visfatin concentration is overwhelmed by preeclampsia.
Why is visfatin differentially regulated in patients with preeclampsia and in those with SGA neonates?
A lingering conundrum in obstetrics is why preeclampsia and pregnancy complicated by an SGA neonate have profoundly different clinical manifestation, despite their common risk factors and similar mechanisms of disease. Several explanations have been proposed to account for the divergence between preeclampsia and SGA including exposure to infection during pregnancy [156-158], differences in the profile of angiogenic and antiangiogenic response to intrauterine insults [30,35], altered activity of the coagulation system [159,160] and altered concentrations of placental growth hormone [161] and pro-inflammatory chemokines such as CSCL10/IP-10 [32].
Ness and Sibai [37] have proposed that underlying maternal metabolic derangements are the cause for the distinct clinical manifestations of these complications of pregnancy. Specifically, increased maternal adiposity, insulin resistance/hyperglycemia or dyslipidemia are associated with preeclampsia, whereas absence of these metabolic complications is associated with SGA. Indeed, obesity [3,162-164] and insulin resistance [165-168] are independent risk factors for preeclampsia and the latter is associated with dyslipidemia [169,170] as well as with metabolic syndrome-related morbidity [171,172] and mortality [173] later in life. In contrast, maternal overweight/obesity has a protective effect for the development of SGA fetuses [162,174].
The cross-sectional nature of this study limited our ability to infer a causal relationship between visfatin and SGA. However, several explanations can account for the differences in maternal circulating visfatin concentrations between patients with preeclampsia and those with an SGA neonate:
High circulating maternal visfatin concentration confers a beneficiary metabolic effect and thus protects the mother from preeclampsia; Compelling evidence suggests that visfatin may have beneficiary metabolic effects. Indeed, injection of visfatin in mice lowered plasma glucose, and visfatin deficient mice have an impaired glucose tolerance suggesting that high concentrations of visfatin may be associated with insulin sensitivity. Thus, it can be postulated that in the presence of common risk factors for preeclampsia and SGA, elevated visfatin concentration will result in increased insulin sensitivity that is associated with a decreased risk for preeclampsia. In contrast, the failure to increase maternal visfatin concentration may be associated with preeclampsia.
Transport from the fetal to maternal compartment: Ibáñez et al. [175] reported that cord blood visfatin concentration in SGA neonates is higher than AGA neonates. Thus, it can be hypothesized that a transport from fetal to maternal circulation can account for the higher concentrations of this adipokine in patients with an SGA neonate. Nevertheless, the relatively high molecular weight of visfatin (52 kDa) and lack of evidence for a specific mechanism of cross-placental transport do not support this explanation. Furthermore, in a study conducted by Malamitsi-Puchner [155], cord blood visfatin concentration did not differ between SGA and AGA neonates.
In summary, this study is the first to compare circulating maternal visfatin concentrations between patients with an SGA neonate and those with preeclampsia. The novel findings reported herein suggest that changes in circulating maternal visfatin concentration participate in the phenotype definition of preeclampsia and SGA. This observation is in line with and lends credence to the report by Ness and Sibai as well as to the growing body of evidence concerning the role of adipokines in complications of pregnancy.
Acknowledgements
This study was supported by the Perinatology Research Branch, Division of Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
REFERENCES
- 1.Romero R. The child is the father of the man. Prenat Neonat Med. 1996;1:8–11. [Google Scholar]
- 2.Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23:325–328. doi: 10.1055/s-2006-947164. [DOI] [PubMed] [Google Scholar]
- 3.Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, Paul RH. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1995;172:642–648. doi: 10.1016/0002-9378(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 4.Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol. 1994;171:410–416. doi: 10.1016/0002-9378(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 5.Zetterstrom K, Lindeberg SN, Haglund B, Hanson U. Chronic hypertension as a risk factor for offspring to be born small for gestational age. Acta Obstet Gynecol Scand. 2006;85:1046–1050. doi: 10.1080/00016340500442654. [DOI] [PubMed] [Google Scholar]
- 6.Chao AS, Huang JY, Lien R, Kung FT, Chen PJ, Hsieh PC. Pregnancy in women who undergo long-term hemodialysis. Am J Obstet Gynecol. 2002;187:152–156. doi: 10.1067/mob.2002.123200. [DOI] [PubMed] [Google Scholar]
