Abstract
Mast cells are derived from the hematopoietic progenitors found in bone marrow and spleen. Committed mast cell progenitors are rare in bone marrow suggesting they are rapidly released into the blood where they circulate and move out into the peripheral tissues. This migration is controlled in a tissue specific manner. Basal trafficking to the intestine requires expression of α4β7 integrin and the chemokine receptor CXCR2 by the mast cell progenitors and expression of MAdCAM-1 and VCAM-1 in the intestinal endothelium; and is also controlled by dendritic cells expressing the transcriptional regulatory protein T-bet. None of these play a role in basal trafficking to the lung. With the induction of allergic inflammation in the lung, there is marked recruitment of committed mast cell progenitors to lung and these cells must express α4β7 and α4β1 integrins. Within the lung there is a requirement for expression of VCAM-1 on the endothelium that is regulated by CXCR2, also expressed on the endothelium. There is a further requirement for expression of the CCR2/CCL2 pathways for full recruitment of the mast cell progenitors to the antigen-inflamed lung.
INTRODUCTION
Mature mast cells are found in many connective tissues where they are rare long-lived cells. These constitutive and connective tissue-associated mast cells have a restricted phenotype based on mediator production and secretory granule proteases that varies somewhat from tissue to tissue. An entirely different phenotype, prominent after a nematode infection, was first identified in the mouse and rat associated with the intestinal mucosa. This rodent mucosal phenotype also shows some variability in granule content. Nonetheless, these findings led to our current paradigm that there are two classes of mast cells, the connective tissue and the mucosal phenotypes. In general, two broad classes of mast cells also have been identified in the human based predominantly on differences in secretory granule protease expression. All phenotypes appear to be derived from a common mast cell progenitor with the final phenotype determined by micro-environmental factors such as cytokines or perhaps by interactions with the extracellular matrix.1-3 In this review we address the issues of how mast cells arise and how they get to their diverse sites of residency. Most of the discussion will be focused on studies conducted in the mouse but wherever possible, differences and similarities in the human will be mentioned.
MAST CELL DEVELOPMENT
The seminal studies by Kitamura and colleagues in the late 1970s showing reconstitution of mast cells in mast cell-deficient mice by the adoptive transfer of wild type bone marrow indicated these cells could be derived from the bone marrow and thus were of hematopoietic origin.4,5 Then, in the early 1980s, the ability to grow cultured mast cells from murine bone marrow using Interleukin (IL)-3 was discovered.6,7 This propelled the studies on understanding the developmental pathway and the observation that the immature mast cell progenitors could be found in peripheral tissues such as in the intestine.8-10 In the last few years, a number of studies by Akashi and colleagues as well as others have defined several of the intermediate steps in what has sometimes been termed the classical model of hematopoiesis.11-14 These define the development of the mast cell in mouse bone marrow occurring along the myeloid pathway. In this model, the initial fate decision made by the hematopoietic stem cell is to commit to the myeloid or lymphoid lineage. This first step in myeloid development is characterized by the down regulation of the Sca-1 antigen. This intermediate has been termed the Common Myeloid Progenitor (CMP) and is distinguished from the Common Lymphoid Progenitor by expression of IL-7R by the latter cells. The CMP intermediate can give rise to either the Megakaryocyte-Erythrocyte Progenitor or to the next step in myeloid development, the Granulocyte Macrophage Progenitor (GMP) which is distinguished by the up regulation in expression of the low affinity IgG receptors, FcγRII/III (identified by the 2.4G2 mAb). The GMP can give rise to macrophages, eosinophils, neutrophils or the novel Basophil-Mast Cell Progenitor (BMCP) first identified in the spleens of C57BL/6 mice. These BMCP, were only found in the spleen of this mouse strain and could be identified as a KIT+, FcγRII/III+, β7 integrinhi, FcεRI− cells, that only gave rise to mast cells or basophils in culture despite using cytokines that give rise to all the myeloid cells after culture of bone marrow cells. Furthermore, transfer of these BMCP into mast cell-deficient mice led to the appearance of mast cells in the spleen and peritoneal cavity, demonstrating their capacity was not limited to in vitro differentiation.12 The isolation of the CMP, GMP and BMCP; and the ability of these cells to differentiate into basophils in culture have been confirmed by others.15
A second mast cell differentiation pathway in adult mice has also been described in which the cells are derived from the multipotential progenitor.16 In these experiments, the authors isolated the mast cell progenitors as lineage negative (including lacking the high affinity IgE receptor, FcεRI), CD27−, β7 integrin+, IL33R (T1/ST2)+. This fraction, as with the BMCP, was able to restore mast cells but not other lineages after transfer into mast cell-deficient mice indicating their lineage potential was restricted. Based on these finding the authors proposed an alternate model of differentiation for the mast cell as occurring via a direct commitment from the multipotential progenitor. This development scheme is more in keeping with the alternative model of hematopoietic development suggested by Kawamoto and others.17-19 This model was first developed using fetal liver-derived cells but has been extended to adult hematopoiesis as well. It suggests that myeloid developmental potential is retained beyond the first commitment step. In fact, in this model, myeloid potential is retained even past the differentiation between B and T-lymphocyte commitment. Support for this comes from many investigations both in vitro and in vivo based on findings such as the demonstration that up to 30% of the macrophages in the thymus are derived from the early T-cell progenitors migrating into the thymus from the bone marrow.17
In either scheme, it is thought the committed mast cell progenitor is released into the blood stream where it migrates to various tissues as discussed further below. While there has been little investigation of the blood-borne mast cell progenitor in adult mice, in 1996, Rodewald et al demonstrated for the first time a committed mast cell progenitor in fetal mouse blood.20 This cell, identified as KIT+, Thy-1lo was unusually prevalent at fetal day 15.5 allowing its identification. The numbers subsequently dropped down suggesting the cells were perhaps seeding tissues at a particular stage in development. Given the difficulty in restoring normal mast cell numbers in many tissues, it may be that there is an important developmental period where mast cells seed tissue such as the skin and provide the indigenous cell population with little turn over.21
The surprising ease with which murine bone marrow gave rise to mast cell lines in vitro was not initially reproducible with human hematopoietic progenitors. It awaited our understanding of the importance of stem cell factor (SCF, also called KIT ligand, KITl) in the growth and differentiation of mast cells before routine in vitro culture of mast cells from human cord blood was established.22-24 This is now an established technique for studying human mast cells in vitro and has been used in many studies such as to demonstrate that the human mast cell progenitor that can be identified in peripheral blood, is closely related to macrophages in development and is increased in the blood of asthmatics.25-27
The critical role of SCF in mast cell development was first suggested by the discovery of mutations in the kit locus, first identified as the W locus, which resulted in a mast cell deficiency of varying degrees. In the absence of full KIT signaling, mast cell maturation is truncated and, depending on the mutation, results in partial or almost complete deficiency of mast cells in the peripheral tissues. The ability to generate early myeloid progenitors is not eliminated, however, leaving the ability to develop mast cells in culture via IL-3 intact.28 Nonetheless, the influence of SCF on development is dramatic, driving maturation of the cells toward a connective tissue phenotype and sustaining the cells both in vitro and in vivo.29-31 Unfortunately, as this receptor is involved in myelopoiesis, gametogenesis and melanogenesis, loss of this receptor results in more than a simple mast cell deficiency. Among other affects, there are decreased numbers of basophils in the bone marrow and BMCP in the spleen; and it also results in a neutropenia which can influence results based on responses involving these two cells,12,32 Thus, caution is necessary in interpreting the results obtained using KIT- or SCF-deficient mice.
