Abstract
Background
Caffeine consumption has been associated with a reduced risk of Parkinson disease. The association is strong and consistent in men, but uncertain in women, possibly because of an interaction with hormone replacement therapy. We sought to confirm these findings using data on Parkinson disease incidence in the CPS II Nutrition Cohort, a large prospective study of men and women.
Methods
We conducted a prospective study of caffeine intake and risk of PD within the Cancer Prevention Study II Nutrition Cohort. Intakes of coffee and other sources of caffeine were assessed at baseline. Incident cases of PD (n = 317; 197 men and 120 women) were confirmed by treating physicians and medical record review. Relative risks (RR) were estimated using proportional hazards models, adjusting for age, smoking and alcohol consumption.
Results
After adjustment for age, smoking and alcohol intake, high caffeine consumption was associated with a reduced risk of PD. The relative risk comparing the 5th to the 1st quintile of caffeine intake was 0.43 (CI: 0.26, 0.71, p-trend = <0.002) in men, and 0.61 (95% CI: 0.34, 1.09; p for trend =0.05) in women. Among women, this association was stronger among never users of hormone replacement therapy (RR=0.32) than among ever users (RR=0.81, p-interaction = 0.15). Consumption of decaffeinated coffee was not associated with PD risk.
Conclusion
Findings from this large prospective study of men and women are consistent with a protective effect of caffeine intake on PD incidence, with an attenuating influence of hormone replacement therapy in women.
Keywords: Parkinson, epidemiology, coffee, caffeine, tea
INTRODUCTION
Coffee and caffeine intake have been associated with a reduced risk of Parkinson disease (PD), especially in men, in a number of prospective1–5 as well as case-control6–8 studies. In women, there is evidence of modification of the association with caffeine by use of post-menopausal estrogens.4, 5 A protective effect of caffeine and its attenuation by estrogen has also been demonstrated in animal models of PD.9 Caffeine’s neuroprotective function is attributed to it’s antagonistic action on adenosine 2A (A2A) receptors in the brain, which are being increasingly targeted as an antiparkinsonian therapy in clincal trials.10 In a large prospective cohort of female nurses,5, 11 no associations were found between coffee or caffeine intake and risk of PD overall, but women who did not use post-menopausal estrogens (HRT) had a reduced risk of PD and users of HRT had an elevated risk. In this study, we assessed the reproducibility these findings with data on PD incidence in the American Cancer Society Cancer Prevention Study II Nutrition Cohort (CPS II – Nutrition), a large prospective cohort of men and women.
METHODS
Study population
The CPS II – Nutrition Cohort is a subgroup of the American Cancer Society CPS II cohort.12 The CPS II Nutrition cohort includes 184,190 participants (86,404 men and 97, 786 women) from 21 U.S. states, who in 1992 reported their medical histories, lifestyle characteristics and dietary habits in response to a mailed questionnaire.12 Starting in 1997, participants completed questionnaires every two years to report incident outcome and updated exposure information. In 2001, as described previously,13 participants in the CPS II Nutrition cohort were asked if they had ever been diagnosed with PD; they were then asked to report a new diagnosis of PD every two years. In this study, we considered 1999 as baseline because coffee and caffeine was comprehensively assessed on the 1999 Food Frequency Questionnaire (see diet assessment, below). For this reason, the number of cases of PD in this study is less than that in previous publications from the same cohort.14, 15 The analytic cohort included 112,122 participants (48,532 men and 63,590 women) who did not have a diagnosis of PD at study baseline in 1999 and returned one or more of the 2001, 2003, 2005 or 2007 questionnaires. The Human Subjects Committee at the Harvard School of Public Health (HSPH) and the Institutional Review Board (IRB) at Emory University approved this study.
Assessment of coffee and caffeine intake
On the 1999 baseline questionnaire, participants responded to questions regarding their consumption of coffee, decaffeinated coffee, chocolate, caffeinated colas, and tea. The questionnaire asked about consumption of a standard serving size of one cup (coffee, tea) or one glass, bottle or can (cola) of beverage in the following categories: never, less than once per month, 1–2 per month, 1 per week, 2–4 per week, 5–6 per week 1 per day 2–3 per day 4 or more per day. We excluded from the study participants who did not return the 1999 baseline questionnaire (N = 32,842) and those who did not return the 2001, 2003 or 2005 survey (N = 10,052). We also excluded participants who were missing coffee intake in 1999 (N = 15,962). We additionally excluded participants who left the entire beverage section blank (N = 448) or had extreme caloric intakes (<800 or >4,200 kcal for men; <600 or >3,500 kcal for women) (N = 12,216). Additional exclusions were made for PD cases, as discussed below. Total caffeine intake was calculated using the U.S. department of Agriculture food composition tables.16 These calculations assumed that the caffeine content was as follows: 137mg and 47 mg per cup of coffee and tea respectively, 46mg per can or bottle of cola and 7mg per serving of chocolate.
