Abstract
BALB/cJ and C57BL/6J inbred mouse strains have been proposed as useful models of low and high levels of sociability (tendency to seek social interaction), respectively, based primarily on behaviors of ~30-day-old mice in the Social Approach Test (SAT). In the SAT, approach and sniffing behaviors of a test mouse toward an unfamiliar stimulus mouse are measured in a novel environment. However, it is unclear whether such results generalize to a familiar environment with a familiar social partner, such as with a littermate in a home cage environment. We hypothesized that C57BL/6J mice would show higher levels of social behaviors than BALB/cJ mice in the home cage environment, particularly at 30 days-of-age. We measured active and passive social behaviors in home cages by pairs of BALB/cJ or C57BL/6J littermates at ages 30, 41, and 69 days. The strains did not differ robustly in their active social behaviors. C57BL/6J mice were more passively social than BALB/cJ mice at 30 days, and C57BL/6J levels of passive social behaviors declined to BALB/cJ levels by 69 days. The differences in passive social behaviors at 30 days-of-age were primarily attributable to differences in huddling. These results indicate that different test conditions (SAT conditions vs. home cage conditions) elicit strain differences in distinct types of behaviors (approach/sniffing vs. huddling behaviors, respectively). Assessment of the more naturalistic social interactions in the familiar home cage environment with a familiar littermate will provide a useful component of a comprehensive assessment of social behaviors in mouse models relevant to autism.
Keywords: mouse, social, behavior, development, genetic, environment
1. Introduction
The high sociability of typically developing infants is evident in their preference for visually investigating faces more than objects, as well as in their development of social smiling, reciprocal and communicative vocalization, and joint attention [1, 2]. This high sociability during infancy and childhood enables children to expand their repertoire of social skills and social cognition as they share their interests, learn social rules of interaction, form relationships, and develop social reciprocity and judgment [3]. By contrast, children with autism spectrum disorders (ASD) often show reduced sociability starting in early childhood, which may hinder the subsequent development of social cognition and social skills [4–6]. Moreover, the profile of social behaviors of individuals with ASD may vary according to the familiarity of the other person and the environment [7–9]. Thus, understanding the altered developmental trajectory of social behaviors in ASD requires understanding how fundamental social interest and behaviors typically develop early in life in multiple environmental contexts and with both familiar and unfamiliar people.
Because of the experimental control they allow, mouse models are useful for investigating the biological basis of fundamental social behavior development. They may also be used to elucidate genetic and environmental influences on these behaviors, and the underlying molecular mechanisms of social behavior development [10]. The Social Approach Test provides a way to measure sociability, or the tendency of a mouse to approach and investigate another mouse [11–14]. In this behavioral assay, which has become perhaps the most widely used test of sociability for assessing mouse models relevant to ASD [15–46], a “test” mouse is placed in a novel arena and allowed to sniff and otherwise investigate an unfamiliar “stimulus” mouse that is confined to a small part of the arena. Mice of the inbred strain C57BL/6J near 30 days of age are highly sociable in this test, while BALB/cJ mice of the same age show relatively low sociability [11, 14, 21, 22, 47].
The Social Approach Test is, by design, a highly controlled test. The test and stimulus mice are separated by a transparent, air-permeable barrier and the stimulus mouse is restricted from moving about the arena, both of which limit naturalistic interaction between the two mice (though in some variants of the Social Approach Test, a period of free interaction is included at the end of the test). The rationale for this high level of control is that it allows the investigator to measure the sociability of a specific mouse, the test mouse, while holding the behavior of the stimulus mouse relatively constant. Furthermore, the test involves handling the mice to place them into a novel environment and to expose them to an unfamiliar stimulus mouse. These elements of stress and novelty may alter the social behaviors of the test mouse relative to a context in which the mouse has not been handled, is in a more familiar environment, and is interacting with a familiar cagemate. Thus, the kind of social behaviors measured in the Social Approach Test, while valid and important in their own right, might be rather different from those that occur in a more familiar environment with a familiar partner and when more naturalistic behaviors are allowed. To conduct a more complete profile of social behaviors in mouse models relevant to ASD, varying the degree of familiarity of both the environment and the stimulus mouse (i.e., the partner, or target, mouse) may be particularly important, because one of the prominent features of ASD is sensitivity to the stress of novel environments and people, or changes in familiar routines [48–50].
Based on previous studies, strain differences in social affiliative behaviors – which excludes aggressive, reproductive, and maternal behaviors – between C57BL/6J and BALB/cJ mice evidently manifest in contexts beyond the Social Approach Test. BALB/cJ mice show lower levels of allogrooming in a novel arena [51], lower social interaction in an assay similar to a resident-intruder paradigm [52], and lower social reward [53] than C57BL/6J mice. In the familiar environment of their home cages with familiar cagemates, BALB/cJ mice allogroom less and sleep and rest alone more than C57BL/6J mice [54]. In the same situation, BALB/cJ mice also do not barber each other or other strains [55], whereas C57BL/6J barber extensively [56] (barbering occurs during formation of dominance hierarchies or around the time of mating). However, the number of studies that have examined home cage social behaviors of these strains is relatively small, and nearly all of these studies, including all of the observations of home cage behaviors, used adult mice. Hence, little is known about the development of social behaviors of C57BL/6J and BALB/cJ mice in home cage environments.
To better understand the development of home cage social behaviors, we observed mice at 30, 41, and 69 days of age. We hypothesized that BALB/cJ mice would show less frequent active and passive social behaviors than C57BL/6J mice and that these strain differences would be more pronounced at 30 days of age, which would parallel results across development in the Social Approach Test [14].
2. Methods
2.1. Husbandry
Progenitor C57BL/6J and BALB/cJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and were mated at the University of Pennsylvania to produce C57BL/6J and BALB/cJ mice. Three separate cohorts of these offspring were observed in their home cages at the 3 ages (30, 41, and 69 days of age). The first day following a litter’s birth was considered postnatal day 1 (P1). Litters were culled to 4 pups usually on P2-5 (and rarely on P6 or P7) to ensure sufficient nutrition for each pup and a more uniform social environment during development. The original, perinatal litter size and litter composition by sex were recorded at this time. Litters were culled as closely as possible to 2 females and 2 males. Mice were ear tagged on P14 – P18 (and on P13, P19, and P20 for one litter each), and weaned on P23 – P26 (and on P22 and P27 for one litter each). Following weaning, same-sex littermates were housed together at 2 per cage.
All mice had access to food and water ad libitum, and were maintained in a 12-hour light-dark cycle (light began at 7:00 a.m.). All animals were treated in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
2.2. Home cage observation procedure
Separate cohorts of mice underwent home cage observations at the ages of 30, 41, or 69 days. A minority of mice were tested on the day before or after the planned day for logistical reasons. The allotment of the 150 mice by strain, age, and sex are reported in Table 1.
Table 1.
Number of mice in each experimental group for home cage observations. The number of mice in each experimental group for the Social Approach Test is reported in parentheses.
| Age | |||
|---|---|---|---|
| 30 days | 41 days | 69 days | |
| C57BL/6J females | 14 (17) | 8 (8) | 10 (13) |
| C57BL/6J males | 16 (19) | 10 (11) | 12 (9) |
| BALB/cJ females | 26 (35) | 4 (9) | 8 (12) |
| BALB/cJ males | 26 (32) | 6 (8) | 10 (11) |
Most mice were tested within 1 – 2 hours of the start of the dark cycle, and all were tested during the first half of the dark cycle. Up to two cages of mice were transported from the colony housing room and placed on opposite sides of the darkened behavioral testing room. The experimenter (A.H.F.) then left the room for 30 min. to allow the mice to acclimate to the behavioral testing room. Upon returning, the experimenter replaced the cage tops and food/water hopper with a flat, transparent plexiglass top with air holes. The experimenter wore gloves the entire time to avoid directly transferring odors to the cage parts or tops. The cameras (Sony DCR-DVD508 Handycam Camcorder, Sony Corporation, Tokyo, Japan) over the cages emitted and detected infrared light, and so were able to record the mice in complete darkness. During acclimation and testing, all visible light sources in the room were switched off. The cameras began recording and the experimenter left the room for about 60 min. The cameras were then switched off, and the mice were returned to the colony housing room.