As noted above, Rodewald et al provided one of the first phenotypic definitions of a committed mast cell progenitor. This Thy-1lo, KIT+ fetal blood cell responded only to SCF and IL-3 together in vitro and lacked any potential for becoming macrophages, granulocytes, erythrocytes or B or T-lymphocytes in several different assays, but gave rise to mast cells at a high frequency. To date a phenotypic definition of committed mast cell progenitors in adult mouse (or human) blood has not been completed, although we have identified committed mast cell progenitors in both the mouse intestine and lung as lineage−, CD45+, CD34+, FcεRI+, β7 integrinhi cells and a c-kit+, CD34+ macrophage/ mast cell progenitor is found in human peripheral blood.12,25,33 Of note is the continued expression of the CD34 marker of murine mast cells throughout their maturation, although this is usually a marker of early hematopoietic progenitors.34,35
BASAL HOMING OF MAST CELL PROGENITORS (FIG. 1)
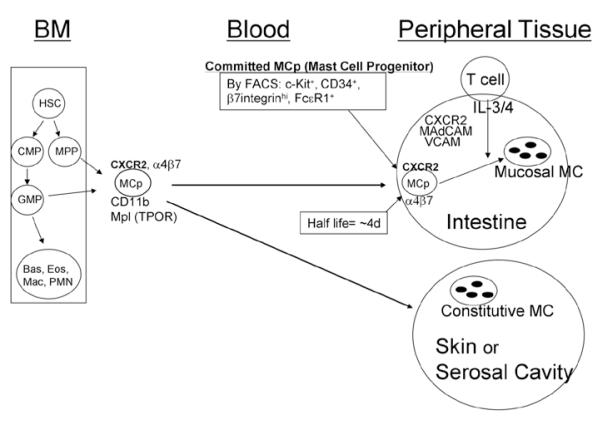
Figure 1.
Development of committed mast cell progenitors (MCp) in the bone marrow (BM) from the hematopoietic stem cell (HSC) and the molecules identified as playing a role in the trafficking of the these cells to the intestine and peritoneal cavity under basal, non-inflamed conditions. Two different developmental pathways have been proposed leading to the production of committed mast cell progenitors. These cells then move out to peripheral tissues via the circulation and migrate into the specific tissue sites such as the intestine and serosal cavity under the control of specific molecules such as the α4 integrins and various chemokine pathways. A role for the C3b and TPO receptors has been shown to affect mast cell numbers in the skin and peritoneal cavity. See text for details.
Most studies of migration and trafficking have focused on movement into the small intestine or peritoneal cavity. This is because of the relatively large number of committed mast cell progenitors or easily obtainable mature mast cells, respectively, found at these peripheral tissue sites in rodents. The pool of committed mast cell progenitors in the intestine is greater than that found in bone marrow (among mononuclear cells isolated from the tissues), although there are many more mononuclear cells and therefore cells within this lineage in the bone marrow. This large intestinal pool of committed mast cell progenitors facilitated the initial studies identifying these cells using clonogenic assays with IL-3 alone or in combination with SCF.8,10,36-38 It also facilitated our investigations on the molecules critical to establishing and maintaining this pool. The appreciation of the immature mast cell progenitor was a direct consequence of the early studies highlighting the ability of these cells to be expanded and maintained with IL-3. This led to studies by several labs showing the identification of mast cell progenitors in blood, lung and intestine.8,36,39 While the early studies used a clonogenic assay that did not distinguish early precursors from the committed mast cell progenitors, the assay did allow the identification of lineage-committed cells and eventually led to the conclusion that a population of committed mast cell progenitors is found in bone marrow, spleen, lung and intestine.12,16,33
The identification of β7 integrins on rodent and human mast cells suggested a role for one or both of the β7 integrins, α4β7 or αEβ7 in homing of mast cells to the intestine based on their involvement in the homing of lymphocytes to the small intestine and associated lymphatic structures.40-43 To test this hypothesis, we used both null strains and blocking antibodies directed against the various integrin chains. These techniques further allowed us to define the critical integrin, the important endothelial ligands, a chemokine receptor and a role for dendritic cells in the establishing and maintaining of the large intestinal pool of committed mast cell progenitors.
Using the β7 integrin null mice, we found a profound deficit in both mast cell progenitors and, as shown by others as well, a deficit in the mature cells in the small intestine.37,44 The deficit in mature mast cells was in both populations located in the submucosal connective tissue and in the mucosa of the intestine. This lack of both phenotypes in the absence of progenitors supported the paradigm that a single committed progenitor gives rise to all phenotypes of mature mast cells. In order to address the issue of which of the β7 integrin molecules was critical in controlling ingress, we established a protocol to allow the use of antibody blocking. To do this as, we took advantage of the observation made a number of years earlier by Kitamura and colleagues, that the progenitors are very sensitive to irradiation.45 This allowed us to eliminate the intestinal pool of mast cell progenitors and then study its reconstitution over the course of the next 2 weeks. We could influence the mast cell progenitor reconstitution via the administration of antibodies to the different integrins. Using anti-α4, anti-β1, anti-β7, anti-αE and anti-α4β7 integrin antibodies, the relevant integrin was defined as α4β7 and not α4β1 or αEβ7. Although after entry, the intestinal-localized mast cell progenitors down regulate expression of α4β7 and up regulate expression αEβ7.46
As the α4β7 integrin molecule can bind to both the mucosal addressin cellular adhesion molecule (MAdCAM)-1 or vascular cell adhesion molecule (VCAM)-1, both of which are expressed within the intestine, we evaluated the involvement of each cellular adhesion molecule. Using both null mice lacking VCAM-1 or antibody blocking directed to MAdCAM-1 or VCAM-1, both molecules were showed to be required for the establishment of the intestinal pool of mast cell progenitors. We also evaluated various chemokine receptor null mice looking for specific chemokine pathways involved in this process. We could find no evidence of any role for CCR2, CCR3, or CCR5, while significant inhibition was observed in CXCR2 null mice.38 This IL-8 receptor is expressed on both circulating leukocytes and on endothelium, suggesting it could be affecting the trafficking either through effects on the circulating mast cell progenitor or on the vascular endothelium.47 Adapting the techniques of sublethal irradiation followed by bone marrow reconstitution of wild type mice that we used for antibody blocking, we reconstituted the various mice with either wild type or CXCR2-deficient bone marrow. These studies demonstrated reduced reconstitution in the wild type mice given CXCR2 null bone marrow, indicating that the circulating progenitor needs to express CXCR2. Further investigation also noted a role for CXCR2 expression by the host, as reconstitution of CXCR2 null mice with wild type bone marrow also resulted in fewer intestinal mast cell progenitors when compared to wild type mice reconstituted with wild type bone marrow in parallel.46 These findings are summarized in Figure 1.