Parkinson Disease Case Ascertainment
Case ascertainment for PD is described in detail in our prior studies of PD incidence in this cohort.14 In summary, we asked all CPS II – Nutrition participants who reported a diagnosis of PD on the 2001, 2003, 2005 or 2007 follow-up questionnaires for permission to contact their treating neurologists and to obtain copies of their medical records to verify the diagnosis. Once permission was obtained, we contacted the treating neurologists and asked them to fill out a diagnostic questionnaire or to send a copy of the patient’s medical record. If the neurologists did not respond, the participant‘s internist was contacted with an analogous request. The questionnaire included questions on cardinal signs of PD (rigidity, postural instability, bradykinesia and rest tremor), response to levodopa treatment as well as the presence of other symptoms or features to support a diagnosis of PD or indicate an alternate diagnosis. The neurologist was asked to confirm the case and rate the certainty of diagnosis as definite, probable or possible; the diagnosis of PD was confirmed as definite or probable in in 68% of the self-reported cases who provided consent. Before 2003, we considered as definite or probable cases of PD those participants for whom the PD diagnosis was considered definite or probable by the treating neurologist or internist, or if on the medical record there was evidence of a final diagnosis of PD made by a neurologist or, also on the medical record, evidence at a neurological exam of at least two of the four cardinal signs of PD (with one of the four being rest tremor of bradykinesia), a progressive course, lack of unresponsiveness to L-dopa or other features suggesting an alternate diagnosis. To confirm cases of PD reported in the 2003, 2005 and 2007 questionnaires, analogous procedures were followed, with the exception that medical records were requested for all cases and were reviewed by a movement disorder specialist (M.A.S.). In case of a conflict between the determination of the movement disorders specialist reviewing the records and the neurologist, the decision of the movement decision specialist was used.
Overall, 1240 participants reported a diagnosis of PD and provided consent to contact their neurologists, whereas 372 confirmed the diagnosis in their reply letter, but denied permission to contact their treating neurologists or refused to participate. The diagnosis of PD was confirmed as definite or probable PD in 839 of the cases that provided consent. After excluding cases with onset before the study baseline (n = 460) or after the cutoff (n = 3), those who reported caloric intake outside the reasonable range (N = 27), did not return the 1999 questionnaire (n = 10), lacked data on caffeine intake (N = 22) we included 317 incident PD cases (197 men and 120 women) in our analyses.
Statistical Analyses
Study follow-up for each participant lasted from the date of return of the 1999 questionnaire to the earlier of the date of return of the latest complete questionnaire (2003, 2003, 2005 or 2007 respectively), date of onset of the first symptoms of PD or date of death. Caffeine intake was analyzed as a categorical variable in quintiles. For tests of trend, the median value in each quintile was used as a continuous variable in order to allow for nonlinear associations.
The a-priori hypothesis tested in this study was that the intake of total caffeine, as well as of caffeinated coffee and tea, but not of decaffeinated beverages, is associated with a decreased risk of Parkinson disease in both men and women, with an effect modification by estrogen use in women. As the analyses were based on an a-priori hypothesis and as other studies have shown an association between caffeine and reduced risk of PD, the p-values presented in this manuscript are not corrected for multiple comparisons. The use of quintiles for caffeine and the categorization of coffee intake were decided a priori.. Cox proportional hazards regression models were used to calculate multivariate relative risks and 95% confidence intervals. All analyses were adjusted for age in months, smoking in quintiles of pack years in 1992 (as pack years in 1999 were not available in this dataset), current smoking in 1999 (yes/no), and alcohol intake in 1999 (men: non-drinker, 0–10g/day, 10–20g/day, 20–30g/day, > 30g/day; women: non-drinker, 0–5g/day, 5–10g/day, 10–15g/day and >15g/day). We conducted additional analyses with adjustment for total caloric intake, pesticide exposure, education, dairy intake, physical activity, use of ibuprofen, and baseline body mass index.
Because prior studies have reported a modification of the risk of PD in women by use of HRT, we performed analyses stratified by the use of HRT at baseline. In the CPS II -Nutrition cohort, women were asked in 1997 whether they had ever used HRT and were asked in 1999 if they had used HRT in the past two years. Thus, we considered never-users women who answered no to both questions, and ever users who answered yes to either question. Women who left both questions blank were excluded from these analyses.
We also examined the association between the consumption of caffeinated coffee, decaffeinated coffee and tea and the risk of PD.
To account for possible confounding effects of smoking on the association between caffeine and coffee intake and risk of PD, we conducted a sensitivity analyses restricted to never smokers. We also performed lag-time analyses excluding the first two years of follow-up because PD patients may change their caffeine consumption prior to diagnosis. Additionally, we performed sensitivity analyses, including 237 cases (156 men and 81 men) who reported PD but did not provide us with consent to contact their treating neurologist or internist (135 of the 372 cases who did not provide consent were prevalent cases or were missing exposure information), as well as sensitivity analyses including as cases participants who were classified as possible cases during the case confirmation process.
RESULTS
Mean age at baseline for all cohort participants was 71 years for men and 69 for women. In the course of study follow-up, we documented a total of 197 male and 120 female incident PD cases; mean age at PD diagnosis was 75 years for men and 74 for women. Compared to study participants with low coffee consumption, study participants with high coffee consumption were more likely to smoke and drink alcohol, had a higher energy intake, and were somewhat less likely to use HRT (women) (Table 1).
Table 1.