2.3. Social Approach Test procedure
On the day following the home cage observations, mice underwent the Social Approach Test, the results of which are reported elsewhere [14]. These results are re-presented here (Figure 4) to facilitate comparisons between the home cage social behaviors and social behaviors in the Social Approach Test.
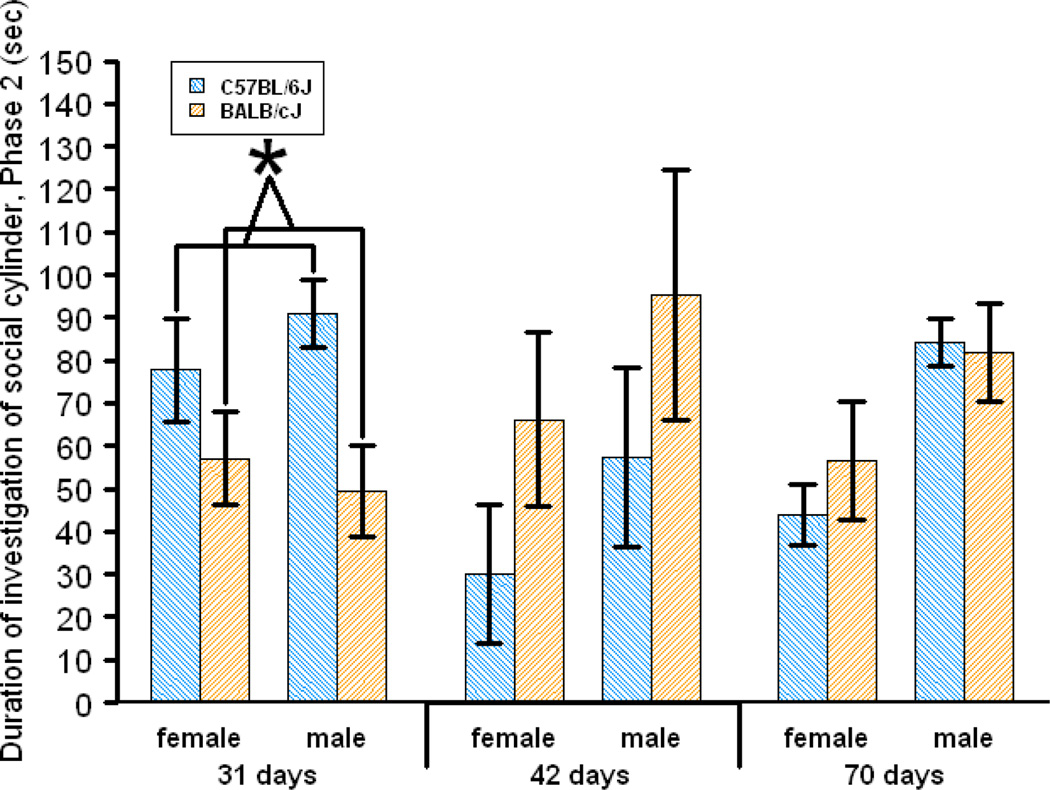
Fig. 4.
Investigation of the social cylinder during Phase 2 of the Social Approach Test. Values are 20% trimmed means ± SE of the trimmed means. * indicates a difference between strains at p < 0.01. These results were originally reported elsewhere [14].
The Social Approach Test procedure was slightly modified from that reported previously [21, 22]. Briefly, each mouse was initially allowed to explore and habituate to a 3-chambered Plexiglas box under dim lighting (<2 lux) for 10 min. (Phase 1). The two end chambers, which were separated by a middle chamber, each contained an empty transparent Plexiglas cylinder in its center. Following Phase 1, an experimenter (A.H.F.) placed a gonadectomized A/J stimulus mouse into one cylinder (the “social cylinder”) while simultaneously placing a novel object (a paper weight) into the other, identical cylinder (the “nonsocial cylinder”). For the next 5 min. (Phase 2), the test mouse could continue to move freely throughout the box and investigate the cylinders. Both cylinders had many holes (1 cm in diameter) in their walls so that the test mouse could sniff the stimulus mouse or novel object inside. For both Phases 1 and 2, the experimenter(s) recorded (either live or later from video) the amount of time the test mouse spent investigating (including sniffing) each cylinder, the amount of time the test mouse spent in each chamber, and the number of times the mouse moved from one chamber to another (i.e., “transitions”). The amount of time that the test mouse sniffed, reared against, and climbed on the social cylinder during Phase 2 was considered an index of sociability, and the sum of these behaviors is referred to below as “social cylinder investigation.” Sniffing of the social cylinder was the overwhelmingly predominant behavior included in social cylinder investigation. Climbing on the walls of the cylinders occurred very rarely. We used social cylinder investigation as the index of sociability because we established previously that sociability scores based on cylinder investigation are more reliable and ecologically valid than those based on how much time the test mouse spends in each chamber of the box [57, 14].
Following Phase 2, the cylinders were removed so that the two mice could interact freely for 5 min and be observed for aggressive interactions (Phase 3). Of the 289 mice tested for sociability in the Social Approach Test, 13 appeared to attack the stimulus mouse during Phase 3 and were eliminated from any further analysis of the Social Approach Test. For the remaining mice, experimenters scored how much time the test mouse spent engaged in active social behaviors towards the stimulus mouse (the test mouse’s head and snout oriented toward, and in close proximity to, the stimulus mouse).[14]
2.4. Home cage behavior analysis
The selection of scored home cage behaviors was a generalization and simplification of a detailed outline of mouse home cage behaviors from a previous study [58]. For each time point at which a mouse was assessed (see section 2.5 below), behavior was scored as belonging to one of several major categories. Broadly, we categorized the behaviors as either social (i.e., affiliative), aggressive, or nonsocial behaviors. Social (affiliative) behaviors were further divided into active and passive behaviors.
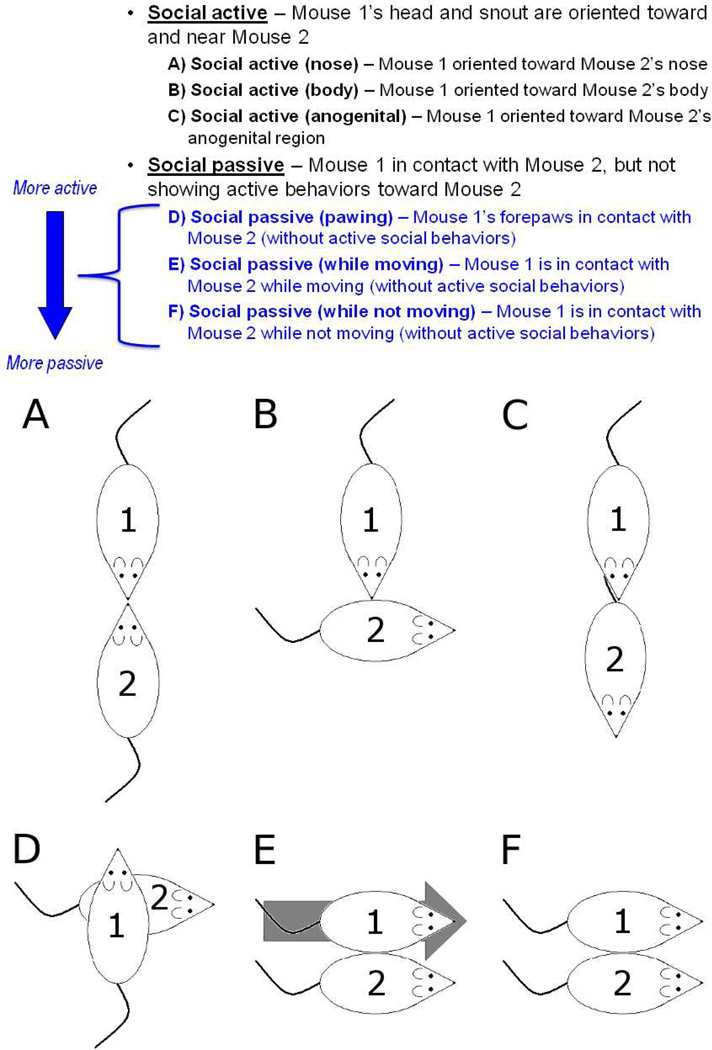
We defined a mouse’s behavior as an active social behavior when that mouse’s head and snout were oriented toward, and were in close proximity to, the other mouse. Also, to be classified as actively social, the mouse had to be moving some part of its body; that is, an actively social mouse was not resting or asleep. Active social behavior was predominantly composed of sniffing and allogrooming, but also subsumed the less frequently observed “crawl under/over” (crawling under or over other mouse and completely crossing from one side of the other mouse to the opposite side) and “push under” (pushing snout under other mouse’s body, followed by resting). Active social behaviors were further subdivided according to whether the mouse was oriented toward the other mouse’s nose, body, or anogenital region (Mouse 1 in Fig. 1a–c).