The process of reconstitution of the pool of mast cell progenitors in the intestine occurred fairly rapidly after sublethal irradiation and reconstitution with wild type bone marrow, achieving about 50% of the normal frequency by one week and almost complete reconstitution by two weeks.37 This rapid replenishment of the pool suggested that this could be an innate process ongoing in normal mice. Confirmation of this hypothesis came with the treatment of normal BALB/c mice with an antibody directed to either the α4β7 integrin or to the CXCR2 receptor. In both cases, administration of the antibodies every other day for about one week resulted in a ~75% depletion of the pool of MCp.38 Since the cells down regulated α4β7 integrin expression and up regulated αEβ7 integrin expression after entering the intestine, the depletion of the pool was a consequence of inhibition of the transmigrating cells attempting to leave the circulation rather that a direct effect on progenitors in the intestines. Support for this interpretation was obtained by monitoring the pool of mast cell progenitors in the lung. This pool is not dependent on either α4 integrins or expression of the CXCR2 receptor; and the size of this pool as well as that in spleen increased when mice were given anti-α4 integrin, suggesting an increase in number of circulating mast cell progenitors as a consequence of preventing them from moving into the intestine. Furthermore, the remarkable depletion of almost 80% after 6 days of treatment with the blocking monoclonal antibody of the pool suggested a half-life for the cells in the intestine of about 4 days.
The innate nature of the basal homing of mast cell progenitors to the intestine was first suggested by Guy-Grand et al in their studies of this process.10 Using germ free mice, they found there was no diminution in the size of progenitor pool in the absence of intestinal flora. Furthermore, the size of intestinal pool was unaltered in athymic mice which lack mature T-cells. We pursued this further using mice lacking the recombinase activating gene (RAG)-2 and the common IL-R gamma chain (common for IL-2, IL-4, IL-7, IL-9 and IL-15 receptors). These double deficient mice lack all mature lymphocytes including natural killer, T, B and natural killer T-cells.48 As in athymic mice, mast cell progenitors homed to the intestine in normal numbers in the absence of all of these other cells types. This was also not a strain specific phenomenon, as both C57BL/6 and BALB/c mice had normal numbers of intestinal mast cell progenitors in the absence of T-cells.37 Given this lack of dependence on the adaptive immune system, we had assumed this was an innate process not dependent on other cell types. Thus, it was surprising to find that in mice lacking the T helper type 1 master developmental regulator, the transcription factor T-bet (Tbx21), there were decreased numbers of mast cell progenitors in both the lung and intestine of both C57BL/6 and BALB/c mice. This also resulted in fewer numbers of mature intestinal mast cells as expected from previous studies.49 These results indicated an innate cell type must be involved and affected developmentally by the loss of T-bet. Yet there was no evidence of expression within the mast cell lineage, despite noting that T-bet null mast cells showed decreased adhesion to VCAM-1 under flow. The demonstration that dendritic cells also expressed T-bet and that loss of this protein had an effect on their role in an arthritis model led us to consider whether this cell type played some role in the formation of the intestinal pool of mast cell progenitors.50 Unexpectedly, the transfer of bone marrow-derived dendritic cells from wild type mice to wild type or T-bet null mice restored the pool of intestinal mast cell progenitors to similar levels in both strains. Thus, while there is no role for lymphocytes in the trafficking of mast cell progenitors to the lung or intestine, dendritic cell functions or development controlled by T-bet play a critical role in the trafficking of these mast cell progenitors to the intestine and development of mature mast cells in this tissue. In subsequent work, we have repeated these experiments, evaluating the much smaller pool of mast cell progenitors in the lung and found no reconstitution of the pulmonary pool in T-bet null mice by wild type bone marrow-derived dendritic cells (Moravia et al, unpublished observations). Thus, while these studies established a critical role for T-bet+ dendritic cells in controlling the numbers of mast cells in the intestine, this is not the case for the lung. It is remarkable that, as of this time, very little is known about what controls the basal pool of mast cell progenitors in the lung.
Other studies have also implicated a role for CD11b and the TPO receptor Mpl on maturation and movement of the mast cell progenitors into the peritoneal cavity and skin. Thus, reduced numbers of mast cells were found in the peritoneal cavity and dorsal skin in CD11b-deficient mice.51 However, there was no defect in the number of mast cell progenitors noted in bone marrow or intestine, suggesting that the loss of this C3b receptor plays a role in maturation or survival of the cells at these sites and does not influence trafficking of the cells. In contrast, because thrombopoietin (TPO) has been shown to increase human mast cell colonies in vitro and increase apoptosis of murine mast cells both in vitro and in vivo, TPO receptor (Mpl) null mice were evaluated for number of mast cell progenitors and mature mast cell in various sites. Using the Chen et al definition of bone marrow mast cell progenitors as FcεRI−, CD27−, β7 integrin+ and IL33R+, loss of Mpl resulted in decreased numbers of mast cell progenitors in the spleen and bone marrow, but increased numbers of mature mast cells based on histological staining and fluorescent staining for KIT and FcεRI.16,52,53 There were also increased numbers of mast cells in the spleen, bone marrow, skin, stomach and peritoneal cavity, suggesting that TPO via its receptor plays an important role in controlling the number of mast cell in several different sites.
RECRUITMENT OF MAST CELL PROGENITORS (FIG. 2)
Figure 2.