Selected Characteristics* of Participants of the CPS II Nutrition Participants, by Quintile of Caffeine Intake
| Quintile of Caffeine Intake |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | |||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Age, y | 70.9 | 70.9 | 70.9 | 70.9 | 70.8 | 69.1 | 69.1 | 69.1 | 69.1 | 69.0 |
| Total Caffeine (mean, mg/day) 1999 | 9.2 | 39.3 | 119.7 | 275.7 | 475.0 | 5.6 | 26.2 | 99.5 | 221.0 | 433.2 |
| Coffee (cups/day) 1999 | 0.01 | 0.06 | 0.58 | 1.71 | 3.16 | 0 | 0.03 | 0.39 | 1.29 | 2.92 |
| Tea (cups/day) 1999 | 0.02 | 0.18 | 0.35 | 0.43 | 0.32 | 0.02 | 0.13 | 0.59 | 0.59 | 0.34 |
| Decaf. Coffee (cups/day) | 0.83 | 1.09 | 0.60 | 0.32 | 0.27 | 0.64 | 1.13 | 0.54 | 0.34 | 0.29 |
| BMI (mean) 1999 | 26.2 | 26.6 | 26.6 | 26.5 | 26.7 | 25.6 | 26.1 | 25.9 | 25.7 | 25.7 |
| Former Smoker % 1992 | 52 | 56 | 58 | 61 | 63 | 32 | 34 | 37 | 39 | 39 |
| Current Smoker % 1992 | 4 | 4 | 6 | 8 | 12 | 4 | 5 | 6 | 8 | 13 |
| Total energy intake, kcal/d | 1717 | 1880 | 1867 | 1939 | 2080 | 1461 | 1611 | 1609 | 1645 | 1743 |
| HRT Ever Users (%) | - | - | - | - | - | 65 | 65 | 65 | 64 | 62 |
| Alcohol intake (mean, g/day) | 7.8 | 8.9 | 11.2 | 13.5 | 12.7 | 3.5 | 3.9 | 5.2 | 6.3 | 6.5 |
| High school graduation, % | 17 | 18 | 16 | 18 | 18 | 31 | 32 | 30 | 29 | 30 |
| College graduation or above, % | 29 | 28 | 29 | 29 | 27 | 13 | 13 | 14 | 14 | 13 |
all variables at baseline in 1999, unless otherwise specified
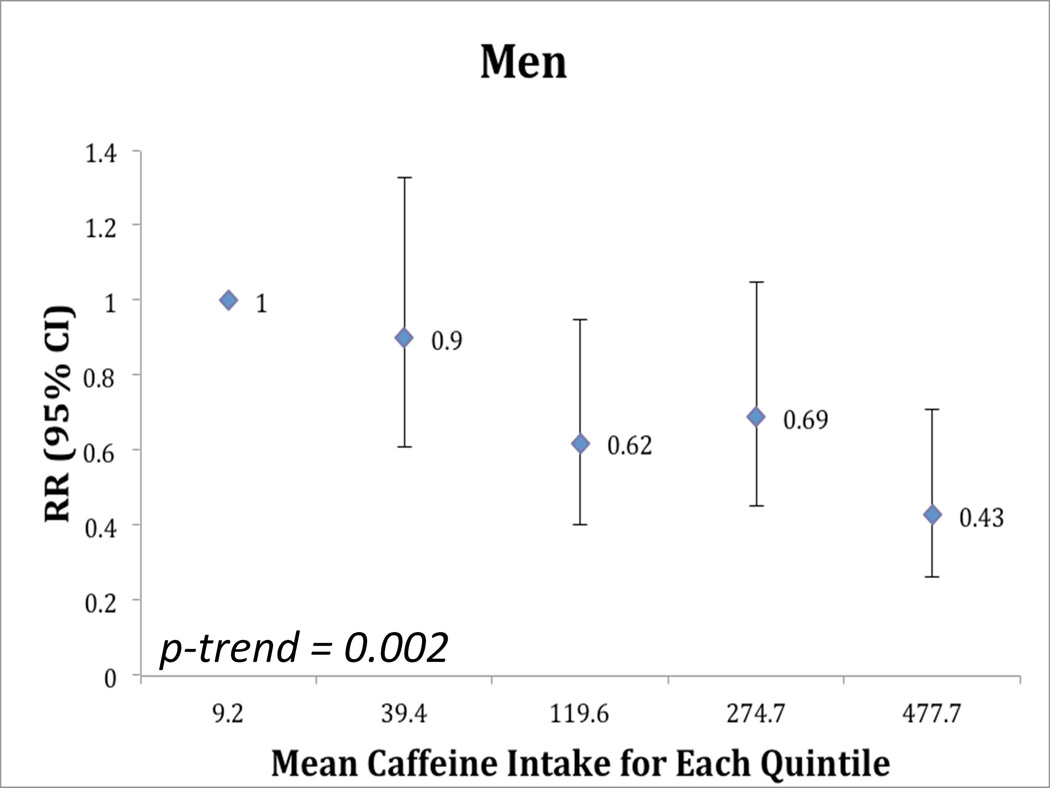
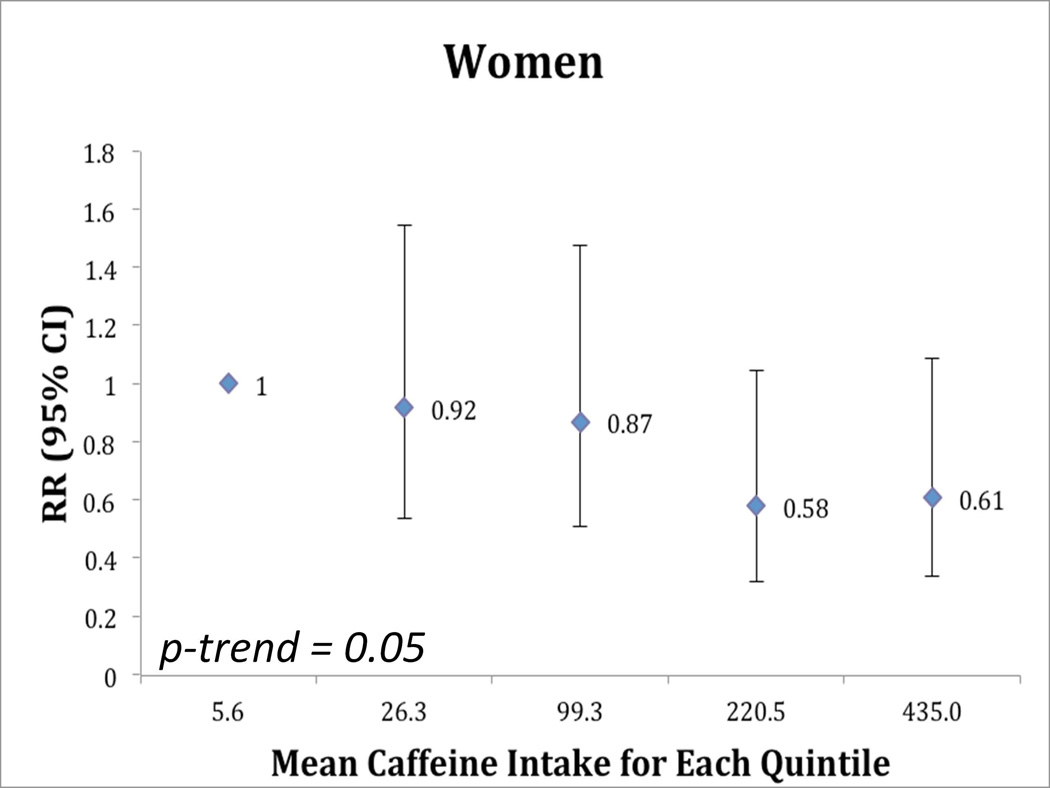