Fig. 1.
Summary of classification of social behaviors. Behaviors may be considered on a gradient from most active to most passive behaviors. “Social active” are the most active behaviors. “Social passive (while not moving)” are the most passive behaviors. “Social passive (pawing)” and “Social passive (while moving)” fall in between the other two categories. (a) Mouse 1 sniffs the nose of Mouse 2, and vice versa, which is categorized as “social active (nose).” (b) Mouse 1 sniffs the body of Mouse 2, which is categorized as social active (body) for Mouse 1. (c) Mouse 1 sniffs the anogenital area of Mouse 2 which is categorized as social active (anogenital) for Mouse 1. (d) Mouse 1 paws Mouse 2, which is categorized as social passive (pawing) for Mouse 1. (e) Mouse 1 “brushes past” or “bumps” Mouse 2, which is categorized as social passive (while moving) for Mouse 1. (f) Mouse 1 huddles with Mouse 2, which is categorized as social passive (while not moving) for both mice.
Passive social behaviors were characterized by contact between the two mice and absence of any orientation of the one mouse toward the other (i.e., contact in the absence of any active social behavior), and these behaviors were subdivided into “pawing,” “passive while moving,” and “passive while not moving.” When pawing, a mouse’s forepaws were in contact with the other mouse but the mouse was not actively sniffing, grooming, or otherwise oriented toward that mouse (Mouse 1 in Fig. 1d). A mouse could also be passively social while moving (i.e., walking, running, or otherwise changing its location in the cage). For example, a mouse that was walking and “bumps” or “brushes past” another mouse (without orienting its head and snout toward the other mouse) was categorized as passively social while moving (Mouse 1 in Fig. 1e). Finally, a mouse could be in passive contact with another mouse while not moving (i.e., in contact while not changing its location in the cage and not orienting toward the other mouse). For example, when both mice were “huddling” (or “socially inactive”), or resting or sleeping while in contact with one another, both mice were passively social while not moving (Mouse 1 and Mouse 2 in Fig. 1f). A passively social mouse could also be the object of the other mouse’s active social behaviors. For example, if one mouse were grooming the second mouse and if the second mouse were oriented away from the first mouse, then the second mouse would be considered passively social at that moment; Mouse 2 in Fig. 1b and Fig. 1c is passively social while not moving and while being the object of Mouse 1’s active social behaviors.
Passive social behaviors were subdivided to distinguish among degrees of passivity in social behaviors. A mouse that was “passive while not moving” was clearly passive. However, “passive while moving” and “pawing” were more ambiguous; these categories might also be considered active. Thus, our classification of social behaviors covered a spectrum from clearly active behaviors (e.g., sniffing, allogrooming) to pawing to “passive while moving” behaviors to clearly passive behaviors (i.e., “passive while not moving”). This spectrum is summarized in Fig. 1.
Behaviors that would be scored as aggressive included attack behaviors (biting, vigorous lunging), tail rattling, or aggressive grooming. Aggressive grooming was defined as vigorous pushing or pouncing behavior that was not sufficiently vigorous to be considered a full aggressive attack.
Several nonsocial behaviors of interest were scored. These included “inactive,” when the mouse was not moving (i.e., changing location) about the cage, and may or may not be sleeping; “rear,” when the mouse assumed a near-vertical orientation, usually with its two forepaws in contact with the cage wall; “autogroom,” when the mouse was grooming itself; “dig,” when the mouse was displacing substantial amounts of bedding with its paws or snout; and “circle,” when the mouse was moving in a tight circular pattern.
2.5. Scoring of home cage behaviors
The home cage behaviors were scored from videos of a 30-min. period beginning 20 min. after the experimenter started the camera and left the room. The behaviors of both mice in each cage were scored simultaneously once every 30 sec. for a total of 61 data points over the 30-min. period for each mouse. If a mouse was not engaged in any of the behaviors described in section 2.4 above at a given scoring time, the mouse was marked as having “no behavior” at that time. A mouse could be engaged in a maximum of one social behavior and one nonsocial behavior at a time.
A total of three raters scored the home cage behaviors. The three raters initially scored 10 videos (10 cages, 20 mice) to establish inter-rater reliability. Inter-rater correlations for each behavior are reported in Table 2. Because the reliabilities for a few behaviors were lower than desired, every subsequent video was scored by at least two raters. Notably, the behaviors with low inter-rater reliabilities (“active (anogenital)” and “passive (pawing)”) were behaviors that occurred relatively little (see section 3. Results). Thus for these behaviors, even minimal disagreements in raters’ scores greatly affected the inter-rater correlations.
Table 2.
Inter-rater reliability (ICC(A,1), Pearson’s r in parentheses) for each home cage behavior on a subset of 10 cages (20 mice). Some correlations are marked “n/a” because at least one rater marked the frequency of the behavior as zero for every mouse. One of two raters identified a single aggressive behavior by a single mouse, though it did not occur at a designated scoring time point. No other mice showed any aggressive behaviors. Aggressive behaviors are not listed; all their correlations are “NA.” Categories of behaviors are in bold. The commonly used Pearson’s r is provided for comparison to other studies.
| Behavior | Rater 1 – Rater 2 | Rater 1 – Rater 3 | Rater 2 – Rater 3 |
|---|---|---|---|
| Social (all) | 0.99 (0.99) | 0.98 (0.99) | 0.98 (0.98) |
| Social – active (all) | 0.93 (0.93) | 0.95 (0.96) | 0.94 (0.95) |
| Social – active (nose) | 0.93 (0.97) | 0.94 (0.95) | 0.95 (0.96) |
| Social – active (body) | 0.77 (0.81) | 0.83 (0.90) | 0.90 (0.91) |
| Social – active (anogenital) | 0.21 (0.33) | 0.37 (0.43) | 0.52 (0.57) |
| Social – passive (all) | 0.93 (0.93) | 0.94 (0.94) | 0.96 (0.96) |
| Social – passive (pawing) | 0.54 (0.57) | 0.82 (0.81) | 0.59 (0.62) |
| Social – passive (moving) | 0.69 (0.73) | 0.77 (0.77) | 0.91 (0.95) |
| Social – passive (not moving) | 0.89 (0.94) | 0.93 (0.93) | 0.95 (0.97) |
| Nonsocial – inactive | NA | 0.65 (0.69) | NA |
| Nonsocial – rear | 0.96 (0.96) | 0.95 (0.95) | 0.98 (0.98) |
| Nonsocial – autogroom | 0.93 (0.93) | 0.87 (0.88) | 0.97 (0.97) |
| Nonsocial – dig | 0.74 (0.83) | 0.86 (0.94) | 0.81 (0.80) |
| Nonsocial – circle | NA | NA | NA |
2.6. Data analysis
The scores from all raters were averaged together into a single score for each behavior of each mouse. Behaviors were analyzed by treating each individual mouse as a single case. The exception was active social investigation directed towards the nose of the other mouse (“active (nose)”), which could occur if and only if the other mouse’s nose was actively oriented towards the first mouse. Thus, the scores of the two mice for this measurement were completely dependent, so each pair of mice in a cage was counted as a single case (i.e., the sample size was reduced by half for this behavior).