- 7.Ramin SM, Vidaeff AC, Yeomans ER, Gilstrap LC., III Chronic renal disease in pregnancy. Obstet Gynecol. 2006;108:1531–1539. doi: 10.1097/01.AOG.0000246790.84218.44. [DOI] [PubMed] [Google Scholar]
- 8.Brenner B. Thrombophilia and pregnancy complications. Pathophysiol Haemost Thromb. 2006;35:28–35. doi: 10.1159/000093540. [DOI] [PubMed] [Google Scholar]
- 9.Lin J, August P. Genetic thrombophilias and preeclampsia: a meta-analysis. Obstet Gynecol. 2005;105:182–192. doi: 10.1097/01.AOG.0000146250.85561.e9. [DOI] [PubMed] [Google Scholar]
- 10.Carmona F, Font J, Cervera R, Munoz F, Cararach V, Balasch J. Obstetrical outcome of pregnancy in patients with systemic Lupus erythematosus. A study of 60 cases. Eur J Obstet Gynecol Reprod Biol. 1999;83:137–142. doi: 10.1016/s0301-2115(98)00312-1. [DOI] [PubMed] [Google Scholar]
- 11.Moroni G, Quaglini S, Banfi G, Caloni M, Finazzi S, Ambroso G, Como G, Ponticelli C. Pregnancy in lupus nephritis. Am J Kidney Dis. 2002;40:713–720. doi: 10.1053/ajkd.2002.35678. [DOI] [PubMed] [Google Scholar]
- 12.Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol. 1977;84:656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- 13.Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to preeclampsia and fetal growth retardation. Br J Obstet Gynaecol. 1981;88:876–881. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 14.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 15.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 17.Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, Kim CJ, Mittal P, Gotsh F, Erez O, et al. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne’s syndrome) J Matern Fetal Neonatal Med. 2006;19:607–613. doi: 10.1080/14767050600922677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 19.Levine RJ, Thadhani R, Qian C, Lam C, Lim KH, Yu KF, Blink AL, Sachs BP, Epstein FH, Sibai BM, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77–85. doi: 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- 20.Levine RJ, Qian C, Maynard SE, Yu KF, Epstein FH, Karumanchi SA. Serum sFlt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous women. Am J Obstet Gynecol. 2006;194:1034–1041. doi: 10.1016/j.ajog.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 21.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 22.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol. 2006;195:255–259. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 24.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 25.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–1544. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 26.Bujold E, Romero R, Chaiworapongsa T, Kim YM, Kim GJ, Kim MR, Espinoza J, Goncalves LF, Edwin S, Mazor M. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med. 2005;18:9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 27.Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, Edwin S, Kim YM, Erez O, Kusanovic JP, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal NeonatalMed. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotsch F, Romero R, Kusanovic JP, Chaiworapongsa T, Dombrowski M, Erez O, Than NG, Mazaki-Tovi S, Mittal P, Espinoza J, et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: soluble Tie-2. J Matern Fetal Neonatal Med. 2008;21:389–402. doi: 10.1080/14767050802046069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, Edwin S, Nien JK, Chaiworapongsa T, et al. The change in concentrations of angiogenic and antiangiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindheimer MD, Romero R. Emerging roles of antiangiogenic and angiogenic proteins in pathogenesis and prediction of preeclampsia. Hypertension. 2007;50:35–36. doi: 10.1161/HYPERTENSIONAHA.107.089045. [DOI] [PubMed] [Google Scholar]
- 32.Gotsch F, Romero R, Friel L, Kusanovic JP, Espinoza J, Erez O, Than NG, Mittal P, Edwin S, Yoon BH, et al. CXCL10/ IP-10: a missing link between inflammation and antiangiogenesis in preeclampsia? J Matern Fetal Neonatal Med. 2007;20:777–792. doi: 10.1080/14767050701483298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–207. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 35.Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, Kusanovic JP, Erez O, Than NG, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med. 2008;21:25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark BA, Halvorson L, Sachs B, Epstein FH. Plasma endothelin levels in preeclampsia: elevation and correlation with uric acid levels and renal impairment. Am J Obstet Gynecol. 1992;166:962–968. doi: 10.1016/0002-9378(92)91372-h. [DOI] [PubMed] [Google Scholar]
- 37.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195:40–49. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 38.Kraayenbrink AA, Dekker GA, van Kamp GJ, van Geijn HP. Endothelial vasoactive mediators in preeclampsia. Am J Obstet Gynecol. 1993;169:160–165. doi: 10.1016/0002-9378(93)90154-b. [DOI] [PubMed] [Google Scholar]
- 39.Schiff E, Ben-Baruch G, Peleg E, Rosenthal T, Alcalay M, Devir M, Mashiach S. Immunoreactive circulating endothelin-1 in normal and hypertensive pregnancies. Am J Obstet Gynecol. 1992;166:624–628. doi: 10.1016/0002-9378(92)91688-7. [DOI] [PubMed] [Google Scholar]