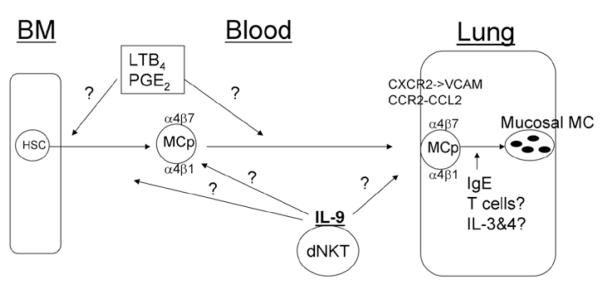
Identification of the molecules and cells controlling (or perhaps controlling, e.g., LTB4, PGE2) inflammation-induced recruitment of MCp to the lung in BALB/c mice and influencing their maturation to mature mucosal mast cells. Question marks indicate pathways not confirmed or established in vivo. See text for details.
Mast cell numbers increase significantly in tissues during inflammatory conditions such as in the joints with arthritis or in the lungs of patients with allergic asthma.54-56 In asthmatics, this is accompanied by an increase in circulating progenitor cells as assessed in a clonogenic assay.27 In mice, the normal number of mast cells in the airways of the common wild type strains C57BL/6 and BALB/c is relatively low, but is significantly increased after the induction of allergic airway inflammation.57,58 This increase may be due to either division of the few mature mast cells in situ or recruitment and maturation of mast cell progenitors in the lung, or both. Because of the constitutive trafficking occurring to maintain the intestinal progenitor pool and previous studies suggesting recruitment to the intestine after helminth infection in mice,10,59 we hypothesized that the increment in mast cells reflects recruitment of mast cell progenitors via transendothelial migration from the blood to the lung. We expected this to be an active process that is induced during the inflammation and is inhibited when the stimuli is stopped involving specific pathways such as found for basal trafficking to the intestine. To test this, both BALB/c and C57BL/6 mice were immunized systemically with ovalbumin (OVA) adsorbed to alum on day 0 and day 7, followed by three consecutive daily OVA aerosol challenges starting 10 days later. Both strains have significantly more mast cell progenitors in the lung after challenge, with a magnitude of ~ 28 fold increase in total number of pulmonary mast cell progenitors in BALB/c mice and ~ 10 fold increase in C57BL/6 mice.60 As reported by others, the histological assessment of inflammation demonstrated that similar OVA treatments did not induce similar degrees of inflammation with C57BL7/6 mice, showing more modest inflammation than in BALB/c.61 The magnitude of the increase in such a short period of time after the initial antigen challenge indicted that recruitment rather than expansion of the small numbers of resident progenitors was the most likely explanation and led to the investigation of specific molecules and cells that controlled the process.
During OVA-induced lung inflammation, VCAM-1 expression on the lung endothelium is up regulated.62 Using endothelial VCAM-1 knock out mice and anti-VCAM-1 treatment before each OVA-aerosol challenge, we showed that mast cell progenitor recruitment is critically dependent on VCAM-1 expression on the endothelium in the lung. In contrast to the homing of mast cell progenitors to intestine, there was no role for MAdCAM-1 in the recruitment to lung, consistent with reports that MAdCAM-1 is not expressed in the lung endothelium.63,64 As both α4β1 and α4β7 integrins can mediate binding to VCAM-1, we investigated the effect of blocking antibodies to these targets administered during the challenge phase. We found using several different antibodies that either integrin could reduce the mast cell progenitor recruitment, suggesting that both these integrins contribute in the adhesion to VCAM-1. Administration of antibody to αE, the other alpha chain that pairs with β7, had no effect, indicating the specificity of the blocking. Moreover, antigen sensitized and challenged β7-integrin deficient mice also had suppressed lung mast cell progenitor recruitment compared to their wild type controls. We concluded that mast cell progenitors express functional α4β1 and a4β7 integrins that interact with VCAM-1 on the lung endothelium to accomplish the transendothelial migration (Fig. 2).
As recruitment also requires attracting the mast cell progenitor to the site of the inflammation, we compared the antigen-induced mast cell progenitor recruitment in several different knock out mouse strains lacking the chemokine receptors CXCR2, CCR5, CCR3 and CCR2. We focused on these based on their known expression by bone marrow derived mast cells (BMMC) and/or human cord blood derived mast cells.24,65,66 Using our established mast cell progenitor recruitment protocol, we found no diminution in mast cell progenitor recruitment in CCR5 and CCR3 knockout mice. CXCR2−/− mice however, had significantly reduced mast cell progenitor recruitment, 66% in total mast cell progenitor content and 53% in the concentration of mast cell progenitors per 106 mononuclear cells.33 To confirm that the need for CXCR2 was due to lack of expression on the mast cell progenitor, we made bone marrow chimeras of wild type mice reconstituted with CXCR2−/− bone marrow. To our surprise, the CXCR2−/− bone marrow could reconstitute the mast cell progenitor recruitment in sublethally irradiated wild type mice, indicating that CXCR2 expression by the circulating mast cell progenitor was not needed. The opposite experiment where bone marrow chimeras of CXCR2−/− mice were produced by being given wild type bone marrow recapitulated the original results with the CXCR2 knockout mice indicating that a nonbone marrow-derived, relatively radioresistent host cell had to express CXCR2 for recruitment of mast cell progenitor to lung. Because CXCR2 is expressed on the lung endothelium, we hypothesized that CXCR2 expression on the lung endothelium regulates VCAM-1 expression. In accordance with this hypothesis, total mRNA levels in the lungs and protein expression of VCAM-1 on the lung endothelium was strongly reduced in CXCR2-deficient mice, suggesting that CXCR2 on the lung endothelium does regulate VCAM-1 expression and thereby the induced influx of mast cell progenitors to this tissue. In support of our observations, endothelial CXCR2 was also shown to, in part, regulate neutrophil influx in LPS-induced lung injury.47
Evaluation of CCR2 null mice also noted reduced recruitment in our model and consistent with this, we found that the chemokine CCL2, the major ligand for CCR2 is chemotactic for bone marrow-derived mast cell progenitors and immature (2 weeks old) BMMC cultured in SCF and IL-3. However, CCL2 was not chemotactic to BMMC cultured in IL-3 alone, even though mast cells cultured in both conditions expressed CCR2 and bound radiolabeled CCL2 with the same affinities.67 This indicates that SCF is an important mediator in coupling CCR2 to downstream signaling events that leads to chemotaxis. When CCL2 was injected intradermally into mice, immature BMMC from IL-3 + SCF cultures administered intravenously migrated to the sites of injection. These data suggested that the CCL2/CCR2 pathway was important in recruitment of mast cell progenitors; and this was supported when we demonstrated that both CCR2- and CCL2-deficient mice had significant reductions in mast cell progenitor recruitment to the lung in our model of allergic airway inflammation. However, when we addressed whether this was due to expression of CCR2 by the mast cell progenitors by evaluating recruitment in sublethally irradiated wild type mice given CCR2−/− bone marrow, we found normal recruitment, showing that CCR2 expression by the circulating progenitors is dispensable. We further confirmed this by analyzing several of the mast cell colonies in the reconstituted wild type mice and found them to all be of donor (CCR2−/−) origin. The opposite experiment, where sublethally irradiated CCR2−/− deficient mice are given wild type bone marrow, still had diminished recruitment, indicating a requirement for expression of the receptor by host tissues. Since mice lacking CCL2, also had diminished mast cell progenitor recruitment to lung, we also evaluated these mice using bone marrow chimeras. We found that the critical source of CCL2 was both in bone marrow-derived cells and stroma-derived cells. Clearly, the CCL2/CCR2 axis is involved in the mechanisms that lead to antigen-induced mast cell progenitor recruitment to lung, but the process is complex and there are missing steps to be sorted out. Despite several attempts to define the critical chemokine receptor(s) and the mechanism(s) by which they act to attract the mast cell progenitor to lung in vivo, the hunt is not over.