Analyses that treated each mouse as a single case were modeled using linear mixed effects models where the strain, sex, and age of the mice were categorical fixed factors and the cage (which contained a pair of same-sex littermates for observation) and litter of the mice were random effects variables. “Cage” was thus hierarchically nested within “litter.” The behavior “active (nose)” was modeled similarly, except that each cage (pair of mice) was a separate case and so “cage” was not a variable.
Analyses were run on the statistical software R [59] with the package ‘lme4’ [60]. All p values of mixed effects models were calculated using a Markov Chain Monte Carlo method supplied by the function ‘pvals.fnc’ and run at 10000 iterations for each analysis. The α level was set at 0.05, but reduced by a Bonferroni correction for multiple testing, where appropriate.
The Social Approach Test was analyzed with conventional analyses of variance (ANOVA) and robust 20% trimmed means ANOVAs using procedures described in Wilcox [61], as outlined in detail previously [14]. Correlations among social behaviors in the Social Approach Test and the home cage were calculated by averaging the scores of each pair of mice that were housed together for the home cage observations. This approach ensured independence among the data points for the purposes of calculating the correlations. Some pairs did not have complete data available in the Social Approach Test and had to be excluded from this analysis. Correlations and corresponding p values were calculated with the ‘cor’ and ‘rcorr.adjust’ functions in R with the packages ‘Rcmdr’ and ‘Hmisc.’
3. Results
3.1. General distributions of home cage behaviors
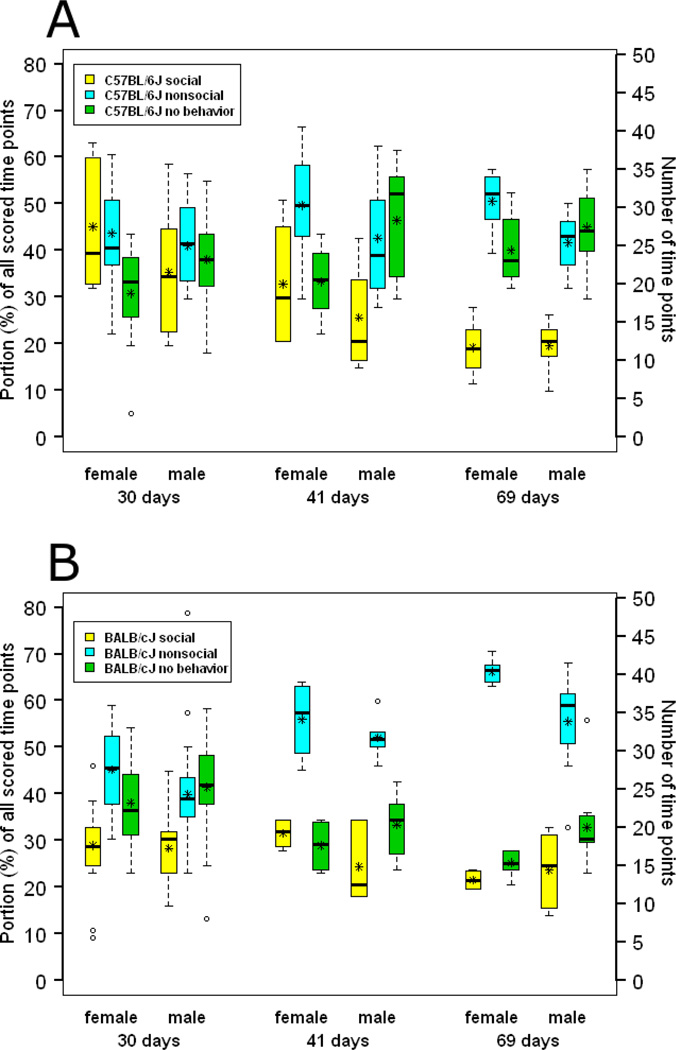
The percentages of time that mice from each experimental group spent on recorded social and nonsocial behaviors are reported in Fig. 2. By the medians of the experimental groups, mice were engaged in social behaviors for about 19% – 39% of the scored time points, and they engaged in nonsocial behaviors for about 39% – 66% of the scored time points. For 25% – 52% of the time points, mice were not engaged in any of the scored behaviors. One of two raters identified a single aggressive behavior, an aggressive groom by a 30-day-old BALB/cJ female, though it did not occur at a designated scoring time point. No other mice showed any aggressive behaviors.
Fig. 2.
Portion (%) of scored time points that mice engaged in social, nonsocial, and “no” behaviors. Boxes enclose the interquartile range (IQR), bold lines through boxes denote medians, stars denotes means, open circles denote outliers beyond 1.5 × IQR, and whiskers denote the range of data points within 1.5 × IQR. (a) C57BL/6J. (b) BALB/cJ.
3.2. Strain differences in social behaviors
To examine strain differences in home cage social behaviors across development, we analyzed active and passive social behaviors at each age. Linear mixed effects (LME) models took ‘strain’, ‘sex’, and their interaction as fixed factors and ‘cage’ and ‘litter’ as random effects variables. Alpha levels were set at 0.017 (0.05 / 3 age groups).
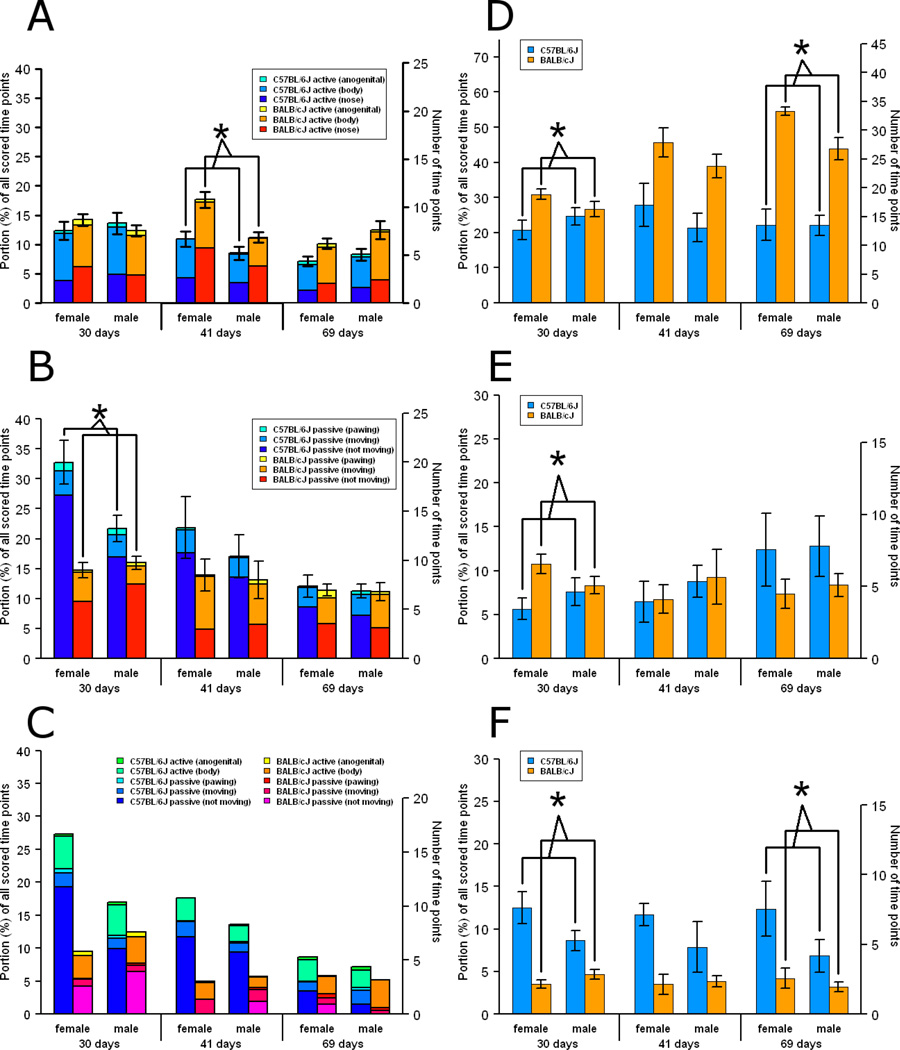
At 30 days, there were no effects of strain or age, nor any interaction between strain and age, on active social behaviors (all ps > 0.22) (Fig. 3a). At 41 days, BALB/cJ mice were more actively social than C57BL/6J mice (main effect of strain: p = 0.005), and female mice were more actively social than male mice (main effect of sex: p = 0.007). This effect was not clearly attributable to any single component (i.e., active social behavior directed toward the nose, body, or anogenital region) of the active social behaviors (all ps > 0.05, α = 0.017). At 69 days, no significant differences were identified (all ps > 0.09).