In men, caffeine intake was associated with lower risk of incident PD (RR = 0.43; 95% CI 0.26, 0.71, comparing men in the highest quintile of caffeine intake to those in the lowest quintile, p-trend = 0.002, Figure 1). Regular (caffeinated) coffee intake was also associated with a reduced risk of incident PD. The RR comparing men who reported consuming 2 or more cups of coffee per day to those who reported never drinking coffee was 0.54 (95% CI: 0.37, 0.80, p-trend =0.0004). Decaffeinated coffee and tea were not associated with decreased risk of PD in men (Table 2). The analyses of decaffeinated coffee and tea were done both with and without adjustment for consumption of caffeinated beverages, and this did not significantly alter the results. In women, we observed a marginally significant reduced risk of PD among women in the highest quintile of caffeine intake compared to women in the lowest quintile (RR = 0.61; 95% CI: 0.34, 1.09, p-trend = 0.05, Figure 1). Caffeinated coffee was associated with marginally reduced risk of PD (p-trend: 0.09), while decaffeinated coffee and tea were not associated with risk of PD in women (Table 2). Although the trend toward the inverse association between caffeine and PD was somewhat stronger among women who never used HRT than women who ever used HRT (Table 3A) the test for interaction between caffeine intake and use of HRT on risk of PD was not statistically significant.
Figure 1.
Relative risk (95% confidence intervals) of Parkinson Disease According to Quintiles of Intake of Caffeine at Baseline (A) Men and (B) Women
**adjusted for age, smoking (pack years in 1992 and yes/no in 1999), and alcohol intake (1999)
Table 2.