- 40.Higgins JR, Papayianni A, Brady HR, Darling MR, Walshe JJ. Circulating vascular cell adhesion molecule-1 in preeclampsia, gestational hypertension, and normal pregnancy: evidence of selective dysregulation of vascular cell adhesion molecule-1 homeostasis in pre-eclampsia. Am J Obstet Gynecol. 1998;179:464–469. doi: 10.1016/s0002-9378(98)70380-1. [DOI] [PubMed] [Google Scholar]
- 41.Friedman SA, de Groot CJ, Taylor RN, Golditch BD, Roberts JM. Plasma cellular fibronectin as a measure of endothelial involvement in preeclampsia and intrauterine growth retardation. Am J Obstet Gynecol. 1994;170:838–841. doi: 10.1016/s0002-9378(94)70295-0. [DOI] [PubMed] [Google Scholar]
- 42.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179:1359–1375. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 43.Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH. Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet Gynecol. 2001;18:441–449. doi: 10.1046/j.0960-7692.2001.00572.x. [DOI] [PubMed] [Google Scholar]
- 44.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiff E, Friedman SA, Baumann P, Sibai BM, Romero R. Tumor necrosis factor-alpha in pregnancies associated with preeclampsia or small-for-gestational-age newborns. Am J Obstet Gynecol. 1994;170:1224–1229. doi: 10.1016/s0002-9378(94)70130-x. [DOI] [PubMed] [Google Scholar]
- 46.Sabatier F, Bretelle F, D’Ercole C, Boubli L, Sampol J, Gnat-George F. Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am J Obstet Gynecol. 2000;183:1558–1563. doi: 10.1067/mob.2000.108082. [DOI] [PubMed] [Google Scholar]
- 47.Kusanovic JP, Romero R, Hassan SS, Gotsch F, Edwin S, Chaiworapongsa T, Erez O, Mittal P, Mazaki-Tovi S, Soto E, et al. Maternal serum soluble CD30 is increased in normal pregnancy, but decreased in preeclampsia and small for gestational age pregnancies. J Matern Fetal Neonatal Med. 2007;20:867–878. doi: 10.1080/14767050701482993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Than NG, Erez O, Wildman DE, Tarca AL, Edwin SS, Abbas A, Hotra J, Kusanovic JP, Gotsch F, Hassan SS, et al. Severe preeclampsia is characterized by increased placental expression of galectin-1. J Matern Fetal Neonatal Med. 2008;21:429–442. doi: 10.1080/14767050802041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–797. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 50.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 51.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 52.Chaiworapongsa T, Gervasi MT, Refuerzo J, Espinoza J, Yoshimatsu J, Berman S, Romero R. Maternal lymphocyte subpopulations (CD45RAþ and CD45ROþ) in preeclampsia. Am J Obstet Gynecol. 2002;187:889–893. doi: 10.1067/mob.2002.127309. [DOI] [PubMed] [Google Scholar]
- 53.Erez O, Romero R, Kim SS, Kim JS, Kim YM, Wildman DE, Than NG, Mazaki-Tovi S, Gotsch F, Pineles B, et al. Over-expression of the thrombin receptor (PAR-1) in the placenta in preeclampsia: a mechanism for the intersection of coagulation and inflammation. J Matern Fetal Neonatal Med. 2008;21:345–355. doi: 10.1080/14767050802034859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Than NG, Romero R, Erez O, Kusanovi JP, Tarca AL, Edwin SS, Kim JS, Hassan SS, Espinoza J, Mittal P, et al. A role for mannose-binding lectin, a component of the innate immune system in pre-eclampsia. Am J Reprod Immunol. 2008;60:333–345. doi: 10.1111/j.1600-0897.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 56.Seeds JW. Impaired fetal growth: definition and clinical diagnosis. Obstet Gynecol. 1984;64:303–310. [PubMed] [Google Scholar]
- 57.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, Hauguel-De MS. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–1685. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 58.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 59.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 60.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 61.Kirwan JP, Hauguel-De MS, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, Kalhan SC, Catalano PM. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Bermejo A, Fernandez-Real JM, Garrido E, Rovira R, Brichs R, Genaro P, Bach C, Cabrero D, Kihara S, Funahashi T, et al. Maternal soluble tumour necrosis factor receptor type 2 (sTNFR2) and adiponectin are both related to blood pressure during gestation and infant’s birthweight. Clin Endocrinol (Oxf) 2004;61:544–552. doi: 10.1111/j.1365-2265.2004.02120.x. [DOI] [PubMed] [Google Scholar]
- 63.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27:799–800. doi: 10.2337/diacare.27.3.799. [DOI] [PubMed] [Google Scholar]
- 64.Frayn KN. Obesity and metabolic disease: is adipose tissue the culprit? Proc Nutr Soc. 2005;64:7–13. doi: 10.1079/pns2004403. [DOI] [PubMed] [Google Scholar]
- 65.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 66.Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Curr Diab Rep. 2005;5:278–281. doi: 10.1007/s11892-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 67.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Wiser A, Schiff E, Sivan E. Maternal serum adiponectin levels during human pregnancy. J Perinatol. 2007;27:77–81. doi: 10.1038/sj.jp.7211639. [DOI] [PubMed] [Google Scholar]