Several other chemoattractants for mast cells may also be involved. In a study aimed at finding the chemoattractant responsible for mast cell progenitor recruitment in a similar model of OVA-induced airway inflammation, BALB/c mice were sensitized weekly with three i.p. injections with OVA in alum followed by five daily intranasal challenges and a final intranasal challenge three days later with OVA. Weller and colleagues took nasal mucosa 20 h after final OVA challenge and generated a mucosal conditional medium that was used to attract immature (2-week-old) BMMC produced in IL-3 alone.68 Purification of the chemoattractant activity in the mucosal conditional medium demonstrated that prostaglandin E2 (PGE2) was inducing the chemotactic response and that an EP3 receptor antagonist could block the response. In addition, LTB4 was shown to be chemotactically active on immature (2-week-old) BMMC.69 Because both mediators are expressed following activation of mast cells, they are most likely to play a role in the recruitment process rather than the basal homing. Furthermore, the demonstration of an effect on 2-week-old cultured mast cells by CCL2, LTB4 and PGE2 with only PGE2 attracting the more mature 10-week-old cultured mast cells, suggests a role for these factors in early recruitment, perhaps from the bone marrow rather than into the tissues.
Mast cell progenitor recruitment to allergen-challenged lung occurs in an antigen-specific manner in previously sensitized mice. Since the use of alum in the sensitization process skews the immune response towards a Th2 response, we hypothesized that Th2 cells control mast cell progenitor recruitment by providing Th2 cytokines that govern the inflammation-induced up-regulation of adhesion molecules and/or necessary production of chemoattractants. In support of this we found that the pulmonary mast cell progenitor recruitment was dependent on CD4+ T-cells in BALB/c mice, since depletion with monoclonal antibody before aerosol challenge inhibited the recruitment but depletion of CD8+ T-cells had no effect.70 Unexpectedly, BALB/c mice lacking IL-4, IL-4R alpha chain or STAT-6 had a normal recruitment response, even though these mouse strains have diminished Th2-responses and no serum IgE.71,72 Further, administration of blocking antibodies for the Th2 cytokines IL-3, IL-4, IL-5, IL-10 or IL-13 during the challenge phase had no effect on the recruitment. These data suggested that a Th2-response is not required for the antigen-induced recruitment of mast cell progenitors to the lung. Similarly IFN-γ−/−, IL-12p40−/−, IL12Rβ1−/− mice or treatment with anti-IFN-γ, anti-IL-12p40 (the IL-12 and IL-23 common chain), anti-IL-6, anti-IL-17A and anti-TGFβ1 did not reduce the recruitment, indicating no role for Th1 or for Th17 cells. Furthermore, anti-CD25 treatment had no effect on the mast cell progenitor recruitment, ruling out a critical role for T-regulatory cells. As natural killer T (NKT) cells have been implicated in the allergic pulmonary allergic response, we next evaluated a role for these cells.73,74 Mast cell progenitor recruitment in CD1d-deficient mice, which lack all NKT cells, had significantly reduced numbers of pulmonary mast cell progenitors. Monoclonal antibody blocking of CD1d during the challenge phase also significantly reduced the mast cell progenitor recruitment compared to wild type mice treated with isotype-matched control immunoglobulins. Blockade or genetic loss of CD1d signaling prevents the activation of both the type 1/ invariant NKT (iNKT) and the type 2 or diverse NKT cells. However, Jα18-deficient mice that specifically lack iNKT cells can be used to differentiate between these two types of CD1d-restricted NKT cells. Surprisingly, we found that the mast cell progenitor recruitment was greater in the antigen-sensitized and challenged Jα18-deficient mice than in the wild type controls, but that anti-CD1d blocking antibody still reduced the mast cell progenitor numbers in this mutant mouse strain, indicating that it is the type 2 NKT cells that is critical for the mast cell progenitor recruitment to the antigen challenged lung.
The screening of possible critical cytokines produced by CD4+ T-cells eliminated many of the mast cell-activation factors we thought might be involved. This led us to IL-9, a mast cell growth factor that is not dependent on STAT-6 for signaling.75 Use of IL-9 deficient mice, as well as anti-IL-9 treatment with monoclonal antibodies during the challenge phase, significantly reduced mast cell progenitor recruitment. One possible source of pulmonary IL-9 is tissue resident mature mast cells. Using three different mast cell-deficient mouse strains (Wsh/Wsh, W/Wv and Sl/Sld), we found that all strains had increases in mast cell progenitor recruitment comparable to their wild type controls, thereby excluding mature mast cells as the critical source of IL-9. Since NKT cells from C57BL/6 mice also had been suggested as a source of IL-9,76 we purified iNKT cells using alpha-Galactosylceramide-CD1d tetramers, from BALB/c mice, cultured them with alpha-GalCer-pulsed bone marrow-derived dendritic cells and evaluated the supernatants for production of IL-9. These cells produced nanogram amounts of IL-9 in vitro, comparable to that produced by cultured polarized Th2 cells. To address if the requirement for IL-9 and for NKT-cells were in the same pathway or parallel, we treated IL-9-deficient and wild type mice with anti-CD1d blocking antibody and CD1d-deficient and wild type mice with anti-IL-9 blocking antibody. No further reduction of the partial suppression of mast cell progenitor recruitment occurred by combining the inhibition of these two factors. These data indicates that IL-9 and the type 2 CD1d-restricted NKT cells function in the same pathway.70
MAST CELL HYPERPLASIA
The increased numbers of mature mast cells associated with parasitic nematode infections and chronic allergic reactions is likely the direct result, at least in part, of the maturation of the increased numbers of mast cell progenitors called into these sites of inflammation. While current studies are exploring this area to better define the contribution of resident versus recruited mast cells, several studies have contributed to our knowledge already. In the CXCR2 null mice, the decreased influx of mast cell progenitors observed after only 3 aerosolized allergen challenges was associated with fewer numbers of interepithelial mast cells observed in the tracheal mucosa a week after the last challenge.33 In contrast, there was no significant difference in the number of submucosal, constitutive mast cells. This finding supports a model in which the recruited mast cell progenitors give rise to the mature mucosal mast cells, but not the connective mast cell. Further work is necessary to establish the relative expansion of the connective tissue mast cells versus the relative contribution of the recruited progenitors to the increased number of mature cells.