Relative Risks (95% confidence intervals) of Parkinson Disease According to Amount of Caffeinated Coffee, Decaffeinated Coffee and Tea at Baseline
| Caffeinated Coffee | |||||
|---|---|---|---|---|---|
| Cases | PY | RR *Crude | RR** | p- trend |
|
| Men | |||||
| Never | 67 | 83259 | 1.00 (Ref) | 1.00 (Ref) | |
| <=1/week | 43 | 62782 | 0.87 (0.59, 1.28) | 0.82 (0.55, 1.21) | |
| 2–6/week | 20 | 36440 | 0.70 (0.42, 1.16) | 0.65 (0.39, 1.07) | |
| 1/day | 21 | 45580 | 0.55 (0.33, 0.90) | 0.50 (0.30, 0.84) | |
| 2+/day | 46 | 104796 | 0.56 (0.38, 0.82) | 0.54 (0.37, 0.80) | 0.0004 |
| Women | |||||
| Never | 44 | 147403 | 1.00 (Ref) | 1.00 (Ref) | |
| <=1/week | 24 | 79007 | 1.03 (0.62, 1.69) | 1.00 (0.61, 1.65) | |
| 2–6/week | 11 | 39177 | 0.94 (0.48, 1.82) | 0.91 (0.47, 1.77 | |
| 1/day | 16 | 70275 | 0.76 (0.43, 1.34) | 0.73 (0.41, 1.31) | |
| 2+/day | 25 | 118640 | 0.70 (0.43, 1.14) | 0.69 (0.42, 1.15) | 0.09 |
|
Decaffeinated Coffee | |||||
| Men | |||||
| Never | 68 | 110633 | 1.00 (Ref) | 1.00 (Ref) | |
| <=1/week | 51 | 92652 | 0.87 (0.60, 1.25 | 0.81 (0.56, 1.17) | |
| 2–6/week | 26 | 41458 | 1.02 (0.65, 1.61 | 0.94 (0.60, 1.49) | |
| 1/day | 23 | 36044 | 1.01 (0.63, 1.63) | 0.95 (0.59, 1.54) | |
| 2+/day | 26 | 51160 | 0.83 (0.53, 1.31) | 0.81 (0.51, 1.27) | 0.68 |
| Women | |||||
| Never | 32 | 143680 | 1.00 (Ref) | 1.00 (Ref) | |
| <=1/week | 37 | 135455 | 1.26 (0.78, 2.03) | 1.21 (0.75, 1.95) | |
| 2–6/week | 18 | 57024 | 1.41 (0.79, 2.52) | 1.34 (0.75, 2.40) | |
| 1/day | 22 | 55212 | 1.78 (1.03, 3.08) | 1.71 (0.99, 2.96 | |
| 2+/day | 11 | 62138 | 0.81 (0.41, 1.61) | 0.80 (0.40, 1.59) | 0.58 |
|
Tea | |||||
| Men | |||||
| Never | 74 | 123254 | 1.00 (Ref) | 1.00 (Ref) | |
| <=1/week | 89 | 140803 | 1.08 (0.79, 1.47) | 1.03 (0.75, 1.40) | |
| 2–6/week | 19 | 35469 | 0.90 (0.54, 1.50) | 0.85 (0.51, 1.41) | |
| 1+/day | 15 | 31916 | 0.74 (0.42, 1.31) | 0.72 (0.40, 1.27) | 0.19 |
| Women | |||||
| Never | 37 | 127958 | 1.00 (Ref) | 1.00 (Ref) | |
| <=1/week | 53 | 205842 | 0.89 (0.59, 1.36) | 0.87 (0.57, 1.33) | |
| 2–6/week | 17 | 59973 | 0.91 (0.51, 1.63) | 0.87 (0.49, 1.56) | |
| 1+/day | 13 | 58036 | 0.77 (0.41, 1.44) | 0.75 (0.40, 1.41) | 0.43 |
Crude: adjusted for age
adjusted for age, smoking (pack years in 1992 and yes/no in 1999), and alcohol intake (1999)
Table 3.
Relative risk (95% confidence intervals) of Parkinson disease by quintiles of caffeine intake at baseline, stratified by (A) history of use of post-menopausal hormones (women only) and (B) smoking history.
| A) Caffeine Intake for Women at Baseline Stratified by Use of HRT (Never – no report HRT in any of 1992, 1997 or 1999, Ever – report HRT at one or more of 1992, 1997 or 1999) | ||||||
|---|---|---|---|---|---|---|
| Quintile of Caffeine | ||||||
| Intake | Cases | PY | RR* Crude | RR** | p-trend | p-int |
| HRT NEVER USERS | ||||||
| Q1 | 11 | 30990 | Ref | Ref | ||
| Q2 | 6 | 31413 | 0.55 (0.20, 1.48) | 0.57 (0.21, 1.55) | ||
| Q3 | 8 | 31345 | 0.77 (0.31, 1.93) | 0.72 (0.28, 1.82) | ||
| Q4 | 7 | 32718 | 0.60 (0.23, 1.57) | 0.60 (0.23, 1.56) | ||
| Q5 | 4 | 34701 | 0.33 (0.10, 1.03) | 0.32 (0.10, 1.03) | 0.09 | |
| HRT EVER USERS | ||||||
| Q1 | 18 | 58102 | Ref | Ref | ||
| Q2 | 21 | 58384 | 1.18 (0.62, 2.22) | 1.17 (0.62, 2.20) | ||
| Q3 | 19 | 60141 | 0.99 (0.52, 1.89) | 0.99 (0.52, 1.90) | ||
| Q4 | 11 | 59479 | 0.60 (0.28, 1,28) | 0.61 (0.28, 1.30) | ||
| Q5 | 15 | 60198 | 0.79 (0.40, 1.58) | 0.82 (0.40, 1.65) | 0.25 | 0.15 |
| B) Caffeine Intake at Baseline Stratified by Smoking history in 1999 | |||||||
|---|---|---|---|---|---|---|---|
| Caffeine Quintile |
Cases | PY | HR *Crude | HR** |
p- trend |
p-int | |
| MALE | |||||||
| Never Smoker | Q1 | 31 | 29178 | Ref | |||
| Q2 | 24 | 27897 | 0.87 (0.51, 1.49) | 0.84 (0.49, 1.45) | |||
| Q3 | 13 | 25237 | 0.53 (0.27, 1.01) | 0.49 (0.25, 0.94) | |||
| Q4 | 14 | 22534 | 0.60 (0.32, 1.14) | 0.55 (0.29 1.06) | |||
| Q5 | 4 | 19186 | 0.22 (0.08, 0.62) | 0.20 (0.07, 0.56) | 0.002 | ||
| Ever Smoker | Q1 | 18 | 32148 | Ref | |||
| Q2 | 22 | 35047 | 1.12 (0.60, 2.10) | 1.09 (0.58, 2.03) | |||
| Q3 | 19 | 37697 | 0.89 (0.46, 1.71) | 0.87 (0.45, 1.67) | |||