- 68.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Efraty Y, Schiff E, Shoham A, Sivan E. Determining the source of fetal adiponectin. J Reprod Med. 2007;52:774–778. [PubMed] [Google Scholar]
- 69.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Gotsch F, Mittal P, Than GN, Nhan-Chang C, Chaiworapongsa T, et al. Adiponectin multimers in maternal plasma. J Matern Fetal Neonatal Med. 2008;21:796–815. doi: 10.1080/14767050802266881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, Pineles BL, Gomez R, Edwin S, Mazor M, et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J PerinatMed. 2007;35:522–531. doi: 10.1515/JPM.2007.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nien JK, Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Pineles BL, Friel LA, Espinoza J, Goncalves L, et al. Resistin: a hormonewhich induces insulin resistance is increased in normal pregnancy. J Perinat Med. 2007;35:513–521. doi: 10.1515/JPM.2007.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, Kanety H. Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab. 2003;88:5656–5660. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- 73.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than NG, Chaiworapongsa T, Nhan-Chang CL, Pacora P, Gotsch F, et al. Maternal visfatin concentration in normal pregnancy. J Perinat Med. 2009;37:206–217. doi: 10.1515/JPM.2009.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ouyang Y, Chen H, Chen H. Reduced plasma adiponectin and elevated leptin in pre-eclampsia. Int J Gynaecol Obstet. 2007;98:110–114. doi: 10.1016/j.ijgo.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 75.Ning Y, Williams MA, Muy-Rivera M, Leisenring WM, Luthy DA. Relationship of maternal plasma leptin and risk of pre-eclampsia: a prospective study. J Matern Fetal Neonatal Med. 2004;15:186–192. doi: 10.1080/14767050410001668293. [DOI] [PubMed] [Google Scholar]
- 76.Kafulafula GE, Moodley J, Ojwang PJ, Kagoro H. Leptin and pre-eclampsia in black African parturients. BJOG. 2002;109:1256–1261. doi: 10.1046/j.1471-0528.2002.02043.x. [DOI] [PubMed] [Google Scholar]
- 77.Chappell LC, Seed PT, Briley A, Kelly FJ, Hunt BJ, Charnock-Jones DS, Mallet AI, Poston L. A longitudinal study of biochemical variables in women at risk of preeclampsia. Am J Obstet Gynecol. 2002;187:127–136. doi: 10.1067/mob.2002.122969. [DOI] [PubMed] [Google Scholar]
- 78.nim-Nyame N, Sooranna SR, Steer PJ, Johnson MR. Longitudinal analysis of maternal plasma leptin concentrations during normal pregnancy and pre-eclampsia. Hum Reprod. 2000;15:2033–2036. doi: 10.1093/humrep/15.9.2033. [DOI] [PubMed] [Google Scholar]
- 79.Teppa RJ, Ness RB, Crombleholme WR, Roberts JM. Free leptin is increased in normal pregnancy and further increased in preeclampsia. Metabolism. 2000;49:1043–1048. doi: 10.1053/meta.2000.7707. [DOI] [PubMed] [Google Scholar]
- 80.McCarthy JF, Misra DN, Roberts JM. Maternal plasma leptin is increased in preeclampsia and positively correlates with fetal cord concentration. Am J Obstet Gynecol. 1999;180:731–736. doi: 10.1016/s0002-9378(99)70280-2. [DOI] [PubMed] [Google Scholar]
- 81.Acromite M, Ziotopoulou M, Orlova C, Mantzoros C. Increased leptin levels in preeclampsia: associations with BMI, estrogen and SHBG levels. Hormones (Athens) 2004;3:46–52. doi: 10.14310/horm.2002.11111. [DOI] [PubMed] [Google Scholar]
- 82.Hendler I, Blackwell SC, Mehta SH, Whitty JE, Russell E, Sorokin Y, Cotton DB. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am J Obstet Gynecol. 2005;193:979–983. doi: 10.1016/j.ajog.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 83.Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in preeclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. 2007;58:21–30. doi: 10.1111/j.1600-0897.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 84.Kocyigit Y, Atamer Y, Atamer A, Tuzcu A, Akkus Z. Changes in serum levels of leptin, cytokines and lipoprotein in pre-eclamptic and normotensive pregnant women. Gynecol Endocrinol. 2004;19:267–273. doi: 10.1080/09513590400018108. [DOI] [PubMed] [Google Scholar]
- 85.Chen D, Dong M, Fang Q, He J, Wang Z, Yang X. Alterations of serum resistin in normal pregnancy and preeclampsia. Clin Sci (Lond) 2005;108:81–84. doi: 10.1042/CS20040225. [DOI] [PubMed] [Google Scholar]
- 86.Cortelazzi D, Corbetta S, Ronzoni S, Pelle F, Marconi A, Cozzi V, Cetin I, Cortelazzi R, Beck-Peccoz P, Spada A. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol (Oxf) 2007;66:447–453. doi: 10.1111/j.1365-2265.2007.02761.x. [DOI] [PubMed] [Google Scholar]
- 87.Haugen F, Ranheim T, Harsem NK, Lips E, Staff AC, Drevon CA. Increased plasma levels of adipokines in preeclampsia: relationship to placenta and adipose tissue gene expression. Am J Physiol Endocrinol Metab. 2006;290:E326–E333. doi: 10.1152/ajpendo.00020.2005. [DOI] [PubMed] [Google Scholar]