Other factors that have been implicated in the formation of the mast cell hyperplasia include the T-cell derived factors IL-3, IL-4, IL-9 and more recently IgE as a survival and anti-apoptotic factor.77-79 Using antibody blocking and null mice, IL-3, IL-4 and IL-9 all have been show to be required for the mucosal mast cell hyperplasia associated with a nematode infection.77,78,80 Much less has been done though on their influence on mucosal mast cells in the lung. The critical role for IL-9 in the recruitment of the mast cell progenitor to the lung was discussed above and these studies also eliminated a role for IL-3 and IL-4 in this process in BALB/c mice. Further studies have suggested that in IL-9-deficient mice, chronic stimulation results in a small decrease in the numbers of mature mucosal mast cells, which may in turn suggest that the early dependence of recruitment is lost with further stimulation. Because of the role for IL-3 and IL-4 in the mature mast cell hyperplasia in the intestine, these bear further investigation in the lung.
Recently, a role for IgE has been found in the mast cell maturation process occurring during inflammation. Using a model of pulmonary inflammation induced by Aspergillus fumigatus extract that leads to remodeling and airway hyperresponsiveness, we evaluated the increase in both mast cell progenitors and the mature mast cells. Surprisingly, in BALB/c mice lacking IgE, recruitment of the immature progenitors was unaltered, but the number of mature pulmonary mast cells was significantly decreased.79 The lack of IgE was further shown to lead to increased numbers of apoptotic cells, indicating that it acts as survival/anti-apoptotic factor.
CONCLUSION AND FUTURE PROSPECTS
During homeostatic conditions, mast cell progenitors arise in the bone marrow and are transported out to peripheral tissues via the blood. It is likely that these circulating progenitors have already made the commitment to the mast cell lineage in the adult animal, but this has not yet been confirmed. Extravasation from the blood stream occurs under basal conditions into certain tissues and is increased with inflammation. The basal homing to the intestine is highly controlled by other innate cells and by several different integrins, cellular adhesion molecules, chemokines and chemokine receptors. Many of these same components are involved in controlling the inflammation-induced recruitment to the lung, but most of the pathways are not fully understood. In addition, the inflammation-induced recruitment is dependent on other unique cells and factors that control the recruitment of these potent effectors in a tissue specific and strain specific manner. Initial studies indicate that these mast cell progenitors mature into mast cells in situ and provide the basis for the mast cell hyperplasia associated with certain inflammatory stimuli. However, this process is in need of much further work to understand the underlying mechanisms to be able to modulate this process and control the pathobiologic consequences of chronic activation of mast cells.
ACKNOWLEDGEMENTS
We would like to thank the many collaborators who have participated in and supported this work. This work has been supported by grants from the National Institutes of Health to MFG (R01-AI083516).
REFERENCES
- 1.Kitamura Y, Yokoyama M, Matsuda H, et al. Spleen colony-forming cell as common precursor for tissue mast cells and granulocytes. Nature. 1981;291(5811):159–160. doi: 10.1038/291159a0. [DOI] [PubMed] [Google Scholar]
- 2.Gurish MF, Pear WS, Stevens RL, et al. Tissue-regulated differentiation and maturation of a v-abl-immortalized mast cell-committed progenitor. Immunity. 1995;3(2):175–186. doi: 10.1016/1074-7613(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe DD, Baram D, Mekori YA. Mast cells Physiol Rev. 1997;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura Y, Shimada M, Hatanaka K, et al. Development of mast cells from grafted bone marrow cells in irradiated mice. Nature. 1977;268(5619):442–443. doi: 10.1038/268442a0. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52(2):447–452. [PubMed] [Google Scholar]
- 6.Nagao K, Yokoro K, Aaronson SA. Continuous lines of basophil/mast cells derived from normal mouse bone marrow. Science. 1981;212(4492):333–335. doi: 10.1126/science.7209531. [DOI] [PubMed] [Google Scholar]
- 7.Razin E, Cordon-Cardo C, Good RA. Growth of a pure population of mouse mast cells in vitro with conditioned medium derived from concanavalin A-stimulated splenocytes. Proc Natl Acad Sci USA. 1981;78(4):2559–2561. doi: 10.1073/pnas.78.4.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crapper RM, Schrader JW. Frequency of mast cell precursors in normal tissues determined by an in vitro assay: antigen induces parallel increases in the frequency of P cell precursors and mast cells. J Immunol. 1983;131(2):923–928. [PubMed] [Google Scholar]
- 9.Schrader JW, Scollay R, Battye F. Intramucosal lymphocytes of the gut: Lyt-2 and thy-1 phenotype of the granulated cells and evidence for the presence of both T-cells and mast cell precursors. J Immunol. 1983;130(2):558–564. [PubMed] [Google Scholar]
- 10.Guy-Grand D, Dy M, Luffau G, et al. Gut mucosal mast cells. Origin, traffic and differentiation. J Exp Med. 1984;160(1):12–28. doi: 10.1084/jem.160.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akashi K, Traver D, Miyamoto T, et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 12.Arinobu Y, Iwasaki H, Gurish MF, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci USA. 2005;102(50):18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwasaki H, Mizuno S, Mayfield R, et al. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J Exp Med. 2005;201(12):1891–1897. doi: 10.1084/jem.20050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91(5):661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 15.Ohmori K, Luo Y, Jia Y, et al. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J Immunol. 2009;182(5):2835–2841. doi: 10.4049/jimmunol.0802870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CC, Grimbaldeston MA, Tsai M, et al. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci USA. 2005;102(32):11408–11413. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamoto H, Katsura Y. A new paradigm for hematopoietic cell lineages: revision of the classical concept of the myeloid-lymphoid dichotomy. Trends Immunol. 2009;30(5):193–200. doi: 10.1016/j.it.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Kincade PW, Owen JJ, Igarashi H, et al. Nature or nurture? Steady-state lymphocyte formation in adults does not recapitulate ontogeny. Immunol Rev. 2002;187:116–125. doi: 10.1034/j.1600-065x.2002.18710.x. [DOI] [PubMed] [Google Scholar]
- 19.Laiosa CV, Stadtfeld M, Graf T. Determinants of lymphoid-myeloid lineage diversification. Annu Rev Immunol. 2006;24:705–738. doi: 10.1146/annurev.immunol.24.021605.090742. [DOI] [PubMed] [Google Scholar]