| Q4 | 24 | 41682 | 1.00 (0.54, 1.85) | 0.95 (0.52, 1.78) | |||
| Q5 | 18 | 47184 | 0.66 (0.34, 1.29) | 0.66 (0.34, 1.30) | 0.24 | 0.70 | |
| FEMALE | |||||||
| Never Smoker | Q1 | 17 | 55999 | Ref | |||
| Q2 | 15 | 53336 | 0.89 (0.44, 1.79) | 0.88 (0.44, 1.78) | |||
| Q3 | 18 | 52625 | 1.06 (0.54, 2.06) | 1.04 (0.53, 2.03) | |||
| Q4 | 11 | 49403 | 0.71 (0.33, 1.52) | 0.69 (0.32, 1.48) | |||
| Q5 | 16 | 45409 | 1.09 (0.55, 2.17) | 1.05 (0.52, 2.11) | 0.90 | ||
| Ever Smoker | Q1 | 10 | 29135 | Ref | |||
| Q2 | 10 | 31976 | 0.84 (0.34, 2.07) | 0.81 (0.32, 2.00) | |||
| Q3 | 8 | 34657 | 0.63 (0.24, 1.68) | 0.62 (0.23, 1.65) | |||
| Q4 | 7 | 38982 | 0.55 (0.21, 1.45) | 0.53 (0.20, 1.40) | |||
| Q5 | 3 | 45514 | 0.21 (0.06, 0.77) | 0.21 (0.06, 0.76) | 0.01 | 0.04 | |
Crude, adjusted for age
adjusted for age, smoking (pack years in 1992 and yes/no in 1999), and alcohol intake (1999)
adjusted for age, and alcohol intake, and, in ever smokers, for smoking (pack years in 1992)
Among men, the lower risk of PD associated with caffeine intake appeared to be stronger in never smokers (RR comparing top to bottom quintile of caffeine = 0.20; 95% CI: 0.07, 0.56; p-trend = 0.002) than in ever smokers (RR = 0.66; 95% CI: 0.34, 1.30; p-trend = 0.24). No association between caffeine and risk of PD was observed in never-smoking women, while in ever-smoking women increased caffeine intake was associated with lower risk of PD. (Table 3B).
Adjustment for total caloric intake, pesticide exposure, education, dairy intake, physical activity, use of ibuprofen and baseline BMI did not significantly alter the results. The results of sensitivity analyses that included participants who did not give consent to contact their treating neurologist did not differ significantly from the main analyses. In these analyses, the RR comparing participants in the highest category of caffeine intake to those in the lowest category was 0.52 (95% CI: 0.32–0.84) in women and 0.48 (95% CI: 0.34–0.69) in men). Likewise, adding to analyses participants who were classified as possible PD by the case confirmation process did not significantly alter the results. The results of lag-time analyses excluding the first 2 years of follow-up were also not significantly different from the primary results. The RR comparing participants in the highest category of caffeine intake to those in the lowest category was 0.69 (95% CI: 0.36–1.32) among women and 0.49 (95% CI: 0.29–0.84) among men.
DISCUSSION
In this study, we found that high caffeine consumption was associated with a reduced risk of PD among men and women. Coffee consumption was associated with significantly reduced risk of PD in men and a marginally reduced risk in women. Tea consumption was also marginally associated with reduced risk of PD, while decaffeinated coffee was not associated with PD risk. The overall results are consistent with other studies. Also, consistent with existing literature5, the majority of the reduction in risk of PD occurred at relatively low levels of caffeine intake. For example, men in the 3rd quintile of caffeine intake (with a mean intake of 119.6 within that quintile), experienced a statistically significant, 38% reduction in risk of PD (95% CI: 0.40, 0.95). We were not able to examine extremely high caffeine intakes because the mean caffeine intake in the top quintile in this study was 478 in men and 435 in women, corresponding to approximately 3 cups of coffee per day.
The strengths of this study include prospective data with 8 years of follow-up, the inclusion of both men and women, a large number of carefully confirmed PD cases and extensive information on potential confounders. As discussed above, one limitation is the relatively low caffeine intake in this population. Other studies, such as the Nurses Health Study5 and the Health Professionals Follow-up Study5, have reported higher caffeine intakes in the top quintile than this study and were thus able to examine the dose-response relationship between caffeine and PD to a higher threshold. The high caffeine intakes in the NHS and HPFS, relative to the CPS II-Nutrition is likely due to the health professions occupation of these cohorts.