Fig. 3.
Portion (%) of scored time points that mice engaged in active or passive social behaviors or in nonsocial behaviors. Values are means ± SE, except for (c) where values are means. * indicates a difference between strains at p < 0.017. (a) Active social behaviors. Each bar representing the total active social behaviors is subdivided by whether the active behavior was directed toward the other mouse’s anogenital region, body, or nose, body. (b) Total passive social behaviors. Each bar representing the total passive social behaviors is subdivided by whether the passive behavior occurred while the mouse was not moving, occurred while the mouse was moving, or was pawing. (c) “Passive while not moving” social behaviors. While the first mouse is passively social while not moving, the second mouse may be sniffing the first mouse’s anogenital region or body, pawing, or otherwise touching the first mouse while moving or while not moving. Each bar consists of and is subdivided by the second mouse’s behavior. Huddling occurs when both mice are engaged in “passive while not moving.” (d) Rearing. (e) Digging. (f) Autogrooming.
At 30 days, C57BL/6J mice showed higher levels of passive social behaviors than BALB/cJ mice (main effect of strain: p = 0.0001, α = 0.017), and the strain differences were larger for females than for males (strain × sex interaction: p = 0.0006, α = 0.017) (Fig. 3b). Analyses of the components of passive social behaviors (“passive while not moving,” “passive while moving,” and “pawing”) showed differences in two of these three behaviors. C57BL/6J mice were “passive while not moving” more than BALB/cJ mice (main effect of strain: p = 0.0001, α = 0.017), and the strain differences were larger for females than for males (strain × sex interaction: p = 0.0001, α = 0.017). C57BL/6J mice also pawed their cage-mates more than BALB/cJ mice did (main effect of strain: p = 0.003, α = 0.017). No significant differences in passive social behaviors were identified at 41 or 69 days of age (all ps > 0.34).
3.3. Developmental trajectories of social behaviors
We asked whether the developmental trajectories of active and passive social behaviors differed between the two strains. An LME model took strain, age, sex, and their interactions as fixed factors and cage and litter as random effects variables with α levels set to 0.025 (0.05 / 2 kinds of social behavior: active and passive). Active social behaviors showed no evidence of differing developmental trajectories by strain (p = 0.58). For passive social behaviors, strain and age interacted (p = 0.0006). Examination of the data (Fig. 3b) suggested that BALB/cJ passive social behaviors were fairly constant with age, while C57BL/6J passive behaviors, and perhaps especially those of the females, decreased with age. This observation was verified in an LME model for each strain (fixed: age, sex, age × sex; random: cage, litter; α = 0.025 (0.05 / 2 kinds of social behavior)): the passive social behaviors for C57BL/6J mice showed an effect of age (p = 0.0001) and an age by sex interaction (p = 0.020), while those for BALB/cJ mice did not (main effect of age: p = 0.16; age × sex interaction: p = 0.63).
3.4. Differences in huddling account for differences in passive social behaviors
C57BL/6J mice, and especially the females, showed higher levels of passive social behaviors than did BALB/cJ mice at 30 days of age, and the bulk of this strain difference was attributable to more C57BL/6J mice being passively social while not moving, as noted above (section 3.2. Strain differences in social behaviors). When a mouse was passively social while not moving, the other mouse could have been socially engaged by actively sniffing the first mouse’s body or anogenital region, or more passively pawing, or otherwise touching the first mouse while moving or while not moving. This last situation is “huddling,” in which both mice are maintaining passive contact with each other while not moving about the cage. The portions of time that the mice spent huddling and engaged in the other four subcomponents of “passive while not moving” behaviors are reported in Fig. 3c. We investigated whether the strain differences and developmental trajectories of passive social behaviors were attributable not just to “passive while not moving” behaviors in general, but more specifically to the subcomponent “huddling” versus other subcomponents (see Table 3 for list of subcomponents).
Table 3.
Composition of differences in total passive social behaviors among mice by subcomponent. A mouse’s passive social behaviors are divided into “pawing,” “passive while moving,” and “passive while not moving.” “Passive while not moving” is further subdivided according to the behavior (indented and in italics) of the other mouse toward the first mouse. The first two columns report the portion (%) of the difference between C57BL/6J and BALB/cJ mice in passive social behaviors at 30 days by subcomponent. The second two columns report the portion (%) of the difference between 30- and 69-day-old C57BL/6J mice in passive social behaviors by subcomponent. The last column reports the correlation (Pearson’s r) of passive social behaviors with each of its subcomponents. Percentages do not always sum to 100% due to rounding.
| Subcomponents of passive social behaviors |
% of 30-day strain difference | % of decline, 30 – 69 days | Correlation with all passive social behaviors (Pearson’s r) |
||
|---|---|---|---|---|---|
| females | males | C57BL/6J females | C57BL/6J males | ||
| Pawing | 5.9 | 7.8 | 6.2 | 3.6 | 0.21 |
| Passive while moving | −5.0 | 11.3 | 3.5 | 2.0 | 0.23 |
| Passive while not moving | |||||
| Active (body) | 7.9 | 10.8 | 7.8 | 18.9 | 0.42 |
| Active (anogenital) | −1.4 | −5.6 | 0.0 | 0.0 | 0.12 |
| Pawing | 3.0 | 2.8 | 2.6 | 0.3 | 0.15 |
| Passive while moving | 5.4 | 10.5 | 3.2 | −5.3 | 0.32 |
| Passive while not moving (huddling) | 84.2 | 62.3 | 76.7 | 81.3 | 0.88 |
Huddling and pawing showed a similar pattern of strain differences and developmental trajectories as was found for all passive social behaviors, but other components of passive social behaviors did not. An LME model (fixed: strain, sex, strain × sex; random: cage, litter; same design as for the corresponding analysis for all passive social behaviors) examined 30-day-old mice with the α level set to 0.0071 (0.05 / 7 subcomponents of passive social behaviors). C57BL/6J mice huddled more than BALB/cJ mice (main effect of strain: p = 0.0001), and the strain differences were larger for females than for males (strain × sex interaction: p = 0.0001) (Fig. 3c). No other subcomponents of passive social behaviors showed the same pattern (all ps > 0.02), except for pawing (main effect of strain: p = 0.003, α = 0.0071), as noted previously (3.2. Strain differences in social behaviors).
The developmental trajectories of huddling were examined with an LME model (fixed: strain, age, sex, and their interactions; random: cage, litter; α = 0.0071). Of the subcomponents, only huddling and pawing showed a strain by age interaction (both ps = 0.0002; all other ps ≥ 0.02), as was found for all passive social behaviors. Additionally, only huddling showed a decline in C57BL/6J mice with age (p = 0.0001), while the corresponding decline for pawing did not quite reach statistical significance (p = 0.013; all other ps > 0.15). The only effect of all passive social behaviors that huddling did not recapitulate was the age by sex interaction in C57BL/6J mice (p = 0.07); no other subcomponent recapitulated this effect, either (all ps > 0.14).
While both huddling and pawing showed a pattern of effects similar to that of all passive social behaviors, huddling constituted a much larger portion of these effects. Of the total difference between C57BL/6J and BALB/cJ mice in passive social behaviors at 30 days, huddling comprised 84% (females) and 62% (males) while no other subcomponent comprised more than 12% (either sex) (Table 3). Of the total decline in passive social behaviors of C57BL/6J mice, huddling comprised 77% (females) and 81% (males) while no other subcomponent comprised more than 19% (either sex) (Table 3). Moreover, huddling and all passive social behaviors correlated very strongly (r = 0.88) whereas the correlations between other subcomponents and all passive social behaviors were not nearly as strong (all rs < 0.43) (Table 3).