- 20.Rodewald HR, Dessing M, Dvorak AM, et al. Identification of a committed precursor for the mast cell lineage. Science. 1996;271(5250):818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- 21.Du T, Friend DS, Austen KF, et al. Tissue-dependent differences in the asynchronous appearance of mast cells in normal mice and in congenic mast cell-deficient mice after infusion of normal bone marrow cells. Clin Exp Immunol. 1996;103(2):316–321. doi: 10.1046/j.1365-2249.1996.d01-610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furitsu T, Saito H, Dvorak AM, et al. Development of human mast cells in vitro. Proc Natl Acad Sci USA. 1989;86(24):10039–10043. doi: 10.1073/pnas.86.24.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kempuraj D, Saito H, Kaneko A, et al. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93(10):3338–3346. [PubMed] [Google Scholar]
- 24.Ochi H, Hirani WM, Yuan Q, et al. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med. 1999;190(2):267–280. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirshenbaum AS, Goff JP, Semere T, et al. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+) and expresses aminopeptidase N (CD13) Blood. 1999;94(7):2333–2342. [PubMed] [Google Scholar]
- 26.Kirshenbaum AS, Kessler SW, Goff JP, et al. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol. 1991;146(5):1410–1415. [PubMed] [Google Scholar]
- 27.Mwamtemi HH, Koike K, Kinoshita T, et al. An increase in circulating mast cell colony-forming cells in asthma. J Immunol. 2001;166(7):4672–4677. doi: 10.4049/jimmunol.166.7.4672. [DOI] [PubMed] [Google Scholar]
- 28.Eklund KK, Ghildyal N, Austen KF, et al. Mouse bone marrow-derived mast cells (mBMMC) obtained in vitro from mice that are mast cell-deficient in vivo express the same panel of granule proteases as mBMMC and serosal mast cells from their normal littermates. J Exp Med. 1994;180(1):67–73. doi: 10.1084/jem.180.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurish MF, Ghildyal N, McNeil HP, et al. Differential expression of secretory granule proteases in mouse mast cells exposed to interleukin 3 and c-kit ligand. J Exp Med. 1992;175(4):1003–1012. doi: 10.1084/jem.175.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai M, Takeishi T, Thompson H, et al. Induction of mast cell proliferation, maturation and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc of Natl Acad Sci USA. 1991;88(14):6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galli SJ, Iemura A, Garlick DS, et al. Reversible expansion of primate mast cell populations in vivo by stem cell factor. J Clin Invest. 1993;91(1):148–152. doi: 10.1172/JCI116164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou JS, Xing W, Friend DS, et al. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. J Exp Med. 2007;204(12):2797–2802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallgren J, Jones TG, Abonia JP, et al. Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc Natl Acad Sci USA. 2007;104(51):20478–20483. doi: 10.1073/pnas.0709651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drew E, Huettner CS, Tenen DG, et al. CD34 expression by mast cells: of mice and men. Blood. 2005;106(5):1885–1887. doi: 10.1182/blood-2005-03-1291. [DOI] [PubMed] [Google Scholar]
- 35.Drew E, Merkens H, Chelliah S, et al. CD34 is a specific marker of mature murine mast cells. Exp Hematol. 2002;30(10):1211–1218. doi: 10.1016/s0301-472x(02)00890-1. [DOI] [PubMed] [Google Scholar]
- 36.Schrader JW, Lewis SJ, Clark-Lewis I, et al. The persisting (P) cell: histamine content, regulation by a T-cell-derived factor, origin from a bone marrow precursor and relationship to mast cells. Proc Natl Acad Sci USA. 1981;78(1):323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurish MF, Tao H, Abonia JP, et al. Intestinal mast cell progenitors require CD49dbeta7 (alpha4beta7 integrin) for tissue-specific homing. J Exp Med. 2001;194(9):1243–1252. doi: 10.1084/jem.194.9.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abonia JP, Austen KF, Rollins BJ, et al. Constitutive homing of mast cell progenitors to the intestine depends on autologous expression of the chemokine receptor CXCR2. Blood. 2005;105(11):4308–4313. doi: 10.1182/blood-2004-09-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonoda T, Ohno T, Kitamura Y. Concentration of mast-cell progenitors in bone marrow, spleen and blood of mice determined by limiting dilution analysis. J Cell Physiol. 1982;112(1):136–140. doi: 10.1002/jcp.1041120120. [DOI] [PubMed] [Google Scholar]
- 40.Gurish MF, Bell AF, Smith TJ, et al. Expression of murine beta 7, alpha 4 and beta 1 integrin genes by rodent mast cells. J Immunol. 1992;149(6):1964–1972. [PubMed] [Google Scholar]
- 41.Yuan Q, Jiang WM, Hollander D, et al. Identity between the novel integrin beta 7 subunit and an antigen found highly expressed on intraepithelial lymphocytes in the small intestine. Biochem Biophys Res Commun. 1991;176(3):1443–1449. doi: 10.1016/0006-291x(91)90448-g. [DOI] [PubMed] [Google Scholar]
- 42.Boyce JA, Mellor EA, Perkins B, et al. Human mast cell progenitors use alpha4-integrin, VCAM-1 and PSGL-1 E-selectin for adhesive interactions with human vascular endothelium under flow conditions. Blood. 2002;99(8):2890–2896. doi: 10.1182/blood.v99.8.2890. [DOI] [PubMed] [Google Scholar]
- 43.Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74(1):185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 44.Artis D, Humphreys NE, Potten CS, et al. Beta7 integrin-deficient mice: delayed leukocyte recruitment and attenuated protective immunity in the small intestine during enteric helminth infection. Eur J Immunol. 2000;30(6):1656–1664. doi: 10.1002/1521-4141(200006)30:6<1656::AID-IMMU1656>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 45.Kitamura Y, Yokoyama M, Sonoda T, et al. Different radiosensitivities of mast-cell precursors in the bone marrow and skin of mice. Rad Res. 1983;93(1):147–156. [PubMed] [Google Scholar]
- 46.Hallgren J, Gurish MF. Pathways of murine mast cell development and trafficking: tracking the roots and routes of the mast cell. Immunol Rev. 2007;217:8–18. doi: 10.1111/j.1600-065X.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- 47.Reutershan J, Morris MA, Burcin TL, et al. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116(3):695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinkai Y, Rathbun G, Lam KP, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 49.Alcaide P, Jones TG, Lord GM, et al. Dendritic cell expression of the transcription factor T-bet regulates mast cell progenitor homing to mucosal tissue. J Exp Med. 2007;204(2):431–439. doi: 10.1084/jem.20060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Fathman JW, Lugo-Villarino G, et al. Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells. J Clin Invest. 2006;116(2):414–421. doi: 10.1172/JCI26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenkranz AR, Coxon A, Maurer M, et al. Impaired mast cell development and innate immunity in Mac-1 (CD11b/CD18, CR3)-deficient mice. J Immunol. 1998;161(12):6463–6467. [PubMed] [Google Scholar]