Caffeine is thought to act on the brain by blocking adenosine receptors. In both laboratory animals and humans, the psychomotor stimulant properties of caffeine have been attributed to its antagonism at the A2A subtype of adenosine receptor expressed at high levels on striatopallidal output neurons.17 Indeed A2A antagonists acting on these receptors can improve bradykinesia and other parkinsonian motor symptoms in rodent and non-human primate models of PD as well as in patients with the disease.18 We thus cannot exclude the possibility that a symptomatic effect of caffeine could reduce the number of confirmed PD cases among those using caffeine. In addition and of particular relevance to the present epidemiological findings, A2A antagonists including caffeine can protect against the degeneration of dopaminergic neurons in multiple neurotoxin models of of PD.19 These animal studies provide further evidence for a biological and potentially neuroprotective effect of caffeine in PD.
A number of cohort studies1, 3–5, 20, 21 have raised the possibility that caffeine may be protective in PD, especially among men.1 In two cohorts of health professionals, the Nurses Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), a significantly reduced risk of PD was found in men but a U-shaped relationship was reported in women.1 The results in women were apparently explained by an interaction between caffeine and use of HRT -- among women who were not users of HRT, the RR was reduced, to an extent similar to that seen in men, while female HRT users with the highest caffeine intakes (those in the top quintile of caffeine intake with a mean caffeine intake of 688 mg/day), were at a 50% increased risk of PD.5 This finding of effect modification by HRT in women was replicated in a study that used PD mortality as an outcome within the 1982 CPS II cohort.4 We did not observe statistically significant effect modification by use of HRT in the current study, although a more marked trend toward a reduction in PD risk with increasing caffeine intake was observed in women who never used HRT. The lack of significant caffeine-HRT interaction in these data may be due to the lower caffeine intake among participants in the CPS II-Nutrition as compared to that in female nurses in the NHS1: the mean caffeine intake among women in the top quintile in this study was 436 grams per day, which is similar to the 4th quintile in the NHS; in the latter, the caffeine-HRT interaction was largely driven by an increased PD risk among women in the 5th quintile of caffeine intake. In an animal model of PD, estrogen was shown to prevent the neuroprotective effect of caffeine,9 further strengthening evidence for an interaction between caffeine and estrogen in their associations with PD risk.
In a prospective cohort of 8004 Japanese-American men in Hawaii with 30 years of follow-up, Ross et al,3 reported that the age-adjusted incidence of PD was decreased from 10.4 per 10,000 person-years in men drinking no coffee to 1.9 per 10,000 person years in men who drank more than 28 ounces of coffee per day (p-trend: 0.001). In that study, Ross et al, also reported that they did not observe effect modification by smoking: high caffeine intake was associated with reduced risk of PD among never, past and current smokers. We observed effect modification by smoking in both men and women: in men, the association between caffeine and PD was more pronounced in never smokers than in ever smokers. In contrast, among women, the association was only present in ever smokers. These findings among women are similar to those previously reported in the NHS and HPFS;1 in that study ever smoker women, but not never-smoking women were at a decreased risk of PD. Tobacco has been shown to strongly induce the CYP1A2 enzyme that metabolizes caffeine22, increasing the metabolism of caffeine in smokers. However, the effect modification may also be due to chance.
Caffeine is a compound that, unlike smoking, is considered generally safe in moderate amounts and could thus have the potential to be safely assessed in a clinical trial of PD. Simon et al23, assessed this relationship in a secondary analysis of two clinical trials of PD progression, where caffeine consumption, although not randomized, was measured in a questionnaire. Although in that study the authors did not find an association between caffeine and PD progression, a larger clinical trial focused specifically on caffeine may be warranted.
In summary, in this prospective study of U.S. men and women, we found a markedly lower risk of developing PD among individuals who regularly consume caffeine. This association was particularly strong in men, but also present in women. The consistently strong inverse association between caffeine use and PD risk in multiple longitudinal studies and the demonstration that caffeine reduces the loss of dopaminergic neurons in animal models of PD suggest that caffeine could have neuroprotective effects in humans as well. The potential benefit of caffeine on PD risk and progression deserves experimental testing in a large clinical trial of individuals with early PD.
Supplementary Material
ACKNOWLEDGMENT
Funding sources for the study: NIH K01 ES019183-01, NIH R01 NS061858
Alberto Ascherio receives research funding from the National Institutes of Health, the Department of Defense, the Michael J. Fox Foundation, and the National Multiple Sclerosis Society. He received prior funding from Autism Speaks and honoraria for two scientific presentations to Merck Serono.
AUTHOR ROLES
Natalia Palacios – Research project: conception, organization, execution. Statistical analysis: design, execution. Manuscript preparation: writing of the first draft.
Xiang Gao – Research project: execution. Statistical analysis: review and critique. Manuscript preparation: review and critique.
Marji McCullough – Research project: execution. Statistical analysis: review and critique. Manuscript preparation – review and critique.
Michael Schwarzschild – Research project: execution. Statistical analysis: review and critique. Manuscript preparation – review and critique.
Roma Shah – Research project: execution. Manuscript preparation – review and critique.
Susan Gapstur – Research project: execution. Manuscript preparation: review and critique.