In summary, the strain difference at 30 days and the developmental trajectory of C57BL/6J mice in all passive social behaviors were also found in huddling. Huddling comprised the bulk of these effects in all passive social behaviors and correlated with passive social behaviors much more strongly than any other subcomponent. Therefore, most of the differences detected in all passive social behaviors among the mice can be accounted for by differences in how much the mice huddled together, while pawing also contributed modestly to these effects.
3.5. Strain differences in nonsocial behaviors
We also examined nonsocial behaviors at each age. Mice were rarely scored as inactive or circling. We therefore did not inferentially analyze these behaviors. For all experimental groups, the median number of scored time points that the mice were inactive was zero, and only 7 mice were inactive for more than 10% of the scored time points. For ‘circle’, only one of two raters scored a single 30-day-old C57BL/6J female as circling once. No other mouse circled at any time.
The remaining nonsocial behaviors – rearing, autogrooming, and digging – were analyzed with LME models (fixed: strain, sex, strain × sex; random: cage, litter). Alpha levels were set at 0.017 (0.05 / 3 age groups). At 30 days, BALB/cJ mice reared (main effect of strain: p = 0.002; Fig. 3d) and dug (main effect of strain: p = 0.010; Fig. 3e) more than C57BL/6J mice. C57BL/6J mice autogroomed more than BALB/cJ mice (main effect of strain: p = 0.0001), and the strain differences were larger in females than in males (strain × sex interaction: p = 0.013) (Fig. 3f). At 41 days, no significant differences were found (all ps > 0.04). At 69 days, BALB/cJ mice reared more than C57BL/6J mice did (main effect of strain: p = 0.0001), and C57BL/6J mice autogroomed more than BALB/cJ mice did (main effect of strain: p = 0.012). There were no significant differences in digging at 69 days (all ps > 0.33).
3.6. Brain weight, body weight, and litter effects on social behaviors
In each strain, we tested whether litter size, litter sex ratio, brain weight, body weight, and brain weight as a proportion of body weight may have influenced home cage social behaviors, as we did for behaviors in the Social Approach Test [14]. Each ‘litter’ or ‘weight’ variable was included as a fixed factor in a separate linear mixed effects model which also took ‘age’ and ‘sex’ as fixed factors and ‘cage’ and ‘litter’ as random effects variables. The dependent variable was the sum of active and passive social behaviors. Alpha levels were set at 0.005 (0.05 / 10 tests across 5 ‘litter’ and ‘weight’ variables and 2 strains). None of the variables showed statistically significant associations with home cage social behaviors.
3.7. Behaviors in the Social Approach Test vs. behaviors in the home cage
The results of the Social Approach Test were originally reported elsewhere [14]. These results are re-presented here in order to facilitate comparisons between the home cage social behaviors and social behaviors in Phase 2 of the Social Approach Test. The numbers of mice in each group are reported in Table 1.
To investigate how the C57BL/6J and BALB/cJ strains differed in their sociability across development in the Social Approach Test, we analyzed each age group (Fig. 4). Alpha levels were corrected to 0.01 for multiple testing. As reported previously [14], at 31 days of age, BALB/cJ mice were less sociable than C57BL/6J mice, F(1, 99) = 8.31, p = 0.0048 (Q(1, 99) = 9.57, p = 0.004), which was consistent with our prior experiments at this age [11, 21, 22, 47]. No strain differences were found at 42 days, F(1, 32) = 4.20, p = 0.049 (Q(1, 32) = 3.82, p = 0.066), nor at 70 days, F(1, 41) = 1.44, p = 0.24 (Q(1, 41) = 0.32, p = 0.58).
To compare social behaviors in the Social Approach Test to those in the home cage, we calculated correlations among these behaviors (Table 4). The only correlation that was significantly different from zero was between the durations of investigation of the test mouse towards the stimulus mouse in Phase 2 versus Phase 3 of the Social Approach Test (Pearson’s r = 0.43, p = 0.0014, α = 0.0083 (0.05 / 6 correlations)), i.e., two variables within the Social Approach Test. Neither active nor passive home cage social behaviors in the home cage correlated with any other social behaviors.
Table 4.
Correlations (Pearson’s r; Spearman’s ρ in parentheses) among social behaviors in the Social Approach Test and the home cage. For the correlation of active and passive social behaviors in the home cage, all 75 pairs of mice were used. For all other correlations, which included the Social Approach Test, only 57 pairs of mice had complete data available. “Social Approach Test Phase 2” refers to social cylinder investigation, the amount of time that the test mouse sniffed, reared against, and climbed on the social cylinder containing the stimulus mouse during Phase 2 of the Social Approach Test. “Social Approach Test Phase 3” refers to how much time the test mouse spent engaged in active social behaviors towards the stimulus mouse after the social cylinder was removed and the stimulus mouse could move freely during Phase 3 of the Social Approach Test. The only Pearson’s r that was significantly different from zero was ‘Social Approach Test Phase 2’ vs. ‘Social Approach Test Phase 3’ (underlined).
| Social Approach Test Phase 2 |
Social Approach Test Phase 3 |
Home cage active social behaviors |
Home cage passive social behaviors |
|
|---|---|---|---|---|
|
Social Approach Test Phase 2 |
1 | |||
|
Social Approach Test Phase 3 |
0.43 (0.45) |
1 | ||
|
Home cage active social behaviors |
0.17 (0.16) |
−0.13 (−0.09) |
1 | |
|
Home cage passive social behaviors |
−0.16 (−0.04) |
−0.18 (−0.28) |
0.10 (0.21) |
1 |
4. Discussion
As we hypothesized, C57BL/6J mice were more passively social than BALB/cJ mice in the home cage environment, and the largest (and only statistically significant) strain differences were at 30 days of age. The levels of C57BL/6J passive social behaviors declined to BALB/cJ levels by 69 days of age, and both the decline and the strain difference at 30 days were mostly attributable to differences in huddling among the mouse groups. Contrary to our hypothesis, 41-day-old BALB/cJ mice were apparently more actively social than 41-day-old C57BL/6J mice in the home cage environment, but this apparent difference may be a false positive, as discussed below.
C57BL/6J mice were more passively social than BALB/cJ mice in home cage environments at 30 days of age, but their passive social levels declined to BALB/cJ levels by 69 days of age. Our previous studies also indicate that at ~30 days of age, C57BL/6J mice are more sociable than BALB/cJ mice in the Social Approach Test, and this strain difference diminishes or disappears by 70 days of age [14, 21, 47]. Thus, a similar strain-difference pattern manifests in what are apparently two different kinds of social behavioral repertoires, which further supports the view that BALB/cJ mice show pervasively low levels of social affiliation at around 30 days of age.
Yet this strain difference in social interaction was not entirely pervasive: it did not appear in active social behaviors in the home cage environment. Instead, the strains were actively social at comparable levels, except at 41 days and principally due to especially high levels of active sociability among BALB/cJ females. This elevated sociability may be the result of pro-affiliative factors that occur specifically around 41 days of age. But, importantly, the 41-day-old BALB/cJ mice were composed of an especially small sample size: 4 females (2 cages) and 6 males (3 cages). Furthermore, most of the elevation appears attributable specifically to a relatively high level of nose-to-nose sniffing among the 41-day-old BALB/cJ females (Fig. 3a). Thus, the elevation may be due to a non-representative sample of a single kind of behavior with only 2 independent cases. Only another, larger sample can distinguish between these two possibilities (i.e., particularly elevated active social behaviors among 41-day-old BALB/cJ females vs. a small, non-representative sample), but a more conservative interpretation is provisionally warranted: C57BL/6J and BALB/cJ mice probably do not differ in their home cage active social behaviors at any age that was tested in this study.
Regardless of whether strain differences truly exist in home cage active social behaviors, active and passive social behaviors clearly showed different patterns of strain differences. Furthermore, the strain-difference pattern and developmental trajectory of the passive – and not the active – social behaviors resembled that of sociability in the Social Approach Test. Initially, this result seems counterintuitive. Cylinder scores of the Social Approach Test almost entirely consist of sniffing, an active social behavior. Yet the strain difference results in the Social Approach Test based on an active behavior resemble strain difference results of passive – not active – behaviors in the home cage environment.