- 52.Martelli F, Ghinassi B, Lorenzini R, et al. Thrombopoietin inhibits murine mast cell differentiation. Stem Cells. 2008;26(4):912–919. doi: 10.1634/stemcells.2007-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghinassi B, Zingariello M, Martelli F, et al. Increased differentiation of dermal mast cells in mice lacking the Mpl gene. Stem Cells Dev. 2009;18(7):1081–1092. doi: 10.1089/scd.2008.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ammit AJ, Bekir SS, Johnson PR, et al. Mast cell numbers are increased in the smooth muscle of human sensitized isolated bronchi. Am J Respir Crit Care Med. 1997;155(3):1123–1129. doi: 10.1164/ajrccm.155.3.9116997. [DOI] [PubMed] [Google Scholar]
- 55.Brightling CE, Bradding P, Symon FA, et al. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 56.Zanini A, Chetta A, Saetta M, et al. Chymase-positive mast cells play a role in the vascular component of airway remodeling in asthma. J Allergy Clin Immunol. 2007;120(2):329–333. doi: 10.1016/j.jaci.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda RK, Miller M, Nayar J, et al. Accumulation of peribronchial mast cells in a mouse model of ovalbumin allergen induced chronic airway inflammation: modulation by immunostimulatory DNA sequences. J Immunol. 2003;171(9):4860–4867. doi: 10.4049/jimmunol.171.9.4860. [DOI] [PubMed] [Google Scholar]
- 58.Yu M, Tsai M, Tam SY, et al. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116(6):1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dillon SB, MacDonald TT. Limit dilution analysis of mast cell precursor frequency in the gut epithelium of normal and Trichinella spiralis infected mice. Parasite Immunol. 1986;8(5):503–511. doi: 10.1111/j.1365-3024.1986.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 60.Abonia JP, Hallgren J, Jones T, et al. Alpha-4 integrins and VCAM-1, but not MAdCAM-1, are essential for recruitment of mast cell progenitors to the inflamed lung. Blood. 2006;108(5):1588–1594. doi: 10.1182/blood-2005-12-012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh B, Shinagawa K, Taube C, et al. Strain-specific differences in perivascular inflammation in lungs in two murine models of allergic airway inflammation. Clin Exp Immunol. 2005;141(2):223–229. doi: 10.1111/j.1365-2249.2005.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzalo JA, Lloyd CM, Kremer L, et al. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T-cells, chemokines and adhesion receptors. J Clin Invest. 1996;98(10):2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151(1):97–110. [PMC free article] [PubMed] [Google Scholar]
- 64.Xu B, Wagner N, Pham LN, et al. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4beta1 integrin/VCAM-1 and LFA-1 adhesion pathways. J Exp Med. 2003;197(10):1255–1267. doi: 10.1084/jem.20010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oliveira SH, Lukacs NW. Stem cell factor and igE-stimulated murine mast cells produce chemokines (CCL2, CCL17, CCL22) and express chemokine receptors. Inflamm Res. 2001;50(3):168–174. doi: 10.1007/s000110050741. [DOI] [PubMed] [Google Scholar]
- 66.Taub D, Dastych J, Inamura N, et al. Bone marrow-derived murine mast cells migrate, but do not degranulate, in response to chemokines. J Immunol. 1995;154(5):2393–2402. [PubMed] [Google Scholar]
- 67.Collington SHJ, Pease JE, Jones T, et al. The role of the CCL2/CCR2 axis in mouse mast cell migration in vitro and in vivo. J Immunol. 2010 doi: 10.4049/jimmunol.0904177. Re-submitted to. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weller CL, Collington SJ, Hartnell A, et al. Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc Natl Acad Sci USA. 2007;104(28):11712–11717. doi: 10.1073/pnas.0701700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weller CL, Collington SJ, Brown JK, et al. Leukotriene B4, an activation product of mast cells, is a chemoattractant for their progenitors. J Exp Med. 2005;201(12):1961–1971. doi: 10.1084/jem.20042407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones TG, Hallgren J, Humbles A, et al. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d-restricted NKT cells. J Immunol. 2009;183(8):5251–5260. doi: 10.4049/jimmunol.0901471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noben-Trauth N, Shultz LD, Brombacher F, et al. An interleukin 4 (IL-4)-independent pathway for CD4+ T-cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci USA. 1997;94(20):10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jankovic D, Kullberg MC, Noben-Trauth N, et al. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164(6):3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 73.Akbari O, Faul JL, Hoyte EG, et al. CD4+ invariant T-cell-receptor+ natural killer T-cells in bronchial asthma. N Engl J Med. 2006;354(11):1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 74.Lisbonne M, Diem S, de Castro Keller A, et al. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171(4):1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 75.Demoulin JB, Uyttenhove C, Van Roost E, et al. A single tyrosine of the interleukin-9 (IL-9) receptor is required for STAT activation, antiapoptotic activity and growth regulation by IL-9. Mol Cell Biol. 1996;16(9):4710–4716. doi: 10.1128/mcb.16.9.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshimoto T, Min B, Sugimoto T, et al. Nonredundant roles for CD1d-restricted natural killer T-cells and conventional CD4+ T-cells in the induction of immunoglobulin E antibodies in response to interleukin 18 treatment of mice. J Exp Med. 2003;197(8):997–1005. doi: 10.1084/jem.20021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Madden KB, Urban JF, Jr, Ziltener HJ, et al. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol. 1991;147(4):1387–1391. [PubMed] [Google Scholar]
- 78.Townsend JM, Fallon GP, Matthews JD, et al. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T-cell development. Immunity. 2000;13(4):573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 79.Mathias CB, Freyschmidt EJ, Caplan B, et al. IgE influences the number and function of mature mast cells, but not progenitor recruitment in allergic pulmonary inflammation. J Immunol. 2009;182(4):2416–2424. doi: 10.4049/jimmunol.0801569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Urban JF, Jr, Noben-Trauth N, Schopf L, et al. Cutting edge: IL-4 receptor expression by nonbone marrow-derived cells is required to expel gastrointestinal nematode parasites. J Immunol. 2001;167(11):6078–6081. doi: 10.4049/jimmunol.167.11.6078. [DOI] [PubMed] [Google Scholar]