Alberto Ascherio – Research project: conception, organization, execution. Statistical anaysis: design, review and critique. Manuscript preparation: review and critique.
Footnotes
Financial Disclosures/Conflicts of Interest:
Natalia Palacios is supported by the NIH K01 award ES019183-01.
Xiang Gao has received research support from the NIH/NINDS and has reported consultant relationship with Teva.
Marji McCullough has nothing to disclose.
Michael Schwarzschild’s work is funded by the NIH grant K24NS060991 and DoD grant W81XWH-11-1-0150.
Roma Shah has nothing to disclose.
Susan Gapstur has nothing to disclose.
REFERENCES
- 1.Ascherio A, Zhang SM, Hernán MA, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 2.Tan EK, Tan C, Fook-Chong SMC, et al. Dose-dependent protective effect of coffee, tea, and smoking in Parkinson's disease: a study in ethnic Chinese. J Neurol Sci. 2003;216:163–167. doi: 10.1016/j.jns.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Ross GW, Abbott RD, Petrovich H, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 2000;283(20):2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 4.Ascherio A, Weisskopf MG, O'Reilly EJ, et al. Coffee consumption, gender, and Parkinson's disease mortality in the Cancer Prevention Study-II cohort: the modifying effects of estrogen. Am J Epidemiol. 2004;160(10):977–984. doi: 10.1093/aje/kwh312. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A, Chen H, Schwarzschild MA, Zhang SM, Colditz GA, Speizer FE. Caffeine, postmenopausal estrogen, and risk of Parkinson's disease. Neurology. 2003;60(5):790–795. doi: 10.1212/01.wnl.0000046523.05125.87. [DOI] [PubMed] [Google Scholar]
- 6.Paganini-Hill A. Risk factors for parkinson's disease: the leisure world cohort study. Neuroepidemiology. 2001;20(2):118–124. doi: 10.1159/000054770. [DOI] [PubMed] [Google Scholar]
- 7.Wirdefeldt K, Gatz M, Pawitan Y, Pedersen NL. Risk and protective factors for Parkinson's disease: A study in Swedish twins. Ann Neurol. 2005;57(1):27–33. doi: 10.1002/ana.20307. [DOI] [PubMed] [Google Scholar]
- 8.Morano A, Jiménez-Jiménez FJ, Molina JA, Antolín MA. Risk-Factors for Parkinson's disease: case -control study in the province of Cáceres, Spain. Acta Neurol Scand. 1994;89:164–170. doi: 10.1111/j.1600-0404.1994.tb01655.x. [DOI] [PubMed] [Google Scholar]
- 9.Xu K, Xu Y, Brown-Jermyn D, et al. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J Neurosci. 2006;26(2):535–541. doi: 10.1523/JNEUROSCI.3008-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu K, Bastia E, Schwarzschild M. Therapeutic potential of adenosine A(2A) receptor antagonists in Parkinson's disease. Pharmacol Ther. 2005;105(3):267–310. doi: 10.1016/j.pharmthera.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Meissner WG, Frasier M, Gasser T, et al. Priorities in Parkinson's disease research. Nat Rev Drug Discov. 10(5):377–393. doi: 10.1038/nrd3430. [DOI] [PubMed] [Google Scholar]
- 12.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Jacobs E, Schwarzschild MA, et al. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol. 2005;58(6):963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- 14.Thacker EL, Chen H, Patel AV, et al. Recreational physical activity and risk of Parkinson's disease. Mov Disord. 2008;23(1):69–74. doi: 10.1002/mds.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thacker EL, O'Reilly EJ, Weisskopf MG, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68(10):764–768. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 17.Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cellular and Molecular Life Sciences. 2004;61(7):857–872. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morelli M, Carta AR, Kachroo A, Schwarzschild MA. Pathophysiological roles for purines: adenosine, caffeine and urate. Prog Brain Res. 2010;183:183–208. doi: 10.1016/S0079-6123(10)83010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kachroo A, Irizarry MC, Schwarzschild MA. Caffeine protects against combined paraquat and maneb-induced dopaminergic neuron degeneration. Exp Neurol. 2010;223(2):657–661. doi: 10.1016/j.expneurol.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu G, Bidel S, Jousilahti P, Antikainen R, Tuomilehto J. Coffee and tea consumption and the risk of Parkinson's disease. Mov Disord. 2007;22(15):2242–2248. doi: 10.1002/mds.21706. [DOI] [PubMed] [Google Scholar]
- 21.Tan LC, Koh WP, Yuan JM, et al. Differential Effects of Black versus Green Tea on Risk of Parkinson's Disease in the Singapore Chinese Health Study. Am J Epidemiol. 2008;167(5):553–560. doi: 10.1093/aje/kwm338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benowitz NL, Peng M, Jacob P. Effects of cigarette smoking and carbon monoxide on chlorzoxazone and caffeine metabolism[ast] Clin Pharmacol Ther. 2003;74(5):468–474. doi: 10.1016/j.clpt.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Simon DK, Swearingen CJ, Hauser RA, et al. Caffeine and progression of Parkinson disease. Clin Neuropharmacol. 2008;31(4):189–196. doi: 10.1097/WNF.0b013e31815a3f03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.