This apparent discrepancy might be resolved by recognizing the very different social situations and environmental contexts between the two behavioral assays. Test mice in the Social Approach Test are paired with an unfamiliar stimulus mouse in a relatively unfamiliar environment. The novelty of this stimulus mouse likely promotes active social sniffing, whereas the familiar littermate in the home cage does not. Instead, any heightened tendency toward social affiliation may manifest as passive social behaviors, such as huddling together, in the home cage. It is possible that genetic variants and neural circuits contribute to a generally low social interaction phenotype in BALB/cJ mice, but that this low social interaction phenotype may manifest in different behaviors depending on the social situation and environment. However, despite the possibility of some mechanisms in common, it is also likely that mechanisms mediating active social investigation in the Social Approach Test and passive social behaviors in the home cage test are not identical. For example, it is notable that we found correlations between sociability in the Social Approach Test and other variables (i.e., brain size, litter size, litter sex composition) [14] that we did not find between home cage social behaviors and these other variables. More importantly, social behaviors in the Social Approach Test and in the home cage did not directly correlate when examined by pairs of mice. Thus, the effects of any genetic variants and neural circuits that may influence active social investigation in the Social Approach Test and passive social behaviors in the home cage may be detectable only at a group level, where individual differences are not as important.
The strain-difference patterns of the nonsocial behaviors in the home cage did not resemble those of social behaviors in either the home cage or the Social Approach Test. In general, BALB/cJ mice reared more than C57BL/6J did, and C57BL/6J mice groomed themselves more than BALB/cJ mice did. For both of these behaviors, there were no significant strain differences at 41 days of age, but this may have been due to the small sample sizes at this age. BALB/cJ mice at 30 days appeared to dig more than C57BL/6J mice, and this difference appeared to be primarily due to the females. However, the difference, as well as the overall levels of digging and autogrooming, was not large. Digging and autogrooming never exceeded 13% of a mouse’s time, on average, whereas the mice reared substantially more. These nonsocial behaviors were of particular interest because they might be considered as repetitive behaviors [62], which are of potential clinical relevance because ASD patients exhibit restricted and repetitive behaviors [3]. Neither strain exceeded the other in all of the nonsocial behaviors, but BALB/cJ mice did show higher levels of rearing, which was the most abundant nonsocial behavior. Additionally, BALB/cJ mice seemed to increase their levels of rearing with age. We did not have an a priori hypothesis about the developmental trajectory of rearing, specifically, and therefore we did not statistically test for such an effect, but this apparent trend may be worth exploring in future studies.
Despite the importance of huddling to the differences between C57BL/6J and BALB/cJ mice in passive social behaviors, we observed relatively little huddling compared to a similar study of home cage behaviors in C57BL/6J mice [63]. Curley et al. (2010) observed that 35- to 45-day-old female and male C57BL/6J mice huddled for about 51% of scored time points. Our 30- and 41-day-old C57BL6/J mice are approximately comparable but huddled for only 9% – 19% of the time. Similarly, mice of Curley et al. (2010) rested alone for 4% – 8% of the time, while our pairs of mice almost never rested separately from each other. The differences in these two behaviors suggest that our mice exhibited an overall higher activity level than the mice reported by Curley et al. (2010). Much of the higher activity in our mice manifested as rearing, which occupied them about 21% – 28% of their time, while Curley et al.’s (2010) mice reared for less than 2% of the time. The higher activity in our mice might be attributable to our observing the mice only during the early part of the dark cycle, when mice tend to show their highest locomotor and social activity levels [64–66]. By contrast, Curley et al. (2010) spread their observations through the day. Additionally, we relocated our mice from the colony room to a separate room for video recording, which might have aroused the mice to explore the partly new environment (novel room, but same cage), even though an hour elapsed between relocation and the start of the observation period.
As noted above, the home cage behaviors might vary when experimental conditions are altered. For example, Panksepp et al. [66] showed that 30- to 35-day-old C57BL/6J and BALB/cJ mice varied in their home cage social behaviors across the circadian cycle. Similarly, different mouse strains might mature at different rates such that differences in social behaviors are observed only at particular ages. Further experiments will be useful in determining how robust our current findings are to other experimental conditions.
The classification scheme used in the present study encompasses nearly all of the social affiliative behaviors included in other studies of home cage behaviors [58]. The most common active social behaviors, sniffing and allogrooming, are subsumed under our “active social” category, along with the less commonly observed behaviors. The primary difference between our study and others is that our behavioral scheme explicitly includes all contact between the mice, even if the contact does not conform to a specific, defined behavior. It does this by including all passive contact while a mouse is moving, rather than being limited to only passive contact while the mice are huddling, or resting together (i.e., not moving). These “passive while moving” behaviors include receiving active social behaviors from the other mouse as well as other, less-defined passive contact, such as “bumping” one another. The disadvantage of our behavioral scheme is that it is not as detailed as those in some other studies. We chose to use more general categories for two reasons. First, it reduced the labor involved in scoring the behaviors, thus making the behavioral scoring procedure potentially widely applicable to assessment of various mouse models in future studies. Second, some behaviors, such as sniffing and allogrooming, were difficult to distinguish in our video recordings. Using more general categories let us avoid especially subjective decisions in distinguishing these behaviors. This approach probably kept our inter-rater reliability relatively high and made our results more reproducible in future studies.
In summary, C57BL/6J and BALB/cJ mice show a differential developmental pattern of passive social behaviors in the home cage similar to their differential developmental pattern of social behaviors in the Social Approach Test. These results show that the relatively low sociability of 30-day-old BALB/cJ mice is pervasive across different kinds of social and environmental contexts. Importantly, the behavioral manifestation of this low sociability can vary depending on the social context: in the Social Approach test, it appears as relatively low levels of sniffing of the unfamiliar stimulus mouse – an active social behavior – whereas in the home cage with a littermate, it appears as relatively low levels of huddling – a passive social behavior. We did not find significant correlations – when viewed by pairs of mice – between social behaviors in the Social Approach Test and the home cage. Moreover, we did not find some of the correlations between home cage social behaviors and brain size, litter size, and litter sex composition that we reported earlier for Social Approach Test behaviors [14]. Thus, although there may be some genetic and neural mechanisms that underlie low BALB/cJ sociability in both the Social Approach Test and the home cage, there are likely to be non-overlapping sets of mechanisms involved as well, in the different social contexts. Future studies are needed to identify these mechanisms. In addition to the commonly used Social Approach Test, an assessment of social interactions in the familiar home cage environment with a familiar littermate can provide a more complete profile of sociability in mouse models relevant to autism.
Research Highlights.