- 88.Kajantie E, Kaaja R, Ylikorkala O, Andersson S, Laivuori H. Adiponectin concentrations in maternal serum: elevated in preeclampsia but unrelated to insulin sensitivity. J Soc Gynecol Investig. 2005;12:433–439. doi: 10.1016/j.jsgi.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Lu D, Yang X, Wu Y, Wang H, Huang H, Dong M. Serum adiponectin, leptin and soluble leptin receptor in preeclampsia. Int J Gynaecol Obstet. 2006;95:121–126. doi: 10.1016/j.ijgo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 90.Naruse K, Yamasaki M, Umekage H, Sado T, Sakamoto Y, Morikawa H. Peripheral blood concentrations of adiponectin, an adipocyte-specific plasma protein, in normal pregnancy and preeclampsia. J Reprod Immunol. 2005;65:65–75. doi: 10.1016/j.jri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 91.Ramsay JE, Jamieson N, Greer IA, Sattar N. Paradoxical elevation in adiponectin concentrations in women with preeclampsia. Hypertension. 2003;42:891–894. doi: 10.1161/01.HYP.0000095981.92542.F6. [DOI] [PubMed] [Google Scholar]
- 92.Suwaki N, Masuyama H, Nakatsukasa H, Masumoto A, Sumida Y, Takamoto N, Hiramatrsu Y. Hypoadiponectinemia and circulating angiogenic factors in overweight patients complicated with pre-eclampsia. Am J Obstet Gynecol. 2006;195:1687–1692. doi: 10.1016/j.ajog.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 93.Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, Than NG, Kim SK, Nhan-Chang CL, et al. Maternal serum adiponectin multimers in preeclampsia. J Perinat Med. 2009;37:349–363. doi: 10.1515/JPM.2009.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vaisbuch E, Romero R, Mazaki-Tovi S, Erez O, Kim SK, Chaiwaropongsa T, Gotsch F, Than NG, Dong Z, Pacora P, et al. Retinol binding protein 4 – a novel association with early-onset preeclampsia. J Perinat Med. 2009 doi: 10.1515/JPM.2009.140. DOI: 10.1515/JPM.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiwaropongsa T, Kim SK, Pacora P, Yeo L, Gotsch F, et al. Maternal serum adiponectin multimers in patients with a small-for-gestational-age newborn. J Perinat Med. 2009 doi: 10.1515/JPM.2009.128. DOI: 10.1515/JPM.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kyriakakou M, Malamitsi-Puchner A, Militsi H, Boutsikou T, Margeli A, Hassiakos D, Kanaka-Gantenbein C, Papassotiriou I, Mastorakos G. Leptin and adiponectin concentrations in intrauterine growth restricted and appropriate for gestational age fetuses, neonates, and their mothers. Eur J Endocrinol. 2008;158:343–348. doi: 10.1530/EJE-07-0692. [DOI] [PubMed] [Google Scholar]
- 97.Verhaeghe J, van BR, Van HE. Maternal body size and birth weight: can insulin or adipokines do better? Metabolism. 2006;55:339–344. doi: 10.1016/j.metabol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 98.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Schiff E, Sivan E. Cord blood adiponectin in large-for-gestational age newborns. Am J Obstet Gynecol. 2005;193:1238–1242. doi: 10.1016/j.ajog.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 99.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than NG, Chaiworapongsa T, Nhan-Chang CL, Pacora P, Gotsch F, et al. Visfatin in human pregnancy: maternal gestational diabetes vis-a-vis neonatal birthweight. J Perinat Med. 2009;37:218–231. doi: 10.1515/JPM.2009.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Yinon Y, Wiser A, Schiff E, Sivan E. Adiponectin and leptin concentrations in dichorionic twins with discordant and concordant growth. J Clin Endocrinol Metab. 2009;94:892–898. doi: 10.1210/jc.2008-2118. [DOI] [PubMed] [Google Scholar]
- 101.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiwaropongsa T, Kim SK, Pacora P, Yeo L, Gotsch F, et al. Maternal serum adiponectin multimers in gestational diabetes. J Perinat Med. 2009 doi: 10.1515/JPM.2009.101. DOI: 10.1515/JPM.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiwaropongsa T, Kim SK, Pacora P, Yeo L, Gotsch F, et al. Dysregulation of maternal serum adiponectin in preterm labor. J Matern Fetal Neonatal Med. 2009;22:887–904. doi: 10.1080/14767050902994655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Chaiwaropongsa T, Mittal P, Kim SK, Pacora P, Gotsch F, Dong Z, et al. Maternal plasma visfatin in preterm labor. J Matern Fetal Neonatal Med. 2009;22:693–704. doi: 10.1080/14767050902994788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, Pineles BL, Gomez R, Edwin S, Mazor M, et al. Adiponectin in severe preeclampsia. J Perinat Med. 2007;35:503–512. doi: 10.1515/JPM.2007.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Erez O, Than GN, Kim SK, Dong M, Gotsch F, Mittal P, Chaiworapongsa T, et al. Retinol binding protein 4: an adipokine associated with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. doi: 10.3109/14767050902994739. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, Erez O, Vaisbuch E, Edwin SS, Than NG, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21:902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, Than NG, Nhan-Chang CL, Hamill N, Vaisbuch E, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med. 2008;36:485–496. doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Mittal P, Gotsch F, Chaiworapongsa T, Than NG, Kim SK, et al. Adiponectin in amniotic fluid in normal pregnancy, spontaneous labor at term, and preterm labor: a novel association with subclinical intrauterine infection/ inflammation. J Matern Fetal Neonatal Med. 2009 doi: 10.3109/14767050903026481. DOI: 10.1080/14767050903026481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mazaki-Tovi S, Romero R, Vaisbuch E, Chaiworapongsa T, Erez O, Mittal P, Kim SK, Gotsch F, Lamont RF, Ogge G, et al. Low circulating maternal adiponectin in patients with pyelonephritis: adiponectin at the crossroads of pregnancy and infection. J Perinat Med. 2009 doi: 10.1515/JPM.2009.134. DOI: 10.1515/JPM.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ, Zhou HD, Wu XP, Liao EY. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. 2007;80:201–210. doi: 10.1007/s00223-006-0155-7. [DOI] [PubMed] [Google Scholar]
- 111.Sethi JK, Vidal-Puig A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol Med. 2005;11:344–347. doi: 10.1016/j.molmed.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haider DG, Schaller G, Kapiotis S, Maier C, Luger A, Wolzt M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49:1909–1914. doi: 10.1007/s00125-006-0303-7. [DOI] [PubMed] [Google Scholar]
- 113.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, Lee YJ. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 114.Lopez-Bermejo A, Chico-Julia B, Fernandez-Balsells M, Recasens M, Esteve E, Casamitjana R, Ricart W, Fernandez-Real JM. Serum visfatin increases with progressive beta-cell deterioration. Diabetes. 2006;55:2871–2875. doi: 10.2337/db06-0259. [DOI] [PubMed] [Google Scholar]
- 115.Chan TF, Chen YL, Lee CH, Chou FH, Wu LC, Jong SB, Tsai EM. Decreased plasma visfatin concentrations in women with gestational diabetes mellitus. J Soc Gynecol Investig. 2006;13:364–367. doi: 10.1016/j.jsgi.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 116.Haider DG, Handisurya A, Storka A, Vojtassakova E, Luger A, Pacini G, Tura A, Wolzt M, Kautzky-Willer A. Visfatin response to glucose is reduced in women with gestational diabetes mellitus. Diabetes Care. 2007;30:1889–1891. doi: 10.2337/dc07-0013. [DOI] [PubMed] [Google Scholar]
- 117.Krzyzanowska K, Krugluger W, Mittermayer F, Rahman R, Haider D, Shnawa N, Schernthaner G. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci (Lond) 2006;110:605–609. doi: 10.1042/CS20050363. [DOI] [PubMed] [Google Scholar]
- 118.Lewandowski KC, Stojanovic N, Press M, Tuck SM, Szosland K, Bienkiewicz M, Vatish M, Lewinski A, Prelevic GM, Randeva HS. Elevated serum levels of visfatin in gestational diabetes: a comparative study across various degrees of glucose tolerance. Diabetologia. 2007;50:1033–1037. doi: 10.1007/s00125-007-0610-7. [DOI] [PubMed] [Google Scholar]
- 119.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 120.Prevention of diabetes mellitus. Report of a WHO study group. World Health Organ Tech Rep Ser. 1994;844:1–100. [PubMed] [Google Scholar]
- 121.ACOG Practice Bulletin Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994) Gestational diabetes. Obstet Gynecol. 2001;98:525–538. [PubMed] [Google Scholar]
- 122.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, Carstens MR, Medina LH, Viviani PG, Rojas IT. [A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000] Rev Med Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 123.ACOG practice bulletin Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 124.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, Joffe G. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177:1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 125.ACOG technical bulletin Hypertension in pregnancy. Number 219–January 1996 (replaces no. 91, February 1986). Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1996;53:175–183. [PubMed] [Google Scholar]
- 126.Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser. 2003;916:1–149. [PubMed] [Google Scholar]
- 127.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol Hum Reprod. 2002;8:399–408. doi: 10.1093/molehr/8.4.399. [DOI] [PubMed] [Google Scholar]
- 129.Nemeth E, Tashima LS, Yu Z, Bryant-Greenwood GD. Fetal membrane distention: I. Differentially expressed genes regulated by acute distention in amniotic epithelial (WISH) cells. Am J Obstet Gynecol. 2000;182:50–59. doi: 10.1016/s0002-9378(00)70490-x. [DOI] [PubMed] [Google Scholar]
- 130.Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26:107–117. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 131.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colonyenhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol. 2002;187:1051–1058. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 132.Ognjanovic S, Tashima LS, Bryant-Greenwood GD. The effects of pre-B-cell colony-enhancing factor on the human fetal membranes by microarray analysis. Am J Obstet Gynecol. 2003;189:1187–1195. doi: 10.1067/s0002-9378(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 133.Ognjanovic S, Ku TL, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor is a secreted cytokine-like protein from the human amniotic epithelium. Am J Obstet Gynecol. 2005;193:273–282. doi: 10.1016/j.ajog.2004.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nemeth E, Millar LK, Bryant-Greenwood G. Fetal membrane distention. II. Differentially expressed genes regulated by acute distention in vitro. Am J Obstet Gynecol. 2000;182:60–67. doi: 10.1016/s0002-9378(00)70491-1. [DOI] [PubMed] [Google Scholar]