C57BL/6J and BALB/cJ mice did not differ in active social behaviors in home cage
C57BL/6J mice were more passively social than BALB/cJ mice at 30 days of age
Strain difference in passive social behaviors mainly due to difference in huddling
C57BL/6J passive social behaviors declined to BALB/cJ levels by adulthood
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants R01MH080718 (E.S.B.), ARRA supplement 3R01MH080718-03S1 (E.S.B.), 5-T32-MH017168 (T.A., training grant supporting A.H.F.), Pennsylvania Department of Health (SAP# 4100043366), Burroughs Wellcome Fund Career Award in the Biomedical Sciences (E.S.B.). We thank Professors Tracy L. Bale, Julie A. Blendy, and Steven A. Thomas for their advice on the project. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, Rogers SJ, Rozga A, Sangha S, Sigman M, Steinfeld MB, Young GS. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49:256–266. e1–2. [PMC free article] [PubMed] [Google Scholar]
- 2.Mundy P, Gwaltney M, Henderson H. Self-referenced processing, neurodevelopment and joint attention in autism. Autism. 2010;14:408–429. doi: 10.1177/1362361310366315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65:946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- 6.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16:231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutgers AH, Bakermans-Kranenburg MJ, van Ijzendoorn MH, van Berckelaer-Onnes IA. Autism and attachment: a meta-analytic review. J Child Psychol Psychiatry. 2004;45:1123–1134. doi: 10.1111/j.1469-7610.2004.t01-1-00305.x. [DOI] [PubMed] [Google Scholar]
- 8.Dissanayake C, Crossley SA. Autistic children's responses to separation and reunion with their mothers. J Autism Dev Disord. 1997;27:295–312. doi: 10.1023/a:1025802515241. [DOI] [PubMed] [Google Scholar]
- 9.Knott F, Lewis C, Williams T. Sibling interaction of children with autism: development over 12 months. J Autism Dev Disord. 2007;37:1987–1995. doi: 10.1007/s10803-006-0347-z. [DOI] [PubMed] [Google Scholar]
- 10.Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 11.Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 13.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 14.Fairless AH, Dow HC, Kreibich AS, Torre M, Kuruvilla M, Gordon E, Morton EA, Tan J, Berrettini WH, Li H, Abel T, Brodkin ES. Sociability and brain development in BALB/cJ and C57BL/6J mice. Behav Brain Res. 2012;228:299–310. doi: 10.1016/j.bbr.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 16.Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Defensor EB, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav Brain Res. 2011;217:302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Fairless AH, Dow HC, Toledo MM, Malkus KA, Edelmann M, Li H, Talbot K, Arnold SE, Abel T, Brodkin ES. Low sociability is associated with reduced size of the corpus callosum in the BALB/cJ inbred mouse strain. Brain Res. 2008;1230:211–217. doi: 10.1016/j.brainres.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deutsch SI, Burket JA, Jacome LF, Cannon WR, Herndon AL. D-Cycloserine improves the impaired sociability of the Balb/c mouse. Brain Res Bull. 2011;84:8–11. doi: 10.1016/j.brainresbull.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Jacome LF, Burket JA, Herndon AL, Cannon WR, Deutsch SI. D-serine improves dimensions of the sociability deficit of the genetically-inbred Balb/c mouse strain. Brain Res Bull. 2011;84:12–16. doi: 10.1016/j.brainresbull.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–188. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etherton MR, Blaiss CA, Powell CM, Sudhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, Sudhof TC, Powell CM. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009;8:114–126. doi: 10.1111/j.1601-183X.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bolliger MF, Sudhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D'Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page DT, Kuti OJ, Prestia C, Sur M. Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci U S A. 2009;106:1989–1994. doi: 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter MD, Shah CR, Muller CL, Crawley JN, Carneiro AM, Veenstra-VanderWeele J. Absence of preference for social novelty and increased grooming in integrin beta3 knockout mice: initial studies and future directions. Autism Res. 2011;4:57–67. doi: 10.1002/aur.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smit-Rigter LA, Wadman WJ, van Hooft JA. Impaired Social Behavior in 5-HT(3A) Receptor Knockout Mice. Front Behav Neurosci. 2010;4:169. doi: 10.3389/fnbeh.2010.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav Neurosci. 2008;122:293–300. doi: 10.1037/0735-7044.122.2.293. [DOI] [PubMed] [Google Scholar]
- 39.Liu ZH, Smith CB. Dissociation of social and nonsocial anxiety in a mouse model of fragile X syndrome. Neurosci Lett. 2009;454:62–66. doi: 10.1016/j.neulet.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahlhaus R, El-Husseini A. Altered neuroligin expression is involved in social deficits in a mouse model of the fragile X syndrome. Behav Brain Res. 2010;208:96–105. doi: 10.1016/j.bbr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Pietropaolo S, Guilleminot A, Martin B, D'Amato FR, Crusio WE. Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS One. 2011;6:e17073. doi: 10.1371/journal.pone.0017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, Tomonaga S, Watanabe Y, Chung YJ, Banerjee R, Iwamoto K, Kato T, Okazawa M, Yamauchi K, Tanda K, Takao K, Miyakawa T, Bradley A, Takumi T. Abnormal behavior in a chromosome-engineered mouse model for human 15q11–13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehninger D, Sano Y, de Vries PJ, Dies K, Franz D, Geschwind DH, Kaur M, Lee YS, Li W, Lowe JK, Nakagawa JA, Sahin M, Smith K, Whittemore V, Silva AJ. Gestational immune activation and Tsc2 haploinsufficiency cooperate to disrupt fetal survival and may perturb social behavior in adult mice. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamilton SM, Spencer CM, Harrison WR, Yuva-Paylor LA, Graham DF, Daza RA, Hevner RF, Overbeek PA, Paylor R. Multiple autism-like behaviors in a novel transgenic mouse model. Behav Brain Res. 2011;218:29–41. doi: 10.1016/j.bbr.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar M, Kim S, Pickup S, Chen R, Fairless AH, Ittyerah R, Abel T, Brodkin ES. Poptani Longitudinal in-vivo diffusion tensor imaging for assessing developmental changes in BALB/cJ mice, a model of reduced sociability relevant to autism. Brain Res. doi: 10.1016/j.brainres.2012.03.041. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomot M, Wicker B. A challenging, unpredictable world for people with Autism Spectrum Disorder. Int J Psychophysiol. 2011 doi: 10.1016/j.ijpsycho.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 49.Corbett BA, Schupp CW, Levine S, Mendoza S. Comparing cortisol, stress, and sensory sensitivity in children with autism. Autism Res. 2009;2:39–49. doi: 10.1002/aur.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearson BL, Defensor EB, Blanchard DC, Blanchard RJ. C57BL/6J mice fail to exhibit preference for social novelty in the three-chamber apparatus. Behav Brain Res. 2010;213:189–194. doi: 10.1016/j.bbr.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Southwick CH, Clark LH. Interstrain differences in aggressive behavior and exploratory activity of inbred mice. Comm Behav Biology. 1968;A:49–59. [Google Scholar]
- 52.Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mondragon R, Mayagoitia L, Lopez-Lujan A, Diaz JL. Social structure features in three inbred strains of mice, C57Bl/6J, Balb/cj, and NIH: a comparative study. Behav Neural Biol. 1987;47:384–391. doi: 10.1016/s0163-1047(87)90500-0. [DOI] [PubMed] [Google Scholar]
- 55.Kalueff AV, Minasyan A, Keisala T, Shah ZH, Tuohimaa P. Hair barbering in mice: implications for neurobehavioural research. Behav Processes. 2006;71:8–15. doi: 10.1016/j.beproc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Sarna JR, Dyck RH, Whishaw IQ. The Dalila effect: C57BL6 mice barber whiskers by plucking. Behav Brain Res. 2000;108:39–45. doi: 10.1016/s0166-4328(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 57.Fairless AH, Shah RY, Guthrie AJ, Li H, Brodkin ES. Deconstructing sociability, an autism-relevant phenotype, in mouse models. Anat Rec (Hoboken) 2011;294:1713–1725. doi: 10.1002/ar.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terranova ML, Laviola G, Alleva E. Ontogeny of amicable social behavior in the mouse: gender differences and ongoing isolation outcomes. Dev Psychobiol. 1993;26:467–481. doi: 10.1002/dev.420260805. [DOI] [PubMed] [Google Scholar]
- 59.R Development Core Team. R: A language and environment for statistical computing. 2010 2.12.1. [Google Scholar]
- 60.Bates D, Maechler M. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-37. 2010 [Google Scholar]
- 61.Wilcox RR. Introduction to Robust Estimation and Hypothesis Testing. Second ed. Amsterdam: Elsevier Academic Press; 2005. p. 588. [Google Scholar]
- 62.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Curley JP, Rock V, Moynihan AM, Bateson P, Keverne EB, Champagne FA. Developmental shifts in the behavioral phenotypes of inbred mice: the role of postnatal and juvenile social experiences. Behav Genet. 2010;40:220–232. doi: 10.1007/s10519-010-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitler MM, Lund R, Sokolove PG, Pittendrigh CS, Dement WC. Sleep and activity rhythms in mice: a description of circadian patterns and unexpected disruptions in sleep. Brain Res. 1977;131:129–145. doi: 10.1016/0006-8993(77)90033-6. [DOI] [PubMed] [Google Scholar]
- 65.de Visser L, van den Bos R, Kuurman WW, Kas MJ, Spruijt BM. Novel approach to the behavioural characterization of inbred mice: automated home cage observations. Genes Brain Behav. 2006;5:458–466. doi: 10.1111/j.1601-183X.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 66.Panksepp JB, Wong JC, Kennedy BC, Lahvis GP. Differential entrainment of a social rhythm in adolescent mice. Behav Brain Res. 2008;195:239–245. doi: 10.1016/j.bbr.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]