- 135.Kendal-Wright CE, Hubbard D, Bryant-Greenwood GD. Chronic stretching of amniotic epithelial cells increases pre-B cell colony-enhancing factor (PBEF/visfatin) expression and protects them from apoptosis. Placenta. 2008;29:255–265. doi: 10.1016/j.placenta.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 136.Esplin MS, Fausett MB, Peltier MR, Hamblin S, Silver RM, Branch DW, Adashi EY, Whiting D. The use of cDNA microarray to identify differentially expressed laborassociated genes within the human myometrium during labor. Am J Obstet Gynecol. 2005;193:404–413. doi: 10.1016/j.ajog.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 137.Dahl TB, Yndestad A, Skjelland M, Oie E, Dahl A, Michelsen A, Damas JK, Tunheim SH, Ueland T, Smith C, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972–980. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- 138.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, Marshall JC. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang YY, Gottardo L, Thompson R, Powers C, Nolan D, Duffy J, Marescotti MC, Avogaro A, Doria A. A visfatin promoter polymorphism is associated with low-grade inflammation and type 2 diabetes. Obesity (Silver Spring) 2006;14:2119–2126. doi: 10.1038/oby.2006.247. [DOI] [PubMed] [Google Scholar]
- 141.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 142.Iqbal J, Zaidi M. TNF regulates cellular NADþ metabolism in primary macrophages. Biochem Biophys Res Commun. 2006;342:1312–1318. doi: 10.1016/j.bbrc.2006.02.109. [DOI] [PubMed] [Google Scholar]
- 143.Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, Williams AS, Bryant-Greenwood G, Jones SA. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006;54:2084–2095. doi: 10.1002/art.21942. [DOI] [PubMed] [Google Scholar]
- 144.Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, Gualillo O. Changes in plasmalevels of fat-derivedhormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mastorakos G, Valsamakis G, Papatheodorou DC, Barlas I, Margeli A, Boutsiadis A, Kouskouni E, Vitoratos N, Papadimitriou A, Papassotiriou I, et al. The role of adipocytokines in insulin resistance in normal pregnancy: visfatin concentrations in early pregnancy predict insulin sensitivity. Clin Chem. 2007;53:1477–1483. doi: 10.1373/clinchem.2006.084731. [DOI] [PubMed] [Google Scholar]
- 146.Morgan SA, Bringolf JB, Seidel ER. Visfatin expression is elevated in normal human pregnancy. Peptides. 2008;29:1382–1389. doi: 10.1016/j.peptides.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 147.Katwa LC, Seidel ER. Visfatin in pregnancy: proposed mechanism of peptide delivery. Amino Acids. 2008 doi: 10.1007/s00726-008-0194-7. DOI: 10.1007/s00726-008-0194-7. [DOI] [PubMed] [Google Scholar]
- 148.Szamatowicz J, Kuzmicki M, Telejko B, Zonenberg A, Nikolajuk A, Kretowski A, Gorska M. Serum visfatin concentration is elevated in pregnant women irrespectively of the presence of gestational diabetes. Ginekol Pol. 2009;80:14–18. [PubMed] [Google Scholar]
- 149.Hu W, Wang Z, Wang H, Huang H, Dong M. Serum visfatin levels in late pregnancy and pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87:413–418. doi: 10.1080/00016340801976012. [DOI] [PubMed] [Google Scholar]
- 150.Akturk M, Altinova AE, Mert I, Buyukkagnici U, Sargin A, Arslan M, Danisman N. Visfatin concentration is decreased in women with gestational diabetes mellitus in the third trimester. J Endocrinol Invest. 2008;31:610–613. doi: 10.1007/BF03345611. [DOI] [PubMed] [Google Scholar]
- 151.Kendal-Wright CE. Stretching, mechanotransduction, and proinflammatory cytokines in the fetal membranes. Reprod Sci. 2007;14:35–41. doi: 10.1177/1933719107310763. [DOI] [PubMed] [Google Scholar]
- 152.Fasshauer M, Waldeyer T, Seeger J, Schrey S, Ebert T, Kratzsch J, Lossner U, Bluher M, Stumvoll M, Faber R, et al. Serum levels of the adipokine visfatin are increased in pre-eclampsia. Clin Endocrinol (Oxf) 2008;69:69–73. doi: 10.1111/j.1365-2265.2007.03147.x. [DOI] [PubMed] [Google Scholar]
- 153.Adali E, Yildizhan R, Kolusari A, Kurdoglu M, Bugdayci G, Sahin HG, Kamaci M. Increased visfatin and leptin in pregnancies complicated by pre-eclampsia. J Matern Fetal Neonatal Med. 2009;22:873–879. doi: 10.1080/14767050902994622. [DOI] [PubMed] [Google Scholar]
- 154.Fasshauer M, Bluher M, Stumvoll M, Tonessen P, Faber R, Stepan H. Differential regulation of visfatin and adiponectin in pregnancies with normal and abnormal placental function. Clin Endocrinol (Oxf) 2007;66:434–439. doi: 10.1111/j.1365-2265.2007.02751.x. [DOI] [PubMed] [Google Scholar]
- 155.Malamitsi-Puchner A, Briana DD, Boutsikou M, Kouskouni E, Hassiakos D, Gourgiotis D. Perinatal circulating visfatin levels in intrauterine growth restriction. Pediatrics. 2007;119:e1314–e1318. doi: 10.1542/peds.2006-2589. [DOI] [PubMed] [Google Scholar]
- 156.Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba’aqeel H, Farnot U, Bergsjo P, Bakketeig L, Lumbiganon P, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194:921–931. doi: 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- 157.von DP, Magee LA. Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction? Acta Obstet Gynecol Scand. 2002;81:642–648. [PubMed] [Google Scholar]
- 158.Hill JA, Devoe LD, Bryans CI., Jr Frequency of asymptomatic bacteriuria in preeclampsia. Obstet Gynecol. 1986;67:529–532. [PubMed] [Google Scholar]
- 159.Erez O, Romero R, Hoppensteadt D, Than NG, Fareed J, Mazaki-Tovi S, Espinoza J, Chaiworapongsa T, Kim SS, Yoon BH, et al. Tissue factor and its natural inhibitor in preeclampsia and SGA. J Matern Fetal Neonatal Med. 2008;21:855–869. doi: 10.1080/14767050802361872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Erez O, Hoppensteadt D, Romero R, Espinoza J, Goncalves L, Nien JK, Kusanovic JP, Fareed J, Gotsch F, Pineles B, et al. Preeclampsia is associated with low concentrations of protein Z. J Matern Fetal Neonatal Med. 2007;20:661–667. doi: 10.1080/14767050701495011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mittal P, Espinoza J, Hassan S, Kusanovic JP, Edwin SS, Nien JK, Gotsch F, Than NG, Erez O, Mazaki-Tovi S, et al. Placental growth hormone is increased in the maternal and fetal serum of patients with preeclampsia. J Matern Fetal Neonatal Med. 2007;20:651–659. doi: 10.1080/14767050701463571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol. 2007;109:419–433. doi: 10.1097/01.AOG.0000253311.44696.85. [DOI] [PubMed] [Google Scholar]
- 163.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, Saade G, Eddleman K, Carter SM, Craigo SD, et al. Obesity, obstetric complications and cesarean delivery rate – a population-based screening study. Am J Obstet Gynecol. 2004;190:1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 164.Eskenazi B, Fenster L, Sidney S. A multivariate analysis of risk factors for preeclampsia. JAMA. 1991;266:237–241. [PubMed] [Google Scholar]
- 165.Joffe GM, Esterlitz JR, Levine RJ, Clemens JD, Ewell MG, Sibai BM, Catalano PM. The relationship between abnormal glucose tolerance and hypertensive disorders of pregnancy in healthy nulliparous women. Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1998;179:1032–1037. doi: 10.1016/s0002-9378(98)70210-8. [DOI] [PubMed] [Google Scholar]
- 166.Solomon CG, Seely EW. Brief review: hypertension in pregnancy: a manifestation of the insulin resistance syndrome? Hypertension. 2001;37:232–239. doi: 10.1161/01.hyp.37.2.232. [DOI] [PubMed] [Google Scholar]
- 167.Wolf M, Sandler L, Munoz K, Hsu K, Ecker JL, Thadhani R. First trimester insulin resistance and subsequent preeclampsia: a prospective study. J Clin Endocrinol Metab. 2002;87:1563–1568. doi: 10.1210/jcem.87.4.8405. [DOI] [PubMed] [Google Scholar]
- 168.Roberts JM, Gammill H. Insulin resistance in preeclampsia. Hypertension. 2006;47:341–342. doi: 10.1161/01.HYP.0000205123.40068.84. [DOI] [PubMed] [Google Scholar]
- 169.Gratacos E, Casals E, Sanllehy C, Cararach V, Alonso PL, Fortuny A. Variation in lipid levels during pregnancy in women with different types of hypertension. Acta Obstet Gynecol Scand. 1996;75:896–901. doi: 10.3109/00016349609055024. [DOI] [PubMed] [Google Scholar]
- 170.Thadhani R, Stampfer MJ, Hunter DJ, Manson JE, Solomon CG, Curhan GC. High body mass index and hypercholesterolemia: risk of hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94:543–550. doi: 10.1016/s0029-7844(99)00400-7. [DOI] [PubMed] [Google Scholar]
- 171.Sibai BM, Sarinoglu C, Mercer BM. Eclampsia. VII. Pregnancy outcome after eclampsia and long-term prognosis. Am J Obstet Gynecol. 1992;166:1757–1761. doi: 10.1016/0002-9378(92)91566-s. [DOI] [PubMed] [Google Scholar]
- 172.Girouard J, Giguere Y, Moutquin JM, Forest JC. Previous hypertensive disease of pregnancy is associated with alterations of markers of insulin resistance. Hypertension. 2007;49:1056–1062. doi: 10.1161/HYPERTENSIONAHA.107.087528. [DOI] [PubMed] [Google Scholar]
- 173.Jonsdottir LS, Arngrimsson R, Geirsson RT, Sigvaldason H, Sigfusson N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995;74:772–776. doi: 10.3109/00016349509021195. [DOI] [PubMed] [Google Scholar]
- 174.Andreasen KR, Andersen ML, Schantz AL. Obesity and pregnancy. Acta Obstet Gynecol Scand. 2004;83:1022–1029. doi: 10.1111/j.0001-6349.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- 175.Ibanez L, Sebastiani G, Lopez-Bermejo A, Diaz M, Gomez-Roig MD, de ZF. Gender specificity of body adiposity and circulating adiponectin, visfatin, insulin, and insulin growth factor-I at term birth: relation to prenatal growth. J Clin Endocrinol Metab. 2008;93:2774–2778. doi: 10.1210/jc.2008-0526. [DOI] [PubMed] [Google Scholar]